Testing the Role of Natural and Sexual Selection on Testes Size Asymmetry in Anurans
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
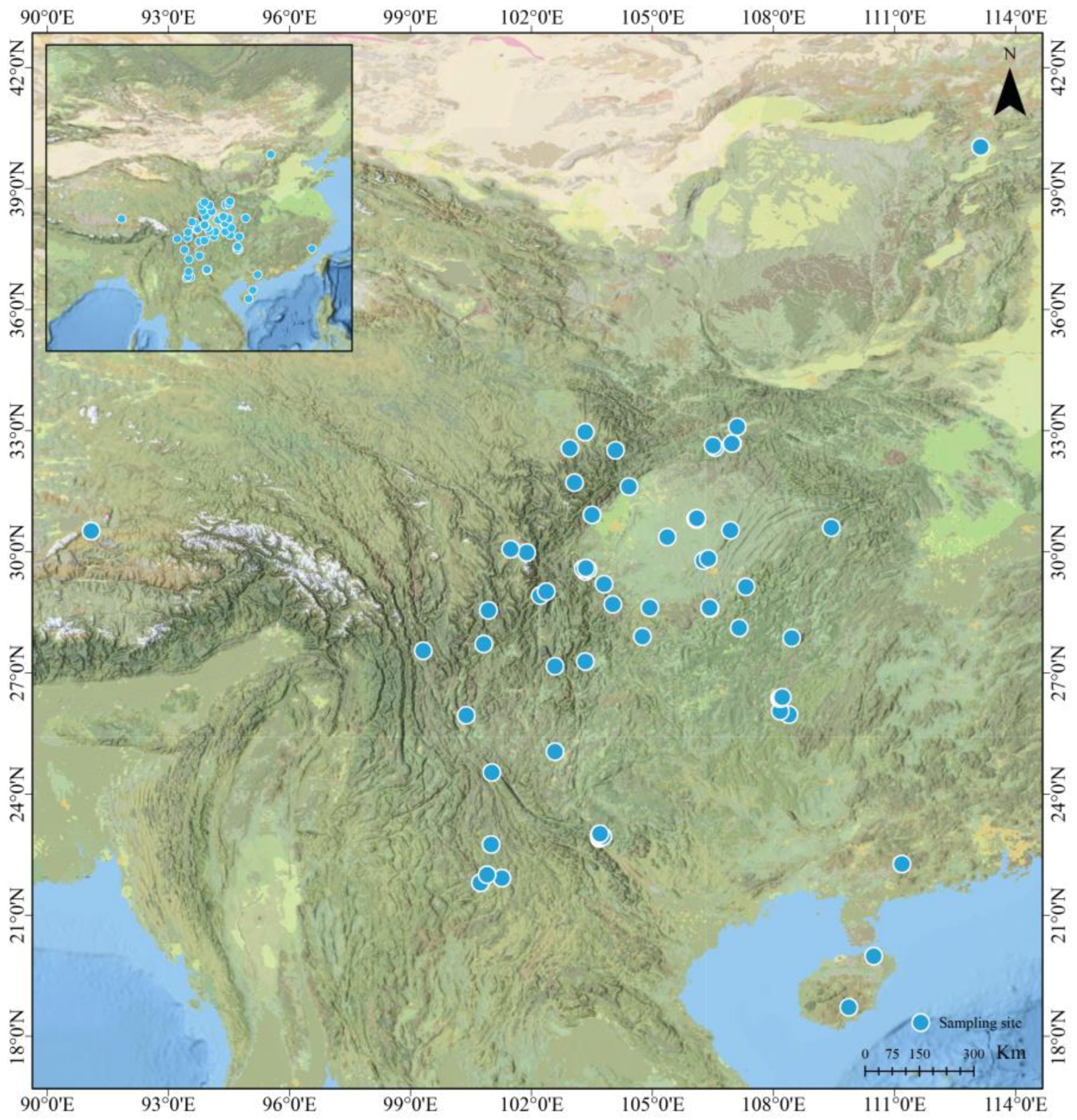
2.1. Data Collection
2.2. Associated Variables
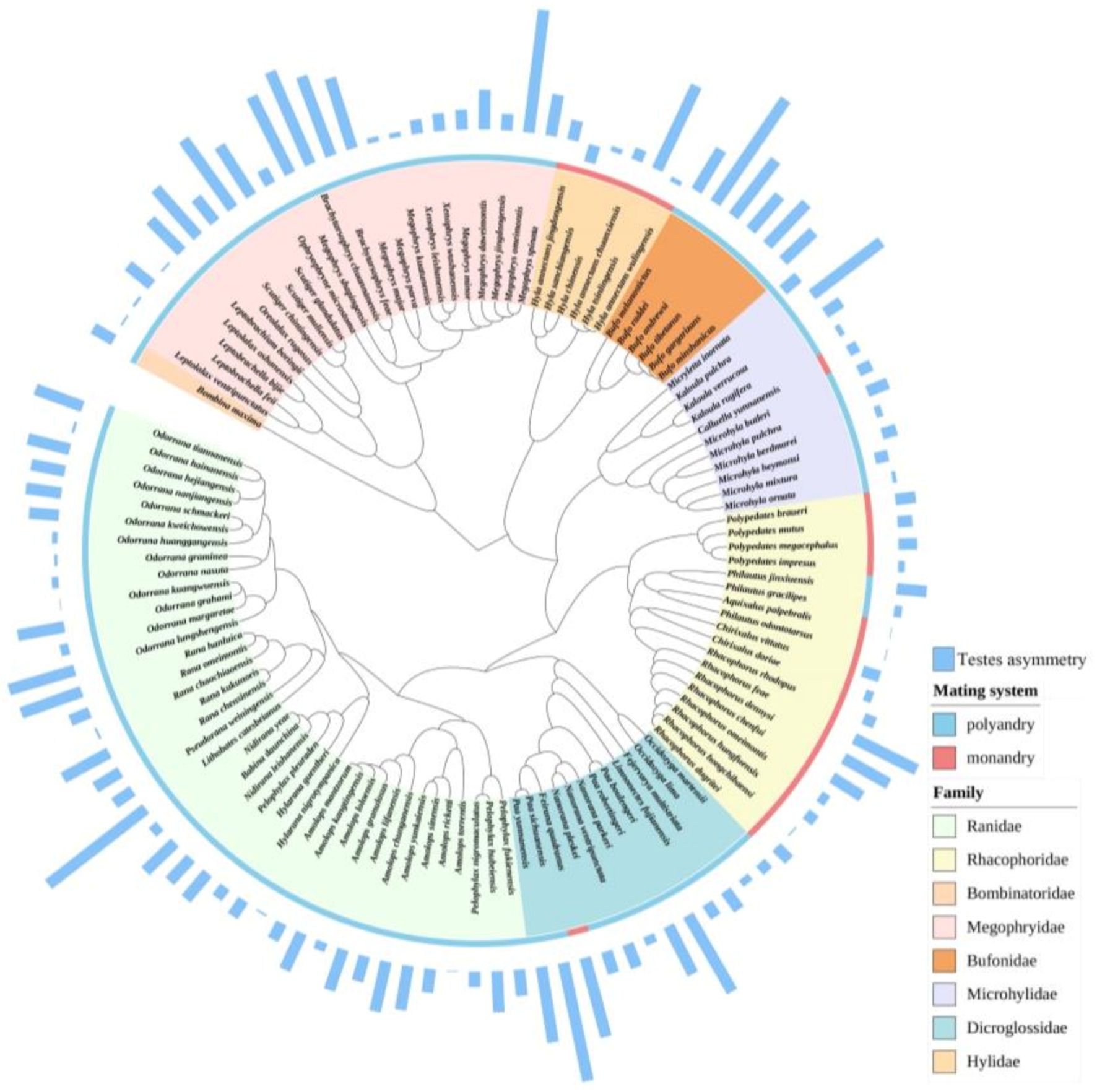
2.3. Phylogeny Reconstruction
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tannenberg, G.W. Dissertatio Inauguralis Sistens Spicilegium Observationum Circa Partes Genitales Masculas Avium; Rosenbusch: Göttingen, German, 1789. [Google Scholar]
- Møller, A.P. Directional selection on directional asymmetry: Testes size and secondary sexual characters in birds. Proc. R. Soc. B 1994, 258, 147–151. [Google Scholar]
- Yu, Z.H. Asymmetrical testicular weights in mammals, birds, reptiles and amphibia. Int. J. Androl. 1998, 21, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Hettyey, A.; Laurila, A.; Herczeg, G.; Jonsson, K.I.; Kovacs, T.; Merilä, J. Does testis weight dechne towards the Subarctic? A case study on the common frog Rana temporaria. Naturwissenschaften 2005, 92, 188–192. [Google Scholar] [PubMed]
- Birkhead, T.R.; Buchanan, K.L.; Devoogd, T.J.; Pellatt, E.J.; Szèkely, T.; Catchpole, C.K. Song, sperm quality and testes asymmetry in the sedge warbler. Anim. Behav. 1997, 53, 965–971. [Google Scholar] [CrossRef]
- Birkhead, T.R.; Fletcher, F.; Pellatt, E.J. Testis asymmetry, condition and sexual selection in birds: An experimental test. Proc. R. Soc. B 1998, 265, 1185–1189. [Google Scholar] [CrossRef]
- Calhim, S.; Birkhead, T. Intraspecific variation in testis asymmetry in birds: Evidence for naturally occurring compensation. Proc. R. Soc. B 2009, 276, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, H. Testicular asymmetry and sex ratio in birds. Biol. Bull. 1927, 52, 197–207. [Google Scholar] [CrossRef]
- Rand, A.L. Testicular asymmetry in the Madagascar coucal. Auk 1933, 50, 219–220. [Google Scholar] [CrossRef]
- Schärer, L.; Vizoso, D.B. Phenotypic plasticity in sperm production rate: There’s more to it than testis size. Evol. Ecol. 2007, 21, 295–306. [Google Scholar] [CrossRef]
- Wu, Q.G.; Liao, W.B. Evidence for directional testes asymmetry in Hyla gongshanensis jindongensis. Acta Herpetol. 2017, 12, 89–93. [Google Scholar]
- Calhim, S.; Pruett-Jones, S.; Webster, M.S.; Rowe, M. Asymmetries in reproductive anatomy: Insights from promiscuous songbirds. Biol. J. Linn. Soc. 2019, 128, 569–582. [Google Scholar] [CrossRef]
- Yue, Y.F.; Jin, L.; Mai, C.L.; Huang, X.F.; Liao, W.B. No evidence for the compensation hypothesis in the swelled vent frog (Feirana quadranus). Asian Herpetol. Res. 2020, 11, 225–229. [Google Scholar]
- Palmer, R.A.; Strobeck, C. Fluctuating asymmetry analyses revisited. In Developmental Instability; Polak, M., Ed.; Oxford University Press: Oxford, UK, 2003; pp. 279–319. [Google Scholar]
- Van Valen, L. A study of fluctuating asymmetry. Evolution 1962, 16, 125–142. [Google Scholar] [CrossRef]
- Graham, J.H.; Freeman, D.C.; Emlen, J.M. Antisymmetry, directional asymmetry, and chaotic morphogenesis. Genetica 1993, 89, 121–137. [Google Scholar] [CrossRef]
- Domm, L.V.; Juhn, M. Compensatory hypertrophy of the testes in brown leghorns. Biol. Bull. 1927, 52, 458–473. [Google Scholar] [CrossRef]
- Graves, G.R. Testicular volume and asymmetry are age-dependent in black-throated blue warblers (Dendroica caerulescens). Auk 2004, 121, 473–485. [Google Scholar] [CrossRef]
- Kimball, R.T.; Ligon, J.D.; Merola-Zwartjes, M. Testicular asymmetry and secondary sexual characters in red jungle fowl. Auk 1997, 114, 221–228. [Google Scholar] [CrossRef]
- Urbach, D.; Bittner, D.; Lenz, T.L.; Bernet, D.; Whali, T.; Wedekind, C. Sperm velocity in an Alpine whitefish: Effects of age, size, condition, fluctuating asymmetry and gonad abnormalities. J. Fish Biol. 2007, 71, 672–683. [Google Scholar] [CrossRef]
- Briskie, J.V.; Montgomerie, R. Testis size, sperm size and sperm competition. In Reproductive biology and Phylogeny of Birds. Part A: Phylogeny, Morphology, Hormones, Fertilization; Jamieson, B.G.M., Ed.; Science Publishers: New York, NY, USA, 2007; pp. 513–551. [Google Scholar]
- Witschi, E. Origin of asymmetry in the reproductive system of birds. Am. J. Anat. 1935, 56, 119–141. [Google Scholar] [CrossRef]
- Lake, P.E. Male Genital Organs. In Form and Function in Birds; Academic Press: London, UK, 1981; pp. 1–61. [Google Scholar]
- Calhim, S.; Montgomerie, R. Testis asymmetry in birds: The influences of sexual and natural selection. J. Avian Biol. 2015, 45, 175–185. [Google Scholar] [CrossRef]
- Kinsky, F.C. The consistent presence of paired ovaries in the kiwi (Apteryx) with some discussion of this condition in other birds. J. Ornithol. 1971, 112, 334–357. [Google Scholar] [CrossRef]
- Pitcher, T.; Dunn, P.O.; Whittingham, L.A. Sperm competition and the evolution of testes size in birds. J. Evol. Biol. 2005, 18, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Lou, S.L.; Liao, W.B.; Jehle, R. Evolution of sperm morphology in anurans: Insights into the roles of mating system and spawning locations. BMC Evol. Biol. 2014, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Lüpold, S.; Linz, G.M.; Rivers, J.W.; Westneat, D.F.; Birkhead, T.R. Sperm competition selects beyond relative testes size in birds. Evolution 2009, 63, 391–402. [Google Scholar] [CrossRef]
- Lüpold, S.; Jin, L.; Liao, W.B. Population density and structure drive differential investment in pre- and postmating sexual traits in frogs. Evolution 2017, 71, 1686–1699. [Google Scholar] [CrossRef]
- Mcwhinnie, R.B.; Sckrabulis, J.P.; Raffel, T.R. Temperature and mass scaling affect cutaneous and pulmonary respiratory performance in a diving frog. Integr. Zool. 2021, 16, 712–728. [Google Scholar] [CrossRef]
- Jiang, Y.; Luan, X.F.; Liao, W.B. Anuran brain size predicts food availability-driven population density. Sci. China Life Sci. 2022. [Google Scholar] [CrossRef]
- Murray, R.L.; Herridge, E.J.; Ness, R.W.; Wiberg, R.A.W.; Bussière, L.F. Competition for access to mates predicts female-specific ornamentation and male investment in relative testis size. Evolution 2020, 74, 1741–1754. [Google Scholar] [CrossRef]
- Liang, T.; Meiri, S.; Shi, L. Sexual size dimorphism in lizards: Rensch’s rule, reproductive mode, clutch size, and line fitting method effects. Integr. Zool. 2022, 17, 787–803. [Google Scholar] [CrossRef]
- Aich, U.; Bonnet, T.; Head, M.L.; Jennions, M.D. Disentangling the effects of male age and mating history: Contrasting effects of mating history on precopulatory mating behavior and paternity success. Evolution 2021, 75, 2867–2880. [Google Scholar] [CrossRef]
- Mai, C.L.; Liao, W.B.; Kotrschal, A.; Lüpold, S. Relative brain size is predicted by the intensity of intrasexual competition in frogs. Am. Nat. 2020, 196, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Kusano, T.; Toda, M.; Fukuyama, K. Testes size and breeding systems in Japanese anurans with special reference to large testes in the treefrog, Rhacophorus arboreus (Amphibia: Rhacophoridae). Behav. Ecol. Sociobiol. 1991, 29, 27–31. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Inter. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Environmental Systems Research Institute (ESRI). ArcGIS Desktop 10.8. Environmental Systems; Environmental Systems Research Institute: Redlands, CA, USA, 2020. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, C.; Liao, W.B. Anuran interorbital space variation: Role of ecological and behavioral factors. Integr. Zool. 2022, 17, 777–786. [Google Scholar] [CrossRef]
- Schliep, K.P. phangorn: Phylogenetic analysis in R. Bioinformatics 2011, 27, 592–593. [Google Scholar] [CrossRef]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef]
- R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 15 November 2022).
- Orme, D.; Freckleton, R.; Thomas, G.; Petzoldt, T.; Fritz, S.; Isaac, N.; Pearse, W. Caper: Comparative Analyses of Phylogenetics and Evolution in R. R Package Version 1.0.1. 2018. Available online: https://cran.r-project.org/package=caper (accessed on 15 November 2022).
- Freckleton, R.P.; Harvey, I.F.; Pagel, M. Phylogenetic analysis and comparative data: A test and review of evidence. Am. Nat. 2002, 160, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.W.; Zhang, L.X.; Lu, X. Global gaps in age data based on skeletochronology for amphibians. Integr. Zool. 2022, 17, 752–763. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, Y.; Jin, L.; Liao, W.B. No evidence for effects of ecological and behavioral factors on eye size evolution in anurans. Front. Ecol. Evol. 2021, 9, 755818. [Google Scholar] [CrossRef]
- Liao, W.B.; Jiang, Y.; Li, D.Y.; Jin, L.; Zhong, M.J.; Qi, Y.; Lüpold, S.; Kotrschal, A. Cognition contra camouflage: How the brain mediates predator–driven crypsis evolution. Sci. Adv. 2022, 8, eabq1878. [Google Scholar] [CrossRef]
- Nakagawa, S.; Cuthill, I. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol. Rev. 2007, 82, 591–605. [Google Scholar] [CrossRef]
- Newton, A. A Dictionary of Birds; Adam and Charles Black: London, UK, 1896. [Google Scholar]
- Dudczak, A.C.; De La Torre, G.M.; Euclydes, L.; Campião, K.M. The roles of anurans in antagonistic networks are explained by life–habit and body–size. Integr. Zool. 2022, 17, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Li, S.R.; Hao, X.; Sun, B.J.; Bi, J.H.; Zhang, Y.P.; Du, W.G. Phenotypic consequences of maternally selected nests: A cross-fostering experiment in a desert lizard. Integr. Zool. 2021, 16, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Donihue, C.M.; Daltry, J.C.; Challenger, S.; Herrel, A. Population increase and changes in behavior and morphology in the Critically Endangered Redonda ground lizard (Pholidoscelis atratus) following the successful removal of alien rats and goats. Integr. Zool. 2021, 16, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q. Under the Pressure of Different Sperm Competition, Adaptive Relationship between Sperm Size and Testicular Tissues in 26 Species of Anura. Master’s Thesis, China West Normal University, Nanchong, China, 2021. [Google Scholar]
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry: Measurement, analysis, patterns. Ann. Rev. Ecol. Syst. 1986, 17, 391–421. [Google Scholar] [CrossRef]
- Byrne, P.G.; Simmons, L.W.; Roberts, J.D. Sperm competition and the evolution of gamete morphology in frogs. Proc. R. Soc. B 2003, 270, 2079–2086. [Google Scholar] [CrossRef]
- Gage, M.J.G.; Freckleton, R.P. Relative testis size and sperm morphometry across mammals: No evidence for an association between sperm competition and sperm length. Proc. R. Soc. B 2003, 270, 625–632. [Google Scholar] [CrossRef]
- Frey, R.; Goymann, W. A single functional testis and long deferent duct papillae: The peculiar male reproductive tract of the classically polyandrous, sex-role reversed black coucal (Centropus grillii). J. Ornithol. 2009, 150, 827–838. [Google Scholar] [CrossRef]
- Zedda, M.; Sathe, V.; Chakraborty, P.; Palombo, M.R.; Farina, V. A first comparison of bone histomorphometry in extant domestic horses (Equus caballus) and a Pleistocene Indian wild horse (Equus namadicus). Integr. Zool. 2021, 15, 448–460. [Google Scholar] [CrossRef]
| Association | Number of Species | λ (95% CL) | Adjusted R2 | Effect Size (95% CL) | Estimate (+SE) | t (P) |
|---|---|---|---|---|---|---|
| 1. Size asymmetry | 116 | 0.127 (0.006, 0403) | 0.452 | |||
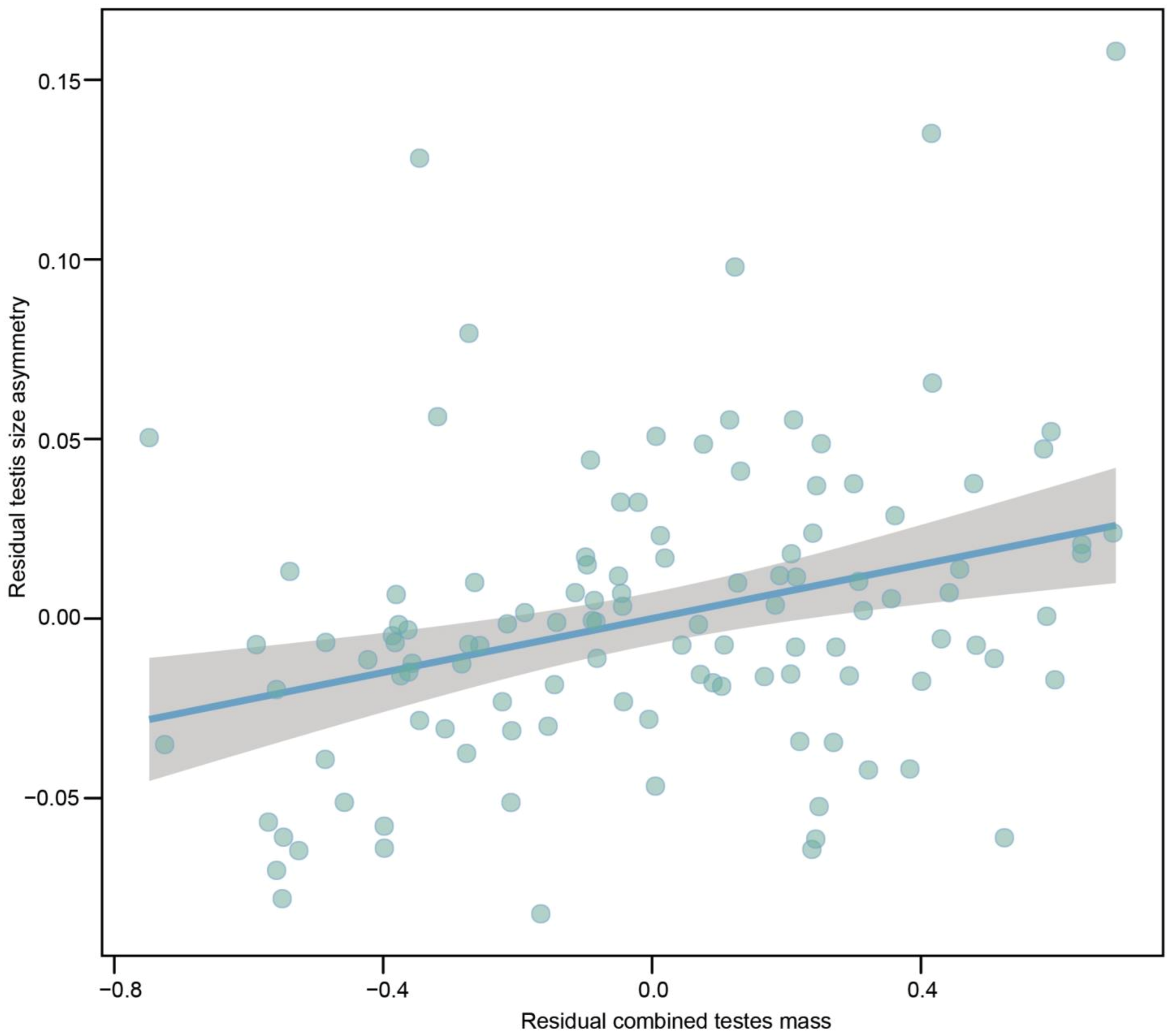
| Combined testes mass | r = 0.322 (0.148, 0.468) | 0.038 (+0.011) | 3.614 (<0.001) | |||
| SVL | r = 0.177 (−0.007, 0.343) | 0.079 (+0.041) | 1.910 (0.059) | |||
| 2. Size asymmetry | 116 | 0.114 (<0.001, 0.456) | 0.525 | |||
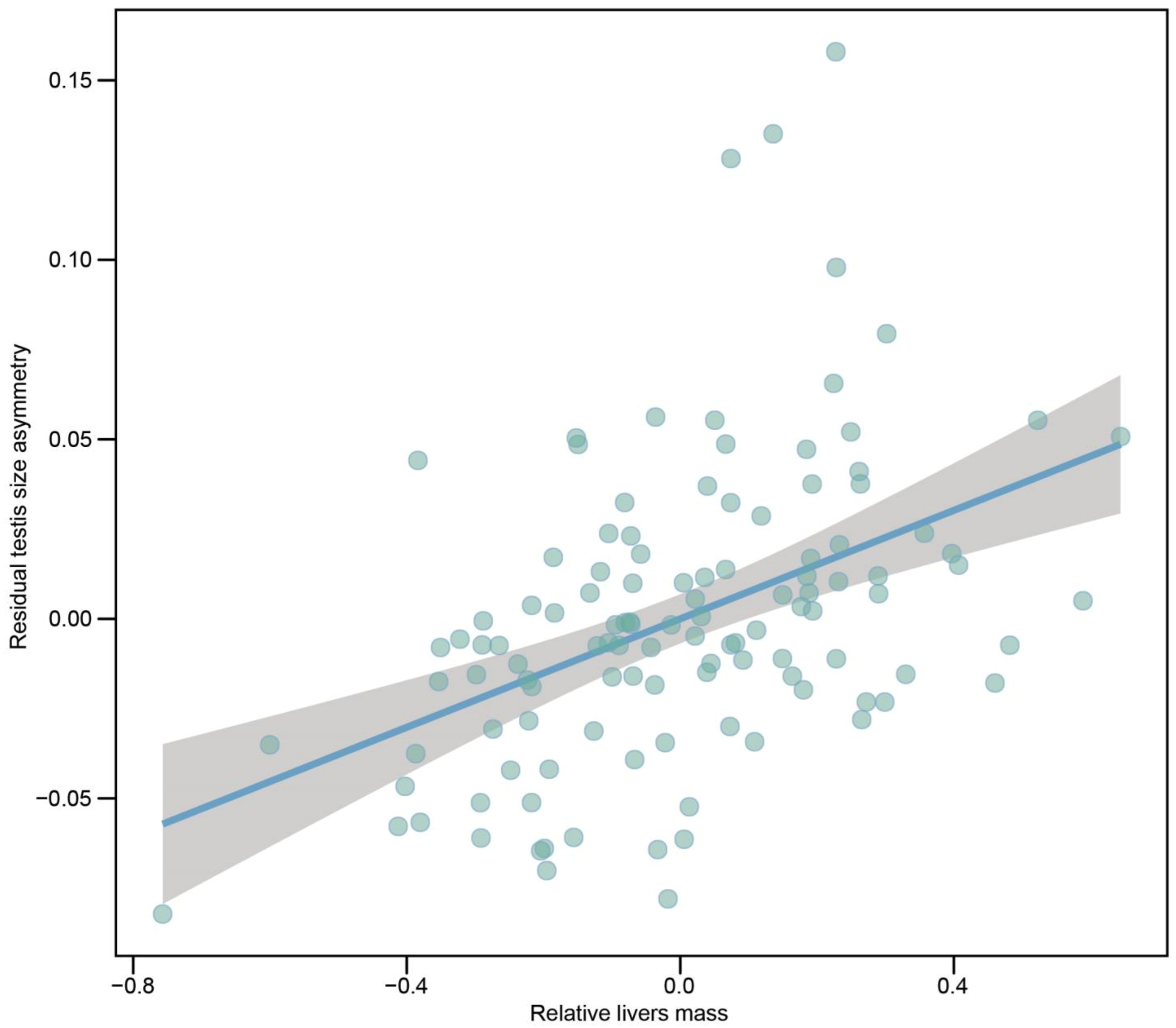
| Livers mass | r = 0.372 (0.203, 0.510) | 0.062 (+0.015) | 4.244 (<0.001) | |||
| Combined testes mass | r = 0.214 (0.031, 0.376) | 0.024 (+0.010) | 2.321 (0.022) | |||
| SVL | r = −0.120 (−0.293, 0.066) | −0.065 (+0.051) | −1.274 (0.205) | |||
| 3. Size asymmetry | 116 | 0.119 (<0.001, 0.421) | 0.401 | |||
| ASR | r = −0.063 (−0.245, 0.126) | −0.009 (+0.014) | −0.653 (0.515) | |||
| SVL | r = 0.622 (0.499, 0.711) | 0.208 (+0.025) | 8.211 (<0.001) | |||
| 4. Size asymmetry | 116 | 0.103 (<0.001, 0.348) | 0.401 | |||
| Mating system | r = −0.124 (−0.296, 0.061) | −0.015 (+0.011) | −1.324 (0.188) | |||
| SVL | r = 0.628 (0.511, 0.713) | 0.201 (+0.023) | 8.581 (<0.001) | |||
| 5. Size asymmetry | 116 | 0.084 (<0.001, 0.363) | 0.505 | |||
| Body fat | r = 0.317 (0.141, 0.464) | 0.038 (+0.109) | 3.531 (<0.001) | |||
| Combined testes mass | r = 0.218 (0.035, 0.379) | 0.025 (+0.011) | 2.361 (0.020) | |||
| SVL | r = −0.070 (−0.247, 0.115) | −0.038 (+0.051) | −0.739 (0.462) | |||
| 6. Size asymmetry | 116 | 0.119 (0.002, 0.409) | 0.466 | |||
| Lung mass | r =0.179 (−0.005, 0.346) | 0.020 (+0.011) | 1.925 (0.057) | |||
| Combined testes mass | r = 0.271 (0.092, 0.425) | 0.033 (+0.011) | 2.977 (0.004) | |||
| SVL | r = 0.063 (−0.122, 0.241) | 0.032 (+0.048) | 0.663 (0.509) | |||
| 7. Size asymmetry | 116 | 0.130 (0.006, 0.407) | 0.448 | |||
| Latitude | r = −0.022 (−0.203, 0.162) | −0.0002 (+0.001) | −0.229 (0.819) | |||
| Combined testes mass | r = 0.316 (0.141, 0.464) | 0.039 (+0.011) | 3.530 (0.001) | |||
| SVL | r = 0.176 (−0.009, 0.343) | 0.079 (+0.042) | 1.889 (0.061) | |||
| 8. Size asymmetry | 116 | 0.125 (0.004, 0.400) | 0.444 | |||
| Precipitation seasonality | r = 0.053 (−0.132, 0.233) | 0.0002 (+0.0003) | 0.558 (0.578) | |||
| Temperature seasonality | r = 0.026 (−0.158, 0.207) | 0.0007 (+0.0002) | 0.273 (0.785) | |||
| Combined testes mass | r = 0.301 (0.123, 0.451) | 0.037 (+0.011) | 3.324 (0.001) | |||
| SVL | r = 0.178 (−0.007, 0.346) | 0.080 (+0.042) | 1.908 (0.059) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Jiang, Y.; Jin, L.; Liao, W. Testing the Role of Natural and Sexual Selection on Testes Size Asymmetry in Anurans. Biology 2023, 12, 151. https://doi.org/10.3390/biology12020151
Chen S, Jiang Y, Jin L, Liao W. Testing the Role of Natural and Sexual Selection on Testes Size Asymmetry in Anurans. Biology. 2023; 12(2):151. https://doi.org/10.3390/biology12020151
Chicago/Turabian StyleChen, Shengnan, Ying Jiang, Long Jin, and Wenbo Liao. 2023. "Testing the Role of Natural and Sexual Selection on Testes Size Asymmetry in Anurans" Biology 12, no. 2: 151. https://doi.org/10.3390/biology12020151
APA StyleChen, S., Jiang, Y., Jin, L., & Liao, W. (2023). Testing the Role of Natural and Sexual Selection on Testes Size Asymmetry in Anurans. Biology, 12(2), 151. https://doi.org/10.3390/biology12020151





