Octopus vulgaris Exhibits Interindividual Differences in Behavioural and Problem-Solving Performance
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Extractive Foraging Implies Sophisticated Problem-Solving Capacity
1.2. Problem Solving Is Essential to Innovate a Predatory Strategy
1.3. Aims of the Study
2. Methods
2.1. Subjects
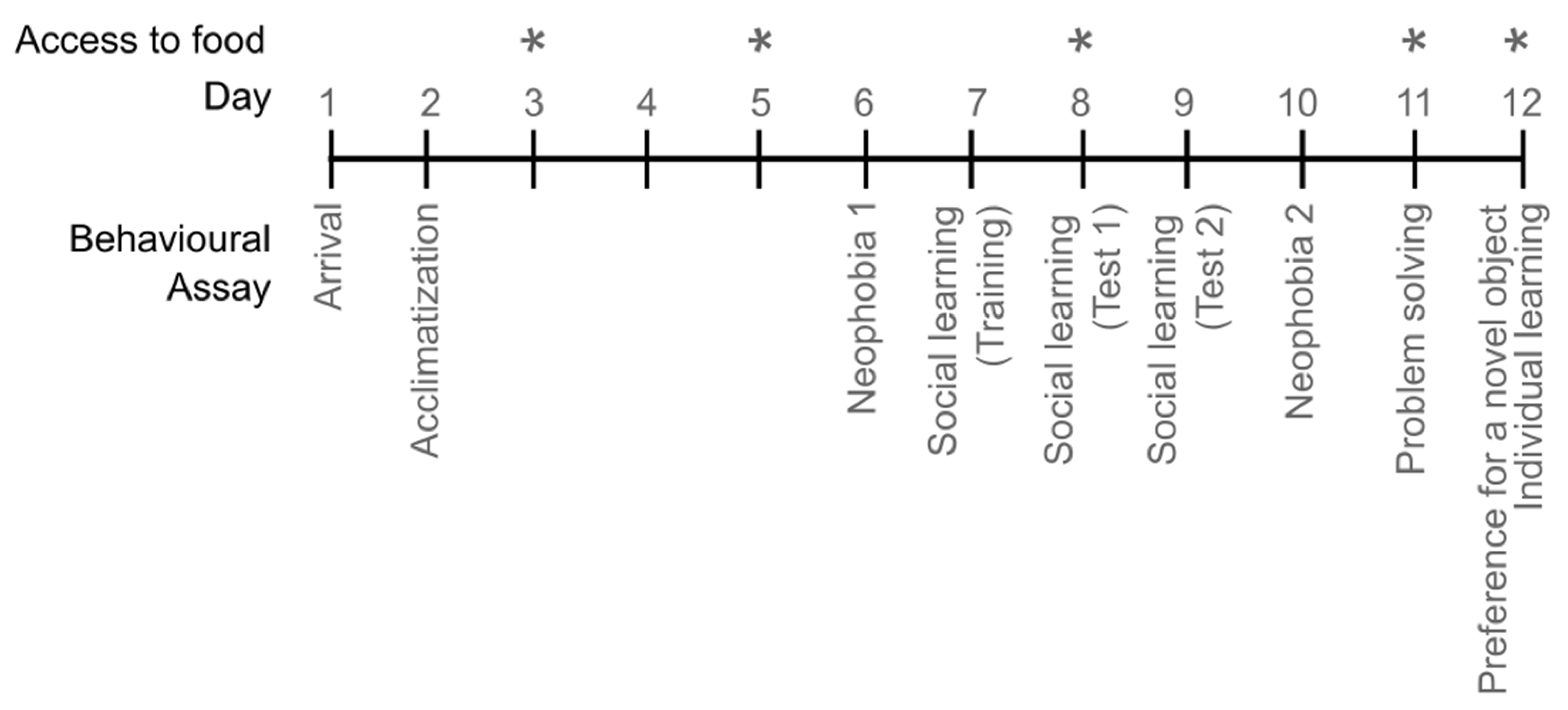
2.2. Experimental Plan
2.3. Data Analysis
3. Results
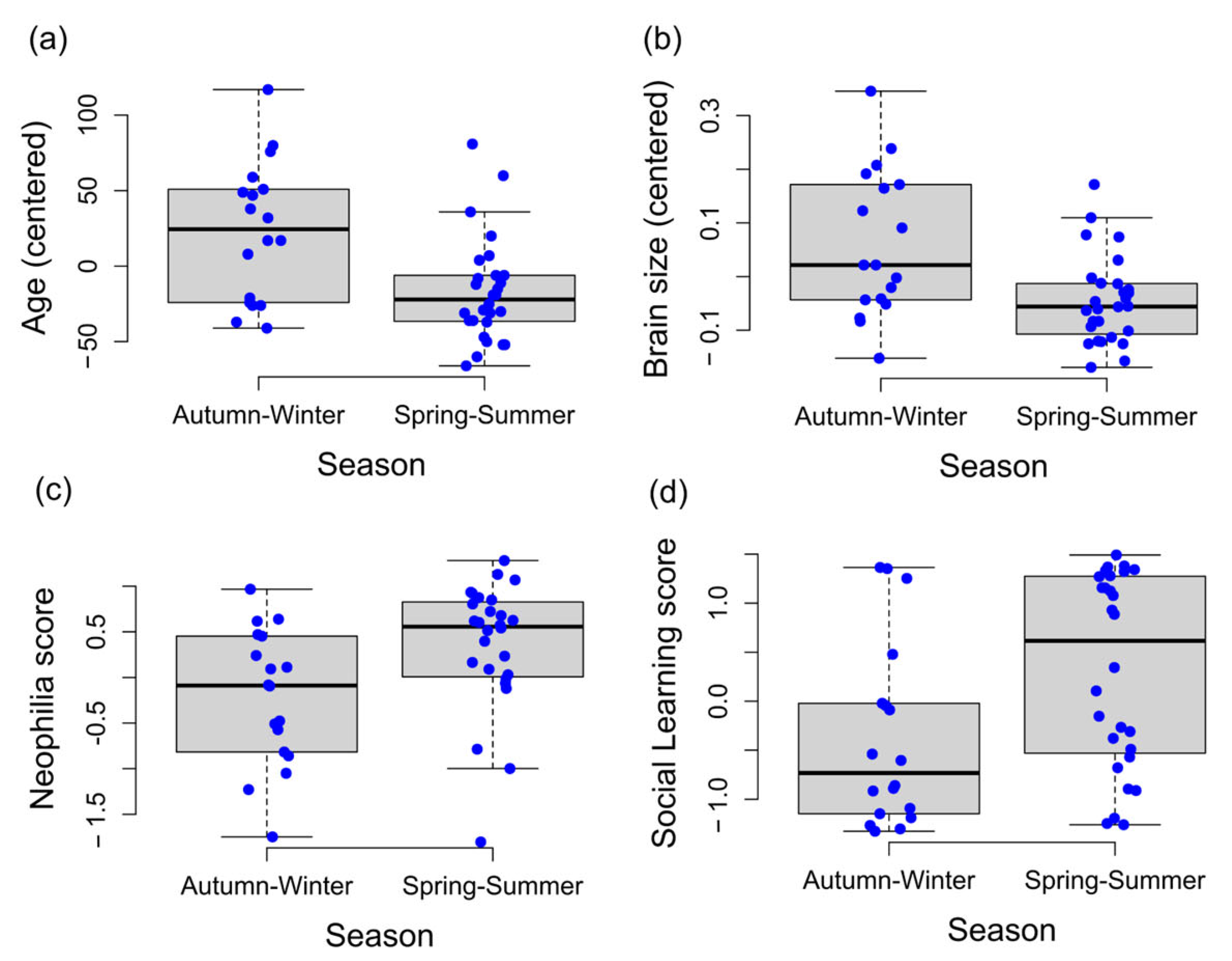
3.1. Morphometric and Behavioural Characteristics Associated with Seasonality
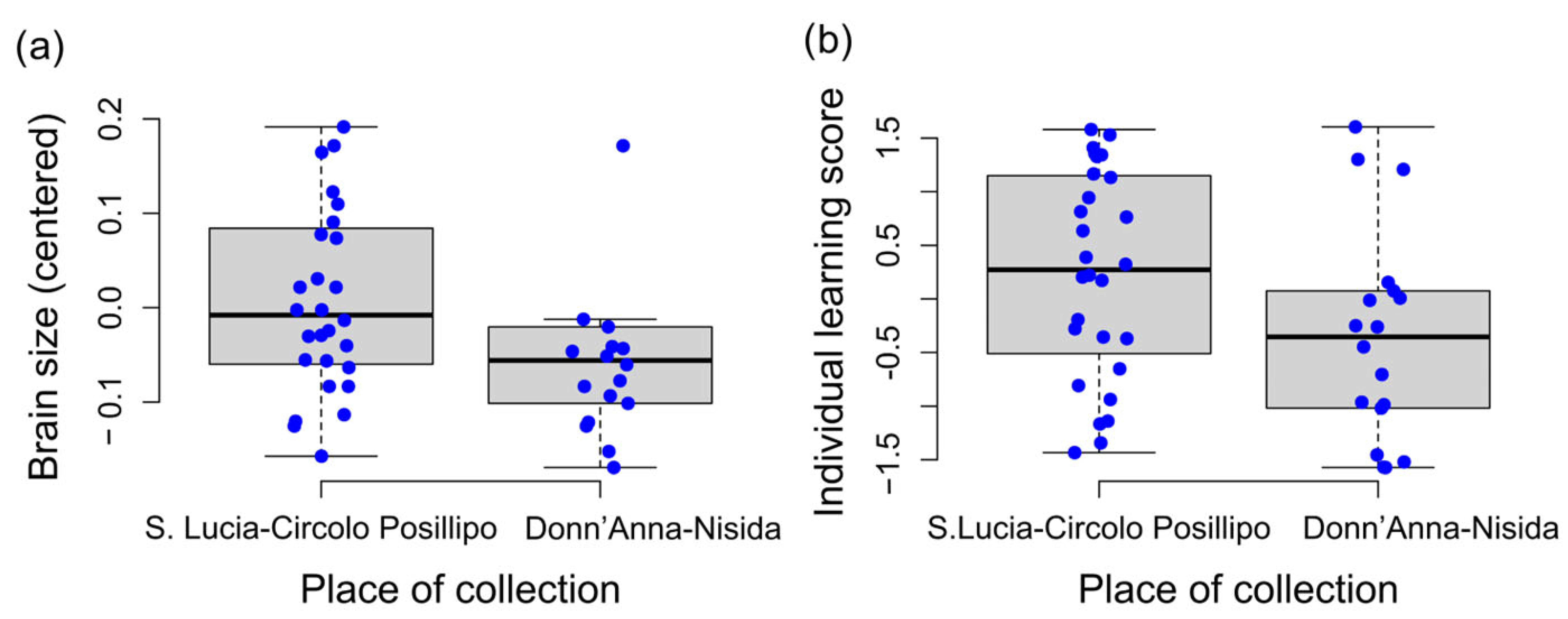
3.2. Morphometric and Behavioural Characteristics Associated with the Location of Origin (Fishing Site)
3.3. Correlations between Morphometric and Individual Behavioural Differences
3.4. Morphometric and Behavioural Characteristics Associated with Problem-Solving Skills
3.5. Grouping Individuals among Features
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiss, A.S. The Epic of the Cephalopod. Discourse 2002, 24, 150–159. [Google Scholar] [CrossRef]
- Caddel, J.; DeVries, M. Eight Arms of Inspiration: The Octopus Art Project; Memento Publishing and Out of Step Books: Los Angeles CA, USA, 2013; p. 336. [Google Scholar]
- Montgomery, S. The Octopus Scientists: Exploring the Mind of a Mollusk; Houghton Mifflin Harcourt: Boston, MA, USA, 2015; p. 71. [Google Scholar]
- Montgomery, S. The Soul of an Octopus: A Surprising Exploration into the Wonder of Consciousness; Atria Books: New York, NY, USA, 2016; p. 272. [Google Scholar]
- Nakajima, R.; Shigeno, S.; Zullo, L.; De Sio, F.; Schmidt, M.R. Cephalopods between science, art, and engineering: A contemporary synthesis. Front. Commun. 2018, 3, 20. [Google Scholar] [CrossRef]
- O’Brien, C.E.; Ponte, G.; Fiorito, G. Octopus. In Encyclopedia of Animal Behavior, 2nd ed.; Choe, J.C., Ed.; Academic Press: Oxford, UK, 2019; pp. 142–148. [Google Scholar] [CrossRef]
- Ponte, G.; Chiandetti, C.; Edelman, D.B.; Imperadore, P.; Pieroni, E.M.; Fiorito, G. Cephalopod Behavior: From Neural Plasticity to Consciousness. Front. Syst. Neurosci. 2022, 15, 787139. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.E.; Roumbedakis, K.; Winkelmann, I.E. The Current State of Cephalopod Science and Perspectives on the Most Critical Challenges Ahead from Three Early-Career Researchers. Front. Physiol. 2018, 9, 700. [Google Scholar] [CrossRef] [PubMed]
- Godfrey-Smith, P. Cephalopods and the evolution of the mind. Pac. Conserv. Biol. 2013, 19, 4–9. [Google Scholar] [CrossRef]
- Godfrey-Smith, P. Other Minds: The Octopus, the Sea, and the Deep Origins of Consciousness; Farrar, Straus and Giroux: New York, NY, USA, 2016. [Google Scholar]
- Godfrey-Smith, P. Octopus experience. Anim. Sentience 2019, 26, 18. [Google Scholar] [CrossRef]
- Boycott, B.B.; Young, J.Z. A memory system in Octopus vulgaris Lamarck. Proc. R. Soc. Lond. Ser. B 1955, 143, 449–480. [Google Scholar] [CrossRef]
- Young, J.Z. Learning and Discrimination in the Octopus. Biol. Rev. 1961, 36, 32–95. [Google Scholar] [CrossRef]
- Maldonado, H. The positive learning process in Octopus vulgaris. Z. Vgl. Physiol. 1963, 47, 191–214. [Google Scholar] [CrossRef]
- Packard, A. The behaviour of Octopus vulgaris. Bull. L’institut Océanographique 1963, 1, 35–49. [Google Scholar]
- Maldonado, H. The positive and negative learning process in Octopus vulgaris Lamarck. Influence of the vertical and median superior frontal lobes. Z. Vergl. Physiol. 1965, 51, 185–203. [Google Scholar] [CrossRef]
- Packard, A. Cephalopods and fish: The limits of convergence. Biol. Rev. 1972, 47, 241–307. [Google Scholar] [CrossRef]
- Packard, A.; Hochberg, F.G. Skin Patterning in Octopus and Other Genera; Elsevier: Amsterdam, The Netherlands, 1977; pp. 191–231. [Google Scholar]
- Wells, M.J. Octopus: Physiology and Behaviour of an Advanced Invertebrate; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1978. [Google Scholar]
- Borrelli, L.; Gherardi, F.; Fiorito, G. A Catalogue of Body Patterning in Cephalopoda; Anton Dohrn Zoological Station, Firenze University Press: Napoli, Italy, 2006. [Google Scholar]
- Hanlon, R.T.; Messenger, J.B. Cephalopod Behaviour, 2nd ed.; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar] [CrossRef]
- Marini, G.; De Sio, F.; Ponte, G.; Fiorito, G. Behavioral Analysis of Learning and Memory in Cephalopods. In Learning and Memory: A Comprehensive Reference, 2nd ed.; Byrne, J.H., Ed.; Volume 1—Learning Theory and Behavior (Menzel, Randolf—Volume Editor); Academic Press & Elsevier: Amsterdam, The Netherlands, 2017; pp. 441–462. [Google Scholar]
- Zarrella, I.; Herten, K.; Maes, G.E.; Tai, S.; Yang, M.; Seuntjens, E.; Ritschard, E.A.; Zach, M.; Styfhals, R.; Sanges, R.; et al. The survey and reference assisted assembly of the Octopus vulgaris genome. Sci. Data 2019, 6, 13. [Google Scholar] [CrossRef]
- Albertin, C.B.; Simakov, O.; Mitros, T.; Wang, Z.Y.; Pungor, J.R.; Edsinger-Gonzales, E.; Brenner, S.; Ragsdale, C.W.; Rokhsar, D.S. The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature 2015, 524, 220–224. [Google Scholar] [CrossRef]
- Liscovitch-Brauer, N.; Alon, S.; Porath, H.T.; Elstein, B.; Unger, R.; Ziv, T.; Admon, A.; Levanon, E.Y.; Rosenthal, J.J.C.; Eisenberg, E. Trade-off between Transcriptome Plasticity and Genome Evolution in Cephalopods. Cell 2017, 169, 191–202.e111. [Google Scholar] [CrossRef]
- Belcaid, M.; Casaburi, G.; McAnulty, S.J.; Schmidbaur, H.; Suria, A.M.; Moriano-Gutierrez, S.; Pankey, M.S.; Oakley, T.H.; Kremer, N.; Koch, E.J.; et al. Symbiotic organs shaped by distinct modes of genome evolution in cephalopods. Proc. Natl. Acad. Sci. USA 2019, 116, 3030–3035. [Google Scholar] [CrossRef]
- Albertin, C.B.; Medina-Ruiz, S.; Mitros, T.; Schmidbaur, H.; Sanchez, G.; Wang, Z.Y.; Grimwood, J.; Rosenthal, J.J.C.; Ragsdale, C.W.; Simakov, O.; et al. Genome and transcriptome mechanisms driving cephalopod evolution. Nat. Commun. 2022, 13, 2427. [Google Scholar] [CrossRef] [PubMed]
- Schmidbaur, H.; Kawaguchi, A.; Clarence, T.; Fu, X.; Hoang, O.P.; Zimmermann, B.; Ritschard, E.A.; Weissenbacher, A.; Foster, J.S.; Nyholm, S.V.; et al. Emergence of novel cephalopod gene regulation and expression through large-scale genome reorganization. Nat. Commun. 2022, 13, 2172. [Google Scholar] [CrossRef]
- Destanović, D.; Schultz, D.T.; Styfhals, R.; Cruz, F.; Gómez-Garrido, J.; Gut, M.; Gut, I.; Fiorito, G.; Simakov, O.; Alioto, T.S.; et al. A chromosome-level reference genome for the common octopus, Octopus vulgaris (Cuvier, 1797). G3 Genes|Genomes|Genet. 2023, jkad220. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-M.; Kang, S.; Ahn, D.-H.; Jung, S.-H.; Rhee, H.; Yoo, J.S.; Lee, J.-E.; Lee, S.; Han, Y.-H.; Ryu, K.-B.; et al. The genome of common long-arm octopus Octopus minor. Gigascience 2018, 7, giy119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liu, Q.; Sun, J.; Liu, S.; Fan, G.; Wang, L.; Zhang, Y.; Seim, I.; An, S.; Liu, X.; et al. The gold-ringed octopus (Amphioctopus fangsiao) genome and cerebral single-nucleus transcriptomes provide insights into the evolution of karyotype and neural novelties. BMC Biol. 2022, 20, 289. [Google Scholar] [CrossRef]
- Li, F.; Bian, L.; Ge, J.; Han, F.; Liu, Z.; Li, X.; Liu, Y.; Lin, Z.; Shi, H.; Liu, C.; et al. Chromosome-level genome assembly of the East Asian common octopus (Octopus sinensis) using PacBio sequencing and Hi-C technology. Mol. Ecol. Resour. 2020, 20, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Vecchione, M.; Sweeney, M.J.; Rothman, P.L. The geographic problem in cephalopod genomics. Front. Mar. Sci. 2022, 9, 1090034. [Google Scholar] [CrossRef]
- Yoshida, M.-A.; Hirota, K.; Imoto, J.; Okuno, M.; Tanaka, H.; Kajitani, R.; Toyoda, A.; Itoh, T.; Ikeo, K.; Sasaki, T.; et al. Gene Recruitments and Dismissals in the Argonaut Genome Provide Insights into Pelagic Lifestyle Adaptation and Shell-like Eggcase Reacquisition. Genome Biol. Evol. 2022, 14, evac140. [Google Scholar] [CrossRef] [PubMed]
- Papini, M.R.; Bitterman, M.E. Appetitive conditioning in Octopus cyanea. J. Comp. Psychol 1991, 105, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Schnell, A.K.; Amodio, P.; Boeckle, M.; Clayton, N.S. How intelligent is a cephalopod? Lessons from comparative cognition. Biol. Rev. 2020, 96, 162–178. [Google Scholar] [CrossRef]
- Schnell, A.K.; Clayton, N.S. Cephalopods: Ambassadors for rethinking cognition. Biochem. Biophys. Res. Commun. 2021, 564, 27–36. [Google Scholar] [CrossRef]
- Mather, J. The Case for Octopus Consciousness: Unity. NeuroSci 2021, 2, 405–415. [Google Scholar] [CrossRef]
- Mather, J. Octopus Consciousness: The Role of Perceptual Richness. NeuroSci 2021, 2, 276–290. [Google Scholar] [CrossRef]
- Mather, J. The Case for Octopus Consciousness: Temporality. NeuroSci 2022, 3, 245–261. [Google Scholar] [CrossRef]
- Boly, M.; Seth, A.; Wilke, M.; Ingmundson, P.; Baars, B.; Laureys, S.; Edelman, D.; Tsuchiya, N. Consciousness in humans and non-human animals: Recent advances and future directions. Front. Psychol. 2013, 4, 625. [Google Scholar] [CrossRef] [PubMed]
- Edelman, D.B.; Seth, A.K. Animal consciousness: A synthetic approach. Trends Neurosci. 2009, 32, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, L. Testing the Contribution of Relative Brain Size and Learning Capabilities on the Evolution of Octopus vulgaris and Other Cephalopods. Ph.D. Thesis, Stazione Zoologica Anton Dohrn, Italy & Open University, Napoli, Italy, 2007. [Google Scholar]
- Tricarico, E.; Borrelli, L.; Gherardi, F.; Fiorito, G. I Know My Neighbour: Individual Recognition in Octopus vulgaris. PLoS ONE 2011, 6, e18710. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.E.; Di Miccoli, V.; Fiorito, G. A preliminary investigation of the response of Octopus vulgaris to experimental stimuli in the wild. J. Molluscan Stud. 2021, 87, eyab032. [Google Scholar] [CrossRef]
- Borrelli, L.; Chiandetti, C.; Fiorito, G. A standardized battery of tests to measure Octopus vulgaris’ behavioural performance. Invertebr. Neurosci. 2020, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, H. The visual attack learning system in Octopus vulgaris. J. Theor. Biol 1963, 5, 470–488. [Google Scholar] [CrossRef] [PubMed]
- Amodio, P.; Andrews, P.; Salemme, M.; Ponte, G.; Fiorito, G. The Use of Artificial Crabs for Testing Predatory Behavior and Health in the Octopus. Altex-Altern. Anim. Exp. 2014, 31, 494–499. [Google Scholar]
- Frasnelli, E.; Vallortigara, G. Individual-level and population-level lateralization: Two sides of the same coin. Symmetry 2018, 10, 739. [Google Scholar] [CrossRef]
- Frasnelli, E.; Ponte, G.; Vallortigara, G.; Fiorito, G. Visual Lateralization in the Cephalopod Mollusk Octopus vulgaris. Symmetry 2019, 11, 1121. [Google Scholar] [CrossRef]
- Carere, C.; Grignani, G.; Bonanni, R.; Gala, M.D.; Carlini, A.; Angeletti, D.; Cimmaruta, R.; Nascetti, G.; Mather, J.A. Consistent individual differences in the behavioural responsiveness of adult male cuttlefish (Sepia officinalis). Appl. Anim. Behav. Sci. 2015, 167, 89–95. [Google Scholar] [CrossRef]
- Mather, J.A.; Anderson, R.C. Personalities of octopuses (Octopus rubescens). J. Comp. Psychol. 1993, 107, 336–340. [Google Scholar] [CrossRef]
- Sinn, D.L.; Perrin, N.A.; Mather, J.A.; Anderson, R.C. Early temperamental traits in an octopus (Octopus bimaculoides). J. Comp. Psychol. 2001, 115, 351–364. [Google Scholar] [CrossRef]
- Sinn, D.L.; Gosling, S.D.; Moltschaniwskyj, N.A. Development of shy/bold behaviour in squid: Context-specific phenotypes associated with developmental plasticity. Anim. Behav. 2008, 75, 433–442. [Google Scholar] [CrossRef]
- Pronk, R.; Wilson, D.R.; Harcourt, R. Video playback demonstrates episodic personality in the gloomy octopus. J. Exp. Biol. 2010, 213, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Zoratto, F.; Cordeschi, G.; Grignani, G.; Bonanni, R.; Alleva, E.; Nascetti, G.; Mather, J.A.; Carere, C. Variability in the “stereotyped” prey capture sequence of male cuttlefish (Sepia officinalis) could relate to personality differences. Anim. Cogn. 2018, 21, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Rosati, A.G. Foraging Cognition: Reviving the Ecological Intelligence Hypothesis. Trends Cogn. Sci. 2017, 21, 691–702. [Google Scholar] [CrossRef]
- Melin, A.D.; Young, H.C.; Mosdossy, K.N.; Fedigan, L.M. Seasonality, extractive foraging and the evolution of primate sensorimotor intelligence. J. Hum. Evol. 2014, 71, 77–86. [Google Scholar] [CrossRef]
- Griffin, A.S.; Guez, D. Innovation and problem solving: A review of common mechanisms. Behav. Process. 2014, 109, 121–134. [Google Scholar] [CrossRef]
- Daniels, S.E.; Fanelli, R.E.; Gilbert, A.; Benson-Amram, S. Behavioral flexibility of a generalist carnivore. Anim. Cogn. 2019, 22, 387–396. [Google Scholar] [CrossRef]
- Yarnall, J.L. Aspects of the behaviour of Octopus cyanea Gray. Anim. Behav. 1969, 17, 747–754. [Google Scholar] [CrossRef]
- Fiorito, G.; Gherardi, F. Prey-handling behaviour of Octopus vulgaris (Mollusca, Cephalopoda) on bivalve preys. Behav. Process. 1999, 46, 75–88. [Google Scholar] [CrossRef]
- Fiorito, G.; von Planta, C.; Scotto, P. Problem solving ability of Octopus vulgaris Lamarck (Mollusca, Cephalopoda). Behav. Neural Biol. 1990, 53, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Mather, J.A. The packaging problem: Bivalve prey selection and prey entry techniques of the octopus Enteroctopus dofleini. J. Comp. Psychol. 2007, 121, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Webster, S.J.; Lefebvre, L. Problem solving and neophobia in a columbiform–passeriform assemblage in Barbados. Anim. Behav. 2001, 62, 23–32. [Google Scholar] [CrossRef]
- Griffin, A.S.; Diquelou, M.; Perea, M. Innovative problem solving in birds: A key role of motor diversity. Anim. Behav. 2014, 92, 221–227. [Google Scholar] [CrossRef]
- Boogert, N.J.; Reader, S.M.; Hoppitt, W.; Laland, K.N. The origin and spread of innovations in starlings. Anim. Behav. 2008, 75, 1509–1518. [Google Scholar] [CrossRef]
- Allison, M.-L.; Reed, R.; Michels, E.; Boogert, N.J. The drivers and functions of rock juggling in otters. R. Soc. Open Sci. 2020, 7, 200141. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.A. Do anvil-using banded mongooses understand means–end relationships? A field experiment. Anim. Cogn. 2010, 13, 325–330. [Google Scholar] [CrossRef]
- Krützen, M.; Mann, J.; Heithaus, M.R.; Connor, R.C.; Bejder, L.; Sherwin, W.B. Cultural transmission of tool use in bottlenose dolphins. Proc. Natl. Acad. Sci. USA 2005, 102, 8939–8943. [Google Scholar] [CrossRef]
- Wheatley, B.P. Cultural Behavior and Extractive Foraging in Macaca fascicularis. Curr. Anthropol. 1988, 29, 516–519. [Google Scholar] [CrossRef]
- Erickson, C.J. Feeding sites for extractive foraging by the aye-aye, Daubentonia madagascariensis. Am. J. Primatol. 1995, 35, 235–240. [Google Scholar] [CrossRef]
- Fiorito, G.; Scotto, P. Observational Learning in Octopus vulgaris. Science 1992, 256, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Amodio, P.; Fiorito, G. Observational and Other Types of Learning in Octopus. In Invertebrate Learning and Memory; Menzel, R., Benjamin, P., Eds.; Academic Press: London, UK, 2013; pp. 293–302. [Google Scholar]
- Biederman, G.B.; Davey, V.A. Social learning in invertebrates. Science 1993, 259, 1627–1628. [Google Scholar] [CrossRef] [PubMed]
- Biederman, G.B.; Vanayan, M. Observational learning in pigeons: The function of quality of observed performance in simultaneous discrimination. Learn. Motiv. 1988, 19, 31–43. [Google Scholar] [CrossRef]
- Fiorito, G.; Biederman, G.B.; Davey, V.A.; Gherardi, F. The role of stimulus preexposure in problem solving by Octopus vulgaris. Anim. Cogn. 1998, 1, 107–112. [Google Scholar] [CrossRef]
- Auersperg, A.M.I.; von Bayern, A.M.P.; Gajdon, G.K.; Huber, L.; Kacelnik, A. Flexibility in Problem Solving and Tool Use of Kea and New Caledonian Crows in a Multi Access Box Paradigm. PLoS ONE 2011, 6, e20231. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zou, Y.; Sun, Y.-H.; Cate, C.T. Problem-solving males become more attractive to female budgerigars. Science 2019, 363, 166–167. [Google Scholar] [CrossRef]
- Jacobson, S.L.; Puitiza, A.; Snyder, R.J.; Sheppard, A.; Plotnik, J.M. Persistence is key: Investigating innovative problem solving by Asian elephants using a novel multi-access box. Anim. Cogn. 2021, 25, 657–669. [Google Scholar] [CrossRef]
- Morton, F.B. Do wild raccoons (Procyon lotor) use tools? Anim. Cogn. 2021, 24, 433–441. [Google Scholar] [CrossRef]
- Ducatez, S.; Audet, J.N.; Lefebvre, L. Problem-solving and learning in Carib grackles: Individuals show a consistent speed–accuracy trade-off. Anim. Cogn. 2015, 18, 485–496. [Google Scholar] [CrossRef]
- Amici, F.; Widdig, A.; Lehmann, J.; Majolo, B. A meta-analysis of interindividual differences in innovation. Anim. Behav. 2019, 155, 257–268. [Google Scholar] [CrossRef]
- Benson-Amram, S.; Holekamp, K.E. Innovative problem solving by wild spotted hyenas. Proc. R. Soc. B Biol. Sci. 2012, 279, 4087–4095. [Google Scholar] [CrossRef] [PubMed]
- Bräuer, J.; Hanus, D.; Pika, S.; Gray, R.; Uomini, N. Old and New Approaches to Animal Cognition: There Is Not “One Cognition”. J. Intell. 2020, 8, 28. [Google Scholar] [CrossRef]
- Reader, S.M.; Laland, K.N. Animal Innovation: An Introduction. In Animal Innovation; Reader, S.M., Laland, K.N., Eds.; Oxford University Press: Oxford, UK, 2003; pp. 3–35. [Google Scholar] [CrossRef]
- Lefebvre, L.; Reader, S.M.; Sol, D. Brains, Innovations and Evolution in Birds and Primates. BBE 2004, 63, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, L.; Sol, D. Brains, Lifestyles and Cognition: Are There General Trends? BBE 2008, 72, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Bandini, E.; Harrison, R.A. Innovation in chimpanzees. Biol. Rev. 2020, 95, 1167–1197. [Google Scholar] [CrossRef] [PubMed]
- Tebbich, S.; Griffin, A.S.; Peschl, M.F.; Sterelny, K. From mechanisms to function: An integrated framework of animal innovation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150195. [Google Scholar] [CrossRef]
- Aplin, L. Understanding the multiple factors governing social learning and the diffusion of innovations. Curr. Opin. Behav. Sci. 2016, 12, 59–65. [Google Scholar] [CrossRef]
- Rawlings, B.S.; Flynn, E.G.; Kendal, R.L. Personality predicts innovation and social learning in children: Implications for cultural evolution. Dev. Sci. 2022, 25, e13153. [Google Scholar] [CrossRef]
- Day, R.L.; Coe, R.L.; Kendal, J.R.; Laland, K.N. Neophilia, innovation and social learning: A study of intergeneric differences in callitrichid monkeys. Anim. Behav. 2003, 65, 559–571. [Google Scholar] [CrossRef]
- Bouchard, J.; Goodyer, W.; Lefebvre, L. Social learning and innovation are positively correlated in pigeons (Columba livia). Anim. Cogn. 2007, 10, 259–266. [Google Scholar] [CrossRef]
- Benson-Amram, S.; Heinen, V.K.; Gessner, A.; Weldele, M.L.; Holekamp, K.E. Limited social learning of a novel technical problem by spotted hyenas. Behav. Process. 2014, 109, 111–120. [Google Scholar] [CrossRef]
- Del Giudice, M.; Crespi, B.J. Basic functional trade-offs in cognition: An integrative framework. Cognition 2018, 179, 56–70. [Google Scholar] [CrossRef]
- Laland, K.N.; Reader, S.M. Foraging innovation in the guppy. Anim. Behav. 1999, 57, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, R.; Mettke-hofmann, C. Ecological Aspects of Neophobia and Neophilia in Birds. In Current Ornithology; Nolan, V., Thompson, C.F., Nolan, V., Thompson, C.F., Eds.; Springer: Boston, MA, USA, 2001; pp. 119–178. [Google Scholar]
- Kummer, H.; Goodall, J.; Weiskrantz, L. Conditions of innovative behaviour in primates. Philos. Trans. R. Soc. London. B Biol. Sci. 1985, 308, 203–214. [Google Scholar] [CrossRef]
- Sih, A.; Cote, J.; Evans, M.; Fogarty, S.; Pruitt, J. Ecological implications of behavioural syndromes. Ecol. Lett. 2012, 15, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Des Roches, S.; Post, D.M.; Turley, N.E.; Bailey, J.K.; Hendry, A.P.; Kinnison, M.T.; Schweitzer, J.A.; Palkovacs, E.P. The ecological importance of intraspecific variation. Nat. Ecol. Evol. 2018, 2, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Royauté, R.; Hedrick, A.; Dochtermann, N.A. Behavioural syndromes shape evolutionary trajectories via conserved genetic architecture. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200183. [Google Scholar] [CrossRef]
- Duncan, M.I.; Bates, A.E.; James, N.C.; Potts, W.M. Exploitation may influence the climate resilience of fish populations through removing high performance metabolic phenotypes. Sci. Rep. 2019, 9, 11437. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, G.L.; Graff, M.; Nishimura, K.K.; Tao, R.; Haessler, J.; Gignoux, C.R.; Highland, H.M.; Patel, Y.M.; Sorokin, E.P.; Avery, C.L.; et al. Genetic analyses of diverse populations improve discovery for complex traits. Nature 2019, 570, 514–518. [Google Scholar] [CrossRef]
- Sanders, G.D. The Cephalopods. In Invertebrate Learning. Cephalopods and Echinoderms; Corning, W.C., Dyal, J.A., Willows, A.O.D., Eds.; Plenum Press: New York, NY, USA, 1975; Volume 3, pp. 1–101. [Google Scholar]
- Finn, J.K.; Tregenza, T.; Norman, M.D. Defensive tool use in a coconut-carrying octopus. Curr. Biol. 2009, 19, R1069–R1070. [Google Scholar] [CrossRef] [PubMed]
- Maselli, V.; Al-Soudy, A.-S.; Buglione, M.; Aria, M.; Polese, G.; Di Cosmo, A. Sensorial Hierarchy in Octopus vulgaris’s Food Choice: Chemical vs. Visual. Animals 2020, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Reader, S.M.; Morand-Ferron, J.; Flynn, E. Animal and human innovation: Novel problems and novel solutions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150182. [Google Scholar] [CrossRef] [PubMed]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Pike, T.W.; Ramsey, M.; Wilkinson, A. Environmentally induced changes to brain morphology predict cognitive performance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170287. [Google Scholar] [CrossRef]
- Quinn, J.L.; Cole, E.F.; Reed, T.E.; Morand-Ferron, J. Environmental and genetic determinants of innovativeness in a natural population of birds. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150184. [Google Scholar] [CrossRef]
- Ponte, G.; Andrews, P.; Galligioni, V.; Pereira, J.; Fiorito, G. Cephalopod Welfare, Biological and Regulatory Aspects: An EU Experience. In The Welfare of Invertebrate Animals; Carere, C., Mather, J., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 209–228. [Google Scholar] [CrossRef]
- Smith, J.A.; Andrews, P.L.; Hawkins, P.; Louhimies, S.; Ponte, G.; Dickel, L. Cephalopod research and EU Directive 2010/63/EU: Requirements, impacts and ethical review. J. Exp. Mar. Biol. Ecol. 2013, 447, 31–45. [Google Scholar] [CrossRef]
- Fiorito, G.; Affuso, A.; Basil, J.; Cole, A.; de Girolamo, P.; D’Angelo, L.; Dickel, L.; Gestal, C.; Grasso, F.; Kuba, M.; et al. Guidelines for the Care and Welfare of Cephalopods in Research—A consensus based on an initiative by CephRes, FELASA and the Boyd Group. Lab. Anim. 2015, 49, 1–90. [Google Scholar] [CrossRef]
- Santarelli, M. La pesca del polpo (Octopus vulgaris) nel golfo di Napoli. Bull. L’institute Océanographique 1932, 597, 1–6. [Google Scholar]
- Canali, E.; Ponte, G.; Belcari, P.; Rocha, F.; Fiorito, G. Evaluating age in Octopus vulgaris: Estimation, validation and seasonal differences. Mar. Ecol.-Prog. Ser. 2011, 441, 141–149. [Google Scholar] [CrossRef]
- Greenberg, R. The role of neophobia in determining the degree of foraging specialization in some migrant warblers. Am. Nat. 1983, 122, 444–453. [Google Scholar] [CrossRef]
- Greenberg, R. Differences in feeding neophobia in the tropical migrant wood warblers Dendroica castanea and D. pensylvanica. J. Comp. Psychol. 1984, 98, 131–136. [Google Scholar] [CrossRef]
- Ihaka, R.; Gentleman, R. R: A Language for Data Analysis and Graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar] [CrossRef]
- Mair, P.; Wilcox, R. Robust statistical methods in R using the WRS2 package. Behav. Res. Methods 2020, 52, 464–488. [Google Scholar] [CrossRef] [PubMed]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-PLUS; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; p. 501. [Google Scholar]
- Fraley, C.; Raftery, A.E. MCLUST Version 3 for R: Normal Mixture Modeling and Model-Based Clustering; Citeseer. 2006. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=880ff0cbd29ac5e712342c8d60d9095633673411 (accessed on 2 December 2023).
- Auersperg, A.M.I.; Gajdon, G.K.; von Bayern, A.M.P. A new approach to comparing problem solving, flexibility and innovation. Commun. Integr. Biol. 2012, 5, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Posada, G.; Iwaniuk, A.N.; Fuxjager, M.J. Extractive foraging behaviour in woodpeckers evolves in species that retain a large ancestral brain. Anim. Behav. 2023, 198, 141–152. [Google Scholar] [CrossRef]
- Kulahci, I.G.; Quinn, J.L. Dynamic Relationships between Information Transmission and Social Connections. Trends Ecol. Evol. 2019, 34, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.L.; Dechanupong, J.; Horpiencharoen, W.; Yindee, M.; Plotnik, J.M. Innovating to solve a novel puzzle: Wild Asian elephants vary in their ability to problem solve. Anim. Behav. 2023, 205, 227–239. [Google Scholar] [CrossRef]
- Sayol, F.; Lapiedra, O.; Ducatez, S.; Sol, D. Larger brains spur species diversification in birds. Evolution 2019, 73, 2085–2093. [Google Scholar] [CrossRef]
- Fristoe, T.S.; Botero, C.A. Alternative ecological strategies lead to avian brain size bimodality in variable habitats. Nat. Commun. 2019, 10, 3818. [Google Scholar] [CrossRef]
- Mazza, V.; Eccard, J.; Zaccaroni, M.; Jacob, J.; Dammhahn, M. The fast and the flexible: Cognitive style drives individual variation in cognition in a small mammal. Anim. Behav. 2018, 137, 119–132. [Google Scholar] [CrossRef]
- Mazza, V.; Jacob, J.; Dammhahn, M.; Zaccaroni, M.; Eccard, J. Individual variation in cognitive style reflects foraging and anti-predator strategies in a small mammal. Sci. Rep. 2019, 9, 10157. [Google Scholar] [CrossRef] [PubMed]
- Daniel, I.B.; Svanbäck, R.; Fordyce, J.A.; Yang, L.H.; Davis, J.M.; Hulsey, C.D.; Forister, M.L. The Ecology of Individuals: Incidence and Implications of Individual Specialization. Am. Nat. 2003, 161, 1–28. [Google Scholar] [CrossRef]
- Ferry-Graham, L.A.; Bolnick, D.I.; Wainwright, P.C. Using Functional Morphology to Examine the Ecology and Evolution of Specialization. Integr. Comp. Biol. 2002, 42, 265–277. [Google Scholar] [CrossRef]
- Snell-Rood, E.C.; Steck, M.K. Behaviour shapes environmental variation and selection on learning and plasticity: Review of mechanisms and implications. Anim. Behav. 2019, 147, 147–156. [Google Scholar] [CrossRef]
- Benson-Amram, S.; Weldele, M.L.; Holekamp, K.E. A comparison of innovative problem-solving abilities between wild and captive spotted hyaenas, Crocuta crocuta. Anim. Behav. 2013, 85, 349–356. [Google Scholar] [CrossRef]
- Benson-Amram, S.; Dantzer, B.; Stricker, G.; Swanson, E.M.; Holekamp, K.E. Brain size predicts problem-solving ability in mammalian carnivores. Proc. Natl. Acad. Sci. USA 2016, 113, 2532–2537. [Google Scholar] [CrossRef]
- Amici, F.; Caicoya, A.L.; Majolo, B.; Widdig, A. Innovation in wild Barbary macaques (Macaca sylvanus). Sci. Rep. 2020, 10, 4597. [Google Scholar] [CrossRef]
- Arseneau-Robar, T.J.M.; Anderson, K.A.; Sicotte, P.; Teichroeb, J.A. Monkeys who experience more feeding competition utilize social information to learn foraging skills faster. Sci. Rep. 2023, 13, 11624. [Google Scholar] [CrossRef]
- Miller, R.; Garcia-Pelegrin, E.; Danby, E. Neophobia and innovation in Critically Endangered Bali myna, Leucopsar rothschildi. R. Soc. Open Sci. 2022, 9, 211781. [Google Scholar] [CrossRef]
- Perry, S.; Carter, A.; Smolla, M.; Akçay, E.; Nöbel, S.; Foster, J.G.; Healy, S.D. Not by transmission alone: The role of invention in cultural evolution. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200049. [Google Scholar] [CrossRef] [PubMed]
- Rowell, M.K.; Pillay, N.; Rymer, T.L. Problem Solving in Animals: Proposal for an Ontogenetic Perspective. Animals 2021, 11, 866. [Google Scholar] [CrossRef] [PubMed]
- Carrada, G.C.; Hopkins, T.S.; Bonaduce, G.; Ianora, A.; Marino, D.; Modigh, M.; Ribera D’Alcalà, M.; Scotto di Carlo, B. Variability in the hydrographic and biological features of the Gulf of Naples. Mar. Ecol. 1980, 1, 105–120. [Google Scholar] [CrossRef]
- Ribera D’Alcalà, M.; Conversano, F.; Corato, F.; Licardo, P.; Mangoni, O.; Marino, D.; Mazzocchi, M.G.; Modigh, M.; Montresor, M.; Nardella, M.; et al. Seasonal patterns in plankton communities in a pluriannual time series at a coastal Mediterranean site (Gulf of Naples): An attempt to discern recurrence and trends. Sci. Mar. 2004, 68, 65–83. [Google Scholar] [CrossRef]
- Colombo, A. La Fauna Sottomarina del Golfo di Napoli; Forzani: Rome, Italy, 1888; pp. 5–107. [Google Scholar]
- Parenzan, P. Ricerche sulle biocenosi del Golfo di Napoli. Atti Soc. Ital. Per Il Prog. Delle Sci. 1933, 21, 3. [Google Scholar]
- Parenzan, P. Contributo alla conoscenza delle elevazioni sottomarine del Golfo di Napoli. Boll. Soc. Nat. Napoli 1954, 63, 45–72. [Google Scholar]
- Gambi, M.C.; D’Ambra, I.; Fiorito, G.; Saggiomo, V. The Archivio Moncharmont: A Pioneering Biodiversity Assessment in the Gulf of Naples (Italy). Oceanography in the Mediterranean and Beyond. In Places, People, Tools: Oceanography in the Mediterranean and Beyond; Proceedings of the Eighth International Congress for the History of Oceanography; Groeben, C., Ed.; Giannini: Napoli, Italy; Pubblicazioni della Stazione Zoologica di Napoli: Napoli, Italy, 2013; pp. 459–467. [Google Scholar]
- Ranzi, S. La distribuzione della vita nel Golfo di Napoli. Atti XI Congr. Geogr. Ital. 1930, 2, 1–4. [Google Scholar]
- Grimpe, G. Pflege, Behandlung und Zucht der Cephalopoden fur zoologische undphysiologische Zwecke. In Handbuch der Biologischen Arbeitsmethoden; Äberhalden, E., Ed.; Verlag Urban & Schwarzenberg: Berlin, Wien, 1928; pp. 331–402. [Google Scholar]
- De Sio, F.; Hanke, F.D.; Warnke, K.; Marazia, C.; Galligioni, V.; Fiorito, G.; Stravidou, I.; Ponte, G. E Pluribus Octo—Building Consensus on Standards of Care and Experimentation in Cephalopod Research; A Historical Outlook. Front. Physiol. 2020, 11, 645. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dissegna, A.; Borrelli, L.; Ponte, G.; Chiandetti, C.; Fiorito, G. Octopus vulgaris Exhibits Interindividual Differences in Behavioural and Problem-Solving Performance. Biology 2023, 12, 1487. https://doi.org/10.3390/biology12121487
Dissegna A, Borrelli L, Ponte G, Chiandetti C, Fiorito G. Octopus vulgaris Exhibits Interindividual Differences in Behavioural and Problem-Solving Performance. Biology. 2023; 12(12):1487. https://doi.org/10.3390/biology12121487
Chicago/Turabian StyleDissegna, Andrea, Luciana Borrelli, Giovanna Ponte, Cinzia Chiandetti, and Graziano Fiorito. 2023. "Octopus vulgaris Exhibits Interindividual Differences in Behavioural and Problem-Solving Performance" Biology 12, no. 12: 1487. https://doi.org/10.3390/biology12121487
APA StyleDissegna, A., Borrelli, L., Ponte, G., Chiandetti, C., & Fiorito, G. (2023). Octopus vulgaris Exhibits Interindividual Differences in Behavioural and Problem-Solving Performance. Biology, 12(12), 1487. https://doi.org/10.3390/biology12121487





