Homogenization of Functional Diversity of Rotifer Communities in Relation to Eutrophication in an Urban River of North China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
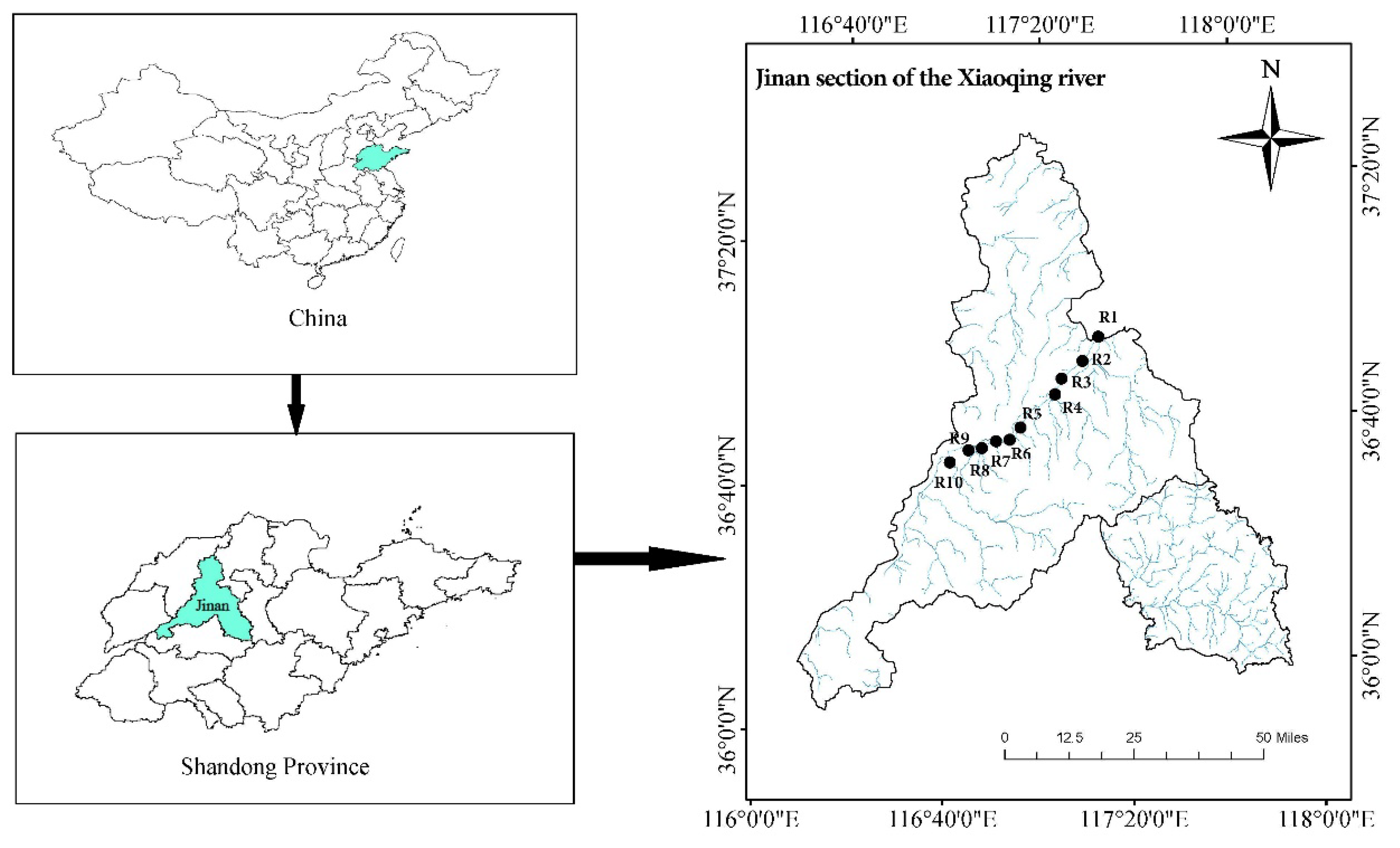
2.1. Study Sites
2.2. Sampling and Analytical Procedure
2.3. Functional Group Categorization
2.4. Statistical Analysis
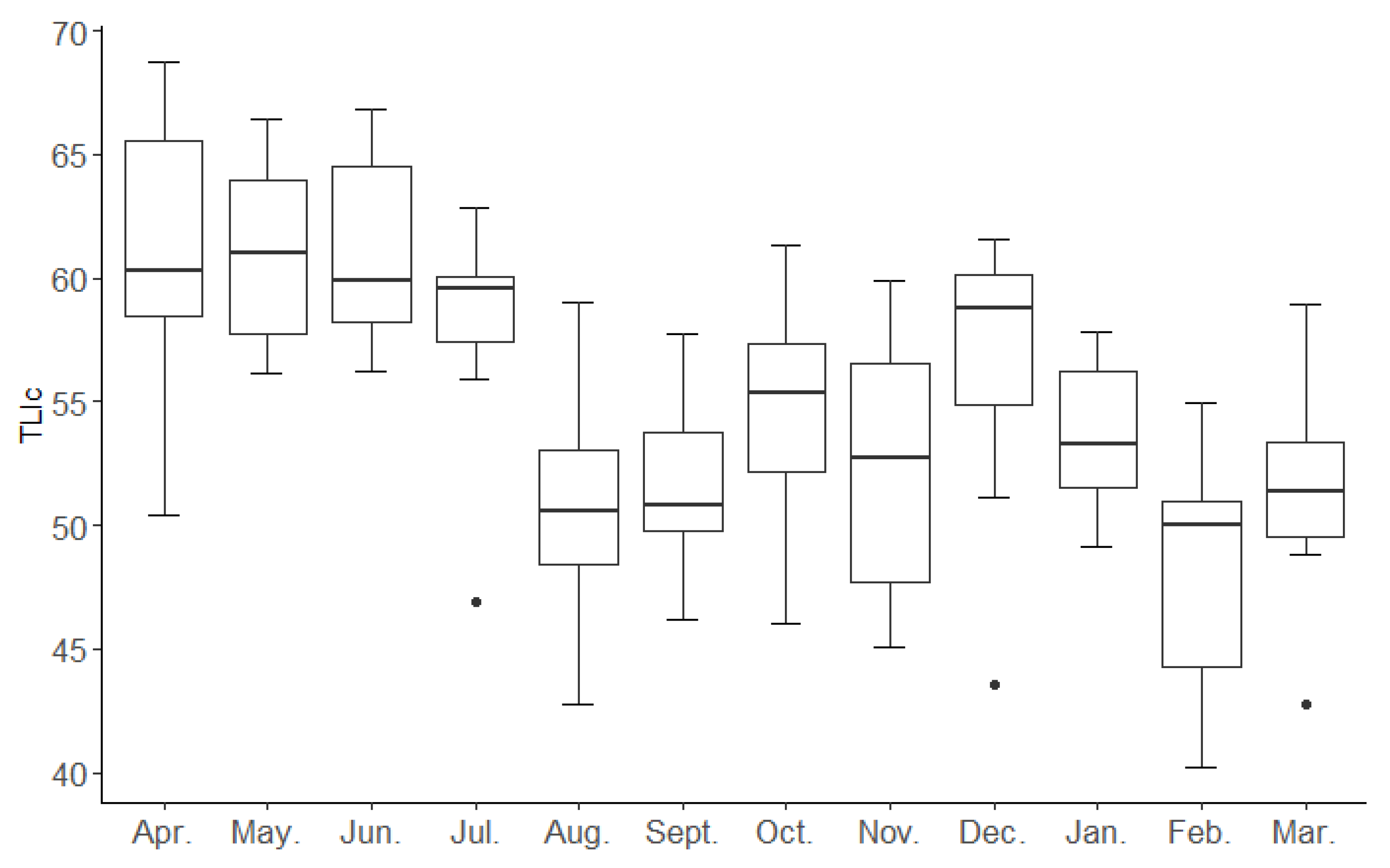
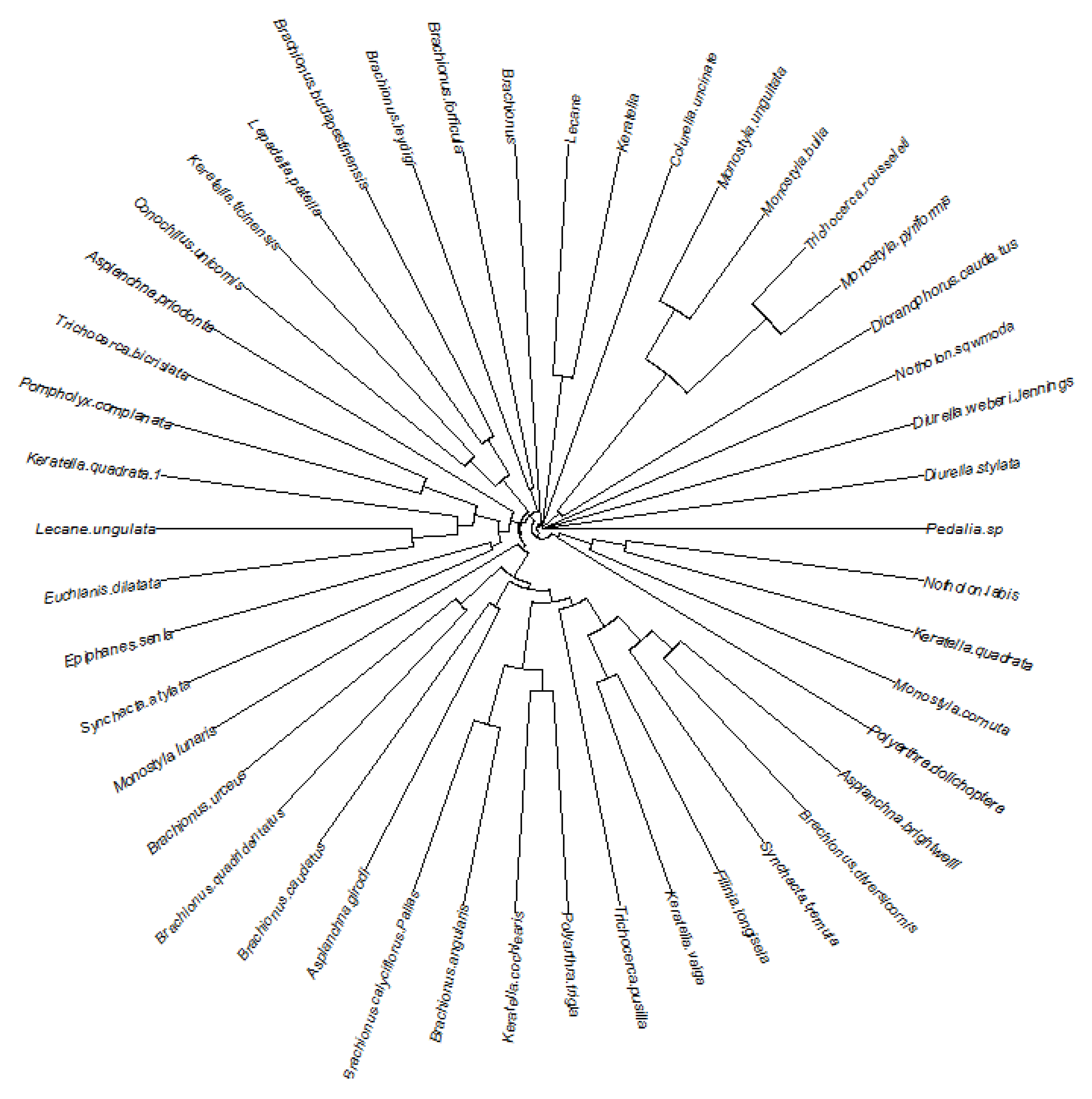
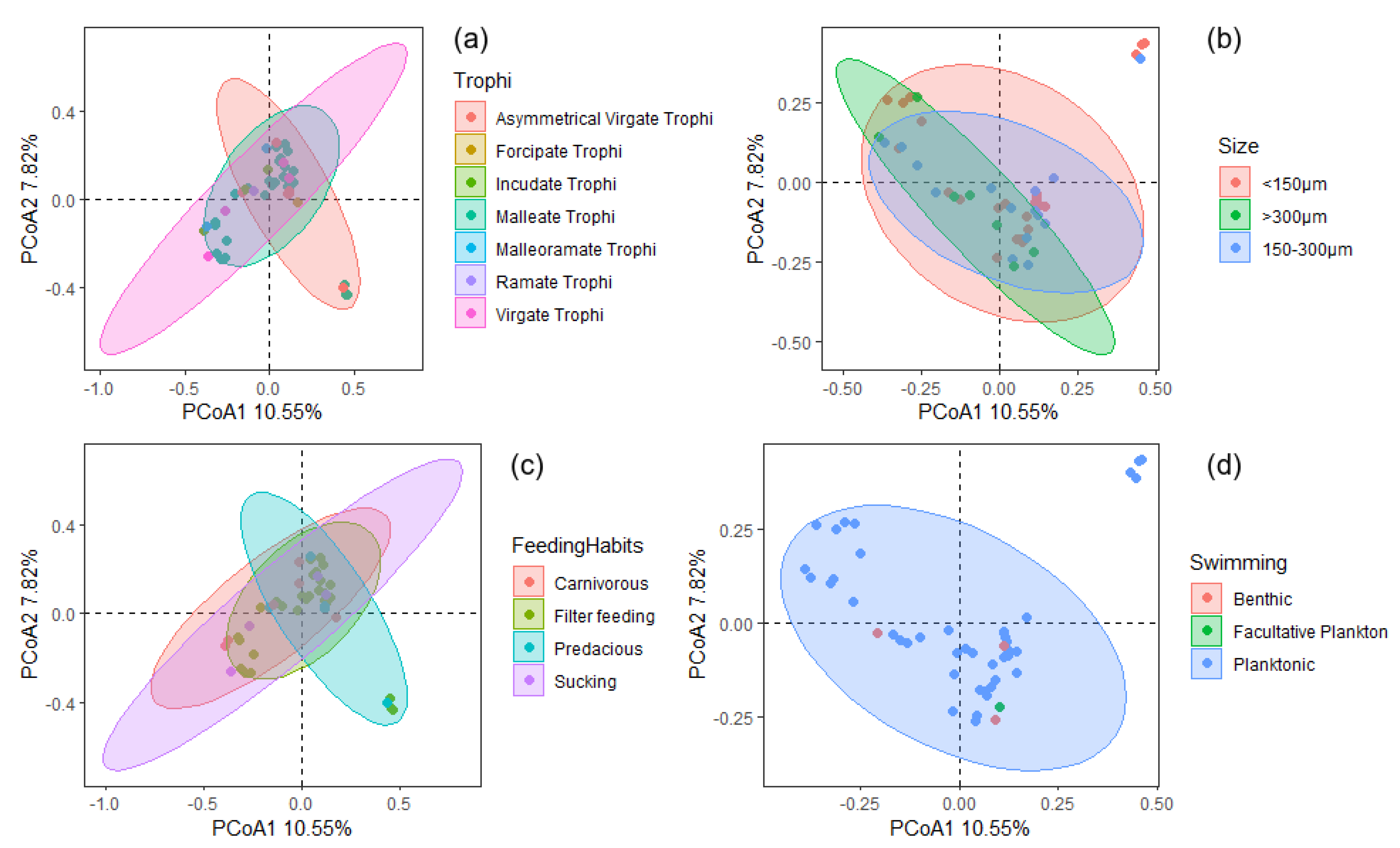
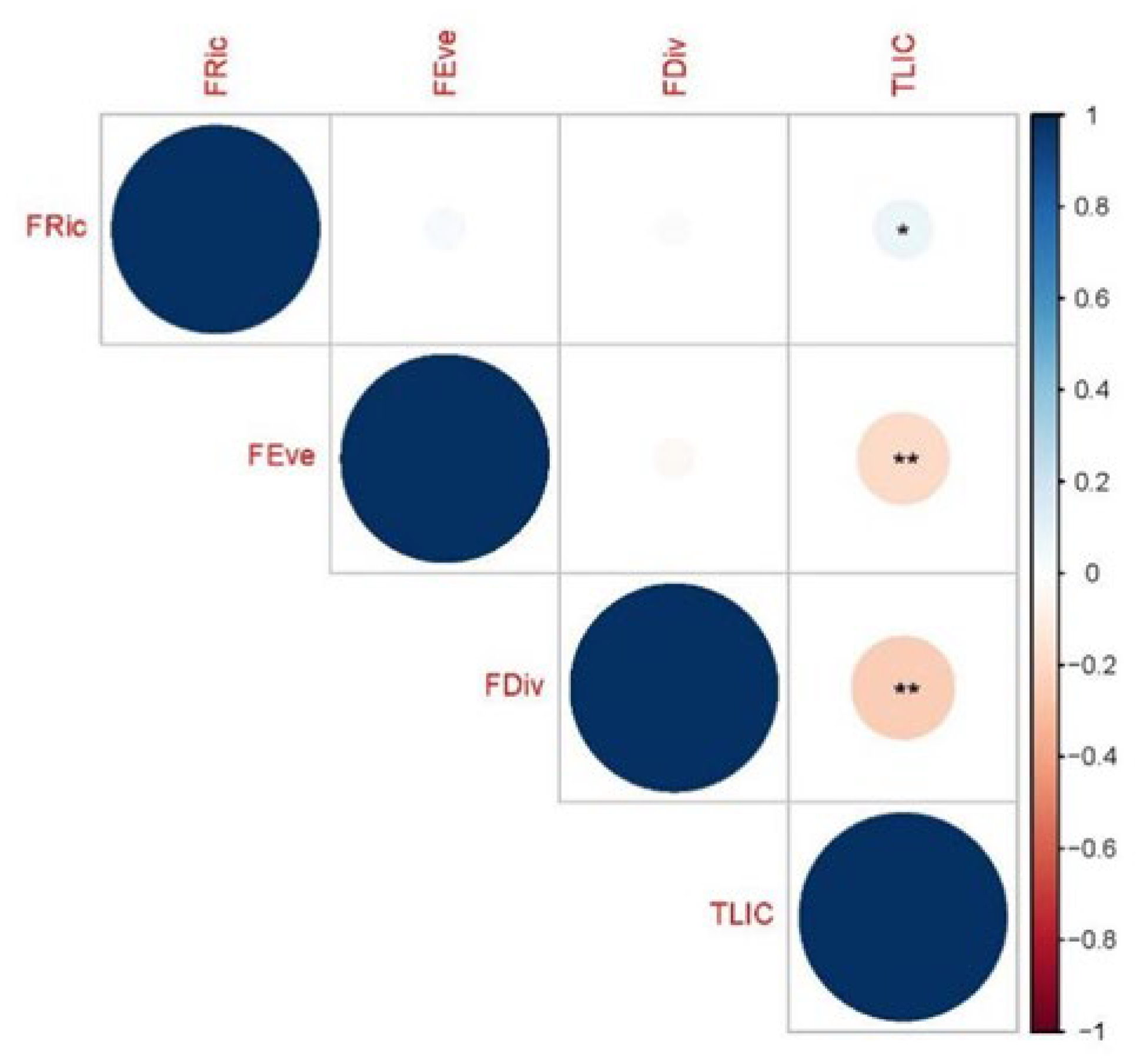
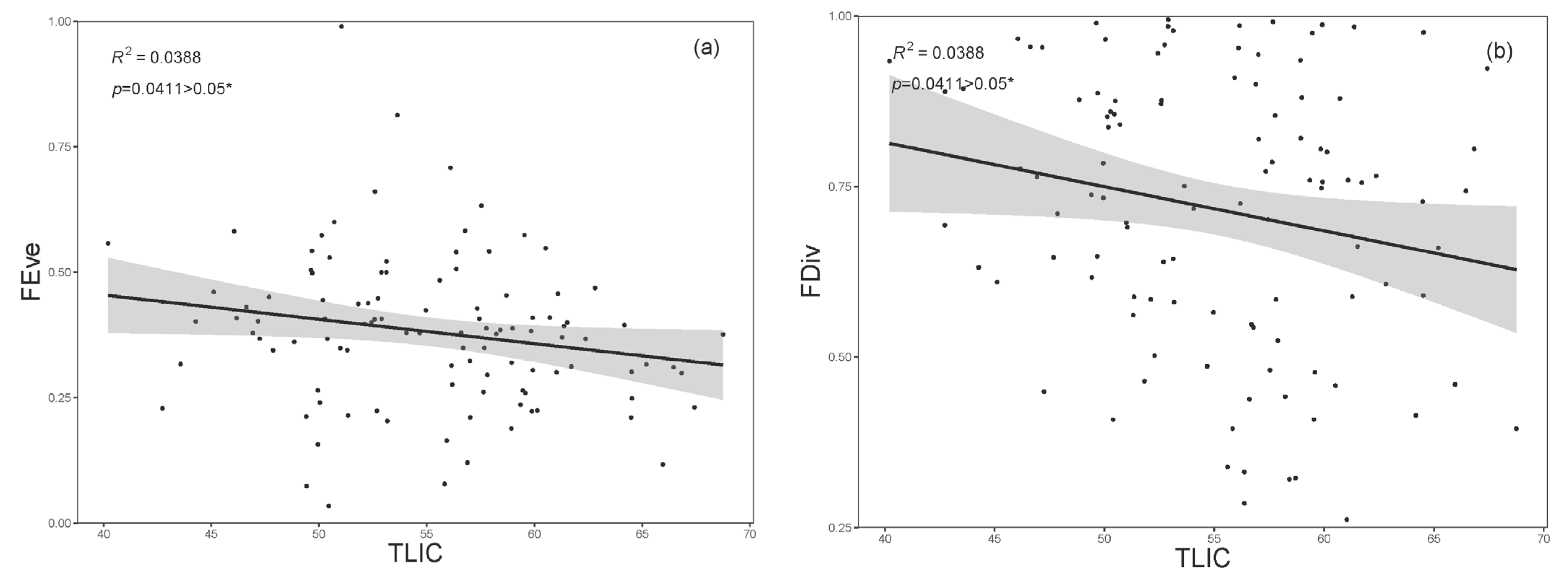
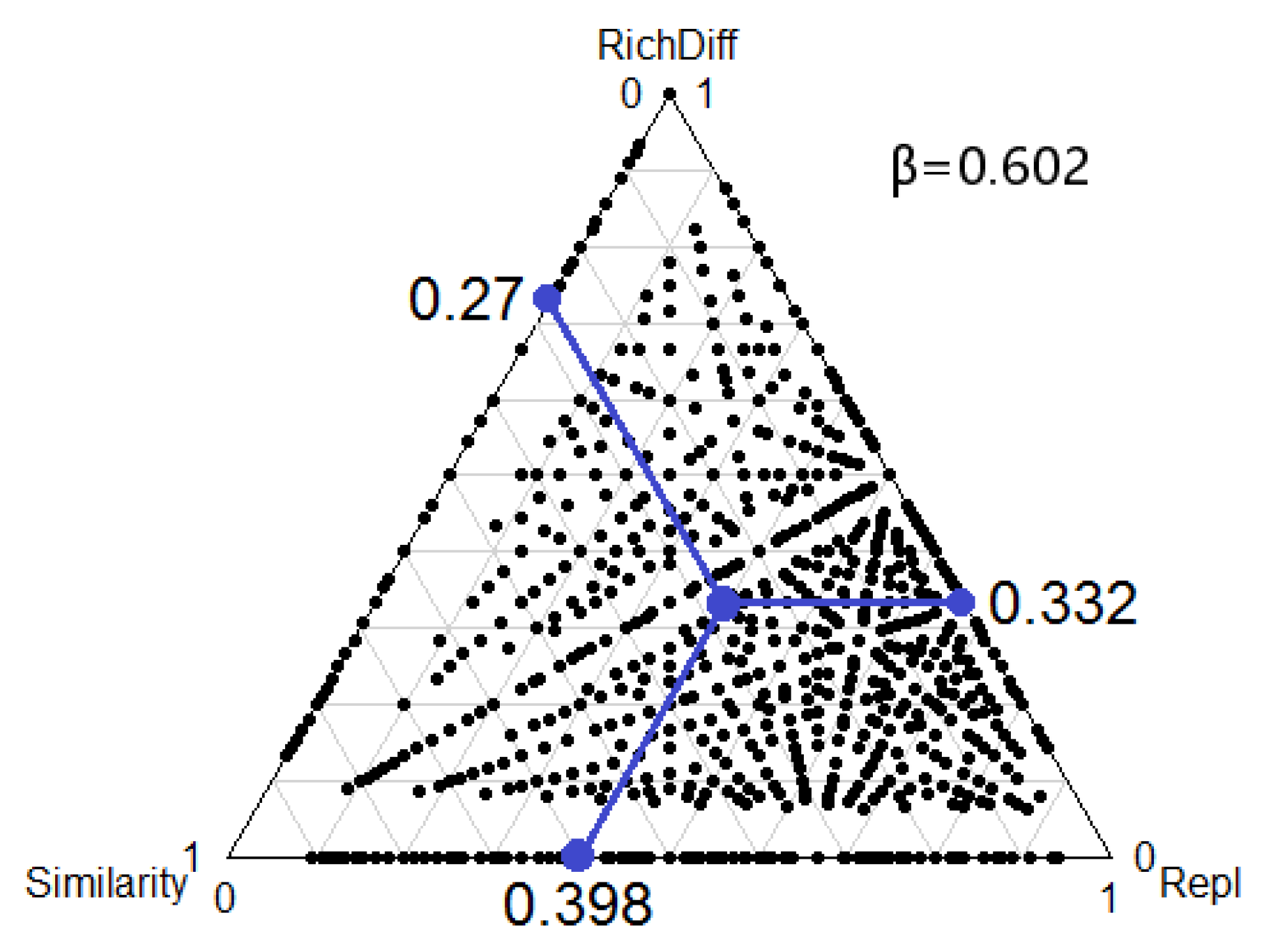
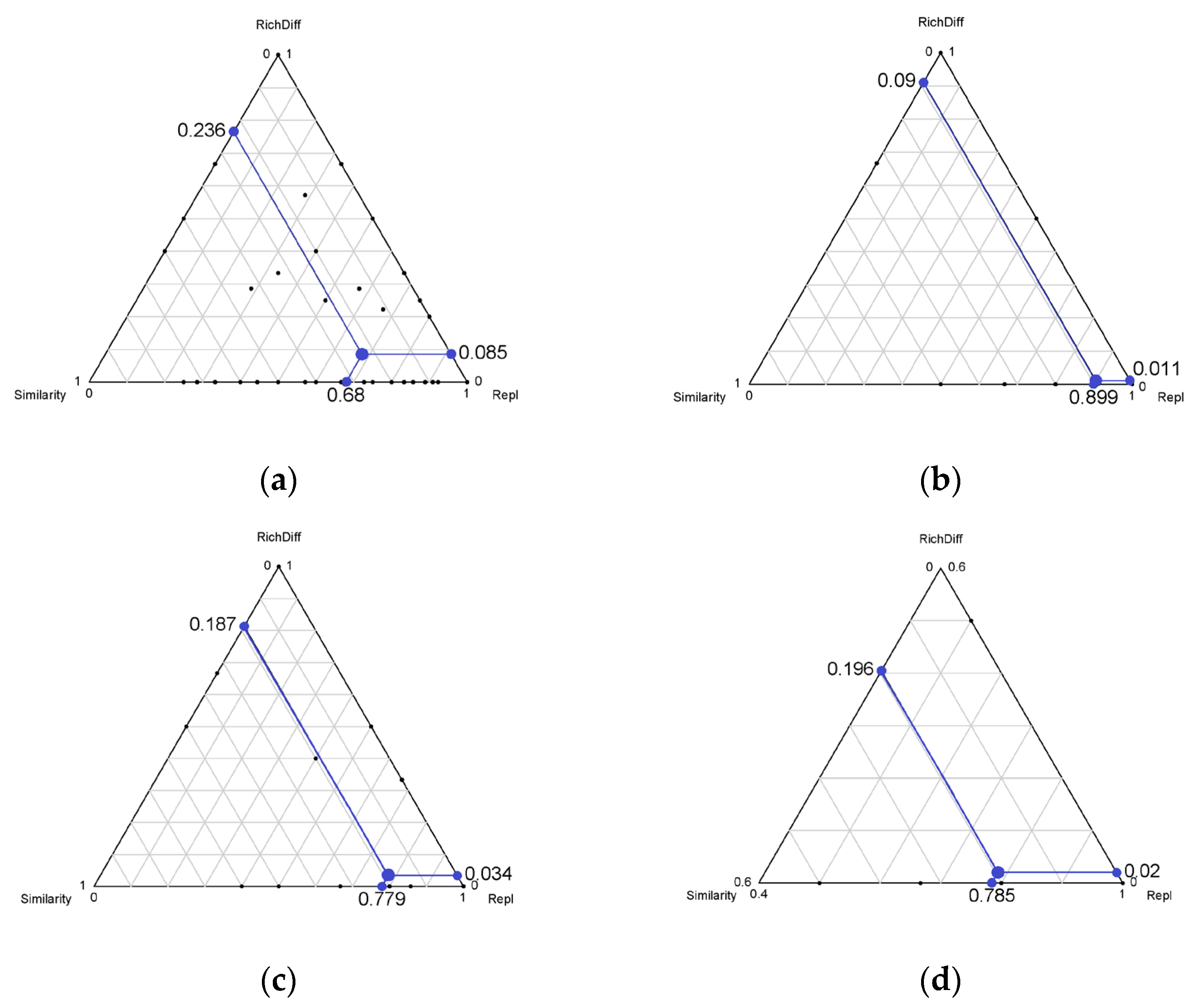
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steffen, W.; Crutzen, P.J.; McNeill, J.R. The Anthropocene: Are Humans Now Overwhelming the Great Forces of Nature. AMBIO A J. Hum. Environ. 2007, 36, 614–621. [Google Scholar] [CrossRef]
- Kumar, P.; Masago, Y.; Mishra, B.K.; Fukushi, K. Evaluating future stress due to combined effect of climate change and rapid urbanization for Pasig-Marikina River, Manila. Groundw. Sustain. Dev. 2018, 6, 227–234. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, J.; Xu, Z.; Wang, X.; Meersmans, J. Ecosystem services supply and demand response to urbanization: A case study of the Pearl River Delta, China. Ecosyst. Serv. 2021, 49, 101274. [Google Scholar] [CrossRef]
- Cerqueira, T.C.; Mendonca, R.L.; Gomes, R.L.; de Jesus, R.M.; da Silva, D.M.L. Effects of urbanization on water quality in a watershed in northeastern Brazil. Env. Monit. Assess. 2019, 192, 65. [Google Scholar] [CrossRef] [PubMed]
- Mangadze, T.; Wasserman, R.J.; Dalu, T. Use of Diatom Communities as Indicators of Conductivity and Ionic Composition in a Small Austral Temperate River System. Water Air Soil Pollut. 2017, 228, 428. [Google Scholar] [CrossRef]
- Freeman, L.A.; Corbett, D.R.; Fitzgerald, A.M.; Lemley, D.A.; Quigg, A.; Steppe, C.N. Impacts of Urbanization and Development on Estuarine Ecosystems and Water Quality. Estuaries Coasts 2019, 42, 1821–1838. [Google Scholar] [CrossRef]
- Shen, J.; Qin, G.; Yu, R.; Zhao, Y.; Yang, J.; An, S.; Liu, R.; Leng, X.; Wan, Y. Urbanization has changed the distribution pattern of zooplankton species diversity and the structure of functional groups. Ecol. Indic. 2021, 120, 106944. [Google Scholar] [CrossRef]
- Knösche, R. Organic Sediment Nutrient Concentrations and their Relationship with the Hydrological Connectivity of Floodplain Waters (River Havel, NE Germany). Hydrobiologia 2006, 560, 63–76. [Google Scholar] [CrossRef]
- Harvey, J.; Gomez-Velez, J.; Schmadel, N.; Scott, D.; Boyer, E.; Alexander, R.; Eng, K.; Golden, H.; Kettner, A.; Konrad, C.; et al. How Hydrologic Connectivity Regulates Water Quality in River Corridors. J. Am. Water Resour. Assoc. 2019, 55, 369–381. [Google Scholar] [CrossRef]
- Liang, D.; Wei, N.; Wang, Q.; Jersabek, C.D.; He, X.; Yang, Y. Influence of Hydrological Heterogeneity on Rotifer Community Structure in Three Different Water Bodies in Shantou Area, Guangdong (China). Zool. Stud. 2019, 58, e23. [Google Scholar]
- Zhu, L.; Chen, Y.; Wang, Y.; Wang, C.; Wei, Y. Ecological assessment of water quality in an urban river replenished with reclaimed water: The phytoplankton functional groups approach. Environ. Res. Commun. 2021, 3, 115006. [Google Scholar] [CrossRef]
- Meyer, J.L.; Paul, M.J.; Taulbee, W.K. Stream ecosystem function in urbanizing landscapes. J. North Am. Benthol. Soc. 2005, 24, 602–612. [Google Scholar] [CrossRef]
- Yang, J.; Wang, F.; Lv, J.; Liu, Q.; Nan, F.; Liu, X.; Xu, L.; Xie, S.; Feng, J. Interactive effects of temperature and nutrients on the phytoplankton community in an urban river in China. Env. Monit. Assess. 2019, 191, 688. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Wang, Q.; Wei, N.; Tang, C.; Sun, X.; Yang, Y. Biological indicators of ecological quality in typical urban river-lake ecosystems: The planktonic rotifer community and its response to environmental factors. Ecol. Indic. 2020, 112, 106127. [Google Scholar] [CrossRef]
- Li, D.; Chen, Q.-B.; Guo, L.; Liu, S.; Wang, Y.; Yang, F.; Lv, B.Y.; Zhang, X.; Dong, Q.; Wang, R. Dynamic Disturbance of Water Environment in Urban Rivers Flowing Through Industrial Areas. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Petsch, D.K. Causes and consequences of biotic homogenization in freshwater ecosystems. Int. Rev. Hydrobiol. 2016, 101, 113–122. [Google Scholar] [CrossRef]
- Olden, J.D.; Rooney, T.P. On defining and quantifying biotic homogenization. Glob. Ecol. Biogeogr. 2006, 15, 113–120. [Google Scholar] [CrossRef]
- Zorzal-Almeida, S.; Bartozek, E.C.R.; Bicudo, D.C. Homogenization of diatom assemblages is driven by eutrophication in tropical reservoirs. Environ. Pollut. 2021, 288, 117778. [Google Scholar] [CrossRef] [PubMed]
- Wengrat, S.; Padial, A.A.; Jeppesen, E.; Davidson, T.A.; Fontana, L.; Costa-Böddeker, S.; Bicudo, D.C. Paleolimnological records reveal biotic homogenization driven by eutrophication in tropical reservoirs. J. Paleolimnol. 2017, 60, 299–309. [Google Scholar] [CrossRef]
- Villéger, S.; Grenouillet, G.; Brosse, S. Functional homogenization exceeds taxonomic homogenization among European fish assemblages. Glob. Ecol. Biogeogr. 2014, 23, 1450–1460. [Google Scholar] [CrossRef]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Environ. 2010, 9, 222–228. [Google Scholar] [CrossRef]
- Mason, N.W.H.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Petchey, O.L.; Gaston, K.J. Functional diversity: Back to basics and looking forward. Ecol. Lett. 2006, 9, 741–758. [Google Scholar] [CrossRef]
- Zhang, Y.; He, N.; Loreau, M.; Pan, Q.; Han, X. Scale dependence of the diversity-stability relationship in a temperate grassland. J. Ecol. 2018, 106, 1227–1285. [Google Scholar] [CrossRef] [PubMed]
- Obertegger, U.; Flaim, G. Taxonomic and functional diversity of rotifers, what do they tell us about community assembly? Hydrobiologia 2018, 823, 79–91. [Google Scholar] [CrossRef]
- Braghin, L.d.S.M.; Almeida, B.d.A.; Amaral, D.C.; Canella, T.F.; Gimenez, B.C.G.; Bonecker, C.C. Effects of dams decrease zooplankton functional β-diversity in river-associated lakes. Freshw. Biol. 2018, 63, 721–730. [Google Scholar] [CrossRef]
- Stegen, J.C.; Hurlbert, A.H. Inferring ecological processes from taxonomic, phylogenetic and functional trait beta-diversity. PLoS ONE 2011, 6, e20906. [Google Scholar] [CrossRef] [PubMed]
- Baselga, A.; Orme, C.D.L. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Marini, L.; Bertolli, A.; Bona, E.; Federici, G.; Martini, F.; Prosser, F.; Bommarco, R. Beta-diversity patterns elucidate mechanisms of alien plant invasion in mountains. Glob. Ecol. Biogeogr. 2013, 22, 450–460. [Google Scholar] [CrossRef]
- Villéger, S.; Grenouillet, G.; Brosse, S. Decomposing functional β-diversity reveals that low functional β-diversity is driven by low functional turnover in European fish assemblages. Glob. Ecol. Biogeogr. 2013, 22, 671–681. [Google Scholar] [CrossRef]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999, 14, 450–453. [Google Scholar] [CrossRef]
- Socolar, J.B.; Gilroy, J.J.; Kunin, W.E.; Edwards, D.P. How Should Beta-Diversity Inform Biodiversity Conservation? Trends Ecol. Evol. 2016, 31, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Obertegger, U.; Manca, M. Response of rotifer functional groups to changing trophic state and crustacean community. J. Limnol. 2011, 70, 231–238. [Google Scholar] [CrossRef]
- Wen, X.; Zhai, P.; Feng, R.; Yang, R.; Xi, Y. Comparative analysis of the spatio-temporal dynamics of rotifer community structure based on taxonomic indices and functional groups in two subtropical lakes. Sci. Rep. 2017, 7, 578. [Google Scholar] [CrossRef]
- Oh, H.J.; Jeong, H.G.; Nam, G.S.; Oda, Y.; Dai, W.; Lee, E.H.; Kong, D.; Hwang, S.J.; Chang, K.H. Comparison of taxon-based and trophi-based response patterns of rotifer community to water quality: Applicability of the rotifer functional group as an indicator of water quality. Anim. Cells Syst. 2017, 21, 133–140. [Google Scholar] [CrossRef]
- GB 3838-2002; Water Quality Standards for Surface Water. China Environmental Science Press: Beijing, China, 2002.
- Obertegger, U.; Flaim, G.; Sommaruga, R. Multifactorial nature of rotifer water layer preferences in an oligotrophic lake. J. Plankton Res. 2008, 30, 633–643. [Google Scholar] [CrossRef]
- Obertegger, U.; Flaim, G. Community assembly of rotifers based on morphological traits. Hydrobiologia 2015, 753, 31–45. [Google Scholar] [CrossRef]
- Station, C.E.M. Lakes (Reservoirs) Eutrophication Assessment Methods and Classification Technology Requirements; China Environmental Monitoring Station: Beijing, China, 2001. [Google Scholar]
- Jin, X.C.; Liu, S.K.; Zhang, Z.S.; Tu, Q.Y.; Xu, N.N. Lake Environment in China; Ocean Press: Beijing, China, 1995. [Google Scholar]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. vegan: Community Ecology Package. R Package Version 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 17 January 2022).
- Laliberté, E.; Legendre, P.; Shipley, B. FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. R Package Version 2014, 1.0-12.1. Available online: https://www.imsbio.co.jp/RGM/R_rdfile?f=FD/man/FD-package.Rd&d=R_CC (accessed on 16 February 2022).
- Barnum, T.R.; Weller, D.E.; Williams, M. Urbanization reduces and homogenizes trait diversity in stream macroinvertebrate communities. Ecol. Appl. 2017, 27, 2428–2442. [Google Scholar] [CrossRef]
- Mouchet, M.A.; Villéger, S.; Mason, N.W.H.; Mouillot, D. Functional diversity measures: An overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 2010, 24, 867–876. [Google Scholar] [CrossRef]
- Dray, S.; Bauman, D.; Blanchet, G.; Borcard, D.; Clappe, S.; Guénard, G.; Jombart, T.; Larocque, G.; Legendre, P.; Madi, N.; et al. adespatial: Multivariate Multiscale Spatial Analysis. R Package Version 0.3-20. 2022. Available online: https://CRAN.R-project.org/package=adespatial (accessed on 19 February 2022).
- Siberchicot, A.; Julien-Laferrière, A.; Dufour, A.B.; Thioulouse, J.; Dray, S. adegraphics: An S4 Lattice-Based Package for the Representation of Multivariate Data. R J. 2017, 9, 198–212. [Google Scholar] [CrossRef]
- Wang, J.; Fu, Z.; Qiao, H.; Liu, F. Assessment of eutrophication and water quality in the estuarine area of Lake Wuli, Lake Taihu, China. Sci. Total Environ. 2019, 650 Pt 1, 1392–1402. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.-B. The ade 4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Legendre, P. Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr. 2014, 23, 1324–1334. [Google Scholar] [CrossRef]
- Marcacci, G.; Westphal, C.; Wenzel, A.; Raj, V.; Nolke, N.; Tscharntke, T.; Grass, I. Taxonomic and functional homogenization of farmland birds along an urbanization gradient in a tropical megacity. Glob. Change Biol. 2021, 27, 4980–4994. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wei, T.; Simko, V. R package ’corrplot’: Visualization of a Correlation Matrix (Version 0.92). 2021. Available online: https://github.com/taiyun/corrplot (accessed on 17 February 2022).
- Dadashpoor, H.; Azizi, P.; Moghadasi, M. Land use change, urbanization, and change in landscape pattern in a metropolitan area. Sci. Total Environ. 2019, 655, 707–719. [Google Scholar] [CrossRef]
- Galir Balkić, A. The importance of environmental differences in the structuring of rotifer functional diversity. J. Limnol. 2019, 78. [Google Scholar] [CrossRef]
- Hillbricht-Ilkowska, A. Response of planktonic rotifers to the eutrophication process and to the autumnal shift of blooms in Lake Biwa, Japan. I. Changes in abundance and composition of rotifers. Jpn. J. Limnol. (rikusuigaku Zasshi) 1983, 44, 93–106. [Google Scholar] [CrossRef]
- da Silva, N.J.; Lansac-Tôha, F.M.; Lansac-Tôha, F.A.; Sales, P.C.L.; de Sousa Rocha, J.d.R. Beta diversity patterns in zooplankton assemblages from a semiarid river ecosystem. Int. Rev. Hydrobiol. 2020, 106, 29–40. [Google Scholar] [CrossRef]
- Brice, M.-H.; Pellerin, S.; Poulin, M.; Pysek, P. Does urbanization lead to taxonomic and functional homogenization in riparian forests? Divers. Distrib. 2017, 23, 828–840. [Google Scholar] [CrossRef]
- Perez Rocha, M.; Bini, L.M.; Gronroos, M.; Hjort, J.; Lindholm, M.; Karjalainen, S.M.; Tolonen, K.E.; Heino, J. Correlates of different facets and components of beta diversity in stream organisms. Oecologia 2019, 191, 919–929. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, L.; Li, K.; Zhang, L.; Cai, Y.; Wang, X.; Heino, J. Nutrient enrichment homogenizes taxonomic and functional diversity of benthic macroinvertebrate assemblages in shallow lakes. Limnol. Oceanogr. 2018, 64, 1047–1058. [Google Scholar] [CrossRef]
- Josué, I.I.P.; Cardoso, S.J.; Miranda, M.; Mucci, M.; Ger, K.A.; Roland, F.; Marinho, M.M. Cyanobacteria dominance drives zooplankton functional dispersion. Hydrobiologia 2018, 831, 149–161. [Google Scholar] [CrossRef]
- Sol, D.; Trisos, C.; Murria, C.; Jeliazkov, A.; Gonzalez-Lagos, C.; Pigot, A.L.; Ricotta, C.; Swan, C.M.; Tobias, J.A.; Pavoine, S. The worldwide impact of urbanisation on avian functional diversity. Ecol. Lett. 2020, 23, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.S.; Ota, A.T.; Fujii, S.; Seino, T.; Kabeya, D.; Okamoto, T.; Ito, M.T.; Kaneko, N.; Hasegawa, M. Concordance and discordance between taxonomic and functional homogenization: Responses of soil mite assemblages to forest conversion. Oecologia 2015, 179, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Flynn, D.F.; Gogol-Prokurat, M.; Nogeire, T.; Molinari, N.; Richers, B.T.; Lin, B.B.; Simpson, N.; Mayfield, M.M.; DeClerck, F. Loss of functional diversity under land use intensification across multiple taxa. Ecol. Lett. 2009, 12, 22–33. [Google Scholar] [CrossRef] [PubMed]
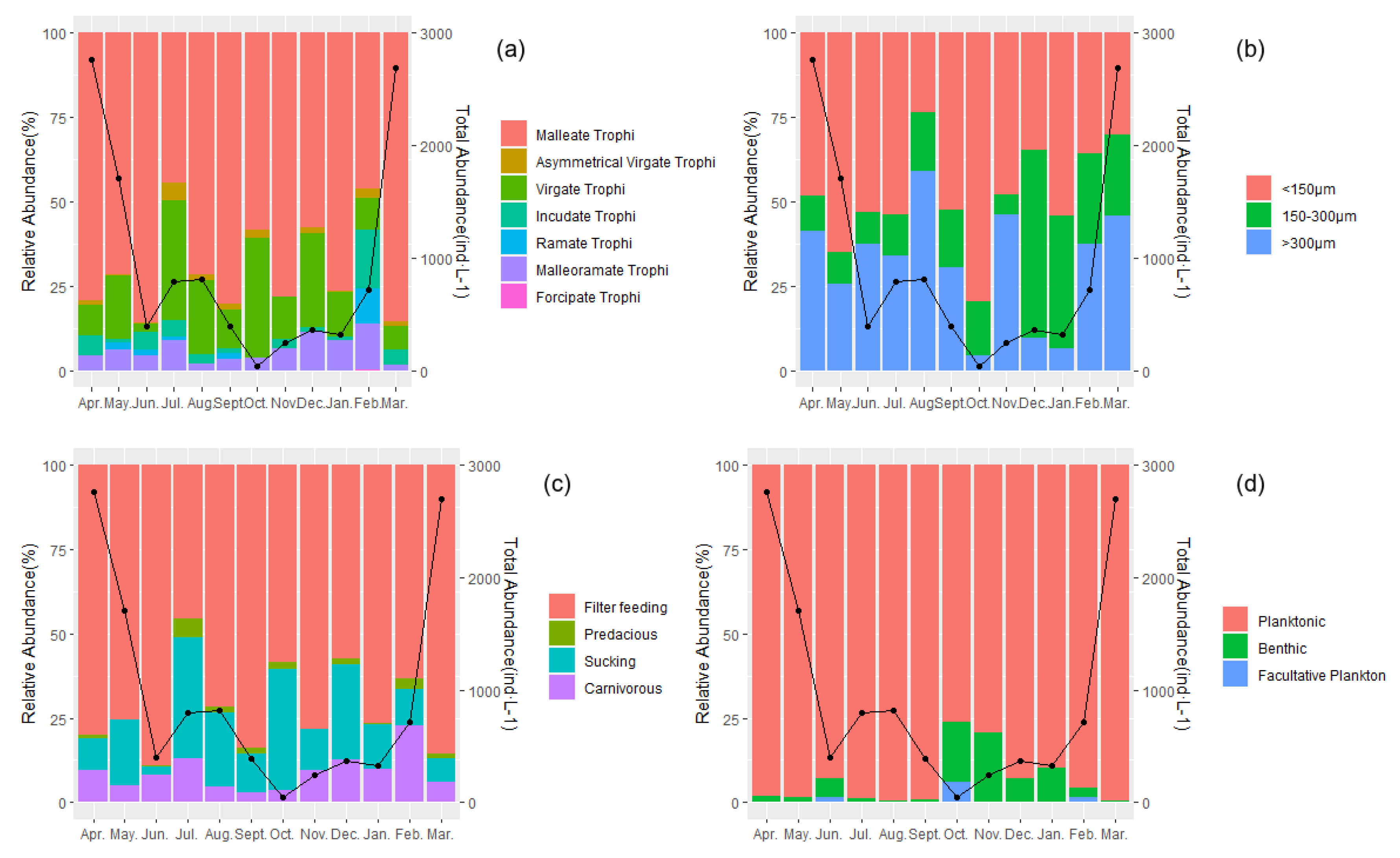
| Trait Category | Trait Modality | Typical Species |
|---|---|---|
| trophi | (1) malleate trophieoram | Brachionus calyciflorus; Keratella valga |
| (2) asymmetrical virgate trophi | Diurella weberi; Trichocerca pusilla | |
| (3) virgate trophi | Polyarthra trigla; Synchaeta tremula | |
| (4) incudate trophi | Asplanchna girodi | |
| (5) ramate trophi | Conochilus unicornis | |
| (6) malleoramate trophi | Pompholyx complanata | |
| (7) forcipate trophi | Dicranophorus caudatus | |
| size | (1) <150 μm | Keratella valga; Brachionus urceus |
| (2) 150–300 μm | Brachionus leydigi; Monostyla lunaris | |
| (3) >300 μm | Asplanchna priodonta; Epiphanes senta | |
| feeding habits | (1) filter-feeding | Brachionus calyciflorus; Conochilus unicornis |
| (2) predacious | Asplanchna brightwelli; Asplanchna priodonta | |
| (3) sucking | Synchaeta atylata; Polyarthra trigla | |
| (4) carnivorous | Trichocerca pusilla; Diurella weberi | |
| swimming | (1) planktonic | Brachionus angularis; Monostyla bulla |
| (2) benthic | Brachionus urceus; Euchlanis dilatata | |
| (3) Facultative plankton | Epiphanes senta |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Yin, X. Homogenization of Functional Diversity of Rotifer Communities in Relation to Eutrophication in an Urban River of North China. Biology 2023, 12, 1488. https://doi.org/10.3390/biology12121488
Wang B, Yin X. Homogenization of Functional Diversity of Rotifer Communities in Relation to Eutrophication in an Urban River of North China. Biology. 2023; 12(12):1488. https://doi.org/10.3390/biology12121488
Chicago/Turabian StyleWang, Bing, and Xuwang Yin. 2023. "Homogenization of Functional Diversity of Rotifer Communities in Relation to Eutrophication in an Urban River of North China" Biology 12, no. 12: 1488. https://doi.org/10.3390/biology12121488
APA StyleWang, B., & Yin, X. (2023). Homogenization of Functional Diversity of Rotifer Communities in Relation to Eutrophication in an Urban River of North China. Biology, 12(12), 1488. https://doi.org/10.3390/biology12121488





