Morphological Variability and Function of Labial Cartilages in Sharks (Chondrichthyes, Elasmobranchii)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Morphological Descriptions
3. Results
3.1. Morphological Descriptions
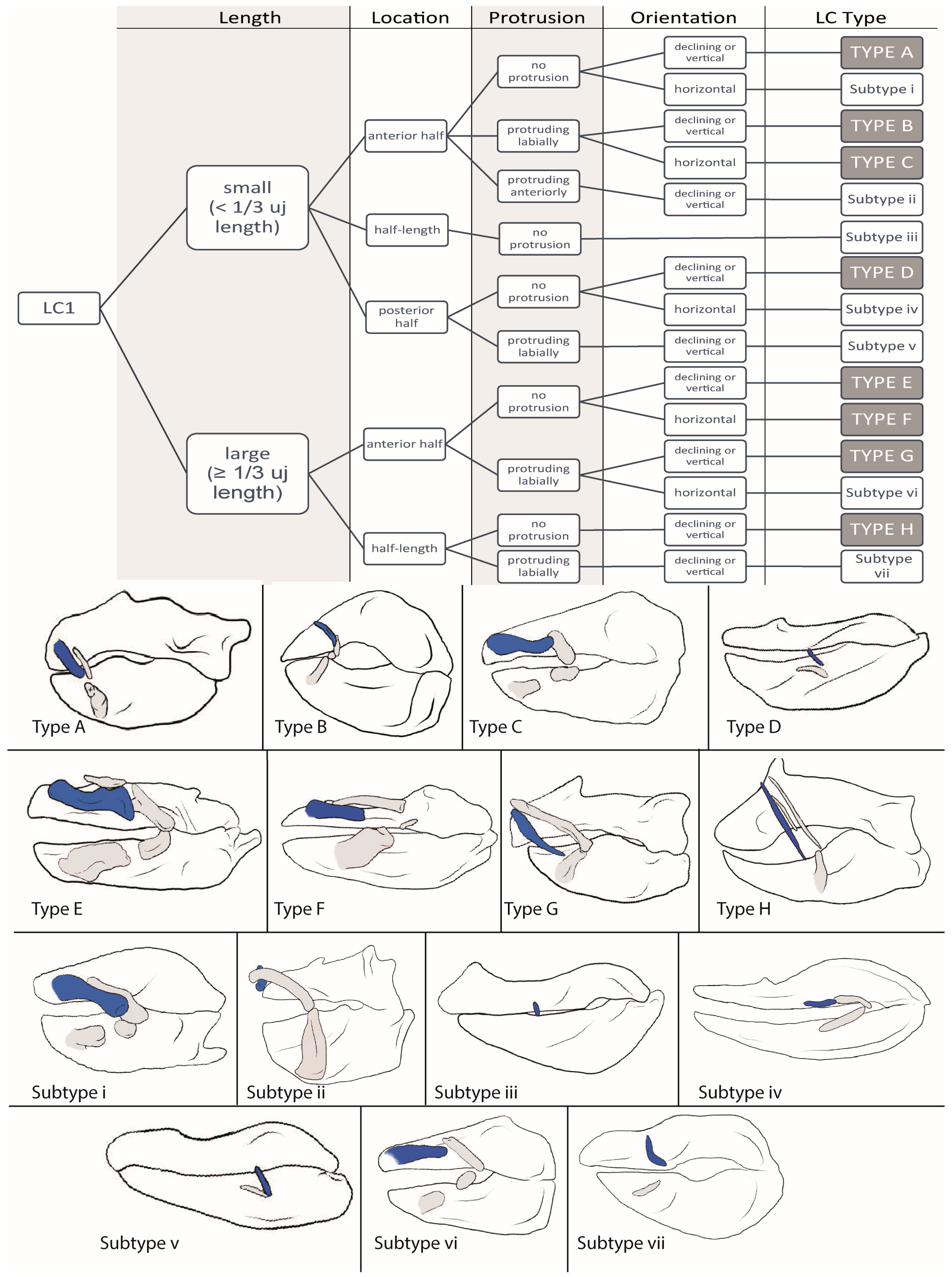
3.1.1. LC1 (Figure 2)
3.1.2. LC2 (Figure 3)

3.1.3. LC2.1 (Figure 4)
3.1.4. LC3.1 (Figure 4)

3.1.5. LC3 (Figure 5)

3.2. Interpretations
3.2.1. LC1
3.2.2. LC2
3.2.3. LC2.1
3.2.4. LC3.1
3.2.5. LC3
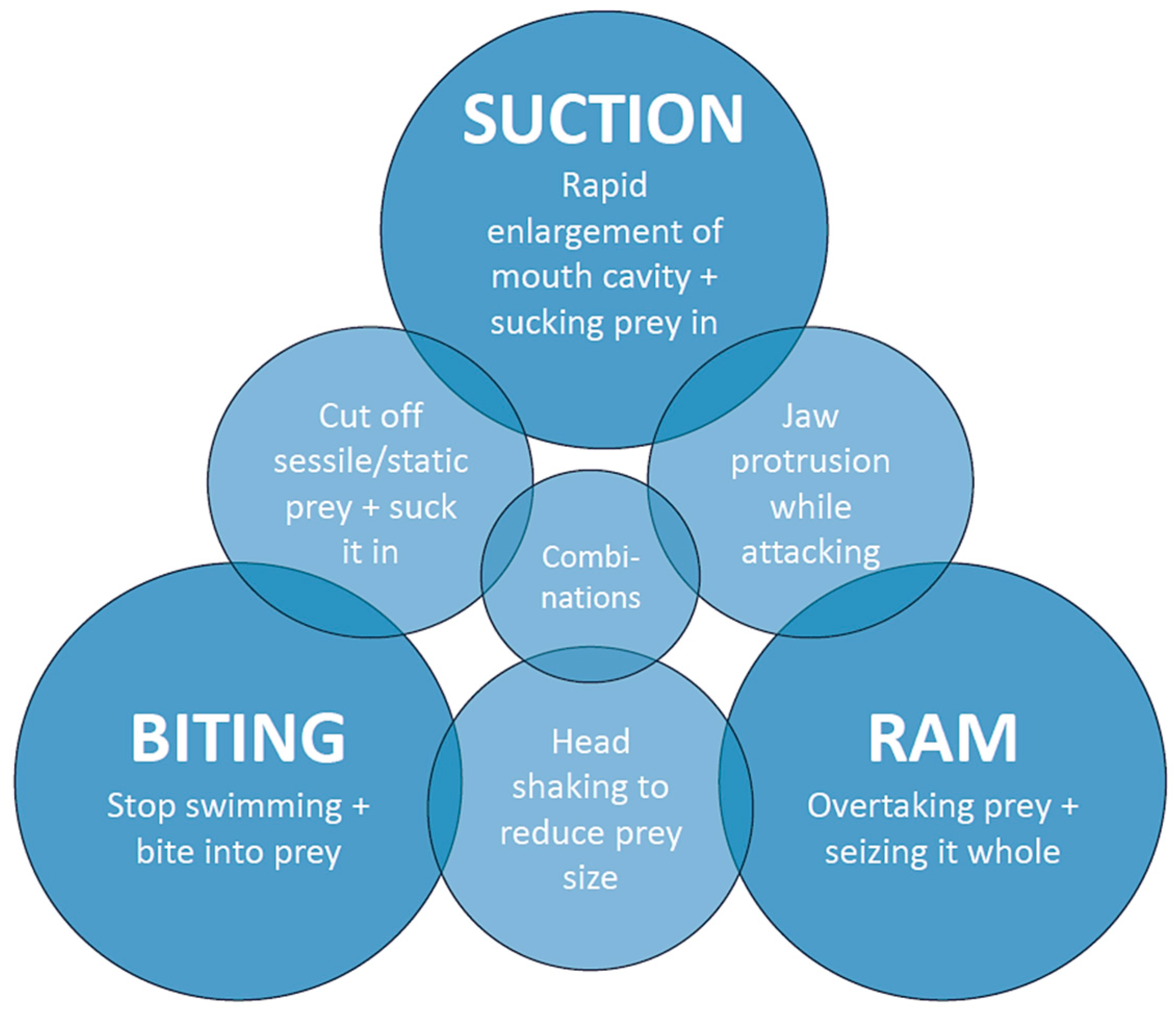
4. Discussion
- (1)
- Number of LC pairs: 0–1 = no suction; 2–3 = suction of different intensity used in combination with ram or biting behavior; and 4–5 = strong suction resulting in suction as the predominant or even exclusive feeding mechanism.
- (2)
- Position of the LCs: The further anterior the LCs 1 and 3 are located, the larger the volume of the mouth cavity. The strongest suction is developed when the LCs are in their extended position and the mouth opening is at its peak [8,53]. LCs 2, 2.1, and 3.1 can be aligned with LCs 1 or 3, or parallel to them, which again influences the mouth volume when the mouth is opened. Aligned LCs provide a volume enhancement, while parallel LCs reinforce the existing LCs and thus strengthen the lateral mouth gape walls, enabling them to bear a stronger suction and possibly can somewhat move along the other LCs.
- (3)
- Orientation of the LCs: The more horizontal the LCs are oriented in their resting position, e.g., when the mouth is closed, the larger their movement when the mouth is opened, since they then move antero-labially [28]. This results in a greater enlargement of the mouth cavity and therefore the volume, increasing the suction force. An LC that protrudes labially provides additional support for a larger mouth volume and therefore a stronger suction.
- (4)
- Size of the LCs: The larger a LC is, the more force it can endure, which influences the possible suction strength.
4.1. Number of Labial Cartilages
4.2. Shapes of Labial Cartilages
4.3. Labial Cartilage Mobility and Adaptations for Special Feeding Strategies
4.4. Labial Cartilage Combinations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maisey, J.G. What is an ‘elasmobranch’? The impact of palaeontology in understanding elasmobranch phylogeny and evolution. J. Fish Biol. 2012, 80, 918–951. [Google Scholar] [CrossRef] [PubMed]
- Cappetta, H. Handbook of Paleoichthyology. Volume 3B. Chondrichthyes II. Mesozoic and Cenozoic Elasmobranchii; Fischer Verlag: Stuttgart, Germany, 1987. [Google Scholar]
- Klug, S. Monophyly, phylogeny and systematic position of the †Synechodontiformes (Chondrichthyes, Neoselachii). Zool. Scr. 2010, 39, 37–49. [Google Scholar] [CrossRef]
- Huber, D.; Wilga, C.D.; Dean, M.; Ferry, L.; Gardiner, J.; Habegger, L.; Papastamatiou, Y.; Ramsay, J.; Whitenack, L. Feeding in Cartilaginous Fishes: An Interdisciplinary Synthesis. In Feeding in Vertebrates: Evolution, Morphology, Behavior, Biomechanics; Bels, V., Whishaw, I.Q., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Dick, J.R.F. On the Carboniferous shark Tristychius arcuatus Agassiz from Scotland. Earth Environ. Sci. Trans. R. Soc. Edinb. 1978, 70, 63–109. [Google Scholar] [CrossRef]
- Maisey, J.G. The Anatomy and Interrelationships of Mesozoic Hybodont Sharks. Am. Mus. Novit. 1982, 2724, 1–48. [Google Scholar]
- Maisey, J.G. Cranial anatomy of Hybodus basanus Egerton from the Lower Cretaceous of England. Am. Mus. Novit. 1983, 2758, 59–64. [Google Scholar]
- Motta, P.J.; Wilga, C.D. Advances in the study of feeding behaviors, mechanisms, and mechanics of sharks. Environ. Biol. Fishes 2001, 60, 131–156. [Google Scholar] [CrossRef]
- Klug, S.; Kriwet, J.; Böttcher, R.; Schweitert, G.; Dietl, G. Skeletal anatomy of the extinct shark Paraorthacodus jurensis (Chondrichthyes; Palaeospinacidae), with comments on synechodontiform and palaeospinacid monophyly. Zool. J. Linn. Soc. 2009, 157, 107–134. [Google Scholar] [CrossRef]
- Coates, M.I.; Tietjen, K.; Olsen, A.M.; Finarelli, J.A. High-performance suction feeding in an early elasmobranch. Sci. Adv. 2019, 5, eaax2742. [Google Scholar] [CrossRef]
- Dean, M.N.; Huber, D.R.; Nanace, H.A. Functional Morphology of Jaw Trabeculation in the Lesser Electric Ray Narcine brasiliensis, With comments on the Evolution of Structural Support in the Batoidea. J. Morphol. 2006, 267, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.N.; Azizi, E.; Summers, A.P. Uniform strain in broad muscles: Active and passive effects of the twisted tendon of the spotted ratfish Hydrolagus collieli. J. Exp. Biol. 2007, 210, 3395–3406. [Google Scholar] [CrossRef]
- Dean, M.N.; Summers, A.P.; Ferry, L.A. Very Low Pressures Drive Ventilatory Flow in Chimaeroid Fishes. J. Morphol. 2012, 273, 461–479. [Google Scholar] [CrossRef]
- Dearden, R.P.; Mansuit, R.; Cuckovic, A.; Herrel, A.; Didier, D.; Tafforeau, P.; Pradel, A. The morphology and evolution of chondrichthyan cranial muscles: A digital dissection of the elephantfish Callorhinchus milii and the catshark Scyliorhinus canicular. J. Anat. 2021, 238, 1081–1105. [Google Scholar] [CrossRef]
- Denison, R.H. Anatomy of the head and pelvic fin of the Whale Shark, Rhineodon. Bull. Am. Mus. Nat. Hist. 1937, 73, 477–515. [Google Scholar]
- Motta, P.J.; Wilga, C.D. Anatomy of the feeding apparatus of the nurse shark, Ginglymostoma cirratum. J. Morphol. 1999, 241, 33–60. [Google Scholar] [CrossRef]
- Gardiner, J.M.; Atema, J.; Hueter, R.E.; Motta, P.J. Modulation of shark prey capture kinematics in response to sensory deprivation. Zoology 2017, 120, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, M.A.; Motta, P.J.; Hueter, R.E. Food capture kinematics on the suction feeding horn shark, Heterodontus francisci. Environ. Biol. Fishes 2001, 62, 415–427. [Google Scholar] [CrossRef]
- Shimada, K.; Rigsby, C.K.; Kim, S.H. Labial cartilages in the smalltooth Sandtiger shark, Odontaspis ferox (Lamniformes: Odontaspididae) and their significance to the phylogeny of lamniform sharks. Anat. Rec. 2009, 292, 813–817. [Google Scholar] [CrossRef]
- Gegenbauer, C. Untersuchungen zur vergleichenden Anatomie der Wirbeltiere—Heft 3: Das Kopfskelett der Selachier, als Grundlage zur Beurtheilung der Genese des Kopfskeletes der Wirbelthiere; W. Engelmann Verlag: Leipzig, Germany, 1872; p. 316 ff. [Google Scholar] [CrossRef]
- Pollard, H.B. Oral cirrhi of siluroids and the origin of the head in vertebrates. Zool. Jahrb. (Abt. Anat.) 1895, 8, 379–424. [Google Scholar]
- Swertzoff, A.N. Etudes sur l’evolution des vertebras inférieurs—1. Morphologie du suelette et de la musculature de la téte des cyclostomes. Arch. Russes Anat. Histol. Embryol. 1916, 1, 1–104. [Google Scholar]
- Goodrich, E.S. Studies on the Structure & Development of Vertebrates; Macmillan & Company: London, UK, 1930. [Google Scholar]
- Smith, B.G. The Bashford Dean Memorial Volume of Archaic Fishes—Article VI: The Anatomy of the Frilled Shark Chlamydoselachus anguineus Garman; American Museum of Natural History: New York, NY, USA, 1937; pp. 330–498. [Google Scholar]
- Veran, M. Are the labial cartilages of Chondrichthyens homologous to the labial bone of the primitive fossil actinopterygians? Geobios 1995, 19, 161–166. [Google Scholar] [CrossRef]
- Klimpfinger, C.; Kriwet, J. Comparative morphology of labial cartilages in sharks (Chondrichthyes, Elasmobranchii). Eur. Zool. J. 2020, 87, 741–753. [Google Scholar] [CrossRef]
- Wu, E.H. Kinematic Analysis of Jaw Protrusion in Orectolobiform Sharks: A New Mechanism for Jaw Protrusion in Elasmobranchs. J. Morphol. 1994, 222, 175–190. [Google Scholar] [CrossRef]
- Motta, P.J.; Hueter, R.E.; Tricas, T.C.; Summers, A.P.; Huber, D.R.; Lowry, D.; Mara, K.R.; Matott, M.P.; Whitenack, L.B.; Wintzer, A.P. Functional Morphology of the Feeding Apparatus, Feeding Constraints, and Suction Performance in the Nurse Shark Ginglymostoma cirratum. J. Morphol. 2008, 269, 1041–1055. [Google Scholar] [CrossRef]
- Brazeau, M.; Kamminga, P.; De Bruin, P.W.; Geleijns, J. X-ray Computed Tomography Library of Shark Anatomy and Lower Jaw Models. On: Figshare. Collection. Available online: https://doi.org/10.6084/m9.figshare.c.3662366.v1 (accessed on 15 November 2023).
- Kamminga, P.; de Bruin, P.W.; Geleijns, J.; Brazeau, M.D. X-ray computed tomography library of shark anatomy and lower jaw surface models. Sci. Data 2017, 4, 170047. [Google Scholar] [CrossRef] [PubMed]
- Chondrichthyan Tree of Life. Available online: https://sharksrays.org/ (accessed on 25 October 2023).
- Corrigan, S.; Naylor, G.; Yang, L. The Chondrichthyan Tree of Life Project: Taking Stock of the World’s Sharks and Rays, Copyright Experiment 2023. Available online: https://experiment.com/projects/the-chondrichthyan-tree-of-life-project-taking-stock-of-the-world-s-sharks-and-rays (accessed on 24 October 2023).
- White, P.J. XV.—The skull and visceral skeleton of the Greenland shark, Læmargus microcephalus. Earth Environ. Sci. Trans. R. Soc. Edinb. 1895, 37, 287–306. [Google Scholar] [CrossRef]
- Summers, A.P.; Ketcham, R.A.; Rowe, T. Structure and Function of the Horn Shark (Heterodontus francisci) Cranium Through Ontogeny: Development of a Hard Prey Specialist. J. Morphol. 2004, 260, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Staggl, M.A.; Ruthensteiner, B.; Straube, N. Head anatomy of a lantern shark wet-collection specimen (Chondrichthyes: Etmopteridae). J. Anat. 2023, 242, 872–890. [Google Scholar] [CrossRef]
- Lavenberg, R.J.; Seigel, J.A. The Pacific’s megamystery-Megamouth. Terra 1985, 23, 29–31. [Google Scholar]
- Capapé, C. Diet of the Angular rough shark Oxynouts centrina (Chondrichthyes: Oxynotidae) off the Languedocian coast (Sothern France, North-Western Mediterranean). Vie Milieu—Life Environ. 2008, 58, 57–61. [Google Scholar]
- Wieczorek, A.M.; Power, A.M.; Browne, P.; Graham, C.T. Stable-isotope analysis reveals the importance of soft-bodied prey in the diet of lesser spotted dogfish Scyliorhinus canicula. J. Fish Biol. 2018, 93, 685–693. [Google Scholar] [CrossRef]
- Scott, B.; Wilga, C.A.D.; Brainerd, E.L. Skeletal kinematics of the hyoid arch in the suction-feeding shark Chiloscyllium plagiosum. J. Exp. Biol. 2019, 222, jeb193573. [Google Scholar] [CrossRef]
- Fouts, W.R.; Nelson, D.R. Prey Capture by the Pacific Angel Shark, Squatina californica: Visually Mediated Strikes an Ambush-Site Characteristics. Copeia 1999, 2, 304–312. [Google Scholar] [CrossRef]
- De Figueiredo Petean, F.; de Carvalho, M.R. Comparative morphology and systematics of the cookiecutter sharks, genus Isistius Gill (1864) (Chondrichthyes: Squaliformes: Dalatiidae). PLoS ONE 2018, 13, e0201913. [Google Scholar] [CrossRef] [PubMed]
- Cade, D.E.; Levenson, J.J.; Cooper, R.; de la Parra, R.; Webb, D.H.; Dove, A.D.M. Whale sharks increase swimming effort while filter feeding, but appear to maintain high foraging efficiencies. J. Exp. Biol. 2020, 223, jeb224402. [Google Scholar] [CrossRef]
- Wilga, C.D. A functional analysis of jaw suspension in elasmobranchs. Biol. J. Linn. Soc. 2002, 75, 483–502. [Google Scholar] [CrossRef]
- Ramsay, J.B. A Comparative Investigation of Cranial Morphology, Mechanics, and Muscle Function in Suction and Bite Feeding Sharks. Ph.D. Thesis., University of Rhode Island, Kingston, RI, USA, 2012. [Google Scholar]
- Denton, J.S.S.; Maisey, J.G.; Grace, M.; Pradel, A.; Doosey, M.H.; Bart, H.L., Jr.; Naylor, G.J.P. Cranial morphology in Mollisquama sp. (Squaliformes; Dalatiidae) and patterns of cranial evolution in dalatiid sharks. J. Anat. 2018, 233, 15–32. [Google Scholar] [CrossRef]
- Nauwelaerts, S.; Wilga, C.; Sanford, C.; Lauder, G. Hydrodynamics of prey capture in sharks: Effects of substrate. J. R. Soc. Interface 2007, 4, 341–345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bels, V.; Whishaw, I.Q. Feeding in Vertebrates: Evolution, Morphology, Behavior, Biomechanics; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Cortés, E.; Papastamatiou, Y.P.; Carlson, J.K.; Ferry-Graham, L.; Wetherbee, B.M. An Overview of the Feeding Ecology and Physiology of Elasmobranch Fishes. In Feeding and Digestive Functions of Fishes; Cyrino, J.E.P., Bureau, D.P., Kapoor, B.G., Eds.; Science Publishers: Enfield, NH, USA, 2008; pp. 393–443. ISBN 9781578083756. [Google Scholar]
- Klimpfinger, C. Variability of Labial Cartilages in Sharks—Functional or Phylogenetic Signal? Morphological Descriptions and Phylogenetic Analysis. Master Thesis, University of Vienna, Vienna, Austria, 2022. Available online: https://ubdata.univie.ac.at/AC16587802 (accessed on 24 October 2023).
- Tricas, T.C. Feeding Ethology of the White Shark, Carcharodon carcharias. Memoirs 1985, 9, 81–91. [Google Scholar]
- Ebert, D.A. Observations on the predatory behaviour of the sevengill shark Notorynchus cepedianus. S. Afr. J. Mar. Sci. 1991, 11, 455–465. [Google Scholar] [CrossRef]
- Moyer, J.K.; Shannon, S.F.; Irschick, D.J. Bite performance and feeding behaviour of the sand tiger shark Carcharias taurus. J. Fish Biol. 2019, 95, 881–892. [Google Scholar] [CrossRef]
- Ferry, L.A.; Paig-Tran, E.M.; Gibbs, A.C. Suction, ram, and biting: Deviations and limitations to the capture of aquatic prey. Integr. Comp. Biol. 2015, 55, 97–109. [Google Scholar] [CrossRef]
- D’Iglio, C.; Savoca, S.; Rinelli, P.; Spanò, N.; Capillo, G. Diet of the Deep-Sea Shark Galeus melastomus Rafinesque 1810, in the Mediterranean Sea: What We Know and What We Should Know. Sustainability 2021, 13, 3962. [Google Scholar] [CrossRef]
- Motta, P.J.; Maslanka, M.; Hueter, R.E.; Davis, R.L.; de la Parra, R.; Mulvany, S.L.; Habegger, M.L.; Strother, J.A.; Mara, K.R.; Gardiner, J.M.; et al. Feeding anatomy, filter-feeding rate, and diet of whale sharks Rhincodon typus during surface ram filter feeding off the Yucatan Peninsula, Mexico. Zoology 2010, 113, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Papastamatiou, Y.P.; Wetherbee, B.M.; O’Sullivan, J.; Goodmanlowe, G.D.; Lowe, C.G. Foraging ecology of cookiecutter sharks (Isistius brasiliensis) on pelagic fishes in Hawaii, inferred from prey bite wounds. Environ. Biol. Fishes 2010, 88, 361–368. [Google Scholar] [CrossRef]
- Day, S.W.; Higham, T.E.; Holzmann, R.; van Wassenberg, S. Morphology, Kinematics, and Dynamics: The Mechanics of Suction Feeding in Fishes. Soc. Integr. Comp. Morphol. 2015, 55, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.C. Isistius brasiliensis, a Squaloid Shark, the Probable Cause of Crater Wounds on Fishes and Cetaceans. Fish. Bull. 1971, 69, 791–798. [Google Scholar]
- Shirai, S.; Nakaya, K. Functional morphology of feeding apparatus of the cookie-cutter shark, Isistius brasiliensis (Elasmobranchii, Dalatiinae). Zool. Sci. 1992, 9, 811–821. [Google Scholar]
- Hoyos-Padilla, M.; Papastamatiou, Y.P.; O’Sullivan, J.; Lowe, C.G. Observation of an attack by a cookiecutter shark (Isistius brasiliensis) on a White Shark (Carcharodon carcharias). Pac. Sci. 2013, 67, 129–134. [Google Scholar] [CrossRef]
- Huber, D.R.; Eason, T.G.; Hueter, R.E.; Motta, P.J. Analysis of the bite force and mechanical design of the feeding mechanism of the durophagous horn shark Heterodontus fancisci. J. Exp. Biol. 2005, 208, 3553–3571. [Google Scholar] [CrossRef]
- Motta, P.J.; Hueter, R.E.; Tricas, T.C.; Summers, A.P. Kinematic Analysis of Suction Feeding in the Nurse Shark, Ginglymostoma cirratum (Orectolobiformes, Ginglymostomatidae). Copeia 2002, 1, 24–38. [Google Scholar] [CrossRef]
- Kryukova, N.V.; Kuznetsov, A.N. Suboccipital muscle of sharpnose sevengill shark Heptranchias perlo and its possible role in prey dissection. Journal of Morphology 2020, 281, 842–861. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.M.; Sullivan, R.; Hedges, K.J. Greenland shark (Somniosus microcephalus) feeding behavior on static fishing gear, effect of SMART (Selective Magnetic and Repellent-Treated) hook deterrent technology, and factors influencing entanglement in bottom longlines. PeerJ 2018, 6. [Google Scholar] [CrossRef]
- Wilga, C.D.; Motta, P.J.; Sanford, C.P. Evolution and ecology of feeding in elasmobranchs. Integr. Comp. Biol. 2007, 47, 55–69. [Google Scholar] [CrossRef]
- Tomita, T.; Sato, K.; Suda, K.; Kawauchi, J.; Nakaya, K. Feeding of the Megamouth Shark (Pisces: Lamniformes: Megachasmidae) Predicted by Its Hyoid Arch: A Biomechanical Approach. J. Morphol. 2011, 272, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Motta, P.J.; Wilga, C.A.D. Anatomy of the Feeding Apparatus of the Lemon Shark, Negaprion brevirostris. J. Morphol. 1995, 226, 309–329. [Google Scholar] [CrossRef]
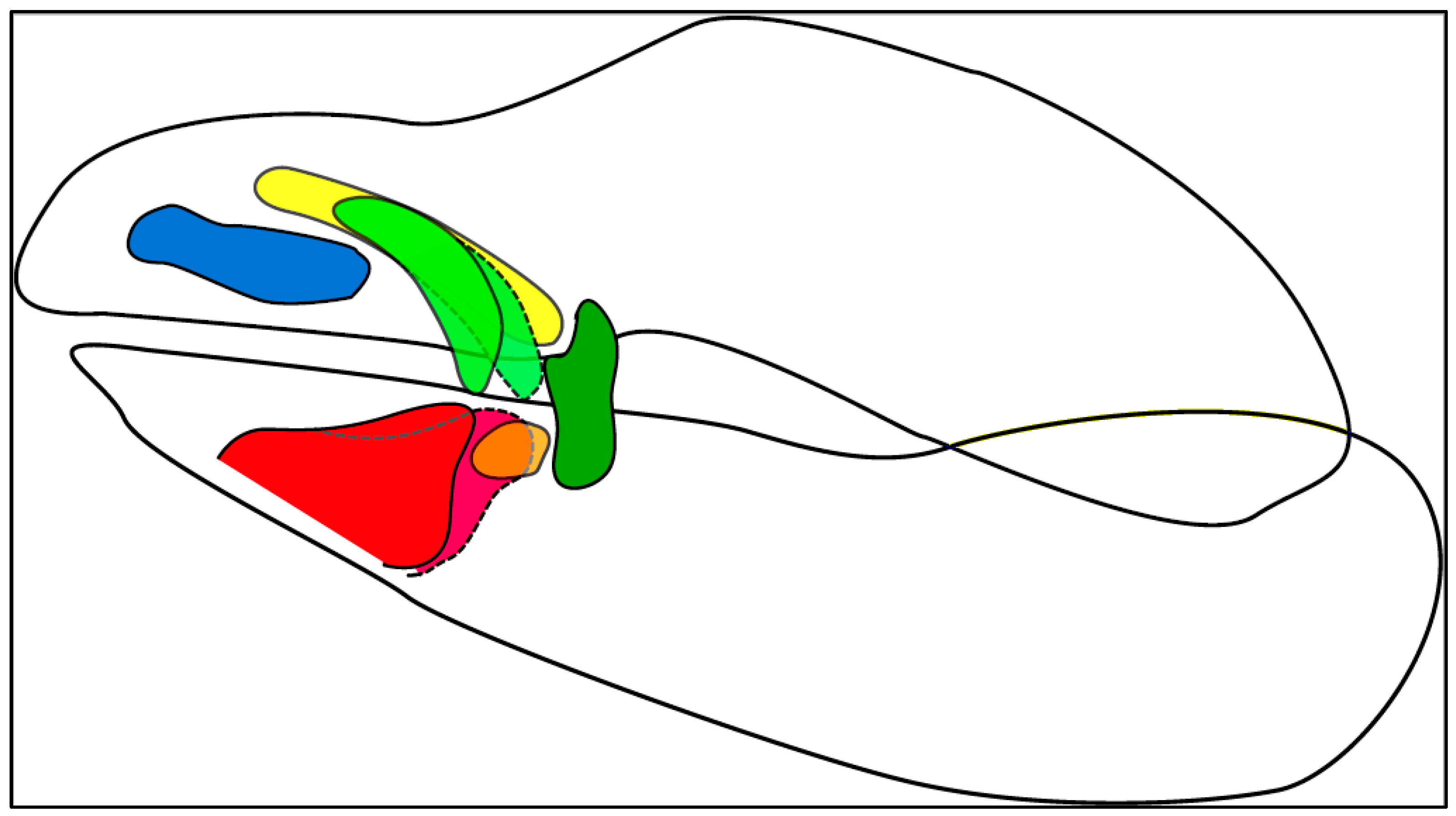
| Color Code | LC No. | Description |
|---|---|---|
| Blue | LC1 | Located/onset at the most anterior position along the upper jaw; sometimes connected to the jaw on the anterior end; mostly oriented along the anterior–posterior-axis; variable shapes |
| Green | LC2 | Located between LC1 and LC3 or between LC1 and LC3.1; never connected to the jaw; oriented antero-posteriorly in differing angles to the dorso-ventral axis; shape is elongated; forking on the anterior end in Orectolobidae |
| Yellow | LC2.1 | A segregated part of the forking of LC2 (see [27]); located dorsally to that of the LC1; never connected to the jaw; orientated antero-posteriorly; slender cartilage only present in Orectolobidae |
| Orange | LC3.1 | A segregated part of the LC3 (see [27]); located in the more posterior position along the lower jaw between LC2 and LC3; oriented antero-posteriorly; mostly short and stout |
| Red | LC3 | Located at the most anterior position along the lower jaw; often connected to the jaw on the anterior ventral end; oriented antero-posteriorly in differing angles to the dorso-ventral axis; variable shape |
| LC1 | LC2 | LC2.1 | LC3.1 | LC3 | Suction Capabilities | Example Species from the Dataset |
|---|---|---|---|---|---|---|
| 1LC only (no matter which) | no suction → biting or ram | Blue shark (Prionace glauca) | ||||
| D/H/iii | - | - | - | G | Lemon shark (Negaprion brevirostris) | |
| V | - | - | - | ii | no suction → extreme jaw protrusion | Goblin shark (Mitsukurina owstonii) |
| H | - | - | - | E | minor to no suction → biting | Small-tooth sand tiger (Odontaspis ferox) |
| H | A | - | B | iii | minor to no suction (uncertain) | Whitetail dogfish (Scymnodalatias albicauda) |
| A/E/F | (i) | - | - | B | minor suction → generalist, mainly biting | Blacktip sawtail catshark (Galeus sauteri) |
| A/E/G | - | - | - | E/F | Smooth-hound (Mustelus mustelus) | |
| G | - | - | - | C | Blackbelly lanternshark (Etmopterus lucifer) | |
| iv | i | - | - | i | Frilled shark (Chlamydoselachus anguineus) | |
| H | A | - | - | E | Barbeled houndshark (Leptocharias smithii) | |
| E | ii | - | - | F | medium to minor suction → probably scavenger | Bramble shark (Echinorhinus brucus) |
| E | A | - | - | A | Mosaic gulper shark (Centrophorus tesselatus) | |
| B/C/G/vi | C | - | (A) | E/F | medium suction + biting or ram → more effective when close to the ground or the surface | Whale shark (Rhincodon typus) |
| A/E/G | A | - | - | A/D/F | medium suction → generalist, mainly benthic prey | Knifetooth dogfish (Scymnodon ringens) |
| A/B/G | A/ii/iii | - | - | C | Angular roughshark (Oxynotus centrina) | |
| B | - | - | - | B | Redspotted catshark (Schroederichthys chilensis) | |
| A/ii | A/B | - | - | A | strong to medium suction → capable of longer-lasting suction (comparable to a suction cup); used for ectoparasitism | Cookie-cutter shark (Isistius brasiliensis) |
| vii | - | - | - | F | strong, short suction → mainly sessile prey | Horn shark (Heterodontus francisci) |
| E/F | C | A | A | B | strong suction → benthic ambush predator | Japanese wobbegong (Orectolobus japonicus) |
| A/E/F/i | B | - | (C) | E | strong suction → benthic ambush predator leaping from the bottom for strikes | Angelshark (Squatina squatina) |
| B/F/i | A | - | B | C/F | strong suction → benthic generalist | Nurse shark (Ginglymostoma cirratum) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimpfinger, C.; Kriwet, J. Morphological Variability and Function of Labial Cartilages in Sharks (Chondrichthyes, Elasmobranchii). Biology 2023, 12, 1486. https://doi.org/10.3390/biology12121486
Klimpfinger C, Kriwet J. Morphological Variability and Function of Labial Cartilages in Sharks (Chondrichthyes, Elasmobranchii). Biology. 2023; 12(12):1486. https://doi.org/10.3390/biology12121486
Chicago/Turabian StyleKlimpfinger, Claudia, and Jürgen Kriwet. 2023. "Morphological Variability and Function of Labial Cartilages in Sharks (Chondrichthyes, Elasmobranchii)" Biology 12, no. 12: 1486. https://doi.org/10.3390/biology12121486
APA StyleKlimpfinger, C., & Kriwet, J. (2023). Morphological Variability and Function of Labial Cartilages in Sharks (Chondrichthyes, Elasmobranchii). Biology, 12(12), 1486. https://doi.org/10.3390/biology12121486






