Near-Infrared Transillumination for Macroscopic Functional Imaging of Animal Bodies
Abstract
Simple Summary
Abstract
1. Introduction
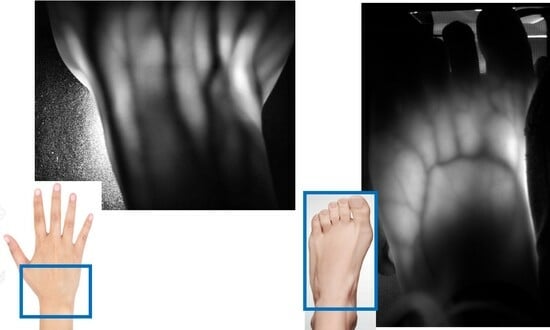
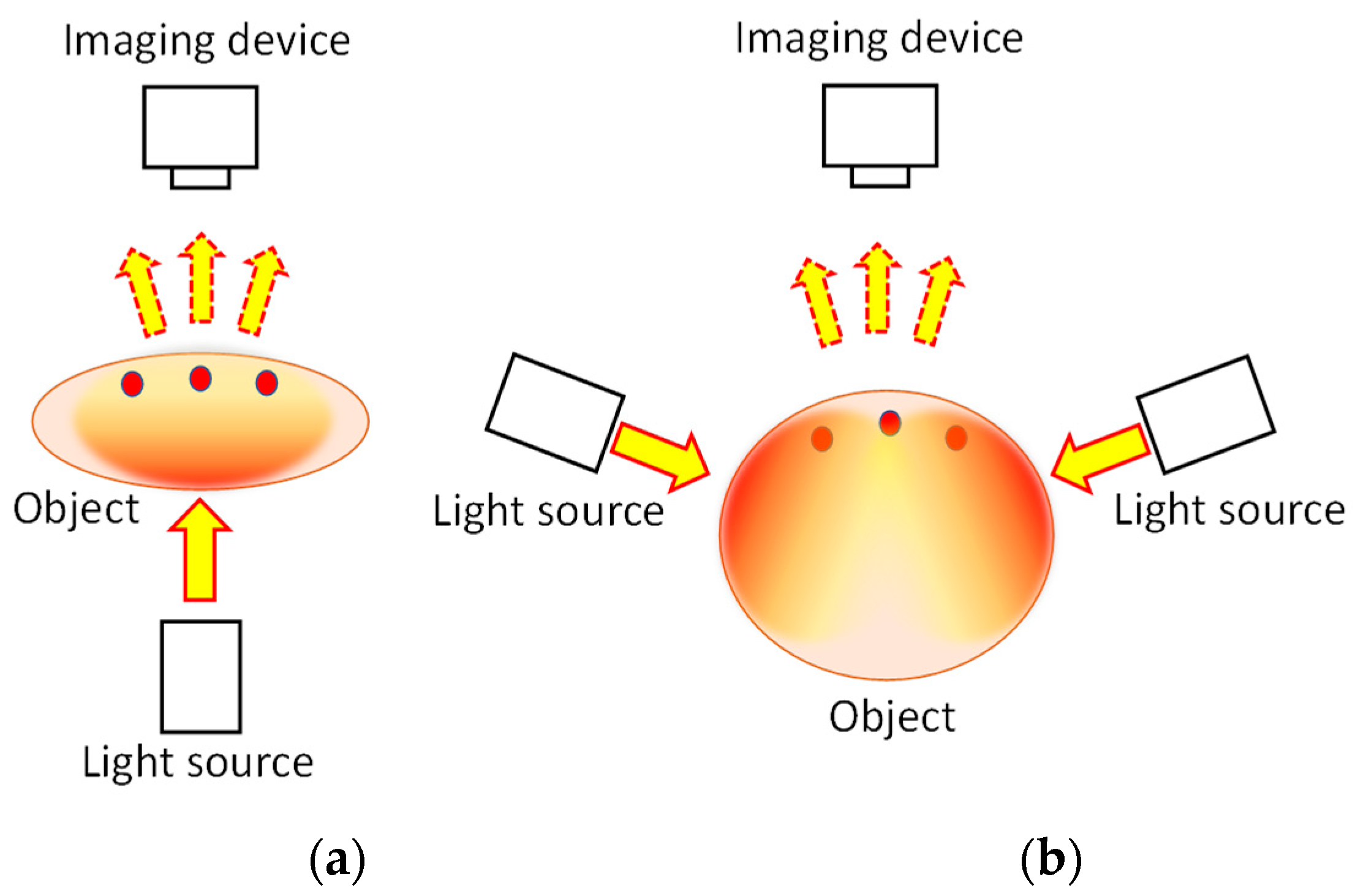
2. Two-Dimensional Transillumination Imaging
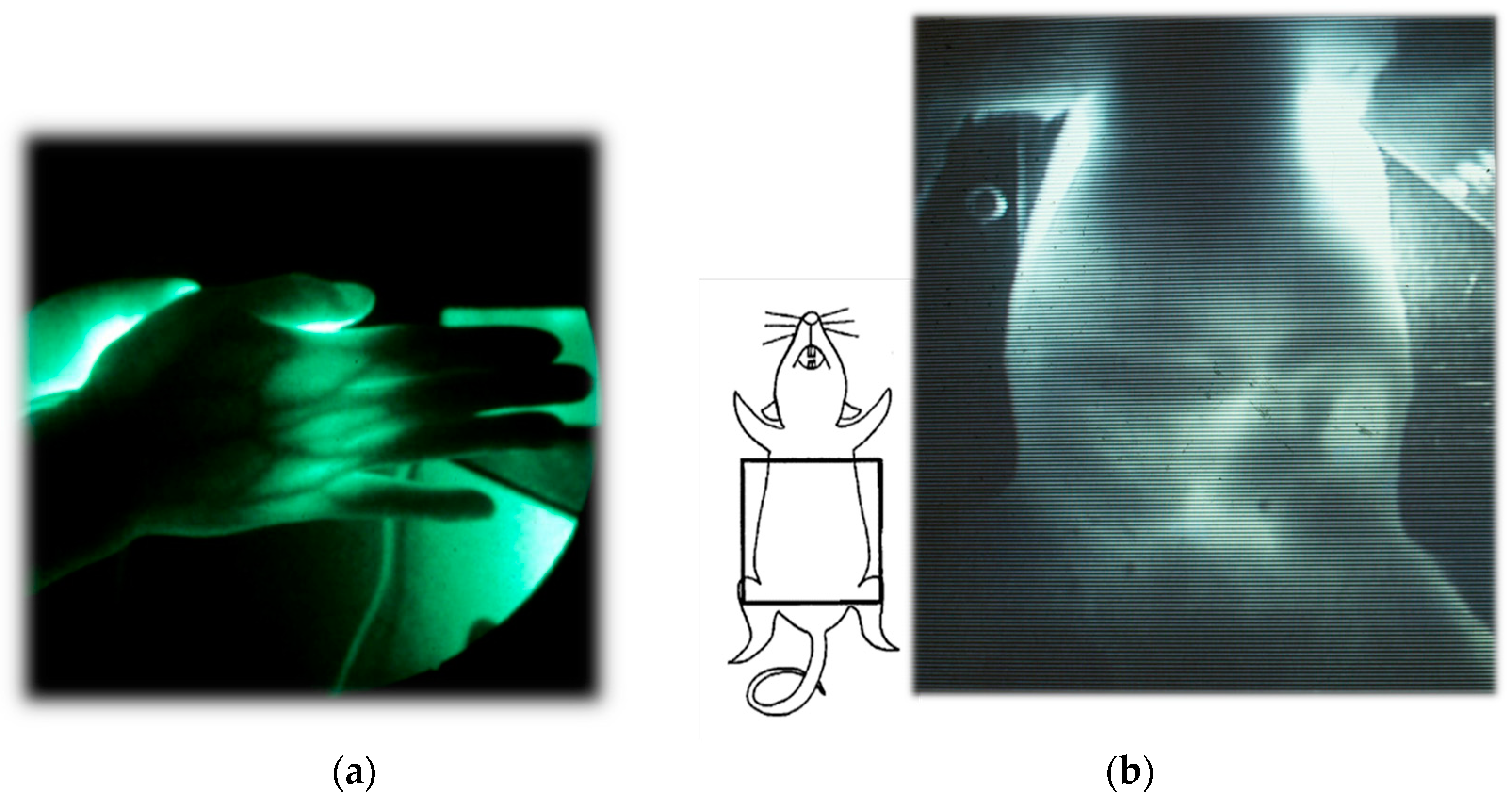
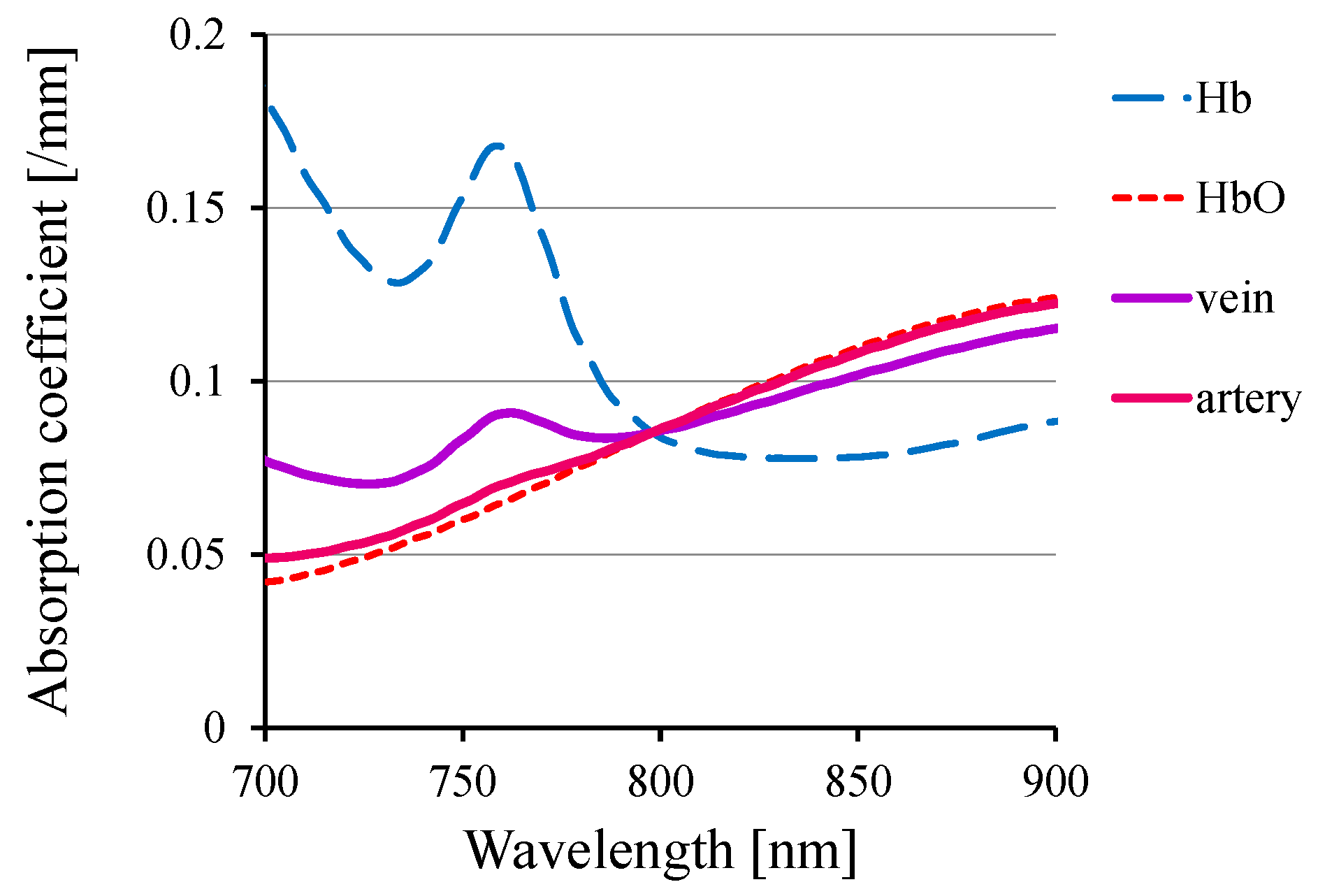
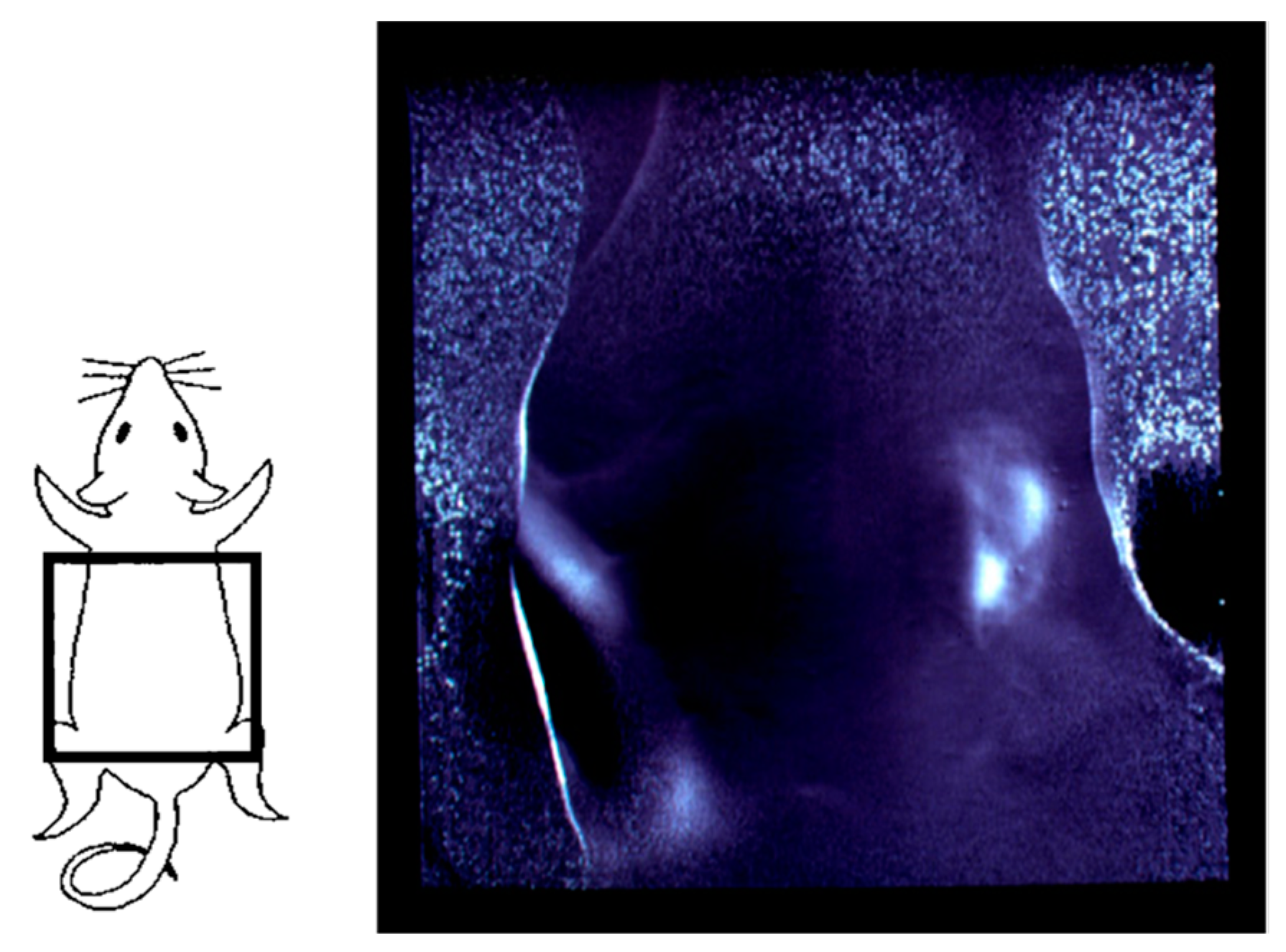
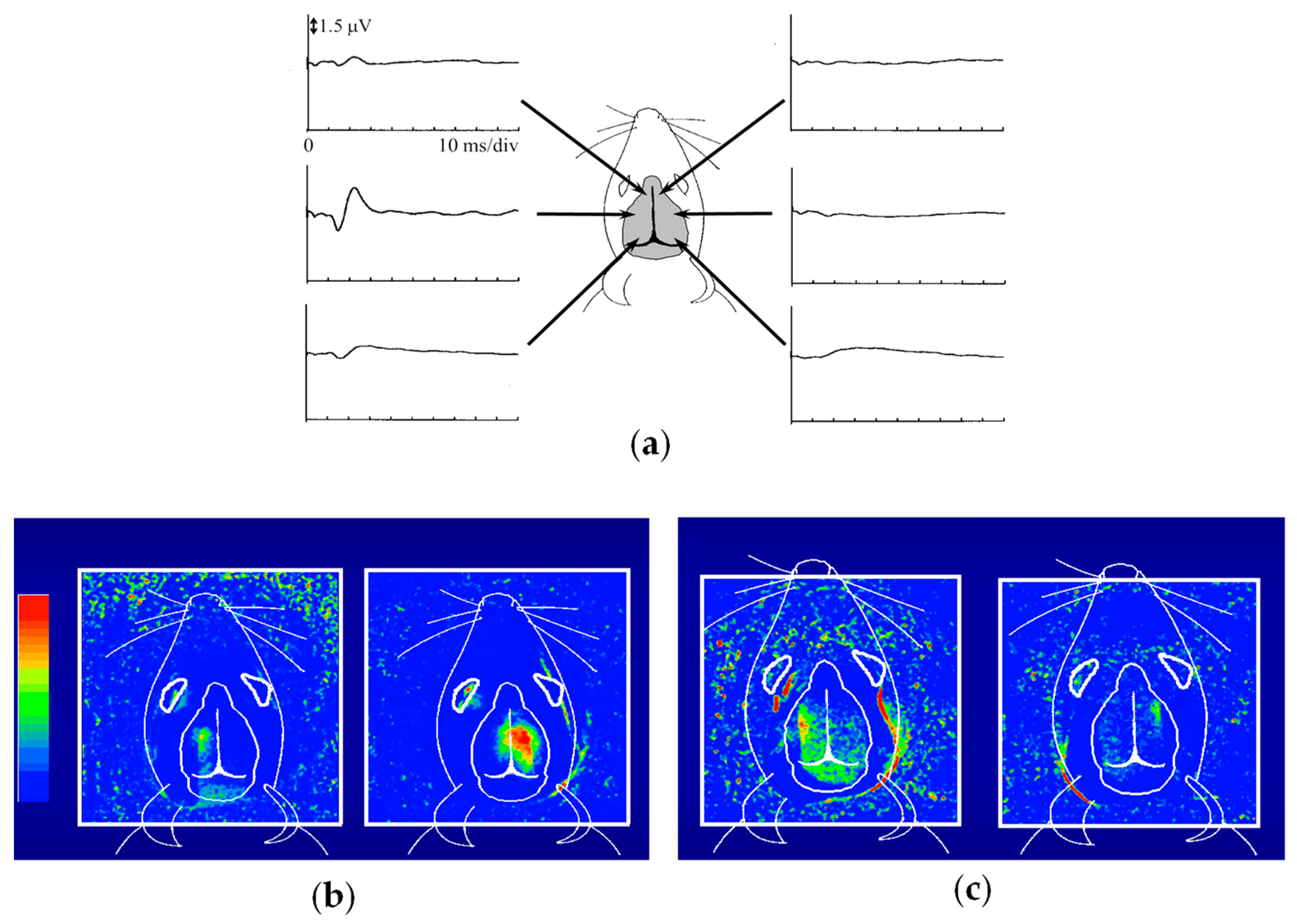
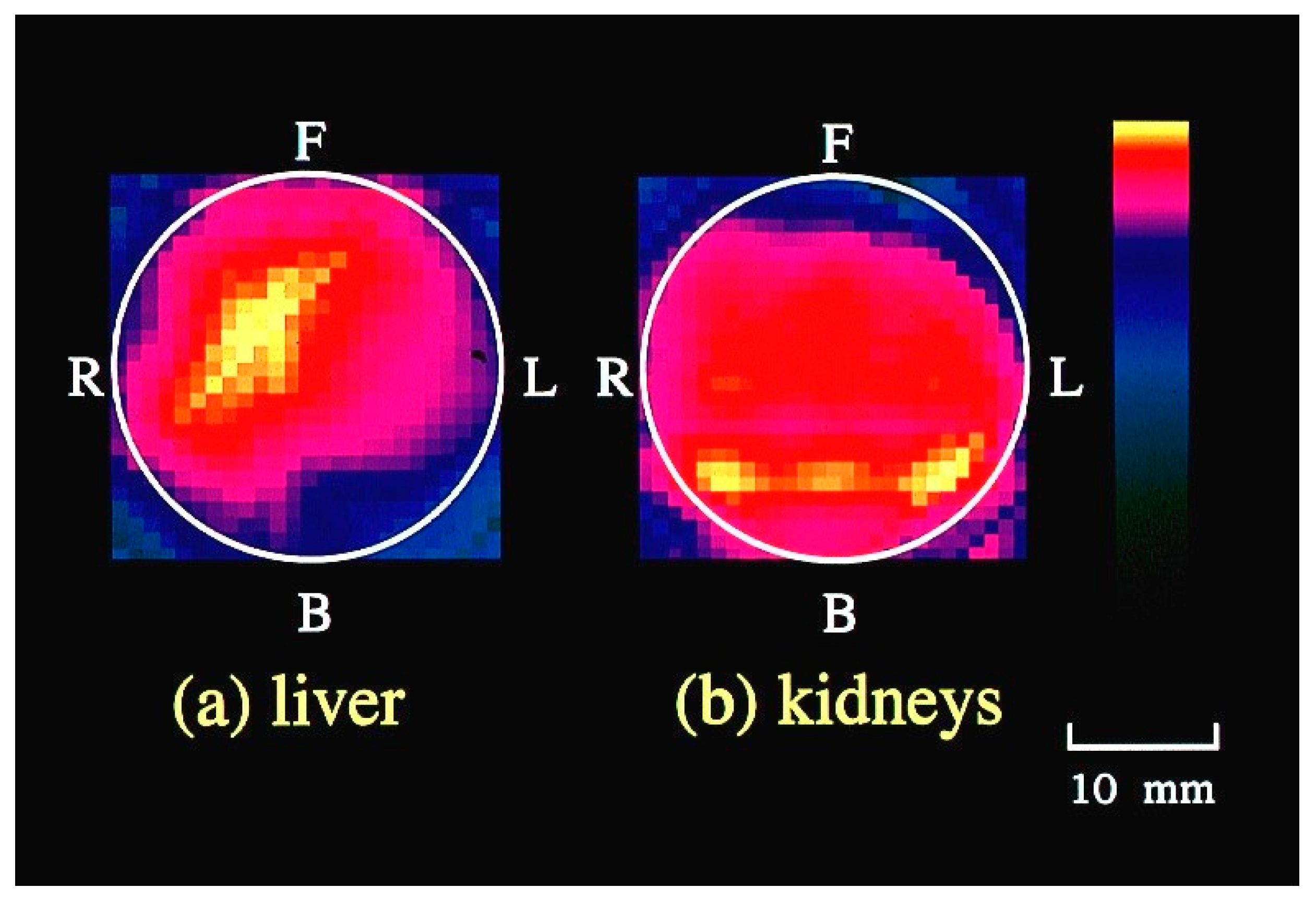
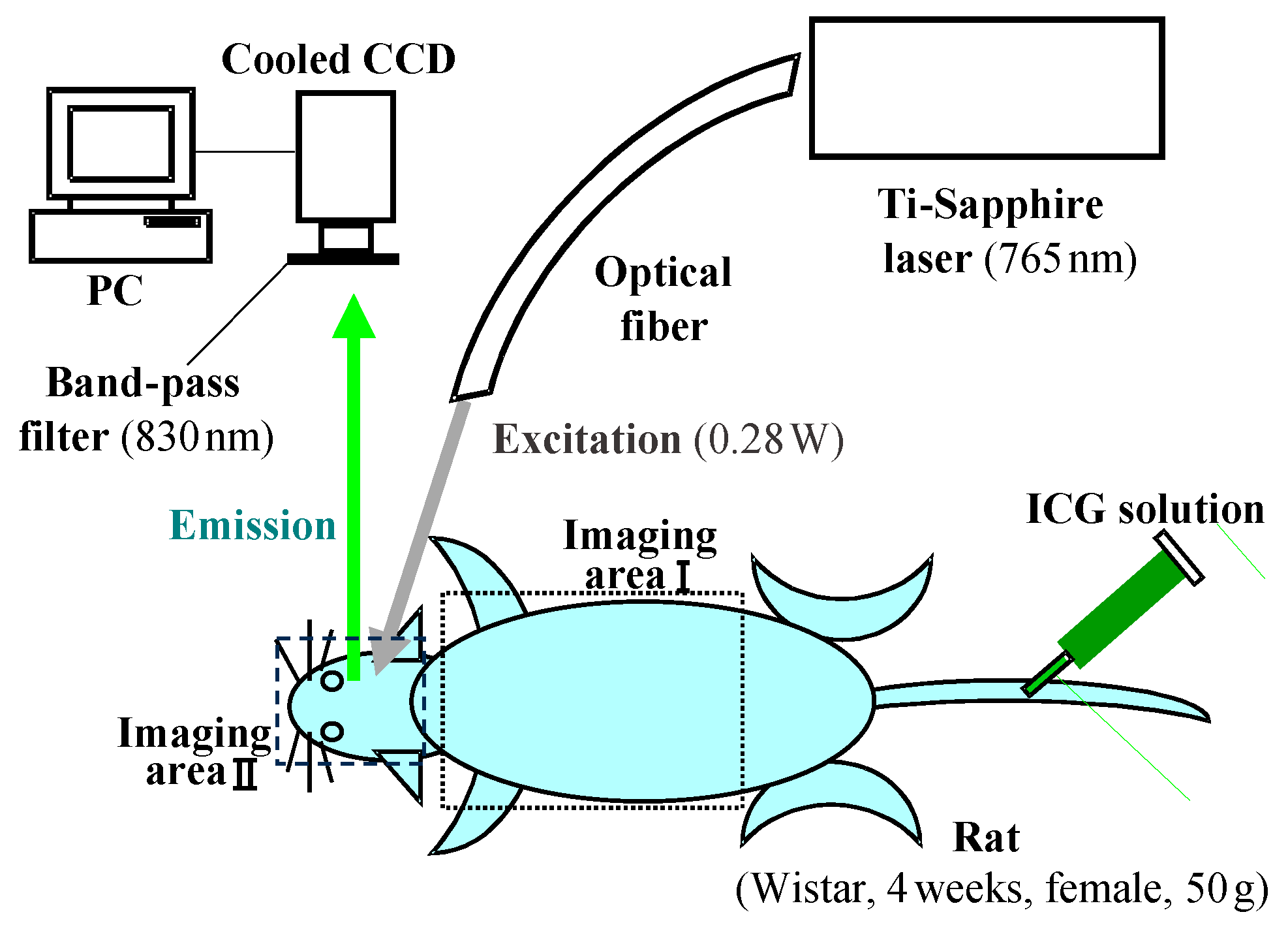
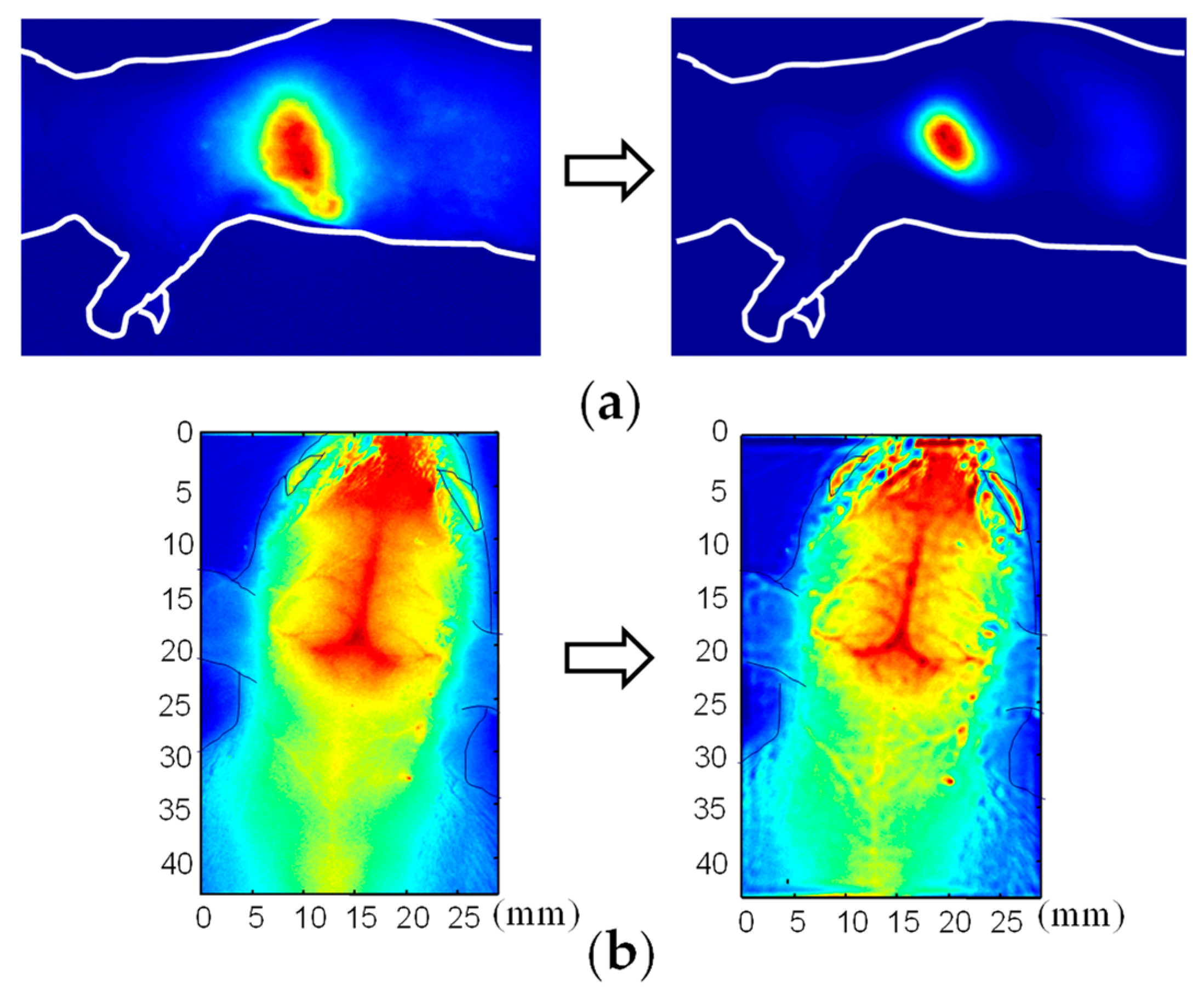
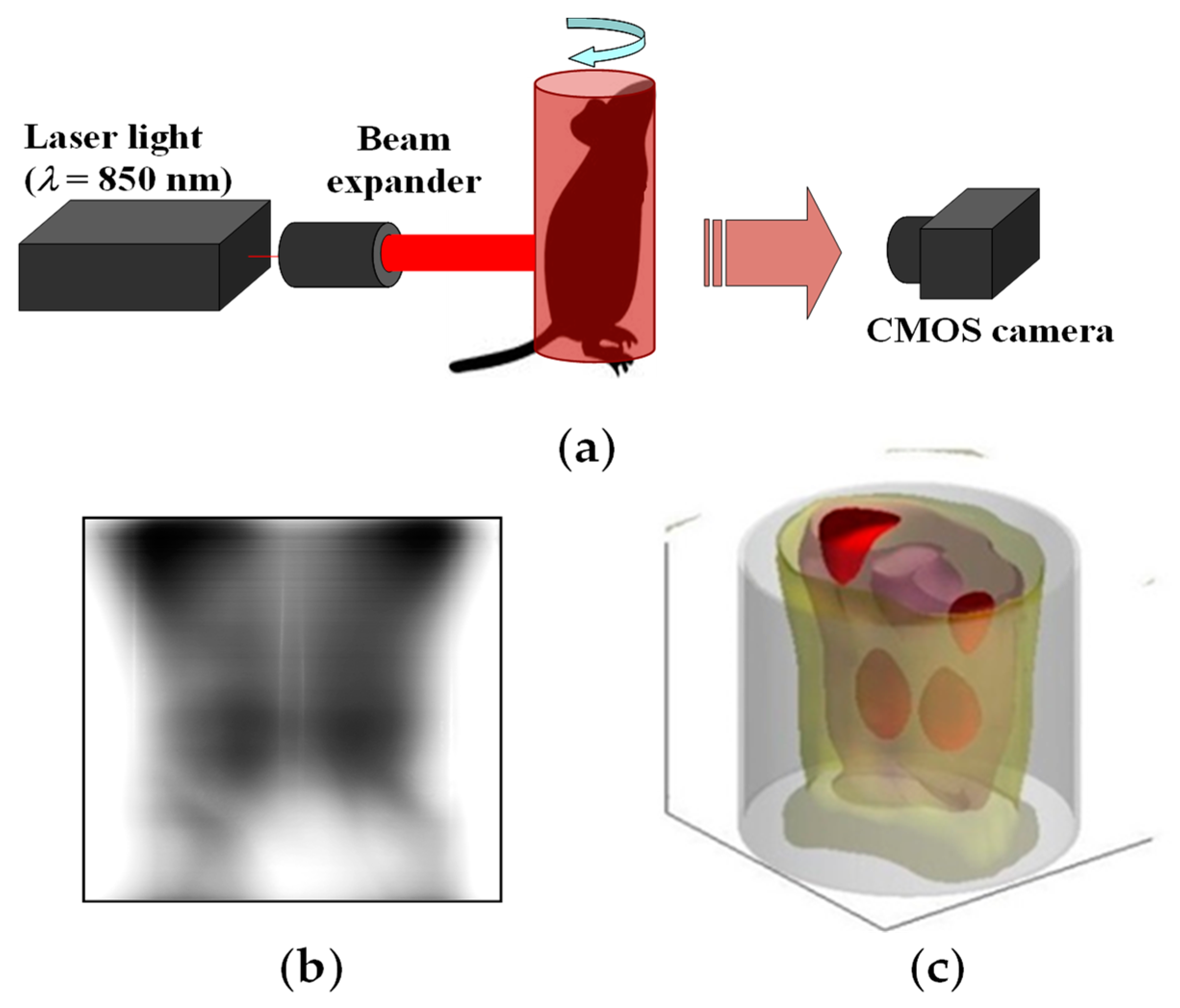
3. Functional Imaging
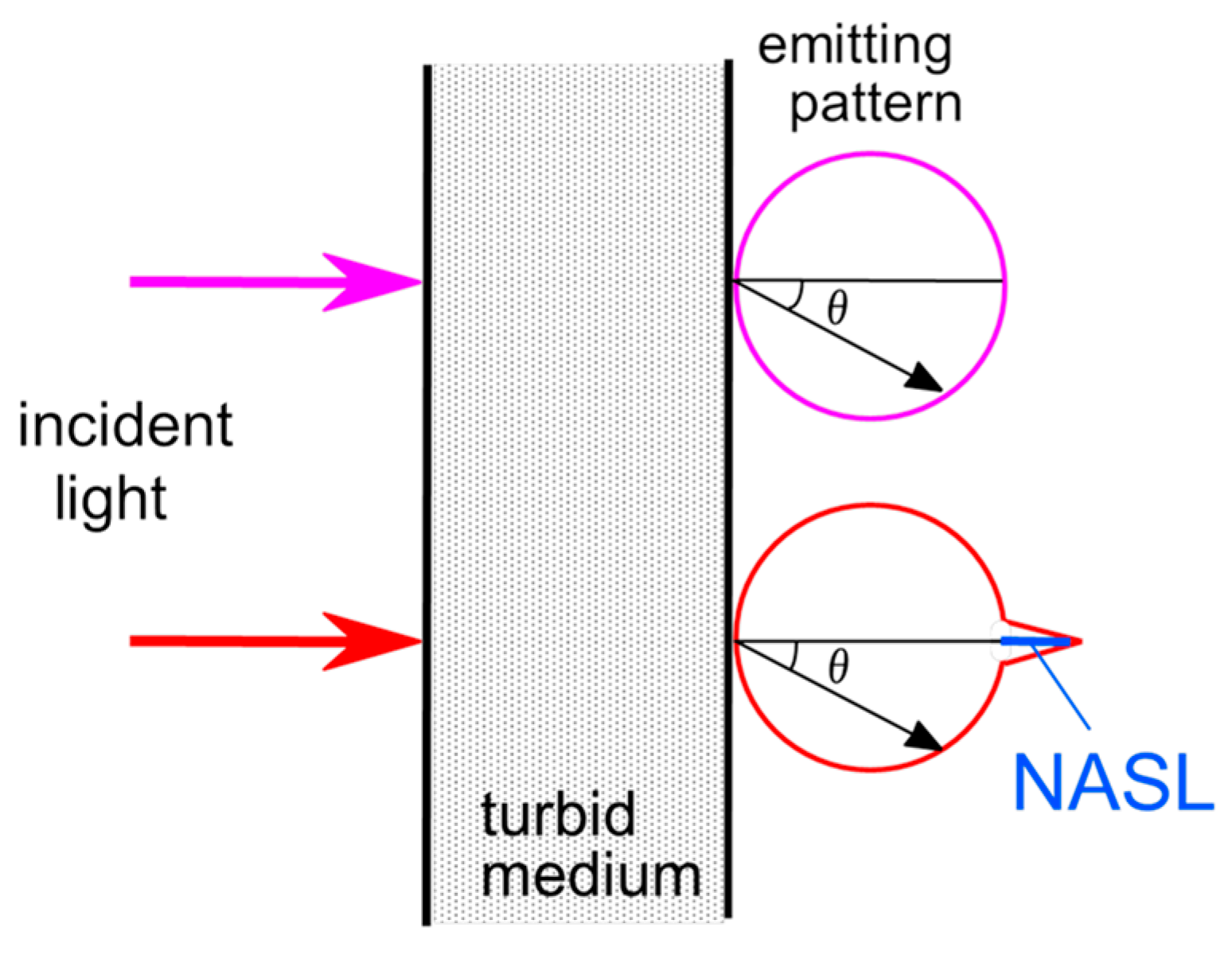
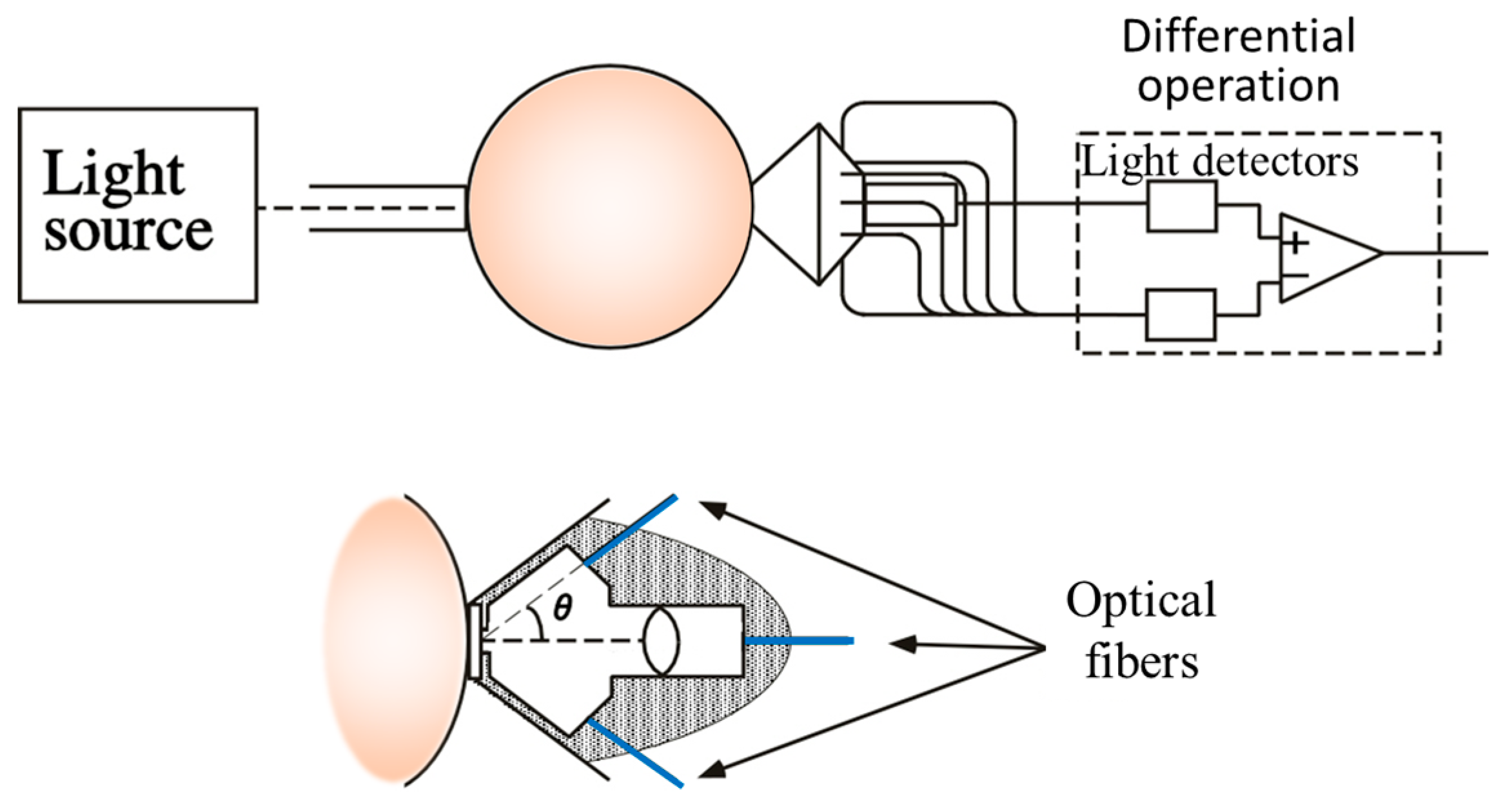
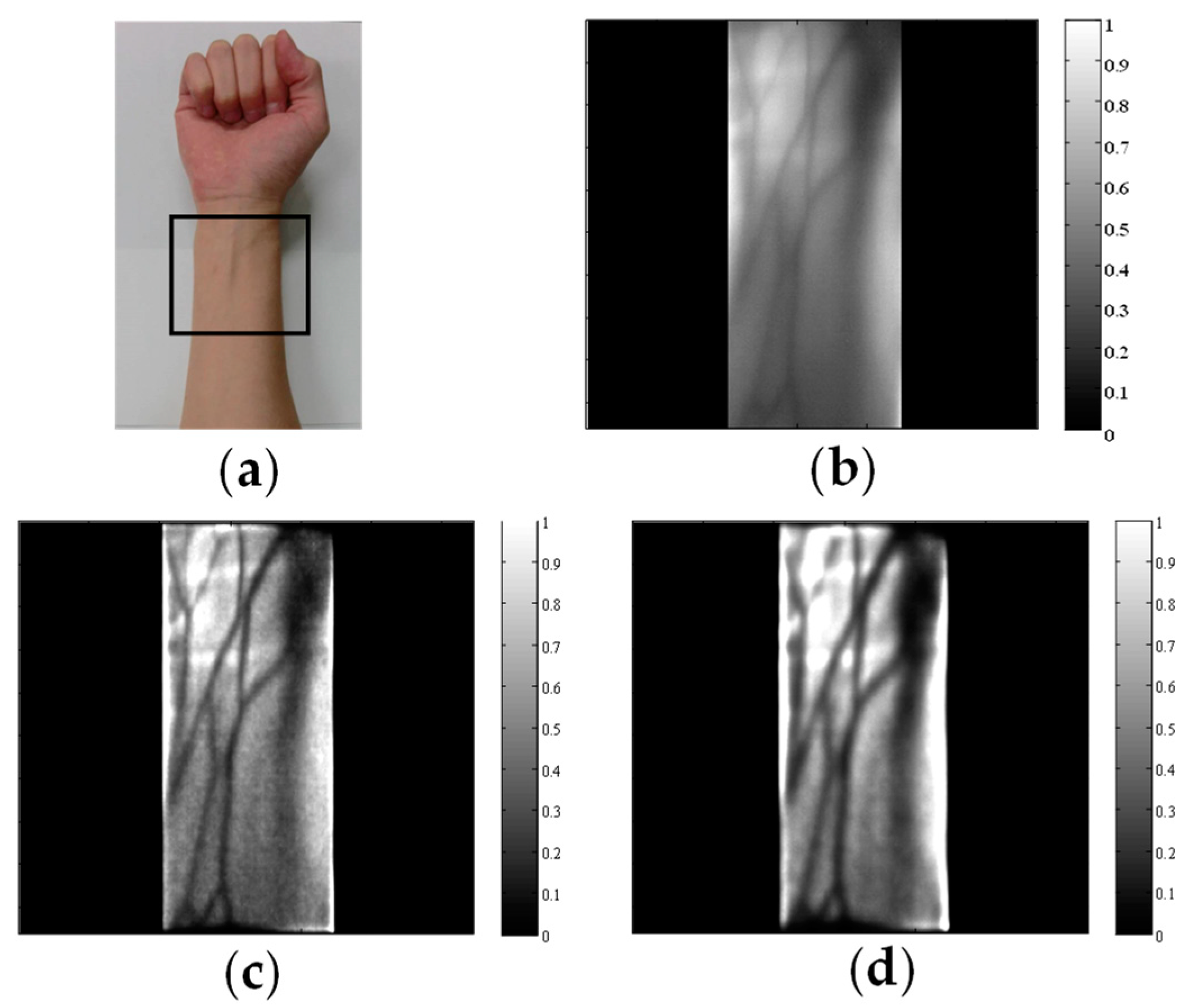
4. Hardware-Based Scattering Suppression
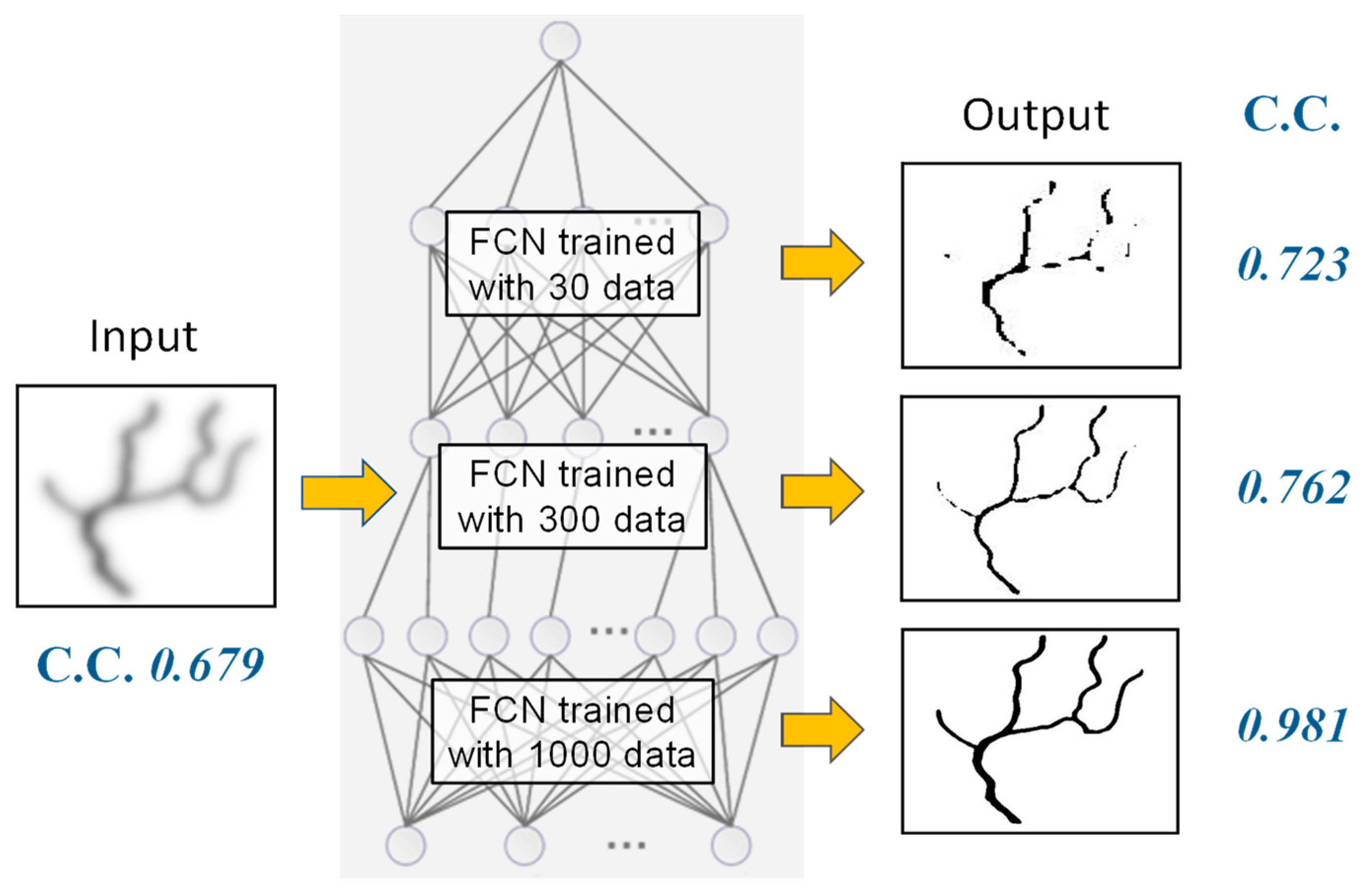
5. Software-Based Scattering Suppression
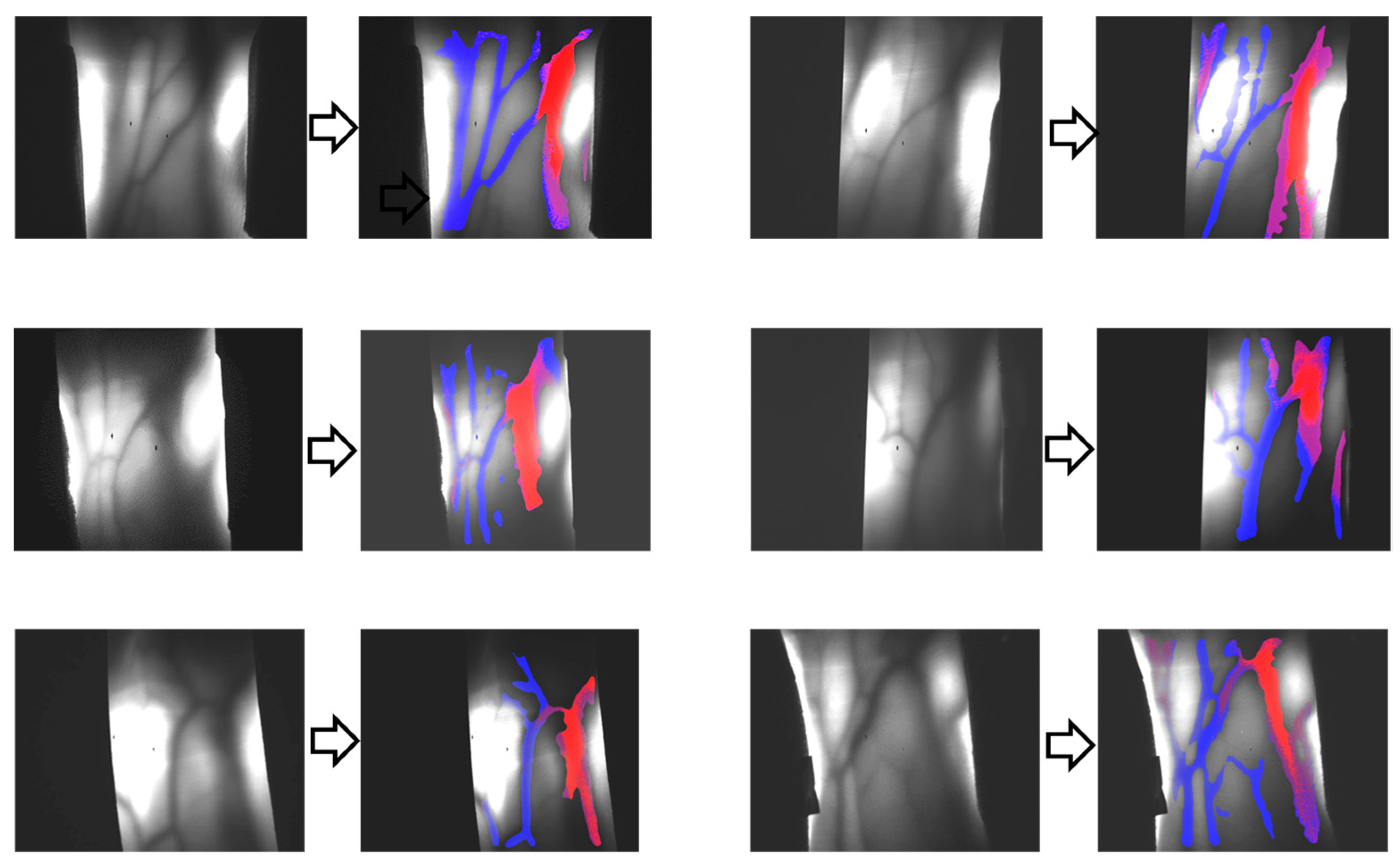
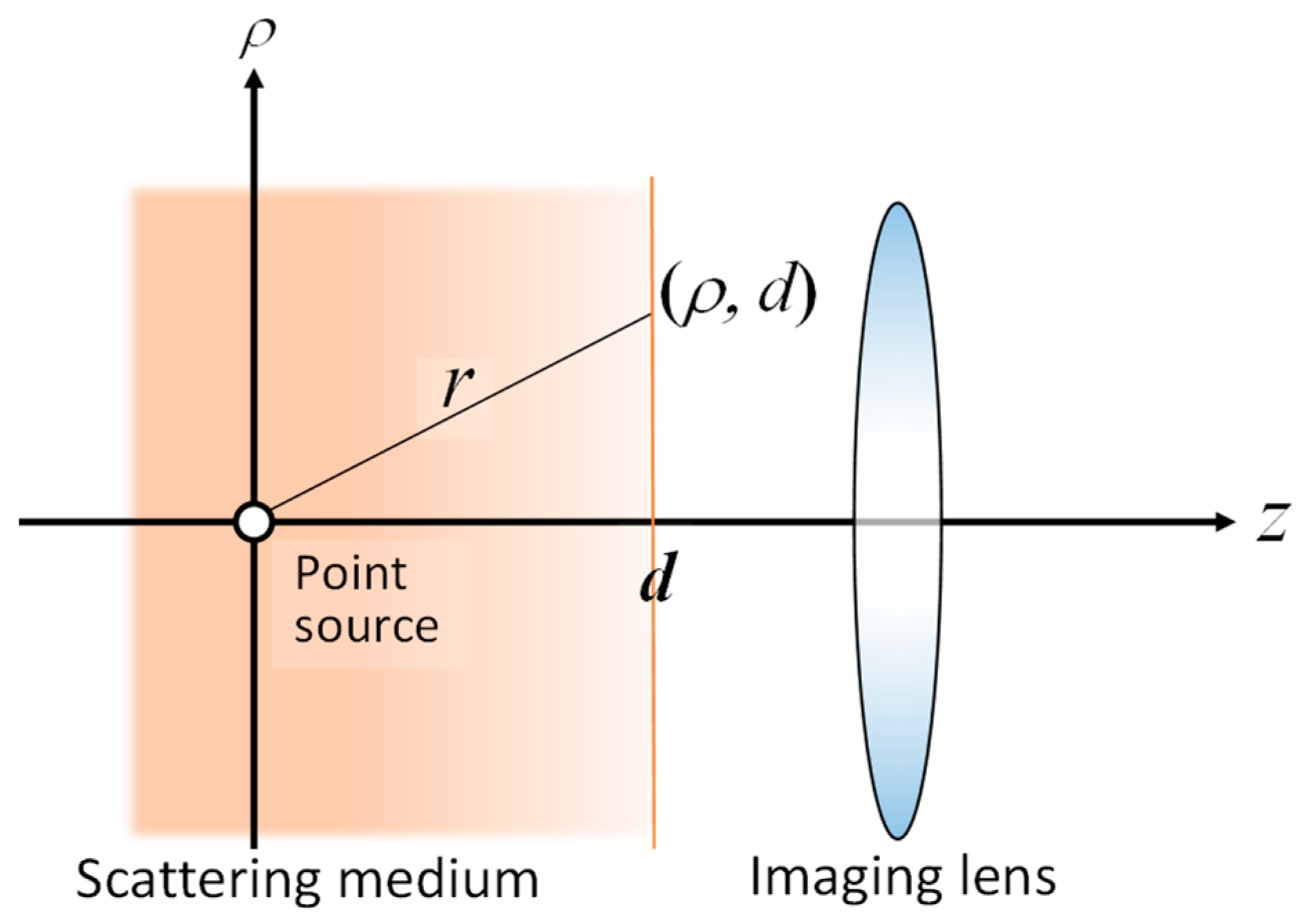
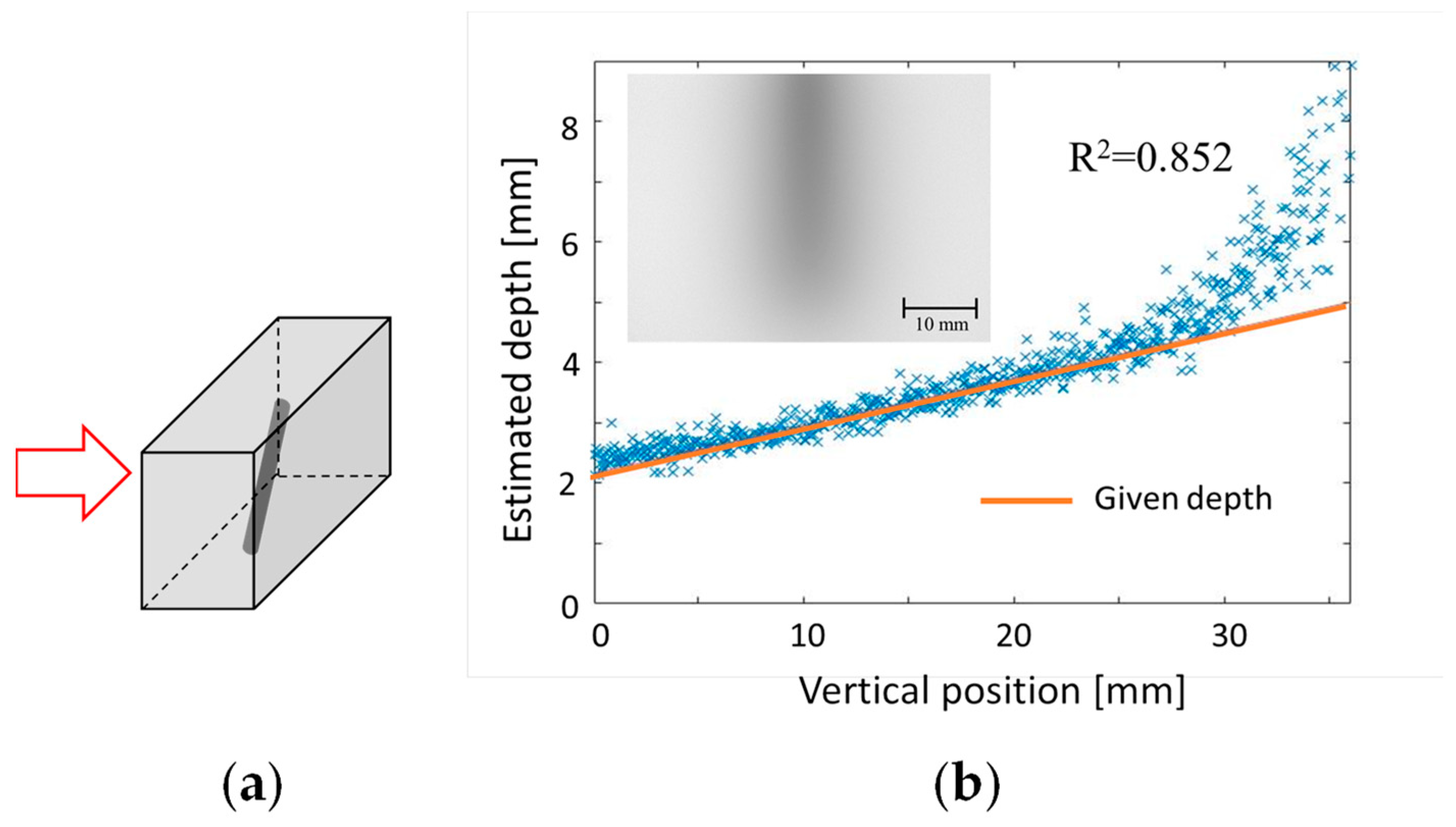
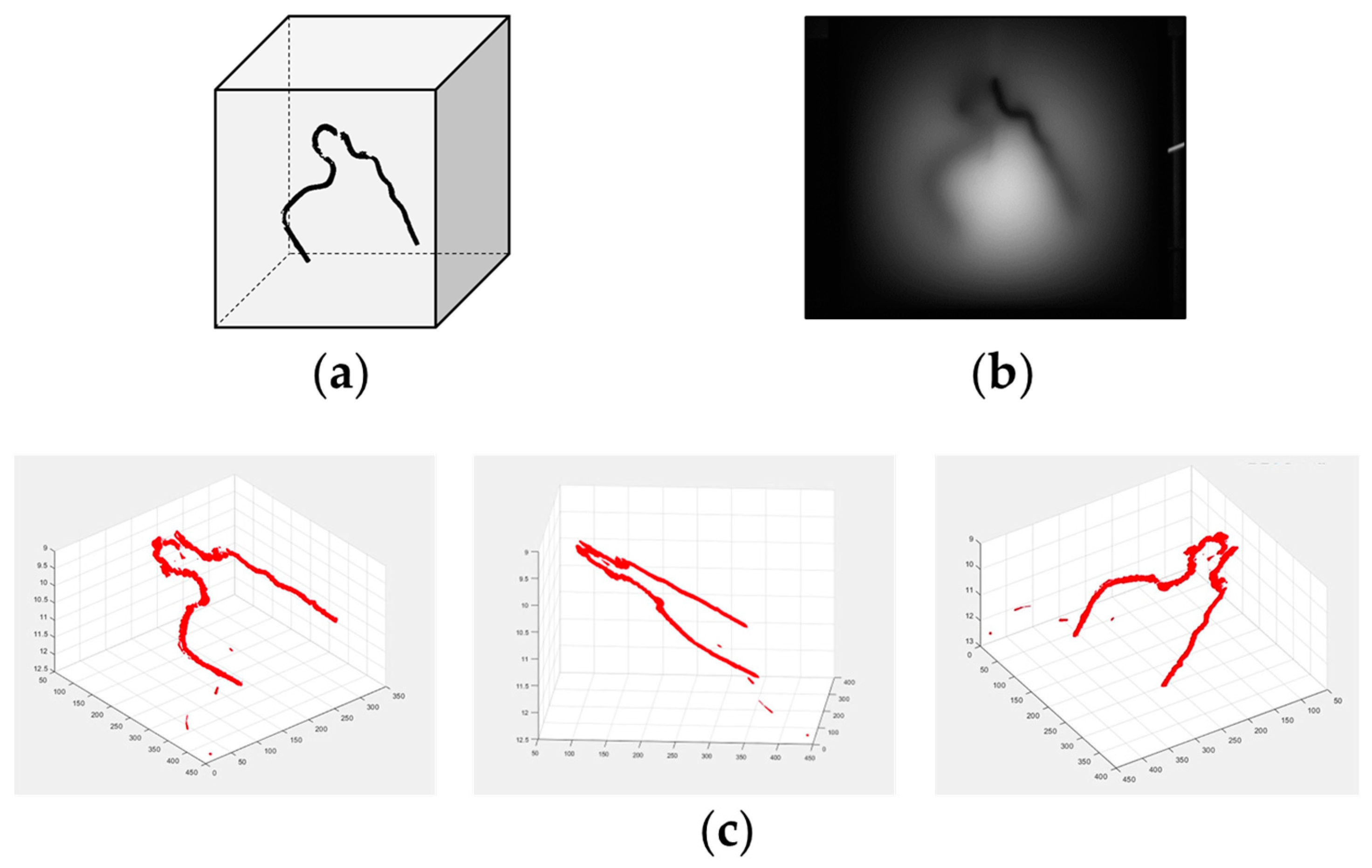
6. 3D Transillumination Imaging
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weissleder, R.; Nahrendorf, M. Advancing biomedical imaging. Proc. Natl. Acad. Sci. USA 2015, 112, 14424–14428. [Google Scholar] [CrossRef] [PubMed]
- Taruttis, A.; van Dam, G.M.; Ntziachristos, V. Mesoscopic and macroscopic optoacoustic imaging of cancer. Cancer Res. 2015, 75, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Pian, Q.; Wang, C.; Chen, X.; Liang, J. Multimodal biomedical optical imaging review: Towards comprehensive investigation of biological tissues. Curr. Mol. Imaging 2014, 3, 72–87. [Google Scholar] [CrossRef]
- Ntziachristos, V. Going deeper than microscopy: The optical imaging frontier in biology. Nat. Methods 2010, 7, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.K.; Mankovich, N.J.; Altmaier, J. A macroscopic and microscopic biomedical image processing system. Proc. Annu. Symp. Comput. Appl. Med. Care 1981, 580–585. [Google Scholar]
- Faragallah, O.S.; El-Hoseny, H.; El-Shafai, W.; El-Rahman, W.A.; El-Sayed, H.S.; El-Rabaie, E.M.; El-Samie, F.E.A.; Geweid, G.G.N. A comprehensive survey analysis for present solutions of medical image fusion and future directions. IEEE Access 2021, 9, 11358–11371. [Google Scholar] [CrossRef]
- Umar, A.A.; Atabo, S.M. A review of imaging techniques in scientific research/clinical diagnosis. MOJ Anat. Physiol. 2019, 6, 175–183. [Google Scholar]
- Bercovich, E.; Javitt, M.C. Medical imaging: From roentgen to the digital revolution, and beyond. Rambam Maimonides Med. J. 2018, 9, e0034. [Google Scholar]
- Kasban, H.; El-Bendary, M.A.M.; Salama, D.H. A comparative study of medical imaging techniques. Int. J. Inf. Sci. Intell. Syst. 2015, 4, 37–58. [Google Scholar]
- Peter, J. Medical Imaging Modalities—An Introduction. In Advanced Imaging in Biology and Medicine; Sensen, C.W., Hallgr’ımsson, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Chapter 10; pp. 225–254. [Google Scholar] [CrossRef]
- Shung, K.K.; Smith, M.; Tsui, B.M.W. Principles of Medical Imaging; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Doi, K. Diagnostic imaging over the last 50 years: Research and development in medical imaging science and technology. Phys. Med. Biol. 2006, 51, R5–R27. [Google Scholar] [CrossRef]
- Hellier, K.; Benard, E.; Scott, C.C.; Karim, K.S.; Abbaszadeh, S. Recent progress in the development of a-Se/CMOS sensors for X-ray detection. Quantum Beam Sci. 2021, 5, 29. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, K.; Huang, X.; Zhao, J.; Xu, K. All silicon microdisplay fabricated utilizing 0.18 μm CMOS-IC with monolithic integration. IEEE Photonics J. 2022, 14, 1–5. [Google Scholar]
- Tuchin, V. Tissue Optics: Light Scattering Methods and Instruments for Medical Diagnosis, 3rd ed.; The Society of Photo-Optical Instrumentation Engineers (SPIE): Bellingham, WA, USA, 2015. [Google Scholar]
- Boas, D.A.; Pitris, C.; Ramanujam, N. Handbook of Biomedical Optics; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Wang, L.V.; Wu, H. Biomedical Optics: Principles and Imaging; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Splinter, R.; Hooper, B.A. An Introduction to Biomedical Optics; Taylor and Francis: New York, NY, USA, 2007. [Google Scholar]
- Vo-Dinh, T. Biomedical Photonics Handbook; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Ntziachristos, V.; Ripoll, J.; Wang, L.V.; Weissleder, R. Looking and listening to light: The evolution of whole-body photonic imaging. Nat. Biotechnol. 2005, 23, 313–320. [Google Scholar] [CrossRef]
- Key, H.; Jackson, P.C.; Wells, P.N.T. New approaches to transillumination imaging. J. Biomed. Eng. 1988, 10, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Gandjbakhche, A.H.; Bonner, R.F.; Nossal, R.; Weiss, G.H. Absorptivity contrast in transillumination imaging of tissue abnormalities. Appl. Opt. 1996, 35, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Gurcan, M.N.; Roysam, B.; Dhawan, A.P.; Wang, L.V. Special Letters Issue on Emerging Technologies in Multiparameter Biomedical Optical Imaging and Image Analysis. IEEE Trans. Biomed. Eng. 2010, 57, 2551–2630. [Google Scholar] [CrossRef][Green Version]
- D’Alessandro, B.; Dhawan, A.P. Depth-dependent hemoglobin analysis from multispectral transillumination images. IEEE Trans. Biomed. Eng. 2010, 57, 2568–2571. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, B.; Dhawan, A.P. Transillumination imaging for blood oxygen saturation estimation of skin lesions. IEEE Trans. Biomed. Eng. 2012, 59, 2660–2667. [Google Scholar] [CrossRef]
- Cutler, M. Transillumination of the breast. Ann. Surg. 1931, 93, 223–234. [Google Scholar] [CrossRef]
- Cai, R.; Kolabas, Z.I.; Pan, C.; Mai, H.; Zhao, S.; Kaltenecker, D.; Voigt, F.F.; Molbay, M.; Ohn, T.-L.; Vincke, C.; et al. Whole-mouse clearing and imaging at the cellular level with vDISCO. Nat. Protoc. 2023, 18, 1197–1242. [Google Scholar] [CrossRef]
- Ueda, H.R.; Ertürk, A.; Chung, K.; Gradinaru, V.; Chédotal, A.; Tomancak, P.; Keller, P.J. Tissue clearing and its applications in neuroscience. Nat. Rev. Neurosci. 2020, 21, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Costantini, I.; Cicchi, R.; Silvestri, L.; Vanzi, F.; Pavone, F.S. In-vivo and ex-vivo optical clearing methods for biological tissues. Biomed. Opt. Express 2019, 10, 5251–5267. [Google Scholar] [CrossRef]
- Cai, R.; Pan, C.; Ghasemigharagoz, A.; Todorov, M.I.; Förstera, B.; Zhao, S.; Bhatia, H.S.; Parra-Damas, A.; Mrowka, L.; Theodorou, D.; et al. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull-meninges connections. Nat. Neurosci. 2019, 22, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Susaki, E.A.; Ueda, H.R. Whole-body and whole-organ clearing and imaging techniques with single-cell resolution: Toward organism-level systems biology in mammals. Cell Chem. Biol. 2016, 23, 137–157. [Google Scholar] [CrossRef]
- Zhu, D.; Larin, K.V.; Luo, Q.; Tuchin, V.V. Recent progress in tissue optical clearing. Laser Photonics Rev. 2013, 7, 732–757. [Google Scholar] [CrossRef]
- Al-Amri, M.D.; El-Gomati, M.; Zubairy, M.S. (Eds.) Optics in Our Time; Springer Nature: Cham, Switzerland, 2016. [Google Scholar]
- Patle, N.; Raj, A.B.; Joseph, C.; Sharma, N. Review of fibreless optical communication technology: History, evolution, and emerging trends. J. Optic. Comm. 2021. [Google Scholar] [CrossRef]
- Sun, X.; Kang, C.H.; Kong, M.; Alkhazragi, O.; Guo, Y.; Ouhssain, M.; Weng, Y.; Jones, H.; Ng, T.K.; Ooi, B.S. A review on practical considerations and solutions in underwater wireless optical communication. J. Light. Technol. 2020, 38, 421–431. [Google Scholar] [CrossRef]
- Kaushal, H.; Kaddoum, G. Optical communication in space: Challenges and mitigation techniques. IEEE Commun. Surv. Tutor. 2017, 19, 57–96. [Google Scholar] [CrossRef]
- Agrawal, G.P. Optical Communication: Its History and Recent Progress. In Optics in Our Time; Al-Amri, M.D., El-Gomati, M., Zubairy, M.S., Eds.; Springer Nature: Cham, Switzerland, 2016; pp. 177–199. [Google Scholar]
- Hoshi, Y. Functional near-infrared optical imaging: Utility and limitations in human brain mapping. Psychophysiology 2003, 40, 511–520. [Google Scholar] [CrossRef]
- Balasubramaniam, G.M.; Wiesel, B.; Biton, N.; Kumar, R.; Kupferman, J.; Arnon, S. Tutorial on the use of deep learning in diffuse optical tomography. Electronics 2022, 11, 305. [Google Scholar] [CrossRef]
- Zuo, C.; Qian, J.; Feng, S.; Yin, W.; Li, Y.; Fan, P.; Han, J.; Qian, K.; Chen, Q. Deep learning in optical metrology: A review. Light Sci. Appl. 2022, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, R.; Goswami, P.K. A comprehensive review on deep learning based image dehazing techniques. In Proceedings of the 2022 11th International Conference on System Modeling & Advancement in Research Trends (SMART), Moradabad, India, 16–17 December 2022; pp. 1392–1397. [Google Scholar] [CrossRef]
- Hu, K.; Weng, C.; Zhang, Y.; Jin, J.; Xia, Q. An overview of underwater vision enhancement: From traditional methods to recent deep learning. J. Mar. Sci. Eng. 2022, 10, 241. [Google Scholar] [CrossRef]
- Tian, L.; Hunt, B.; Bell, M.A.L.; Yi, J.; Smith, J.T.; Ochoa, M.; Intes, X.; Durr, N.J. Deep learning in biomedical optics. Lasers Surg. Med. 2021, 53, 748–775. [Google Scholar] [CrossRef] [PubMed]
- Gröhl, J.; Schellenberg, M.; Dreher, K.; Maier-Hein, L. Deep learning for biomedical photoacoustic imaging: A review. Photoacoustics 2021, 22, 100241. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Qiao, H.; Dai, Q.; Ma, C. Deep learning in photoacoustic imaging: A review. J. Biomed. Opt. 2021, 26, 040901. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ye, J.C.; Man, B.D. Deep learning for tomographic image reconstruction. Nat. Mach. Intell. 2020, 2, 737–748. [Google Scholar] [CrossRef]
- Belthangady, C.; Royer, L.A. Applications, promises, and pitfalls of deep learning for fluorescence image reconstruction. Nat. Methods 2019, 16, 1215–1225. [Google Scholar] [CrossRef]
- Cheng, S.; Li, H.; Luo, Y.; Zheng, Y.; Lai, P. Artificial intelligence-assisted light control and computational imaging through scattering media. J. Innov. Opt. Health Sci. 2019, 12, 1930006. [Google Scholar] [CrossRef]
- Shimizu, K.; Xian, S.; Guo, J. Reconstructing a deblurred 3D structure in a turbid medium from a single blurred 2D image—For near-infrared transillumination imaging of a human body. Sensors 2022, 22, 5747. [Google Scholar] [CrossRef]
- Shimizu, K.; Kitama, M. Fundamental study on near-axis scattered light and its application to optical computed tomography. Opt. Rev. 2000, 7, 383–388. [Google Scholar] [CrossRef]
- Wang, L.; Ho, P.P.; Liu, C.; Zhang, G.; Alfano, R.R. Ballistic 2-D imaging through scattering walls using an ultrafast optical Kerr gate. Science 1991, 253, 769–771. [Google Scholar] [CrossRef] [PubMed]
- Das, B.B.; Yoo, K.M.; Alfano, R.R. Ultrafast time-gated imaging in thick tissues: A step toward optical mammography. Opt. Lett. 1993, 18, 1092–1094. [Google Scholar] [CrossRef] [PubMed]
- Das, B.B.; Yoo, K.M.; Alfano, R.R. Snake Photon Imaging in Thick Tissues in Visible and Near Infrared Radiation Spectral Region. Adv. Opt. Imaging Photon Migr. 1994, 21, 166–169. [Google Scholar]
- Freund, I.; Kaveh, M.; Rosenbluh, M. Dynamic Multiple Scattering: Ballistic Photons and the Breakdown of the Photon-Diffusion Approximation. Phys. Rev. Lett. 1988, 60, 1130–1133. [Google Scholar] [CrossRef]
- Chen, N.G.; Bai, J. Estimation of quasi-straightforward propagating light in tissues. Phys. Med. Biol. 1999, 44, 1669–1676. [Google Scholar] [CrossRef]
- Vasefi, F.; Najiminaini, M.; Ng, E.; Kaminska, B.; Chapman, G.H.; Carson, J.J.L. Angular domain transillumination imaging optimization with an ultrafast gated camera. J. Biomed. Opt. 2010, 15, 061710. [Google Scholar] [CrossRef]
- Gopal, V.; Mujumdar, S.; Ramachandran, H.; Sood, A.K. Imaging in turbid media using quasi-ballistic photons. Opt. Commun. 1999, 170, 331–345. [Google Scholar] [CrossRef]
- Shimizu, K.; Mouri, M.; Yamamoto, K. Trans-body imaging of physiological functions with light. In Optical Methods in Biomedical and Environmental Sciences; Ohzu, H., Komatsu, S., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1994; pp. 63–66. [Google Scholar]
- Uhl, A.; Busch, C.; Marcel, S.; Veldhuis, R. (Eds.) Handbook of Vascular Biometrics; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Yang, L.; Yang, G.; Yin, Y.; Zhou, L. A survey of finger vein recognition. In Biometric Recognition; Sun, Z., Shan, S., Sang, H., Zhou, J., Wang, Y., Yuan, W., Eds.; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Kamiyama, H.; Kitama, M.; Shimizu, H.O.; Yamashita, M.; Kojima, Y.; Shimizu, K. Fundamental study for optical transillumination imaging of arteriovenous fistula. Adv. Biomed. Eng. 2021, 10, 1–10. [Google Scholar] [CrossRef]
- Zhao, M.; Hao, Y.; Zhang, C.; Zhai, R.; Liu, B.; Liu, W.; Wang, C.; Jafri, S.H.M.; Razak, A.; Papadakis, R.; et al. Advances in two-dimensional materials for optoelectronics applications. Crystals 2022, 12, 1087. [Google Scholar] [CrossRef]
- Xu, K. Silicon electro-optic micro-modulator fabricated in standard CMOS technology as components for all silicon monolithic integrated optoelectronic systems. J. Micromech. Microeng. 2021, 31, 054001. [Google Scholar] [CrossRef]
- Matsuda, Y.; Namita, T.; Kato, Y.; Shimizu, K. Arteriovenous discrimination by spectroscopic analysis of transillumination images. IEICE Tech. Rep. 2012, 111, 151–156. [Google Scholar]
- Taka, Y.; Sakatani, K.; Kato, Y.; Shimizu, K. Non—Invasive lmaging of absorption changes in rat brain by NIR transillumination. Med. Imaging Tech. 1999, 7, 545–555. [Google Scholar]
- Tandon, S.; Kambi, N.; Jain, N. Overlapping representations of the neck and whiskers in the rat motor cortex revealed by mapping at different anaesthetic depths. Eur. J. Neurosci. 2008, 27, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Watanabe, H.; Sarkisyan, D.; Andersen, M.S.; Nosova, O.; Galatenko, V.; Carvalho, L.; Lukoyanov, N.; Thelin, J.; Schouenborg, J.; et al. Hindlimb motor responses to unilateral brain injury: Spinal cord encoding and left-right asymmetry. Brain Commun. 2020, 2, fcaa055. [Google Scholar] [CrossRef]
- Toida, M.; Kondo, M.; Ichimura, T.; Inaba, H. Two-dimensional coherent detection imaging in multiple scattering media based on the directional resolution capability of the optical heterodyne method. Appl. Phys. B 1991, 52, 391–394. [Google Scholar] [CrossRef]
- Devaraj, B.; Usa, M.; Chan, K.P.; Akatsuka, T.; Inaba, H. Recent advances in coherent detection imaging (CDI) in biomedicine: Laser tomography of human tissues in vivo and in vitro. IEEE J. Sel. Top. Quantum Electron. 1996, 2, 1008–1016. [Google Scholar] [CrossRef]
- Muller, G.; Chance, B.; Alfano, R.R.; Arridge, S.; Beuthan, J.; Gratton, E.; Kaschke, M.; Masters, B.; Svanberg, S.; van der Zee, P. (Eds.) Medical Optical Tomography: Functional Imaging and Monitoring; SPIE: Bellingham, WA, USA, 1993. [Google Scholar]
- Alfano, R.R. (Ed.) OSA Proceedings on Advances in Optical Imaging and Photon Migration; Optical Society of America: Washington, DC, USA, 1994. [Google Scholar]
- Alfano, R.R.; Fujimoto, J.G. (Eds.) OSA Trends in Optics and Photonics on Advantages in Optical Imaging and Photon Migration; Optical Society of America: Washington, DC, USA, 1996. [Google Scholar]
- Chan, K.P.; Devaraj, B.; Yamada, M.; Inaba, H. Coherent detection techniques in optical imaging of tissues. Phys. Med. Biol. 1997, 42, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Kato, Y.; Shimizu, K. Extraction of near-axis scattered light for transillumination imaging. Appl. Opt. 2009, 48, D36–D44. [Google Scholar] [CrossRef]
- Takagi, K.; Kakinuma, H.; Kato, Y.; Shimizu, K. CW transillumination imaging by extracting weakly scattered light from strongly diffused light. Opt. Express 2009, 17, 8332–8342. [Google Scholar] [CrossRef]
- Shimizu, K.; Tochio, K.; Kato, Y. Improvement of transcutaneous fluorescent images with a depth-dependent point-spread function. Appl. Opt. 2005, 44, 2154–2161. [Google Scholar] [CrossRef]
- Tran, T.N.; Yamamoto, K.; Namita, T.; Kato, Y.; Shimizu, K. Three-dimensional transillumination image reconstruction for small animal with new scattering suppression technique. Biomed. Opt. Express 2014, 5, 1321–1335. [Google Scholar] [CrossRef]
- Yamamoto, K.; Tran, T.N.; Namita, T.; Kato, Y.; Shimizu, K. Image improvement of absorbing structure in turbid medium for optical transillumination imaging of biological body. IEICE Tech. Rep. 2014, 113, 115–120. [Google Scholar]
- Van, T.N.P.; Tran, T.N.; Inujima, H.; Shimizu, K. Three-dimensional imaging through turbid media using deep learning: NIR transillumination imaging of animal bodies. Biomed. Opt. Express 2021, 12, 2873–2887. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Shelhamer, E.; Darrell, T. Fully convolutional networks for semantic segmentation. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Boston, MA, USA, 7–12 June 2015; pp. 3431–3440. [Google Scholar]
- Djavanshir, G.R.; Chen, X.; Yang, W. A Review of artificial intelligence’s neural networks (deep learning) applications in medical diagnosis and prediction. IT Prof. 2021, 23, 58–62. [Google Scholar] [CrossRef]
- Cai, L.; Gao, J.; Zhao, D. A review of the application of deep learning in medical image classification and segmentation. Ann. Transl. Med. 2020, 8, 713. [Google Scholar] [CrossRef]
- Qureshi, I.; Yan, J.; Abbas, Q.; Shaheed, K.; Riaz, A.B.; Wahid, A.; Khan, M.W.J.; Szczuko, P. Medical image segmentation using deep semantic-based methods: A review of techniques, applications and emerging trends. Inf. Fusion 2023, 90, 316–352. [Google Scholar] [CrossRef]
- Jütte, L.; Yang, Z.; Sharma, G.; Roth, B. Focus stacking in non-contact dermoscopy. Biomed. Phys. Eng. Express 2022, 8, 065022. [Google Scholar] [CrossRef]
- Zhang, X. Deep Learning-Based Multi-Focus Image Fusion: A Survey and a Comparative Study. IEEE Trans. Pattern Anal. Mach. Intell. 2022, 44, 4819–4838. [Google Scholar] [CrossRef]
- Brecko, J.; Mathys, A.; Dekoninck, W.; Leponce, M.; VandenSpiegel, D.; Semal, P. Focus stacking: Comparing commercial top-end set-ups with a semi-automatic low budget approach. A possible solution for mass digitization of type specimens. ZooKeys 2014, 464, 1–23. [Google Scholar] [CrossRef]
- Galantucci, L.M.; Lavecchia, F.; Percoco, G. Multistack close range photogrammetry for low cost submillimeter metrology. J. Comput. Inf. Sci. Eng. 2013, 13, 044501. [Google Scholar] [CrossRef]
- Zhang, C.; Bastian, J.; Shen, C.; van den Hengel, A.; Shen, T. Extended depth-of-field via focus stacking and graph cuts. In Proceedings of the 2013 IEEE International Conference on Image Processing, Melbourne, Australia, 15–18 September 2013; pp. 1272–1276. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, K. Near-Infrared Transillumination for Macroscopic Functional Imaging of Animal Bodies. Biology 2023, 12, 1362. https://doi.org/10.3390/biology12111362
Shimizu K. Near-Infrared Transillumination for Macroscopic Functional Imaging of Animal Bodies. Biology. 2023; 12(11):1362. https://doi.org/10.3390/biology12111362
Chicago/Turabian StyleShimizu, Koichi. 2023. "Near-Infrared Transillumination for Macroscopic Functional Imaging of Animal Bodies" Biology 12, no. 11: 1362. https://doi.org/10.3390/biology12111362
APA StyleShimizu, K. (2023). Near-Infrared Transillumination for Macroscopic Functional Imaging of Animal Bodies. Biology, 12(11), 1362. https://doi.org/10.3390/biology12111362