Biogenic Selenium Nanoparticles: Anticancer, Antimicrobial, Insecticidal Properties and Their Impact on Soybean (Glycine max L.) Seed Germination and Seedling Growth
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Fungal Strain
2.2. Synthesis of SeNPs
2.3. Characterization of SeNPs
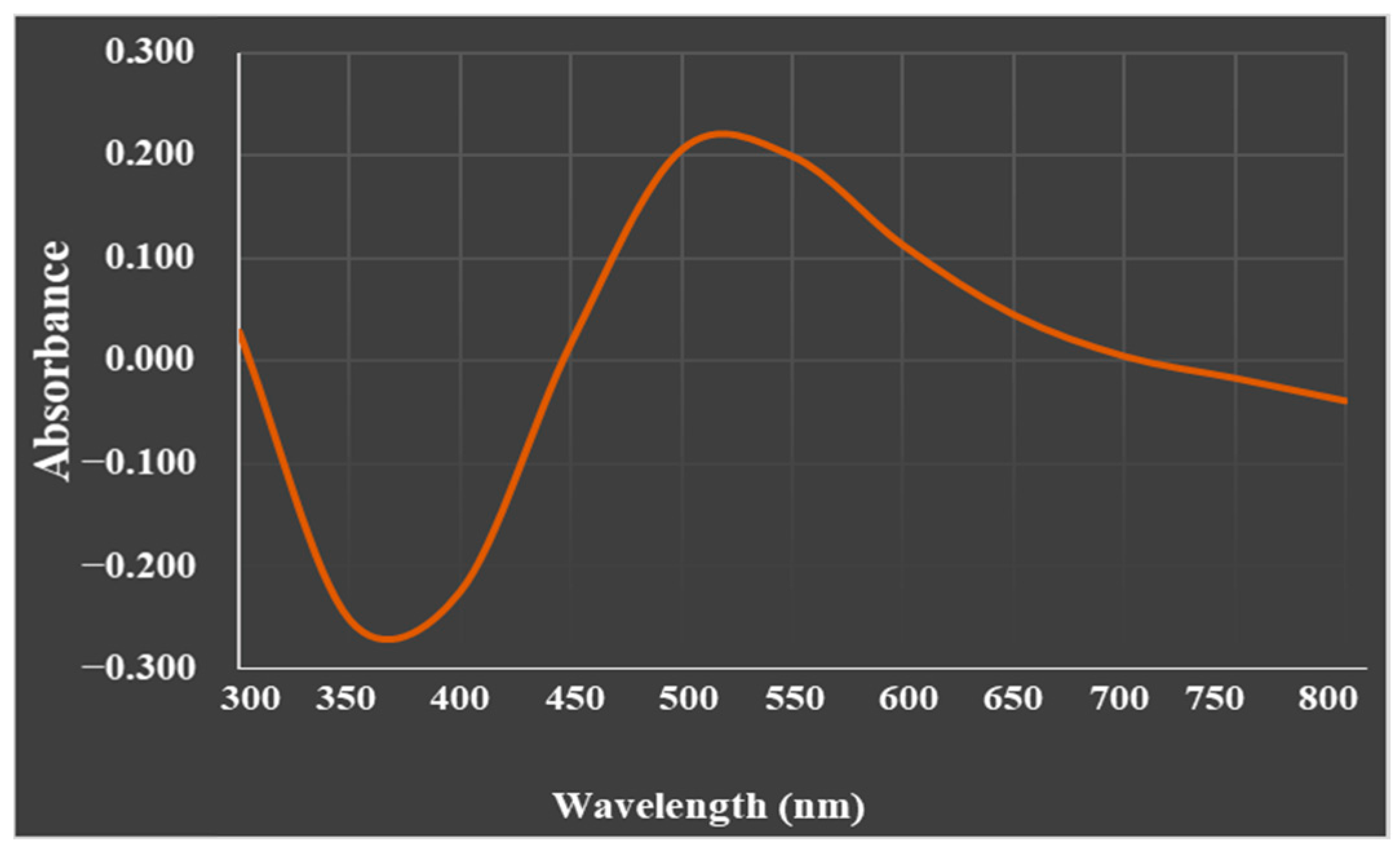
2.3.1. UV-Spectral Analysis
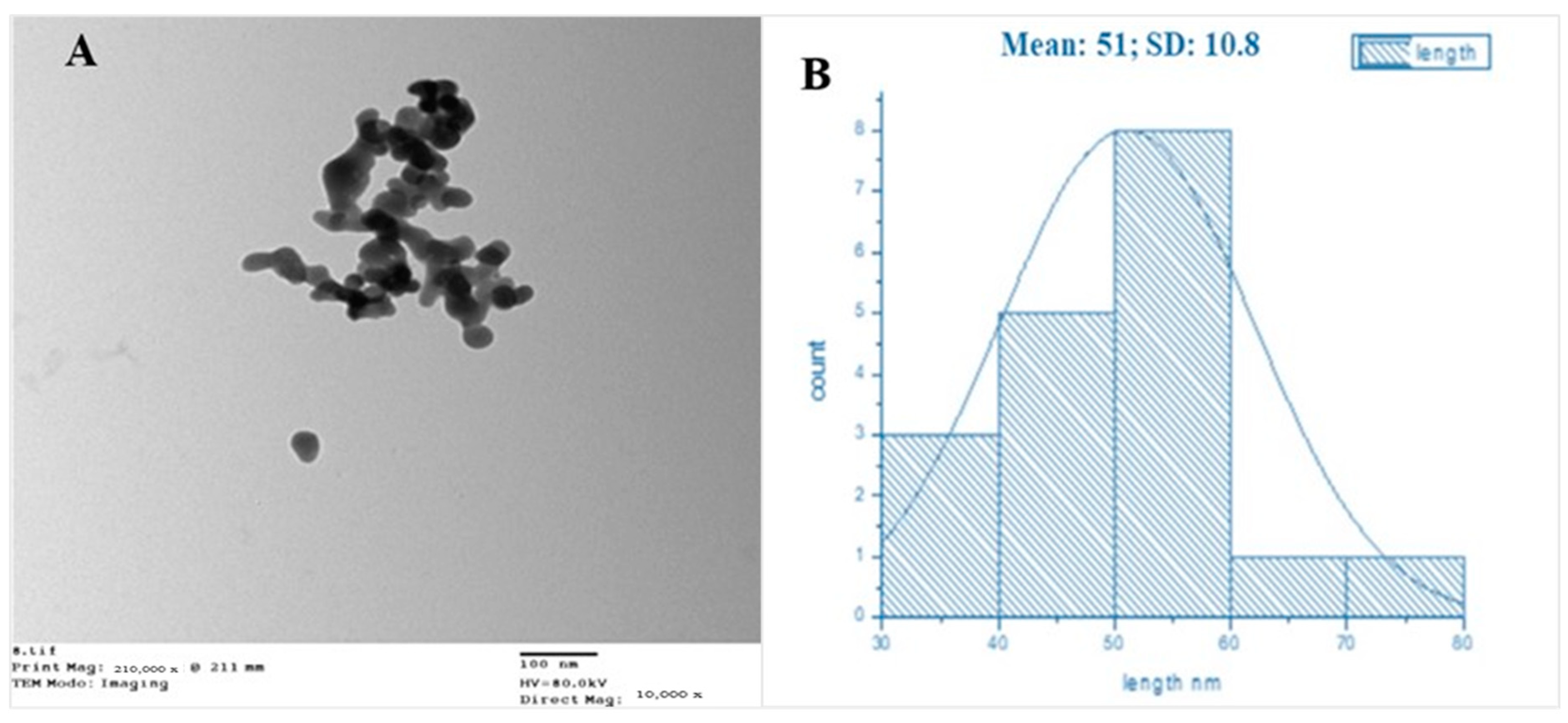
2.3.2. Transmission Electron Microscopy (TEM)
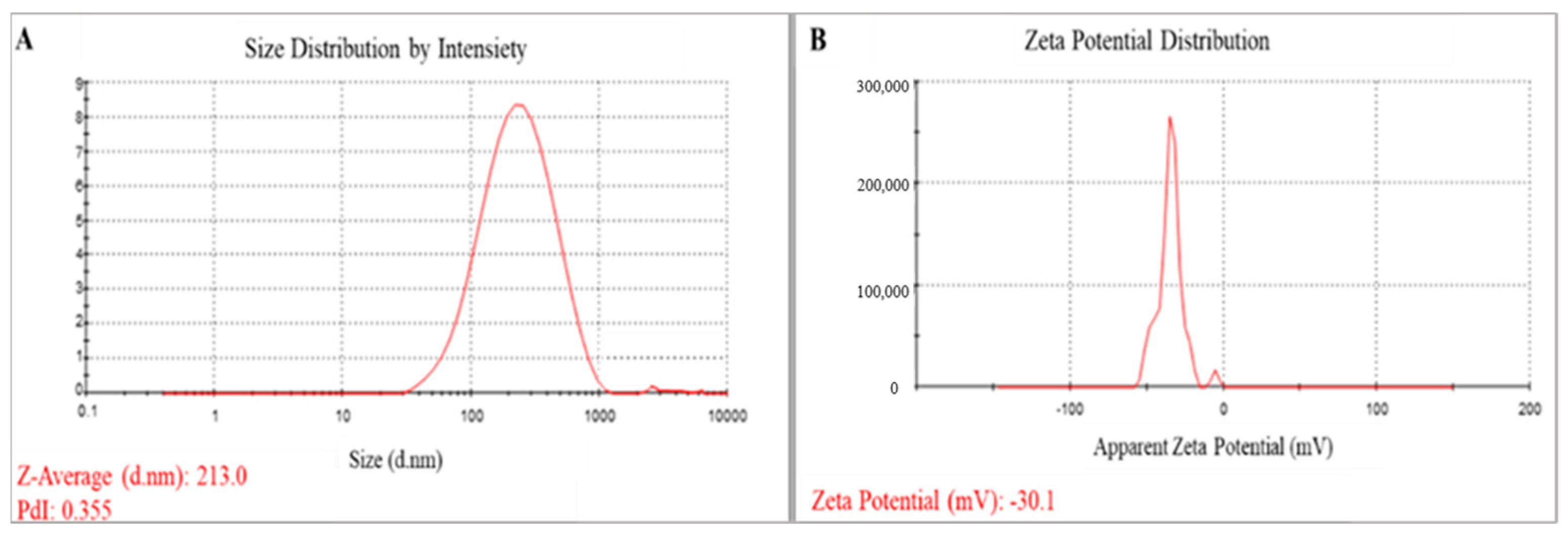
2.3.3. Dynamic Light Scattering (DLS)
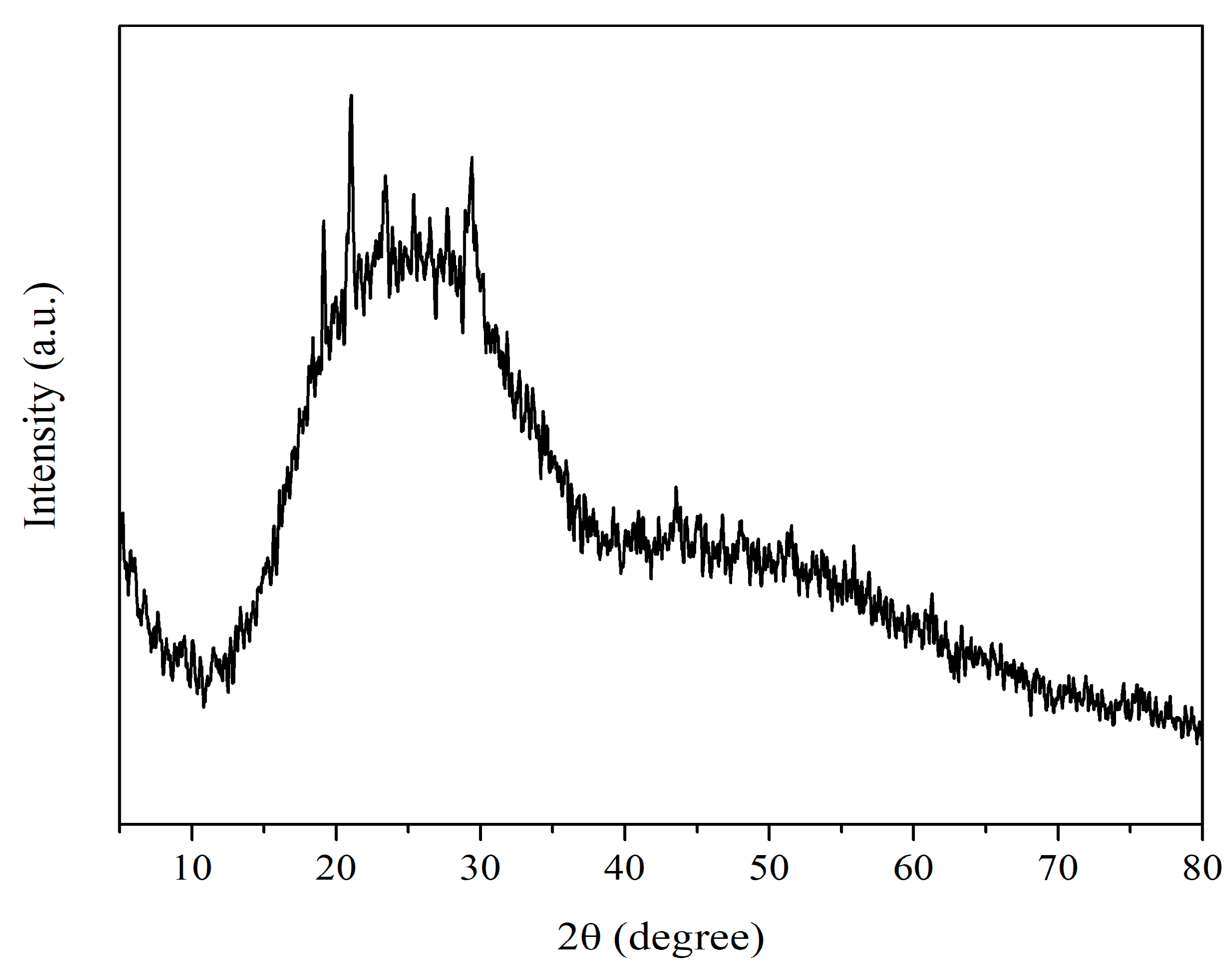
2.3.4. X-ray Diffraction (XRD)
2.3.5. Fourier Transform Infrared Spectroscopy
2.4. Biological Applications of SeNPs
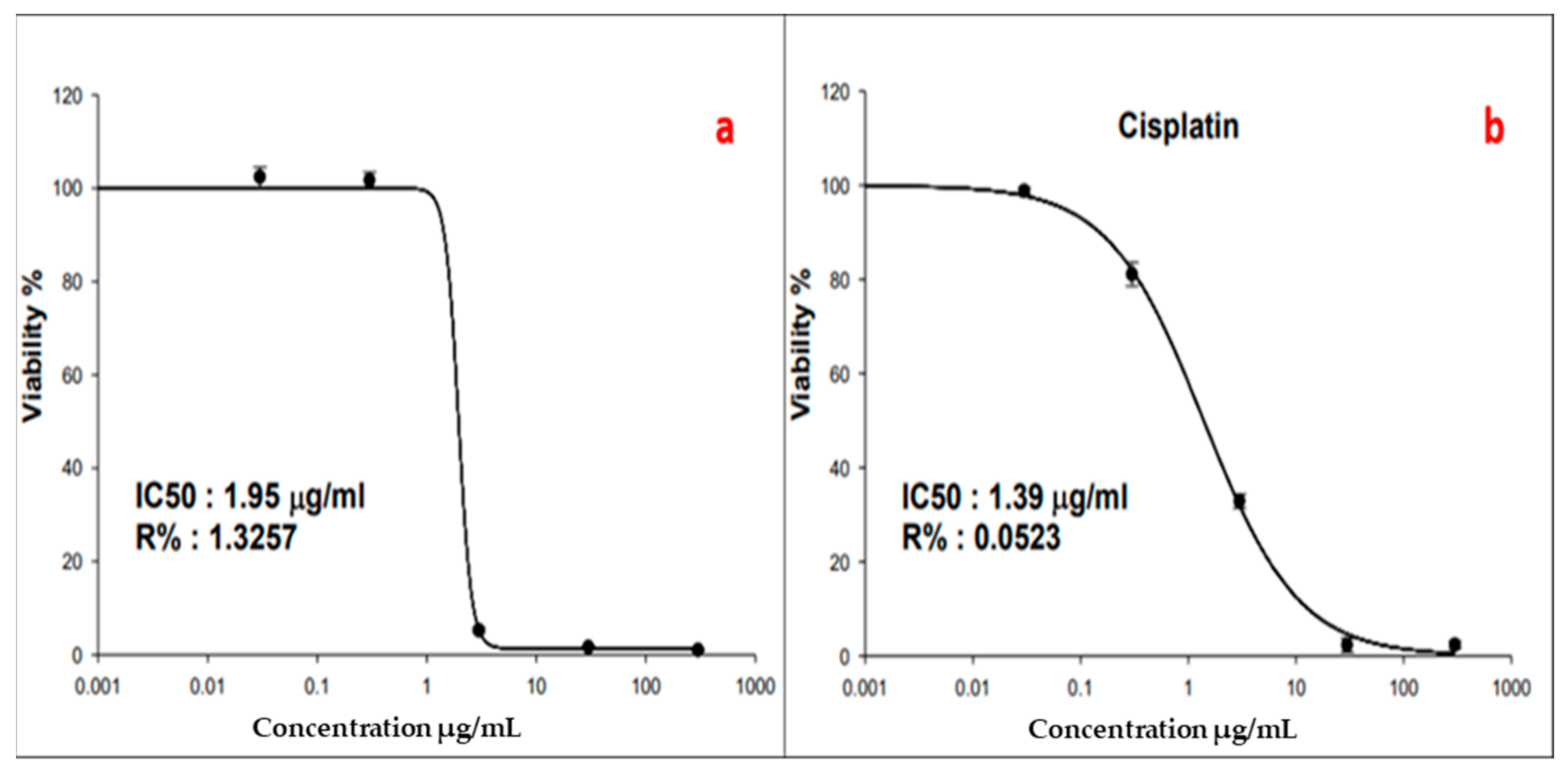
2.4.1. Anticancer Effect Evaluation
- Sulforhodamine B (SRB) Assay
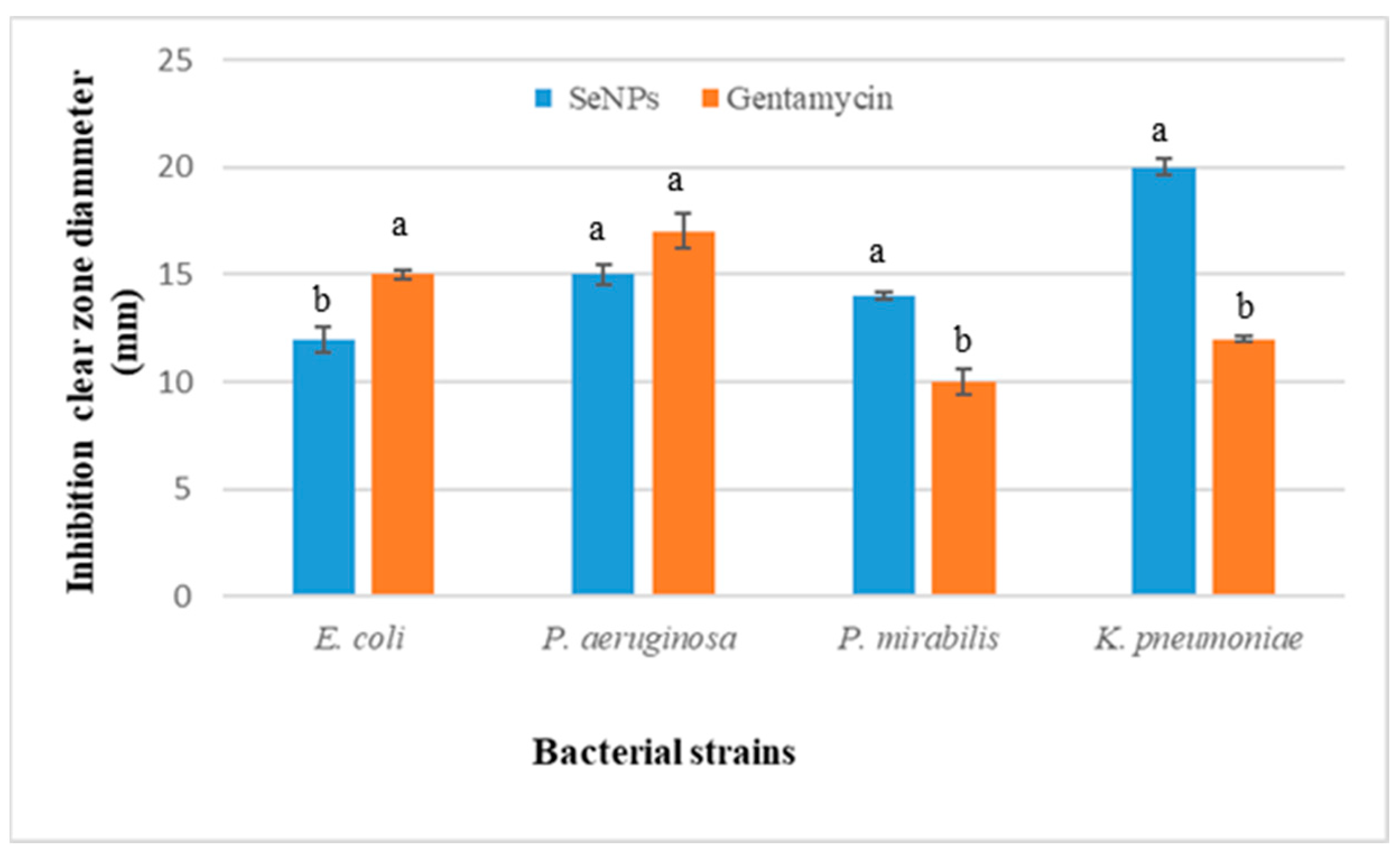
2.4.2. Antibacterial Activity
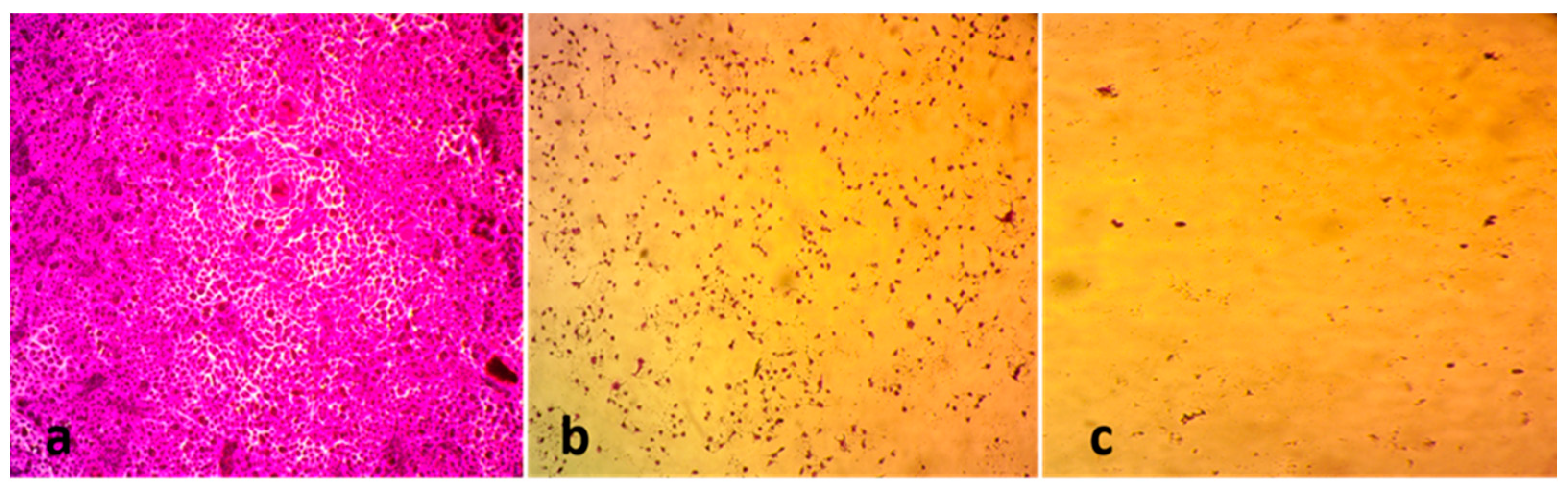
2.4.3. Effect of SeNPs on Glycine max Seed Germination and Seedling Growth
2.4.4. Effect of SeNPs on Plant Pathogenic Fungi
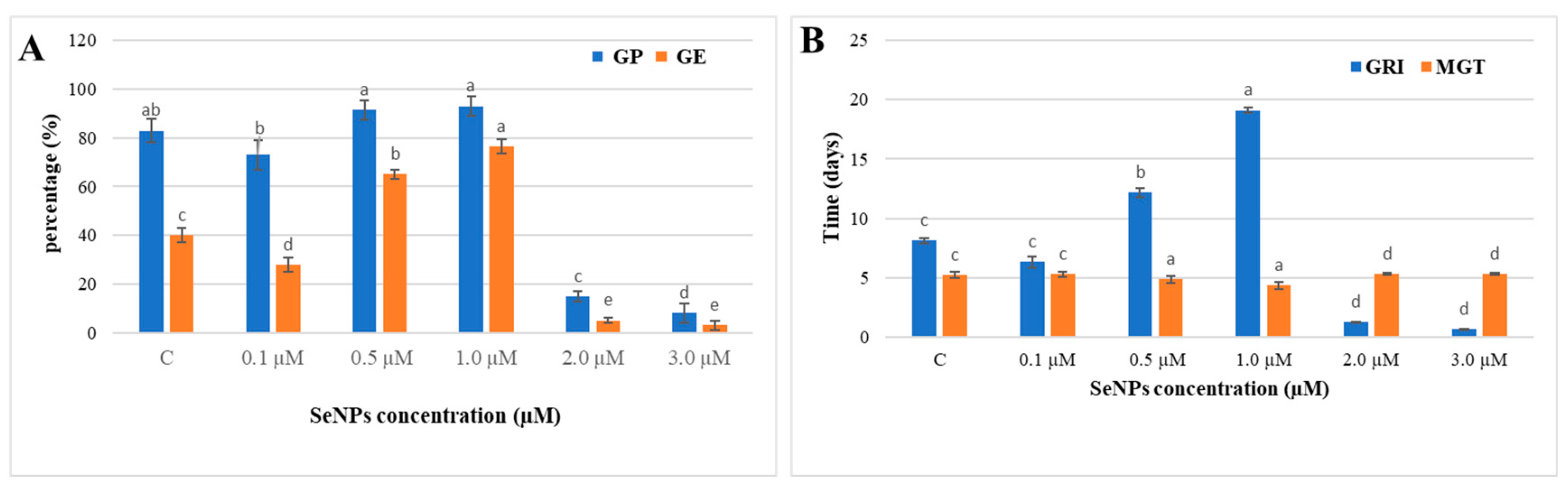
2.4.5. Insecticidal Activity of SeNPs against the Egyptian Cotton Leafworm
2.5. Statistical Analysis
3. Results
3.1. SeNPs Characterization
3.1.1. Visual Color
3.1.2. UV–Visible spectroscopy
3.1.3. Transmission Electron Microscopy (TEM)
3.1.4. Particle Size and Zeta Potential
3.1.5. X-ray Diffraction (XRD)
3.1.6. FTIR analysis
3.2. Biological Activities
3.2.1. Anticancer Activity
3.2.2. Antibacterial Activities
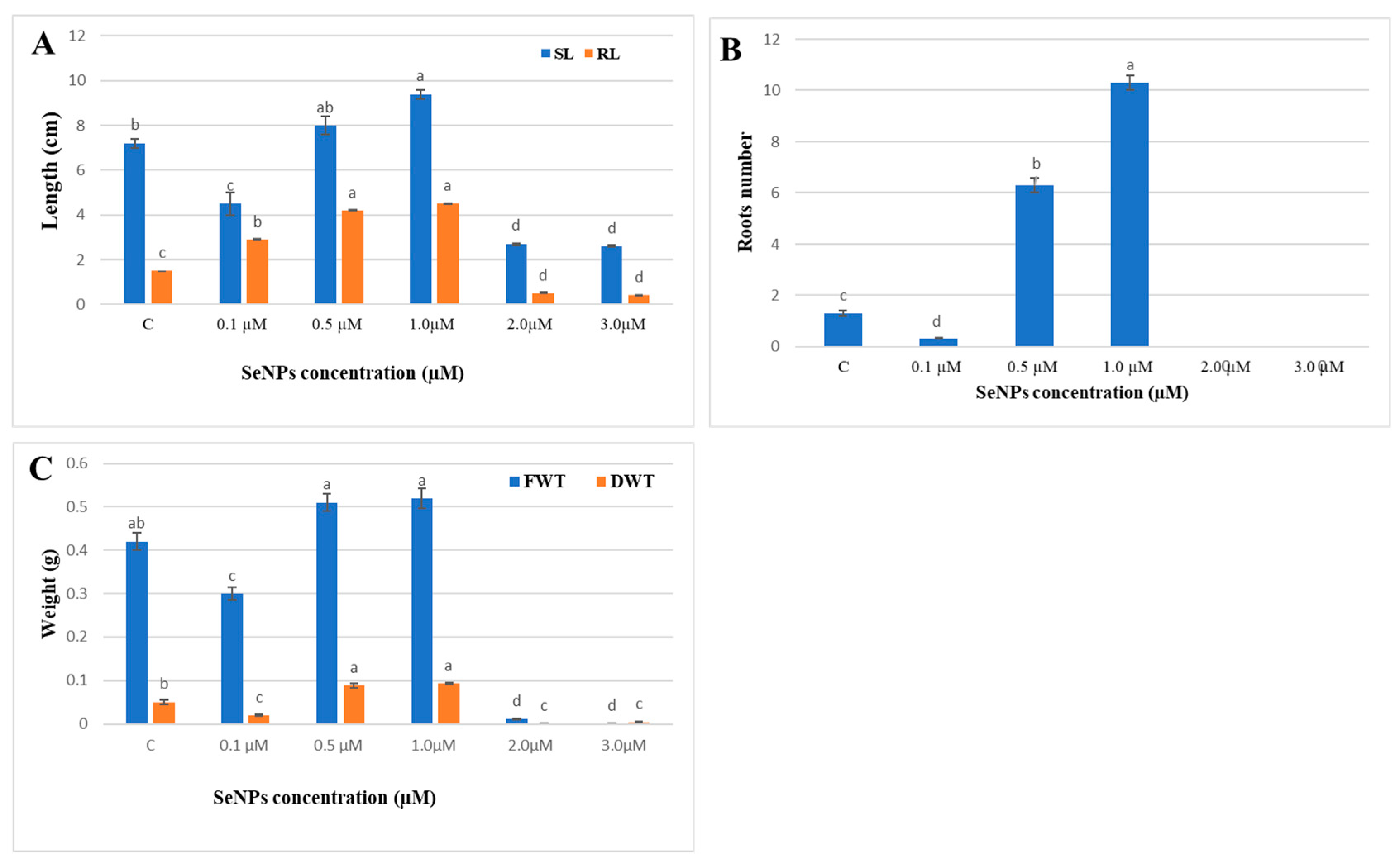
3.2.3. Effect of SeNPs on Glycine max Seed Germination
3.2.4. Antifungal Activities against Plant Pathogens
3.2.5. Insecticidal Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.who.int/news-room/fact-sheets/detail/pesticide-residues-in-food (accessed on 1 April 2023).
- Tsalidis, G.A. Human Health and Ecosystem Quality Benefits with Life Cycle Assessment Due to Fungicides Elimination in Agriculture. Sustainability 2022, 14, 846. [Google Scholar] [CrossRef]
- Chen, J.; Lü, S.; Zhang, Z.; Zhao, X.; Li, X.; Ning, P.; Liu, M. Environmentally Friendly Fertilizers: A Review of Materials Used and Their Effects on the Environment. Sci. Total Environ. 2018, 613–614, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green Synthesis of Metal Nanoparticles Using Microorganisms and Their Application in the Agrifood Sector. J. Nanobiotechnol. 2021, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Kapinder; Dangi, K.; Verma, A.K. Efficient & Eco-Friendly Smart Nano-Pesticides: Emerging Prospects for Agriculture. Mater. Today Proc. 2021, 45, 3819–3824. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.V.N.; Jeevanantham, S.; Yaashikaa, P.R.; George, C.S. A Review on Biosynthesis of Metal Nanoparticles and Its Environmental Applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef] [PubMed]
- Zohra, T.; Khalil, A.T.; Saeed, F.; Latif, B.; Salman, M.; Ikram, A.; Ayaz, M.; Murthy, H.C.A. Green Nano-Biotechnology: A New Sustainable Paradigm to Control Dengue Infection. Bioinorg. Chem. Appl. 2022, 2022, 3994340. [Google Scholar] [CrossRef]
- Silva, L.P.; Bonatto, C.C.; Polez, V.L.P. Green Synthesis of Metal Nanoparticles by Fungi: Current Trends and Challenges. In Advances and Applications through Fungal Nanobiotechnology. Fungal Biology; Prasad, R., Ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Shafiq, T.; Uzair, M.; Iqbal, M.J.; Zafar, M.; Hussain, S.J.; Shah, S.A.A. Green Synthesis of Metallic Nanoparticles and Their Potential in Bio-Medical Applications. Nano Biomed. Eng. 2021, 13, 191–206. [Google Scholar] [CrossRef]
- Ashtekar, N.; Anand, G.; Thulasiram, H.V.; Rajeshkumar, K.C. Genus Penicillium: Advances and Application in the Modern Era. New Future Dev. Microb. Biotechnol. Bioeng. 2021, 201–213. [Google Scholar] [CrossRef]
- Kavitha, K.; Vijaya, N.; Arthanareeswari, M.; Rajendran, S.; Al-Hashem, A.; Subramania, A. Nanomaterials for Antifungal Applications. Nanotoxic. Prev. Antibact. Appl. Nanomater. 2020, 385–398. [Google Scholar] [CrossRef]
- Yassin, M.A.; Elgorban, A.M.; El-Samawaty, A.E.R.M.A.; Almunqedhi, B.M.A. Biosynthesis of Silver Nanoparticles Using Penicillium Verrucosum and Analysis of Their Antifungal Activity. Saudi J. Biol. Sci. 2021, 28, 2123–2127. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.D.; Eid, A.M.; Abdel-Rahman, M.A.; Hamza, M.F. Light Enhanced the Antimicrobial, Anticancer, and Catalytic Activities of Selenium Nanoparticles Fabricated by Endophytic Fungal Strain, Penicillium Crustosum EP-1. Sci. Rep. 2022, 12, 11834. [Google Scholar] [CrossRef] [PubMed]
- Serov, D.A.; Khabatova, V.V.; Vodeneev, V.; Li, R.; Gudkov, S.V. A Review of the Antibacterial, Fungicidal and Antiviral Properties of Selenium Nanoparticles. Materials 2023, 16, 5363. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, K.S. Role of Nano-Selenium in Health and Environment. J. Biotechnol. 2021, 325, 152–163. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Tryfonopoulou, E.; Aindelis, G.; Ypsilantis, P.; Sarafidis, C.; Kalogirou, O.; Chlichlia, K. Biogenic Selenium Nanoparticles Produced by: Lactobacillus Casei ATCC 393 Inhibit Colon Cancer Cell Growth in Vitro and in Vivo. Nanoscale Adv. 2021, 3, 2516–2528. [Google Scholar] [CrossRef]
- Mosallam, F.M.; El-Sayyad, G.S.; Fathy, R.M.; El-Batal, A.I. Biomolecules-Mediated Synthesis of Selenium Nanoparticles Using Aspergillus Oryzae Fermented Lupin Extract and Gamma Radiation for Hindering the Growth of Some Multidrug-Resistant Bacteria and Pathogenic Fungi. Microb. Pathog. 2018, 122, 108–116. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, L.; Zhou, K.; Ding, L.; Zeng, J.; Zhang, W. Anti-Oxidant and Anti-Endothelial Dysfunctional Properties of Nano-Selenium in Vitro and in Vivo of Hyperhomocysteinemic Rats. Int. J. Nanomed. 2020, 15, 4501–4521. [Google Scholar] [CrossRef] [PubMed]
- Geoffrion, L.D.; Hesabizadeh, T.; Medina-Cruz, D.; Kusper, M.; Taylor, P.; Vernet-Crua, A.; Chen, J.; Ajo, A.; Webster, T.J.; Guisbiers, G. Naked Selenium Nanoparticles for Antibacterial and Anticancer Treatments. ACS Omega 2020, 5, 2660–2669. [Google Scholar] [CrossRef]
- Bano, I.; Skalickova, S.; Sajjad, H.; Skladanka, J.; Horky, P. Uses of Selenium Nanoparticles in the Plant Production. Agronomy 2021, 11, 2229. [Google Scholar] [CrossRef]
- Lee, C.; Choi, M.-S.; Kim, H.-T.; Yun, H.-T.; Lee, B.; Chung, Y.-S.; Kim, R.W.; Choi, H.-K. Soybean [Glycine max (L.) Merrill]: Importance as A Crop and Pedigree Reconstruction of Korean Varieties. Plant. Breed. Biotechnol. 2015, 3, 179–196. [Google Scholar] [CrossRef]
- Pagano, M.C.; Miransari, M. The Importance of Soybean Production Worldwide. In Abiotic and Biotic Stresses in Soybean Production: Soybean Production: Volume 1; Academic Press: Cambridge, MA, USA, 2016; Volume 5, pp. 1–26. [Google Scholar] [CrossRef]
- Modgil, R.; Tanwar, B.; Goyal, A.; Kumar, V. Soybean (Glycine max). In Oilseeds: Health Attributes and Food Applications; Tanwar, B., Goyal, A., Eds.; Springer: Singapore, 2021; pp. 1–46. ISBN 978-981-15-4194-0. [Google Scholar]
- Singh, H.; Jassal, R.K.; Kang, J.S.; Sandhu, S.S.; Kang, H.; Grewal, K. Seed Priming Techniques in Field Crops-A Review. Agric. Rev. 2015, 36, 251–264. [Google Scholar] [CrossRef]
- Mohamed Zeid, I.; El Lateef Gharib, F.A.; Mohamed Ghazi, S.; Zakaria Ahmed, E. Promotive Effect of Ascorbic Acid, Gallic Acid, Selenium and Nano-Selenium on Seed Germination, Seedling Growth and Some Hydrolytic Enzymes Activity of Cowpea (Vigna Unguiculata) Seedling. J. Plant Physiol. Pathol. 2019, 7, 1. [Google Scholar] [CrossRef]
- Ren, C.H.; Bilyeu, K.D.; Beuselinck, P.R. Composition, Vigor, and Proteome of Mature Soybean Seeds Developed under High Temperature. Crop. Sci. 2009, 49, 1010–1022. [Google Scholar] [CrossRef]
- Oh, B.J.; Park, W.M. Histopathological Observation and Identification of Fusarium Spp. Causing Soybean Sprout Rot. Hangug Sigmul Byeongri Haghoeji 1996, 471–475. [Google Scholar]
- Ashry, N.A.; Ghonaim, M.M.; Mohamed, H.I.; Mogazy, A.M. Physiological and Molecular Genetic Studies on Two Elicitors for Improving the Tolerance of Six Egyptian Soybean Cultivars to Cotton Leaf Worm. Plant Physiol. Biochem. 2018, 130, 224–234. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Alkordy, M.W.; Atta, A.A.A.A. Effect of Host Plants on Biology of Spodoptera littoralis (Boisd.). Egypt. Acad. J. Biol. Sci. A Èntomol. 2019, 12, 65–73. [Google Scholar]
- Hamama, H.M.; Fergani, Y.A. Toxicity and Oxidative Stress Induced in Spodoptera littoralis (Boisduval)(Lepidoptera: Noctuidae) Treated with Some Insecticides. Afr. Entomol. 2019, 27, 523–531. [Google Scholar] [CrossRef]
- Morad, M.Y.; El-Sayed, H.; Elhenawy, A.A.; Korany, S.M.; Aloufi, A.S.; Ibrahim, A.M. Myco-Synthesized Molluscicidal and Larvicidal Selenium Nanoparticles: A New Strategy to Control Biomphalaria Alexandrina Snails and Larvae of Schistosoma Mansoni with an In Silico Study on Induced Oxidative Stress. J. Fungi 2022, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; Fouda, M.M.G.; Fouda, A.; Awad, M.A.; Al-Olayan, E.M.; Allam, A.A.; Shaheen, T.I. Antibacterial, Cytotoxicity and Larvicidal Activity of Green Synthesized Selenium Nanoparticles Using Penicillium Corylophilum. J. Clust. Sci. 2021, 32, 351–361. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. JNCI J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Allam, R.M.; Al-Abd, A.M.; Khedr, A.; Sharaf, O.A.; Nofal, S.M.; Khalifa, A.E.; Mosli, H.A.; Abdel-Naim, A.B. Fingolimod Interrupts the Cross Talk between Estrogen Metabolism and Sphingolipid Metabolism within Prostate Cancer Cells. Toxicol. Lett. 2018, 291, 77–85. [Google Scholar] [CrossRef]
- Afzal, O.; Hassan, F.U.; Ahmed, M.; Shabbir, G.; Ahmed, S. TEMPERATURE AFFECTS GERMINATION INDICES OF SAFFLOWER (Carthamus tinctorius L.). J. Anim. Plant. Sci. 2022, 32, 1691–1702. [Google Scholar] [CrossRef]
- Hesami, M.; Pepe, M.; Monthony, A.S.; Baiton, A.; Phineas Jones, A.M. Modeling and Optimizing in Vitro Seed Germination of Industrial Hemp (Cannabis Sativa L.). Ind. Crops Prod. 2021, 170, 113753. [Google Scholar] [CrossRef]
- Dehnavi, A.R.; Zahedi, M.; Ludwiczak, A.; Perez, S.C.; Piernik, A. Effect of Salinity on Seed Germination and Seedling Development of Sorghum (Sorghum bicolor (L.) Moench) Genotypes. Agronomy 2020, 10, 859. [Google Scholar] [CrossRef]
- Naglah, A.M.; Moustafa, G.O.; Elhenawy, A.A.; Mounier, M.M.; El-Sayed, H.; Al-Omar, M.A.; Almehizia, A.A.; Bhat, M.A. Nα-1, 3-Benzenedicarbonyl-Bis-(Amino Acid) and Dipeptide Candidates: Synthesis, Cytotoxic, Antimicrobial and Molecular Docking Investigation. Drug Des. Dev. Ther. 2015, 15, 1315–1332. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971; p. 333. [Google Scholar]
- Chhipa, H. Chapter 5-Mycosynthesis of Nanoparticles for Smart Agricultural Practice: A Green and Eco-Friendly Approach. In Green Synthesis, Characterization and Applications of Nanoparticles; Shukla, A.K., Iravani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–109. ISBN 978-0-08-102579-6. [Google Scholar]
- Islam, S.N.; Naqvi, S.M.A.; Raza, A.; Jaiswal, A.; Singh, A.K.; Dixit, M.; Barnwal, A.; Gambhir, S.; Ahmad, A. Mycosynthesis of Highly Fluorescent Selenium Nanoparticles from Fusarium Oxysporum, Their Antifungal Activity against Black Fungus Aspergillus Niger, and in-Vivo Biodistribution Studies. 3 Biotech 2022, 12, 309. [Google Scholar] [CrossRef]
- El-Sayed, E.-S.R.; Abdelhakim, H.K.; Ahmed, A.S. Solid-State Fermentation for Enhanced Production of Selenium Nanoparticles by Gamma-Irradiated Monascus Purpureus and Their Biological Evaluation and Photocatalytic Activities. Bioprocess Biosyst. Eng. 2020, 43, 797–809. [Google Scholar] [CrossRef]
- Saied, E.; Mekky, A.E.; Al-Askar, A.A.; Hagag, A.F.; El-bana, A.A.; Ashraf, M.; Walid, A.; Nour, T.; Fawzi, M.M.; Arishi, A.A.; et al. Aspergillus Terreus-Mediated Selenium Nanoparticles and Their Antimicrobial and Photocatalytic Activities. Crystals 2023, 13, 450. [Google Scholar] [CrossRef]
- Hussein, H.G.; El-Sayed, E.-S.R.; Younis, N.A.; Hamdy, A.E.H.A.; Easa, S.M. Harnessing Endophytic Fungi for Biosynthesis of Selenium Nanoparticles and Exploring Their Bioactivities. AMB Express 2022, 12, 68. [Google Scholar] [CrossRef]
- Wadhwani, S.A.; Gorain, M.; Banerjee, P.; Shedbalkar, U.U.; Singh, R.; Kundu, G.C.; Chopade, B.A. Green Synthesis of Selenium Nanoparticles Using Acinetobacter Sp. SW30: Optimization, Characterization and Its Anticancer Activity in Breast Cancer Cells. Int. J. Nanomed. 2017, 12, 6841–6855. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Azab, M.S. An Eco-Friendly Approach to Textile and Tannery Wastewater Treatment Using Maghemite Nanoparticles (γ-Fe2O3-NPs) Fabricated by Penicillium Expansum Strain (K-W). J. Environ. Chem. Eng. 2021, 9, 104693. [Google Scholar] [CrossRef]
- Salem, S.S. Bio-Fabrication of Selenium Nanoparticles Using Baker’s Yeast Extract and Its Antimicrobial Efficacy on Food Borne Pathogens. Appl. Biochem. Biotechnol. 2022, 194, 1898–1910. [Google Scholar] [CrossRef] [PubMed]
- Piacenza, E.; Presentato, A.; Ferrante, F.; Cavallaro, G.; Alduina, R.; Chillura Martino, D.F. Biogenic Selenium Nanoparticles: A Fine Characterization to Unveil Their Thermodynamic Stability. Nanomaterials 2021, 11, 1195. [Google Scholar] [CrossRef] [PubMed]
- Barabadi, H.; Kobarfard, F.; Vahidi, H. Biosynthesis and Characterization of Biogenic Tellurium Nanoparticles by Using Penicillium Chrysogenum PTCC 5031: A Novel Approach in Gold Biotechnology. Iran. J. Pharm. Res. IJPR 2018, 17, 87–97. [Google Scholar] [PubMed]
- Rajkumar, K.; Sandhya, M.V.S.; Koganti, S.; Burgula, S. Selenium Nanoparticles Synthesized Using Pseudomonas Stutzeri (Mh191156) Show Antiproliferative and Anti-Angiogenic Activity against Cervical Cancer Cells. Int. J. Nanomed. 2020, 15, 4523–4540. [Google Scholar] [CrossRef]
- Amin, M.A.; Ismail, M.A.; Badawy, A.A.; Awad, M.A.; Hamza, M.F.; Awad, M.F.; Fouda, A. The Potency of Fungal-Fabricated Selenium Nanoparticles to Improve the Growth Performance of Helianthus Annuus l. And Control of Cutworm Agrotis Ipsilon. Catalysts 2021, 11, 1551. [Google Scholar] [CrossRef]
- Barzegarparay, F.; Najafzadehvarzi, H.; Pourbagher, R.; Parsian, H.; Ghoreishi, S.M.; Mortazavi-Derazkola, S. Green synthesis of novel selenium nanoparticles using Crataegus monogyna extract (SeNPs@CM) and investigation of its toxicity, antioxidant capacity, and anticancer activity against MCF-7 as a breast cancer cell line. Biomass Convers. Biorefinery 2023, 1–10. [Google Scholar] [CrossRef]
- Marandi, A.; Ashrafi, F.; Bakhtiari, N. Preparation and Evaluation of Anti-Cancer Effect of Lactobacillus Casei-Containing Niosome on Breast Cancer Cells Viability. Iran. J. Sci. 2023, 47, 1029–1038. [Google Scholar] [CrossRef]
- Cui, D.; Ma, J.; Liang, T.; Sun, L.; Meng, L.; Liang, T.; Li, Q. Selenium Nanoparticles Fabricated in Laminarin Polysaccharides Solutions Exert Their Cytotoxicities in HepG2 Cells by Inhibiting Autophagy and Promoting Apoptosis. Int. J. Biol. Macromol. 2019, 137, 829–835. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Li, Y.; Ma, L.; Lin, Z.; Xu, J.; Guo, Y. Preparation and Anti-Tumor Activity of Selenium Nanoparticles Based on a Polysaccharide from Paeonia Lactiflora. Int. J. Biol. Macromol. 2023, 232, 123261. [Google Scholar] [CrossRef]
- Chen, T.; Wong, Y.S.; Zheng, W.; Bai, Y.; Huang, L. Selenium Nanoparticles Fabricated in Undaria Pinnatifida Polysaccharide Solutions Induce Mitochondria-Mediated Apoptosis in A375 Human Melanoma Cells. Colloids Surf. B Biointerfaces 2008, 67, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Z.; Dai, C.; Wang, P.; Fan, S.; Yu, B.; Qu, Y. Antibacterial Properties and Mechanism of Selenium Nanoparticles Synthesized by Providencia Sp. DCX. Environ. Res. 2021, 194, 110630. [Google Scholar] [CrossRef] [PubMed]
- Lesnichaya, M.; Perfileva, A.; Nozhkina, O.; Gazizova, A.; Graskova, I. Synthesis, Toxicity Evaluation and Determination of Possible Mechanisms of Antimicrobial Effect of Arabinogalactane-Capped Selenium Nanoparticles. J. Trace Elem. Med. Biol. 2022, 69, 126904. [Google Scholar] [CrossRef] [PubMed]
- Tuan, P.A.; Sun, M.; Nguyen, T.N.; Park, S.; Ayele, B.T. Molecular Mechanisms of Seed Germination. In Sprouted Grains: Nutritional Value, Production, and Applications; AACC International Press: Eagan, MN, USA, 2019; pp. 1–24. [Google Scholar] [CrossRef]
- Ikram, M.; Raja, N.I.; Javed, B.; Mashwani, Z.-U.; Hussain, M.; Hussain, M.; Ehsan, M.; Rafique, N.; Malik, K.; Sultana, T.; et al. Foliar Applications of Bio-Fabricated Selenium Nanoparticles to Improve the Growth of Wheat Plants under Drought Stress. Green Process. Synth. 2020, 9, 706–714. [Google Scholar] [CrossRef]
- Garza-García, J.J.O.; Hernández-Díaz, J.A.; Zamudio-Ojeda, A.; León-Morales, J.M.; Guerrero-Guzmán, A.; Sánchez-Chiprés, D.R.; López-Velázquez, J.C.; García-Morales, S. The Role of Selenium Nanoparticles in Agriculture and Food Technology. Biol. Trace Elem. Res. 2022, 200, 2528–2548. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Blinov, A.V.; Serov, A.V.; Gvozdenko, A.A.; Kravtsov, A.A.; Nagdalian, A.A.; Raffa, V.V.; Maglakelidze, D.G.; Blinova, A.A.; Kobina, A.V.; et al. Effect of Selenium Nanoparticles on Germination of Hordéum Vulgáre Barley Seeds. Coatings 2021, 11, 862. [Google Scholar] [CrossRef]
- Cheng, B.; Wang, C.; Yue, L.; Chen, F.; Cao, X.; Lan, Q.; Liu, T.; Wang, Z. Selenium Nanomaterials Improve the Quality of Lettuce (Lactuca sativa L.) by Modulating Root Growth, Nutrient Availability, and Photosynthesis. NanoImpact 2023, 29, 100449. [Google Scholar] [CrossRef]
- Hussein, H.A.A.; Darwesh, O.M.; Mekki, B.B. Environmentally Friendly Nano-Selenium to Improve Antioxidant System and Growth of Groundnut Cultivars under Sandy Soil Conditions. Biocatal. Agric. Biotechnol. 2019, 18, 101080. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Mohamed, I.A.A.; Wang, Z.; Wang, C.; Tabl, K.M.; Khatab, A.; Kuai, J.; Wang, J.; Wang, B.; et al. Mitigation of the Salinity Stress in Rapeseed (Brassica napus L.) Productivity by Exogenous Applications of Bio-Selenium Nanoparticles during the Early Seedling Stage. Environ. Pollut. 2022, 310, 119815. [Google Scholar] [CrossRef]
- Fenille, R.C.; Luiz De Souza, N.; Kuramae, E.E. Characterization of Rhizoctonia solani Associated with Soybean in Brazil. Eur. J. Plant Pathol. 2002, 108, 783–792. [Google Scholar] [CrossRef]
- Chang, X.; Dai, H.; Wang, D.; Zhou, H.; He, W.; Fu, Y.; Ibrahim, F.; Zhou, Y.; Gong, G.; Shang, J.; et al. Identification of Fusarium Species Associated with Soybean Root Rot in Sichuan Province, China. Eur. J. Plant Pathol. 2018, 151, 563–577. [Google Scholar] [CrossRef]
- Joshi, S.M.; De Britto, S.; Jogaiah, S.; Ito, S.I. Mycogenic Selenium Nanoparticles as Potential New Generation Broad Spectrum Antifungal Molecules. Biomolecules 2019, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Bafghi, M.H.; Darroudi, M.; Zargar, M.; Zarrinfar, H.; Nazari, R. Biosynthesis of Selenium Nanoparticles by Aspergillus Flavus and Candida Albicans for Antifungal Applications. Micro Nano Lett. 2021, 16, 656–669. [Google Scholar] [CrossRef]
- Martínez, A.; Apip, C.; Meléndrez, M.F.; Domínguez, M.; Sánchez-Sanhueza, G.; Marzialetti, T.; Catalán, A. Dual Antifungal Activity against Candida Albicans of Copper Metallic Nanostructures and Hierarchical Copper Oxide Marigold-like Nanostructures Grown in Situ in the Culture Medium. J. Appl. Microbiol. 2021, 130, 1883–1892. [Google Scholar] [CrossRef]
- Yazhiniprabha, M.; Vaseeharan, B. In Vitro and in Vivo Toxicity Assessment of Selenium Nanoparticles with Significant Larvicidal and Bacteriostatic Properties. Mater. Sci. Eng. C 2019, 103, 109763. [Google Scholar] [CrossRef] [PubMed]
- Cittrarasu, V.; Kaliannan, D.; Dharman, K.; Maluventhen, V.; Easwaran, M.; Liu, W.C.; Balasubramanian, B.; Arumugam, M. Green Synthesis of Selenium Nanoparticles Mediated from Ceropegia Bulbosa Roxb Extract and Its Cytotoxicity, Antimicrobial, Mosquitocidal and Photocatalytic Activities. Sci. Rep. 2021, 11, 1032. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Al-Otaibi, W.A.; Saber, T.; AlMotwaa, S.M.; Alshallash, K.S.; Elhady, M.; Badr, N.F.; Abdel-Rahman, M.A. Antimicrobial, Antiviral, and In-Vitro Cytotoxicity and Mosquitocidal Activities of Portulaca Oleracea-Based Green Synthesis of Selenium Nanoparticles. J. Funct. Biomater. 2022, 13, 157. [Google Scholar] [CrossRef]
- Lashin, I.; Fouda, A.; Gobouri, A.A.; Azab, E.; Mohammedsaleh, Z.M.; Makharita, R.R. Antimicrobial and in Vitro Cytotoxic Efficacy of Biogenic Silver Nanoparticles (Ag-nps) Fabricated by Callus Extract of Solanum incanum L. Biomolecules 2021, 11, 341. [Google Scholar] [CrossRef]




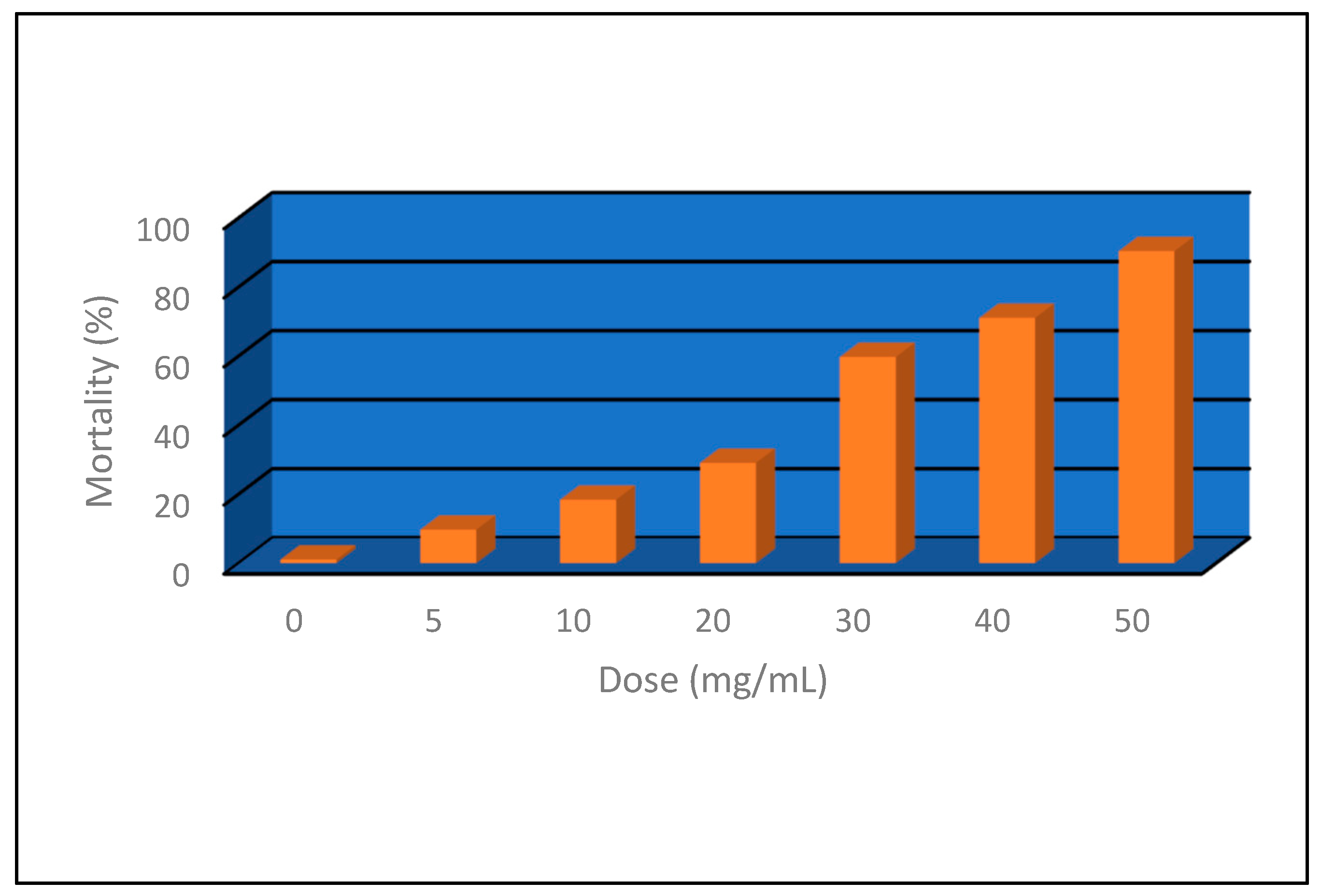
| Lethal Conc. (LC) | Conc. (mg/mL) | 95% Fiducial Limits (mg/mL) | Slope | Probability (P) | Regression Analysis (R2) | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| 25 50 90 | 12.36 23.08 75.66 | 6.49 15.57 66.56 | 15.41 34.02 188.95 | 2.49 +/− 0.12 | 0.0004 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelsalam, A.; El-Sayed, H.; Hamama, H.M.; Morad, M.Y.; Aloufi, A.S.; Abd El-Hameed, R.M. Biogenic Selenium Nanoparticles: Anticancer, Antimicrobial, Insecticidal Properties and Their Impact on Soybean (Glycine max L.) Seed Germination and Seedling Growth. Biology 2023, 12, 1361. https://doi.org/10.3390/biology12111361
Abdelsalam A, El-Sayed H, Hamama HM, Morad MY, Aloufi AS, Abd El-Hameed RM. Biogenic Selenium Nanoparticles: Anticancer, Antimicrobial, Insecticidal Properties and Their Impact on Soybean (Glycine max L.) Seed Germination and Seedling Growth. Biology. 2023; 12(11):1361. https://doi.org/10.3390/biology12111361
Chicago/Turabian StyleAbdelsalam, Asmaa, Heba El-Sayed, Heba M. Hamama, Mostafa Y. Morad, Abeer S. Aloufi, and Rehab M. Abd El-Hameed. 2023. "Biogenic Selenium Nanoparticles: Anticancer, Antimicrobial, Insecticidal Properties and Their Impact on Soybean (Glycine max L.) Seed Germination and Seedling Growth" Biology 12, no. 11: 1361. https://doi.org/10.3390/biology12111361
APA StyleAbdelsalam, A., El-Sayed, H., Hamama, H. M., Morad, M. Y., Aloufi, A. S., & Abd El-Hameed, R. M. (2023). Biogenic Selenium Nanoparticles: Anticancer, Antimicrobial, Insecticidal Properties and Their Impact on Soybean (Glycine max L.) Seed Germination and Seedling Growth. Biology, 12(11), 1361. https://doi.org/10.3390/biology12111361







