Characterization of Spontaneous Melanization by Fluorescence Spectroscopy: A Basis for Analytical Application to Biological Substrates
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
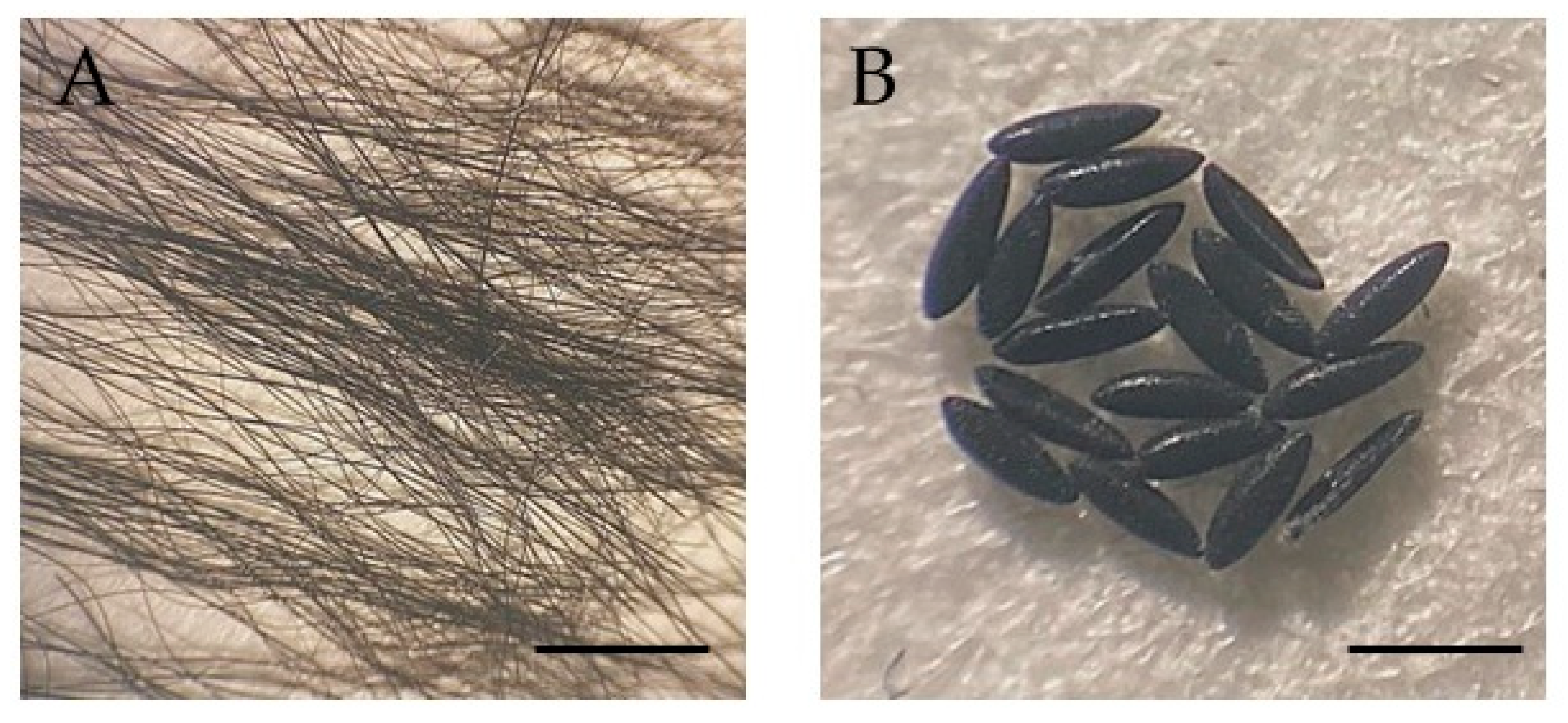
2.2. Biological Samples
2.3. Spectrofluorometric Analysis
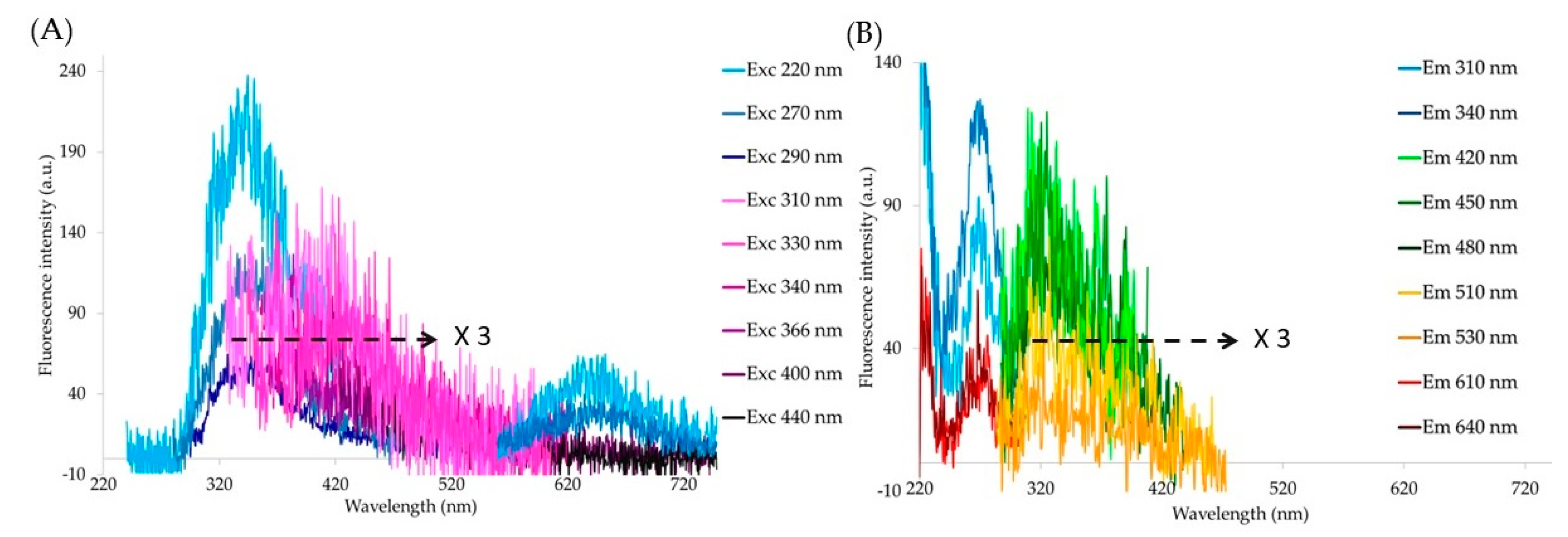
3. Results
Synthetic Melanin and Pure Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hong, L.; Wakamatsu, K.; Ito, S.; Adhyaru, B.; Cheng, C.-Y.; Bowers, C.R.; Simon, J.D. Comparison of structural and chemical properties of black and red human hair melanosomes. Photochem. Photobiol. 2005, 81, 135–144. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining chitin, chitosan and their melanin complexes from insects. Int. J. Biol. Macromol. 2021, 167, 1319–1328. [Google Scholar] [CrossRef]
- Derby, C.D. Cephalopod ink: Production, chemistry, functions and applications. Mar. Drugs 2014, 12, 2700. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A. Melanogenesis in the ink gland of Sepia officinalis. Pigment Cell Res. 2003, 16, 517–522. [Google Scholar] [CrossRef]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef]
- Solano, F. Photoprotection and skin pigmentation: Melanin-related molecules and some other new agents obtained from natural sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef]
- Hill, H.Z.; Li, W.; Xin, P.; Mitchell, D.L. Melanin: A two edged sword? Pigment Cell Res. 1997, 10, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Nasti, T.H.; Timares, L. MC1R, eumelanin and pheomelanin: Their role in determining the susceptibility to skin cancer. Photochem. Photobiol. 2015, 91, 188–200. [Google Scholar] [CrossRef]
- Felix, C.C.; Hyde, J.S.; Sarna, T.; Sealy, R.C. Interactions of melanin with metal ions. Electron spin resonance evidence for chelate complexes of metal ions with free radicals. J. Am. Chem. Soc. 1978, 100, 3922–3926. [Google Scholar] [CrossRef]
- Zucca, F.A.; Giaveri, G.; Gallorini, M.; Albertini, A.; Toscani, M.; Pezzoli, G.; Lucius, R.; Wilms, H.; Sulzer, D.; Ito, S.; et al. The neuromelanin of human substantia nigra: Physiological and pathogenic aspects. Pigment Cell Res. 2004, 17, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, J.A. The antimicrobial properties of melanocytes, melanosomes and melanin and the evolution of black skin. J. Theor. Biol. 2001, 211, 101–113. [Google Scholar] [CrossRef]
- Nappi, A.J.; Christensen, B.M. Melanogenesis and associated cytotoxic reactions: Applications to insect innate immunity. Insect Biochem. Mol. Biol. 2005, 35, 443–459. [Google Scholar] [CrossRef]
- Burkhart, C.G.; Burkhart, C.N. The mole theory: Primary function of melanocytes and melanin may be antimicrobial defense and immunomodulation (not solar protection). Int. J. Dermatol. 2005, 44, 340–342. [Google Scholar] [CrossRef]
- Whitten, M.M.A.; Coates, C.J. Re-evaluation of insect melanogenesis research: Views from the dark side. Pigment Cell Melanoma Res. 2017, 30, 386–401. [Google Scholar] [CrossRef]
- Sugumaran, M.; Barek, H. Critical analysis of the melanogenic pathway in insects and higher animals. Int. J. Mol. Sci. 2016, 17, 1753. [Google Scholar] [CrossRef]
- Sarna, T.; Pilas, B.; Land, E.J.; Truscott, T.G. Interaction of radicals from water radiolysis with melanin. Biochim. Biophys. Acta 1986, 883, 162–167. [Google Scholar] [CrossRef]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, melanin, and melanogenesis: The yin and yang relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef] [PubMed]
- Ito, S. A chemist’s view of melanogenesis. Pigment Cell Res. 2003, 16, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Bertazzo, A.; Costa, C.V.L.; Allegri, G.; Favretto, D.; Traldi, P. Application of matrix-assisted laser desorption/ionization mass spectrometry to the detection of melanins formed from Dopa and dopamine. J. Mass Spectrom. 1999, 34, 922–929. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K.; Ozeki, H. Chemical analysis of melanins and its application to the study of the regulation of melanogenesis. Pigment Cell Res. 2000, 13 (Suppl. 8), 103–109. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Chemical degradation of melanins: Application to identification of dopamine-melanin. Pigment Cell Res. 1998, 11, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Lerche, C.M.; Olsen, P.; Nissen, C.V.; Philipsen, P.A.; Wulf, H.C. A novel LC-MS/MS method to quantify eumelanin and pheomelanin and their relation to UVR sensitivity—A study on human skin biopsies. Pigment Cell Melanoma Res. 2019, 32, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Simon, J.D. Isolation and biophysical studies of natural eumelanins: Applications of imaging technologies and ultrafast spectroscopy. Pigment Cell Res. 2003, 16, 606–618. [Google Scholar] [CrossRef]
- Mostert, A.B. Melanin, the what, the why and the how: An introductory review for materials scientists interested in flexible and versatile polymers. Polymers 2021, 13, 1670. [Google Scholar] [CrossRef]
- Pralea, I.E.; Moldovan, R.C.; Petrache, A.M.; Ilieș, M.; Hegheș, S.C.; Ielciu, I.; Nicoară, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From extraction to advanced analytical methods: The challenges of melanin analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef] [PubMed]
- Gallas, J.M.; Eisner, M. Fluorescence of melanin-dependence upon excitation wavelength and concentration. Photochem. Photobiol. 1987, 45, 595–600. [Google Scholar] [CrossRef]
- Meredith, P.; Riesz, J. Radiative relaxation quantum yields for synthetic eumelanin. Photochem. Photobiol. 2004, 79, 211–216. [Google Scholar] [CrossRef]
- Nighswander-Rempel, S.P.; Riesz, J.; Gilmore, J.; Bothma, J.P.; Meredith, P. Quantitative fluorescence excitation spectra of synthetic eumelanin. J. Phys. Chem. B 2005, 109, 20629–20635. [Google Scholar] [CrossRef]
- Teale, F.W.; Weber, G. Ultraviolet fluorescence of the aromatic amino acids. Biochem. J. 1957, 65, 476–482. [Google Scholar] [CrossRef]
- Teale, F.W. The ultraviolet fluorescence of proteins in neutral solution. Biochem. J. 1960, 76, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Ghisaidoobe, A.B.T.; Chung, S.J. Intrinsic tryptophan fluorescence in the detection and analysis of proteins: A focus on Förster resonance energy transfer techniques. Int. J. Mol. Sci. 2014, 15, 22518–22538. [Google Scholar] [CrossRef]
- Macías, P.; Pinto, M.C.; Gutiérrez-Mérino, C. Long-wavelength fluorescence of tyrosine and tryptophan solutions. Biochem. Int. 1987, 15, 961–969. [Google Scholar] [PubMed]
- Stark, K.B.; Gallas, J.M.; Zajac, G.W.; Eisner, M.; Golab, J.T. Spectroscopic study and simulation from recent structural models for eumelanin: I. Monomer, dimers. J. Phys. Chem. B 2003, 107, 3061–3067. [Google Scholar] [CrossRef]
- Stark, K.B.; Gallas, J.M.; Zajac, G.W.; Eisner, M.; Golab, J.T. Spectroscopic study and simulation from recent structural models for eumelanin: II. Oligomers. J. Phys. Chem. B 2003, 107, 11558–11562. [Google Scholar] [CrossRef]
- Rzepecki, L.M.; Waite, J.H. Aβ-dehydro-3,4-dihydroxyphenylalanine derivatives: Rate and mechanism of formation. Arch. Biochem. Biophys. 1991, 285, 27–36. [Google Scholar] [CrossRef]
- Lee, B.P.; Dalsin, J.L.; Messersmith, P.B. Synthesis and gelation of DOPA-modified poly(ethylene glycol) hydrogels. Biomacromolecules 2002, 3, 1038–1047. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Wang, Y.; Chan, H.W.; Wang, L.; Chan, W. Mass spectrometric and spectrophotometric analyses reveal an alternative structure and a new formation mechanism for melanin. Anal. Chem. 2015, 87, 7958–7963. [Google Scholar] [CrossRef]
- Gauden, M.; Pezzella, A.; Panzella, L.; Napolitano, A.; D’Ischia, M.; Sundström, V. Ultrafast excited state dynamics of 5,6-dihydroxyindole, a key eumelanin building block: Nonradiative decay mechanism. J. Phys. Chem. B 2009, 113, 12575–12580. [Google Scholar] [CrossRef]
- Nighswander-Rempel, S.P.; Mahadevan, I.B.; Rubinsztein-Dunlop, H.; Meredith, P. Time-resolved and steady-state fluorescence spectroscopy of eumelanin and indolic Polymers. Photochem. Photobiol. 2007, 83, 1449–1454. [Google Scholar] [CrossRef]
- Pigoli, C.; Gibelli, L.R.; Caniatti, M.; Moretti, L.; Sironi, G.; Giudice, C. Bleaching melanin in formalin-fixed and paraffin-embedded melanoma specimens using visible light: A pilot study. Eur. J. Histochem. 2019, 63, 3071. [Google Scholar] [CrossRef]
- Leupold, D.; Szyc, L.; Stankovic, G.; Hofmann, M.; Scholz, M.; Forschner, A. Dermatofluoroscopy is also for redheads a sensitive method of early melanoma detection. Dermatology 2020, 236, 508–516. [Google Scholar] [CrossRef]
- Dimitrow, E.; Riemann, I.; Ehlers, A.; Koehler, M.J.; Norgauer, J.; Elsner, P.; König, K.; Kaatz, M. Spectral fluorescence lifetime detection and selective melanin imaging by multiphoton laser tomography for melanoma diagnosis. Exp. Dermatol. 2009, 18, 509–515. [Google Scholar] [CrossRef] [PubMed]
- König, K.; Ehlers, A.; Stracke, F.; Riemann, I. In vivo drug screening in human skin using femtosecond laser multiphoton tomography. Skin Pharmacol. Physiol. 2006, 19, 78–88. [Google Scholar] [CrossRef]
- Teuchner, K.; Ehlert, J.; Freyer, W.; Leupold, D.; Altmeyer, P.; Stücker, M.; Hoffmann, K. Fluorescence studies of melanin by stepwise two-photon femtosecond laser excitation. J. Fluoresc. 2000, 10, 275–281. [Google Scholar] [CrossRef]
- Krasieva, T.B.; Stringari, C.; Liu, F.; Sun, C.-H.; Kong, Y.; Balu, M.; Meyskens, F.L.; Gratton, E.; Tromberg, B.J. Two-photon excited fluorescence lifetime imaging and spectroscopy of melanins In Vitro and In Vivo. J. Biomed. Opt. 2013, 18, 031107. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, R.; Wessler, G.; Scholz, M.; Leupold, D.; Stankovic, G.; Buder, S.; Stücker, M.; Hoffmann, K. Early diagnosis of melanotic melanoma based on laser-induced melanin fluorescence. J. Biomed. Opt. 2009, 14, 034033. [Google Scholar] [CrossRef]
- Lapierre-Landry, M.; Carroll, J.; Skala, M.C. Imaging retinal melanin: A review of current technologies. J. Biol. Eng. 2018, 12, 29. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Pfau, M.; Fleckenstein, M.; Staurenghi, G.; Sparrow, J.R.; Bindewald-Wittich, A.; Spaide, R.F.; Wolf, S.; Sadda, S.R.; Holz, F.G. Fundus autofluorescence imaging. Prog. Retin Eye Res. 2021, 81, 100893. [Google Scholar] [CrossRef]
- Keilhauer, C.N.; Delori, F.C. Near-infrared autofluorescence imaging of the fundus: Visualization of ocular melanin. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3556–3564. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Monteiro, A. Melanin pathway genes regulate color and morphology of butterfly wing scales. Cell Rep. 2018, 24, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Stavenga, D.G.; Leertouwer, H.L.; Hariyama, T.; de Raedt, H.A.; Wilts, B.D. Sexual dichromatism of the damselfly Calopteryx japonica caused by a melanin-chitin multilayer in the male wing veins. PLoS ONE 2012, 7, e49743. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, X.; Christensen, B.M. Dopachrome conversion activity in Aedes aegypti: Significance during melanotic encapsulation of parasites and cuticular tanning. Insect Biochem. Mol. Biol. 1994, 24, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.K.; Li, J.; Christensen, B.M. Cloning and characterization of a dopachrome conversion enzyme from the yellow fever mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 2001, 31, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Croce, A.C.; Scolari, F. Autofluorescent biomolecules in Diptera: From structure to metabolism and behavior. Molecules 2022, 27, 4458. [Google Scholar] [CrossRef] [PubMed]
- Gebru, A.; Jansson, S.; Ignell, R.; Kirkeby, C.; Prangsma, J.C.; Brydegaard, M. Multiband modulation spectroscopy for the determination of sex and species of mosquitoes in flight. J. Biophotonics 2018, 11, e201800014. [Google Scholar] [CrossRef]
- Nansen, C. Penetration and scattering—Two optical phenomena to consider when applying proximal remote sensing technologies to object classifications. PLoS ONE 2019, 13, e0204579. [Google Scholar] [CrossRef] [PubMed]
- Scolari, F.; Girella, A.; Croce, A.C. Imaging and spectral analysis of autofluorescence patterns in larval head structures of mosquito vectors. Eur. J. Histochem. 2022, 66, 3462. [Google Scholar] [CrossRef]
- Farnesi, L.C.; Vargas, H.C.M.; Valle, D.; Rezende, G.L. Darker eggs of mosquitoes resist more to dry conditions: Melanin enhances serosal cuticle contribution in egg resistance to desiccation in Aedes, Anopheles and Culex vectors. PLoS Negl. Trop. Dis. 2017, 11, e0006063. [Google Scholar] [CrossRef]
- Anderson, P.J.B.; Rogers, Q.R.; Morris, J.G. Cats require more dietary phenylalanine or tyrosine for melanin deposition in hair than for maximal growth. J. Nutr. 2002, 132, 2037–2042. [Google Scholar] [CrossRef]
- Prota, G. Melanins and Melanogenesis; Academic Press: San Diego, CA, USA, 1992; p. 290. [Google Scholar]
- Pudar, D.; Puggioli, A.; Balestrino, F.; Sy, V.; Carrieri, M.; Bellini, R.; Petrić, D. Effect of cage size on Aedes albopictus wing length, survival and egg production. Heliyon 2021, 7, e07381. [Google Scholar] [CrossRef]
- Marquardt, D.W. An Algorithm for least-squares estimation of nonlinear parameters. SIAM J. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Hutnik, C.M.L.; Szabo, A.G. Long-wavelength fluorescence of tyrosine and tryptophan: A classic example of second order diffraction. Biochem. Int. 1988, 16, 587–591. [Google Scholar] [PubMed]
- Meredith, P.; Powell, B.J.; Riesz, J.; Nighswander-Rempel, S.P.; Pederson, M.R.; Moore, E.G. Towards structure–property–function relationships for eumelanin. Soft Matter. 2006, 2, 37–44. [Google Scholar] [CrossRef]
- Slawinski, J.; Puzyna, W.; Slawinska, D. Chemiluminescence during photooxidation of melanins and soil humic acids arising from a singlet oxygen mechanism. Photochem. Photobiol. 1978, 28, 459–463. [Google Scholar] [CrossRef]
- Kohl, F.R.; Grieco, C.; Kohler, B. Ultrafast spectral hole burning reveals the distinct chromophores in eumelanin and their common photoresponse. Chem. Sci. 2020, 11, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Kendall, T.; Yip, P.; Davy, A.; Sefcik, J.; Sutter, J.U. Influence of ions and pH on the formation of solid- and liquid-like melanin. ACS Omega 2020, 5, 25059–25068. [Google Scholar] [CrossRef]
- Meng, S.; Kaxiras, E. Theoretical models of eumelanin protomolecules and their optical properties. Biophys. J. 2008, 94, 2095–2105. [Google Scholar] [CrossRef]
- Noh, M.Y.; Mun, S.; Kramer, K.J.; Muthukrishnan, S.; Arakane, Y. Yellow-y Functions in egg melanization and chorion morphology of the Asian tiger mosquito, Aedes albopictus. Front. Cell Dev. Biol. 2021, 9, 769788. [Google Scholar] [CrossRef]
- Novellino, L.; Napolitano, A.; Prota, G. Isolation and characterization of mammalian eumelanins from hair and irides. Biochim. Biophys. Acta Gen. Subj. 2000, 1475, 295–306. [Google Scholar] [CrossRef]
- Palladini, G.; di Pasqua, L.G.; Cagna, M.; Croce, A.C.; Perlini, S.; Mannucci, B.; Profumo, A.; Ferrigno, A.; Vairetti, M. MCD diet rat model induces alterations in zinc and iron during NAFLD progression from steatosis to steatohepatitis. Int. J. Mol. Sci. 2022, 23, 6817. [Google Scholar] [CrossRef] [PubMed]
- Arakane, Y.; Noh, M.Y.; Asano, T.; Kramer, K.J. Tyrosine metabolism for insect cuticle pigmentation and sclerotization. In Extracellular Composite Matrices in Arthropods; Springer International Publishing: Cham, Switzerland, 2016; pp. 165–220. [Google Scholar]
- Rajpurohit, S.; Peterson, L.M.; Orr, A.J.; Marlon, A.J.; Gibbs, A.G. An experimental evolution test of the relationship between melanism and desiccation survival in insects. PLoS ONE 2016, 11, e0163414. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.Y.; Kim, S.H.; Gorman, M.J.; Kramer, K.J.; Muthukrishnan, S.; Arakane, Y. Yellow-g and yellow-G2 proteins are required for egg desiccation resistance and temporal pigmentation in the Asian tiger mosquito, Aedes albopictus. Insect Biochem. Mol. Biol. 2020, 122, 103386. [Google Scholar] [CrossRef] [PubMed]
- Massey, J.H.; Chung, D.; Siwanowicz, I.; Stern, D.L.; Wittkopp, P.J. The yellow gene influences Drosophila male mating success through sex comb melanization. Elife 2019, 8, e49388. [Google Scholar] [CrossRef]
- Ito, K.; Yoshikawa, M.; Fujii, T.; Tabunoki, H.; Yokoyama, T. Melanin pigmentation gives rise to black spots on the wings of the silkworm Bombyx mori. J. Insect Physiol. 2016, 91–92, 100–106. [Google Scholar] [CrossRef]
- Vincent, J.F.V.; Wegst, U.G.K. Design and mechanical properties of insect cuticle. Arthropod. Struct. Dev. 2004, 33, 187–199. [Google Scholar] [CrossRef]
- Andersen, S.O. 6—Cuticular sclerotization and tanning. In Insect Molecular Biology and Biochemistry; Gilbert, L.I., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2012; pp. 167–192. [Google Scholar] [CrossRef]
- Reinhardt, K.; Breunig, H.G.; König, K. Autofluorescence lifetime variation in the cuticle of the bedbug Cimex lectularius. Arthropod. Struct. Dev. 2017, 46, 56–62. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croce, A.C.; Scolari, F. Characterization of Spontaneous Melanization by Fluorescence Spectroscopy: A Basis for Analytical Application to Biological Substrates. Biology 2023, 12, 433. https://doi.org/10.3390/biology12030433
Croce AC, Scolari F. Characterization of Spontaneous Melanization by Fluorescence Spectroscopy: A Basis for Analytical Application to Biological Substrates. Biology. 2023; 12(3):433. https://doi.org/10.3390/biology12030433
Chicago/Turabian StyleCroce, Anna Cleta, and Francesca Scolari. 2023. "Characterization of Spontaneous Melanization by Fluorescence Spectroscopy: A Basis for Analytical Application to Biological Substrates" Biology 12, no. 3: 433. https://doi.org/10.3390/biology12030433
APA StyleCroce, A. C., & Scolari, F. (2023). Characterization of Spontaneous Melanization by Fluorescence Spectroscopy: A Basis for Analytical Application to Biological Substrates. Biology, 12(3), 433. https://doi.org/10.3390/biology12030433






