Water-Level Fluctuation Control of the Trophic Structure of a Yangtze River Oxbow
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
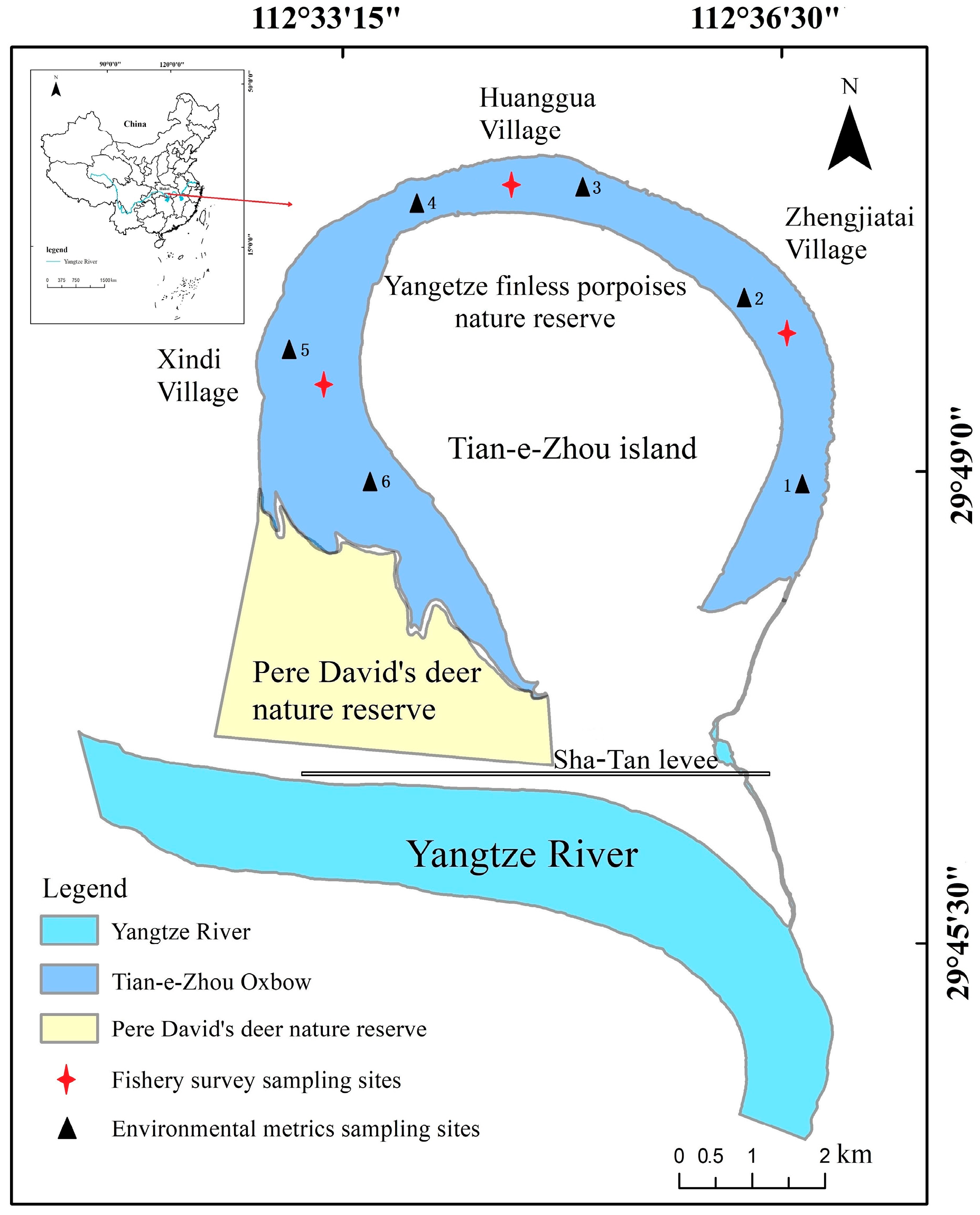
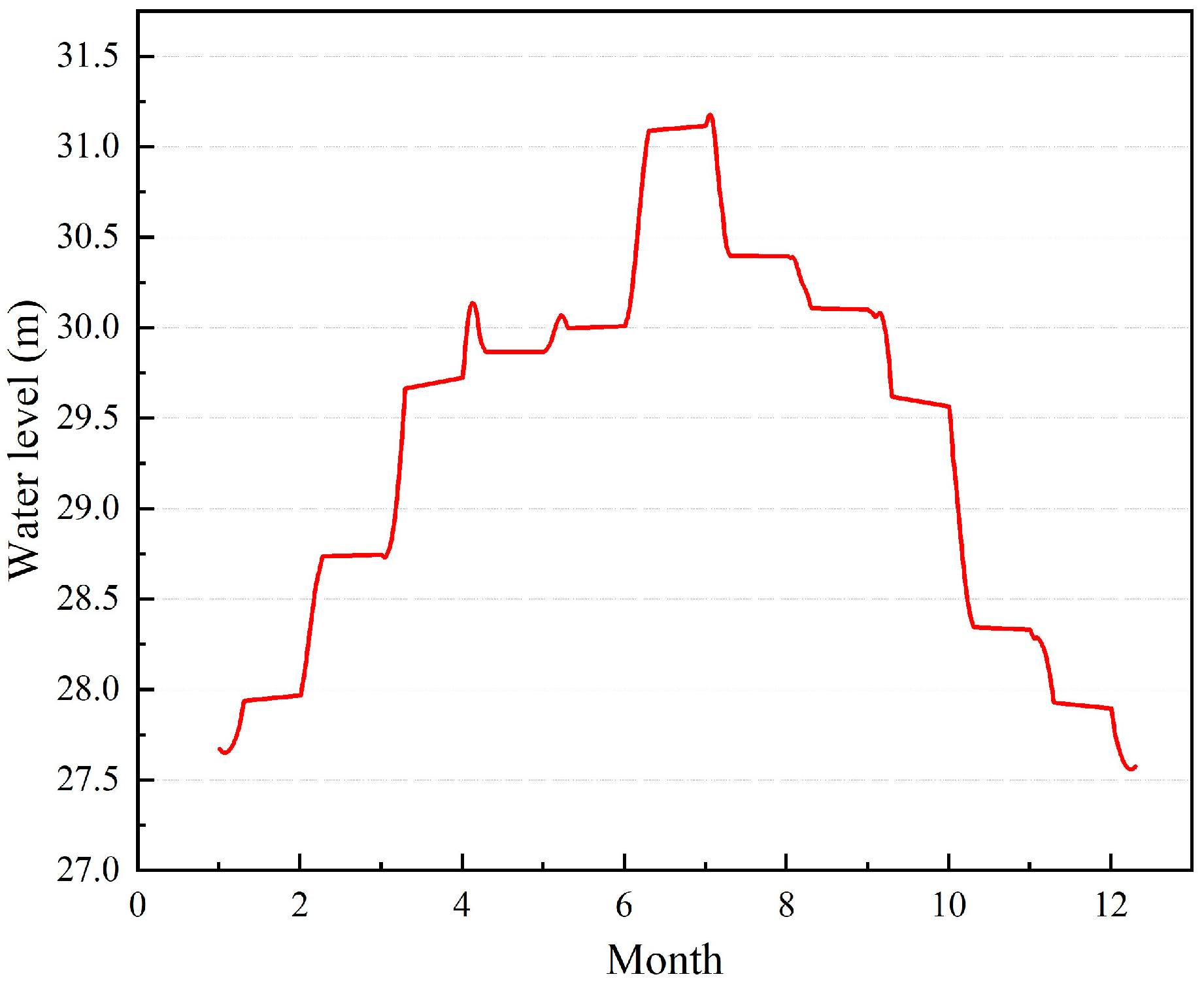
2.1. Study Area
2.2. Sample Collection and Preparation
2.3. Stable Isotope Analysis
2.4. Data Analysis
3. Results
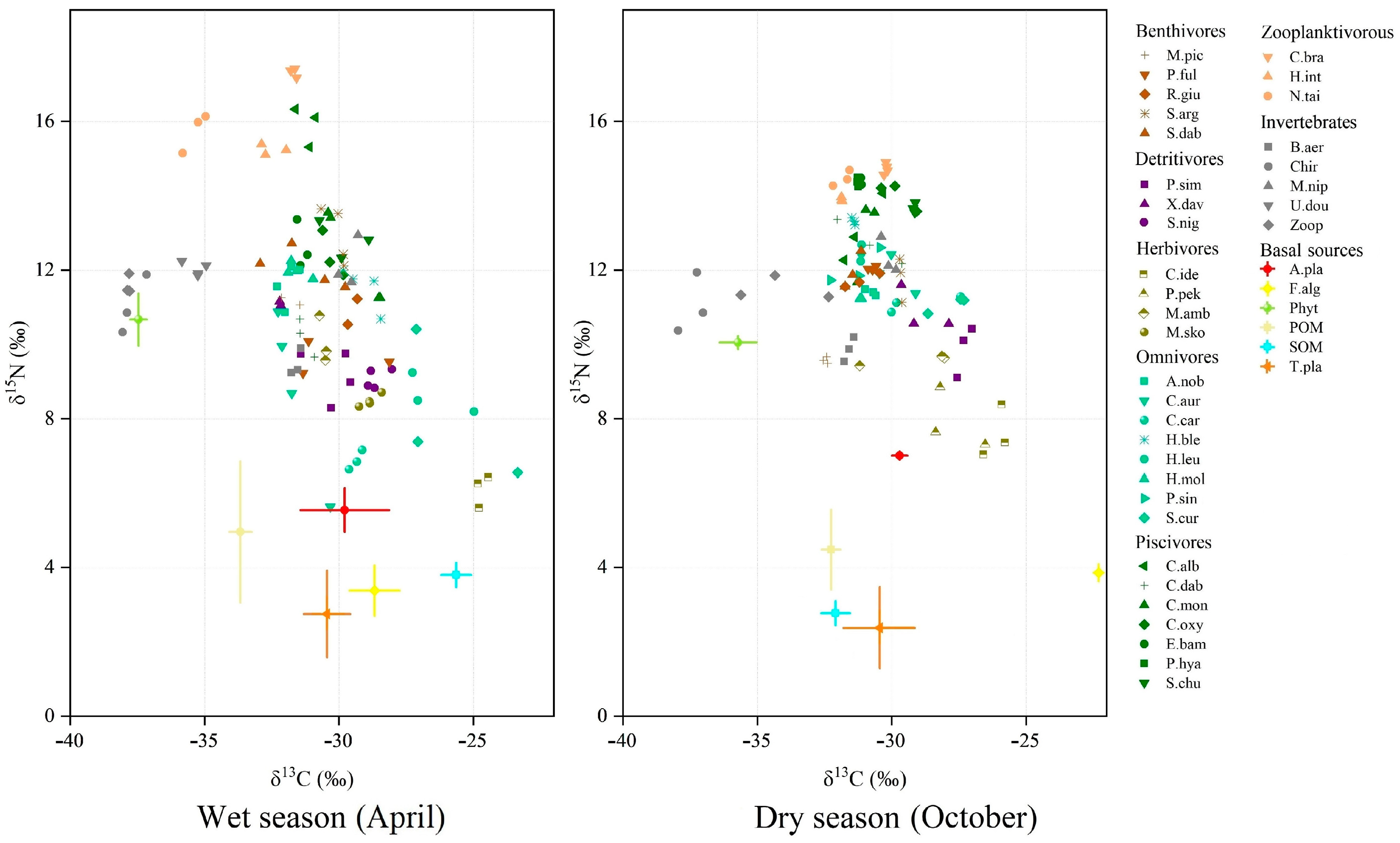
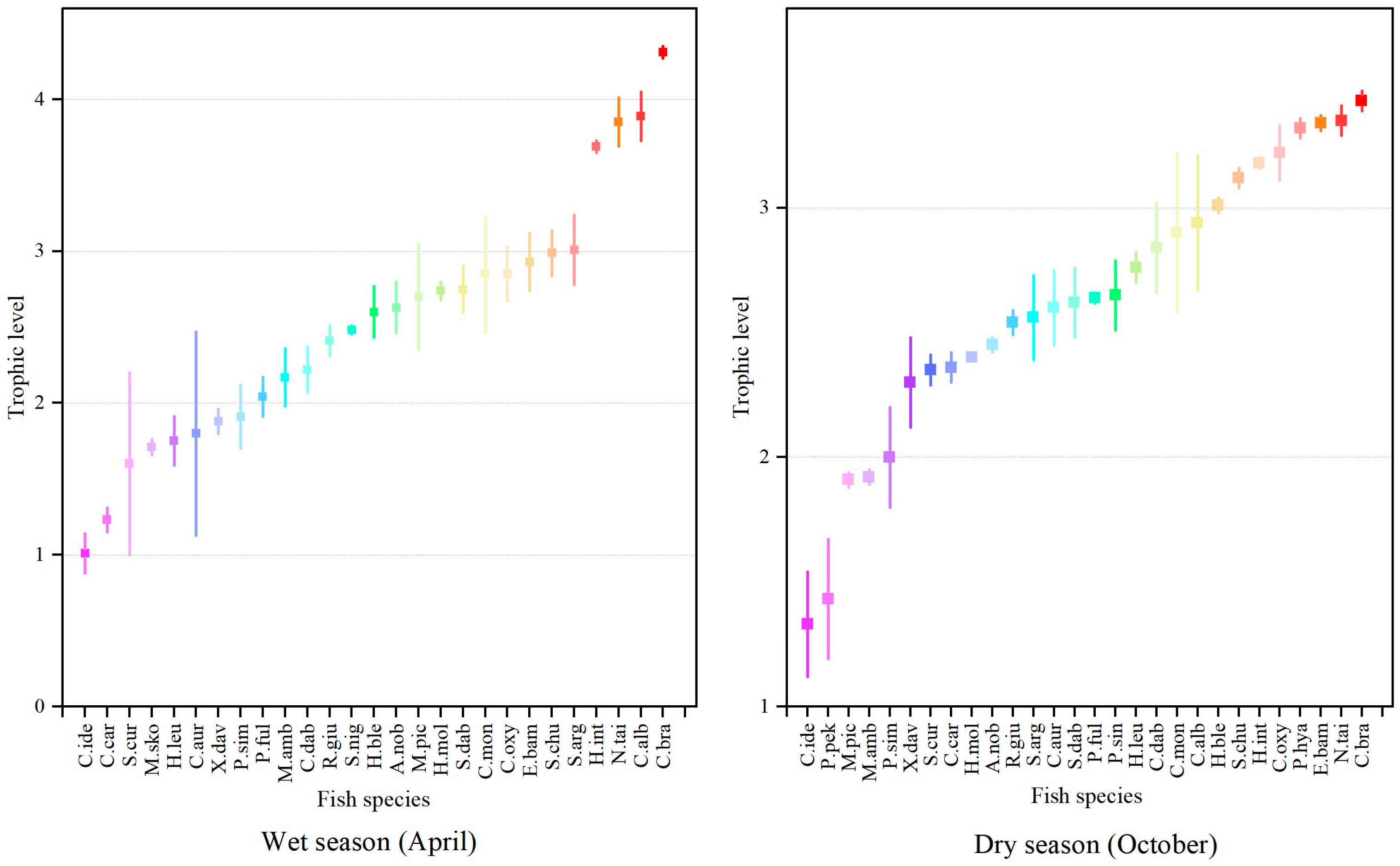
3.1. Isotopic Values and Trophic Levels
3.2. Trophic Niche
3.3. Contributions of Basal Resources to Consumers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Leveque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Penas, F.J.; Barquin, J. Assessment of large-scale patterns of hydrological alteration caused by dams. J. Hydrol. 2019, 572, 706–718. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Zhang, G.X.; Wu, Y.F.; Xu, Y.J.; Dai, C.L. Dam Effects on Downstream Riparian Wetlands: The Nenjiang River, Northeast China. Water 2019, 11, 2038. [Google Scholar] [CrossRef]
- Liermann, C.R.; Nilsson, C.; Robertson, J.; Ng, R.Y. Implications of Dam Obstruction for Global Freshwater Fish Diversity. Bioscience 2012, 62, 539–548. [Google Scholar] [CrossRef]
- Mao, Z.G.; Gu, X.H.; Cao, Y.; Luo, J.H.; Zeng, Q.F.; Chen, H.H.; Jeppesen, E. Pelagic energy flow supports the food web of a shallow lake following a dramatic regime shift driven by water level changes. Sci. Total Environ. 2021, 756, 143642. [Google Scholar] [CrossRef]
- Wantzen, K.M.; Rothhaupt, K.O.; Mortl, M.; Cantonati, M.; Laszlo, G.T.; Fischer, P. Ecological effects of water-level fluctuations in lakes: An urgent issue. Hydrobiologia 2008, 613, 1–4. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Q.J.; Wang, X.X.; Ji, H.; Quigley, E.J.; Sharbatmaleki, M.; Li, S.M.; Xi, B.D.; Sun, B.A.; Li, C.L. Effects of hydrological and climatic variables on cyanobacterial blooms in four large shallow lakes fed by the Yangtze River. Environ. Sci. Ecotechnol. 2021, 5, 100069. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef]
- Jiang, T.; Kundzewicz, Z.W.; Su, B. Changes in monthly precipitation and flood hazard in the Yangtze River Basin, China. Int. J. Climatol. 2008, 28, 1471–1481. [Google Scholar] [CrossRef]
- Essington, T.E.; Beaudreau, A.H.; Wiedenmann, J. Fishing through marine food webs. Proc. Natl. Acad. Sci. USA 2006, 103, 3171–3175. [Google Scholar] [CrossRef]
- Mills, E.L.; Leach, J.H.; Carlton, J.T.; Secor, C.L. Exotic species in the great-lakes- A history of biotic crises and anthropogenic introductions. J. Great Lakes Res. 1993, 19, 1–54. [Google Scholar] [CrossRef]
- Qi, T.; Ban, X.; Du, H.; Guo, W.X.; Du, Y.; Long, A.Y.; Nan, L.Y.; Shi, X.T.; Zheng, C.Y. Impact of alteration of hydrologic rigime during fish sensitive hydrological period on fish resources in middle reaches of Yangtze River. Resour. Environ. Yangtze Basin 2022, 31, 2621–2632. [Google Scholar]
- Wang, Y.K.; Rhoads, B.L.; Wang, D. Assessment of the flow regime alterations in the middle reach of the Yangtze River associated with dam construction: Potential ecological implications. Hydrol. Process. 2016, 30, 3949–3966. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yu, X.B.; Li, W.H.; Xu, J.; Chen, Y.W.; Fan, N. Potential influence of water level changes on energy flows in a lake food web. Chin. Sci. Bull. 2011, 56, 2794–2802. [Google Scholar] [CrossRef]
- Que, Y.F.; Xie, J.Y.; Xu, J.; Li, W.T.; Wang, E.Z.; Zhu, B. Ecological Influences of Water-Level Fluctuation on Food Web Network. Water 2021, 13, 2371. [Google Scholar] [CrossRef]
- Ma, J.S.; Chen, W.Q.; Chen, M.J.; Zhong, K.E.; Yao, N.; Zhang, X.M.; Zhang, H.; Erik, J.; Zhou, Q. Water level fluctuations associated with hydrological connectivity consolidate the food web stability of the largest Chinese freshwater lake via mediating trophodynamics and trophic structure. Ecol. Indic. 2023, 153, 110372. [Google Scholar] [CrossRef]
- Durante, L.; Wing, S.; Ingram, T.; Sabadel, A.; Shima, J. Changes in trophic structure of an exploited fish community at the centennial scale are linked to fisheries and climate forces. Sci. Rep. 2022, 12, 4309. [Google Scholar] [CrossRef]
- Iglesias, C.; Meerhoff, M.; Johansson, L.S.; Gonzalez-Bergonzoni, I.; Mazzeo, N.; Pacheco, J.P.; Teixeira-de Mello, F.; Goyenola, G.; Lauridsen, T.L.; Sondergaard, M.; et al. Stable isotope analysis confirms substantial differences between subtropical and temperate shallow lake food webs. Hydrobiologia 2017, 784, 111–123. [Google Scholar] [CrossRef]
- Davenport, S.R.; Bax, N.J. A trophic study of a marine ecosystem off southeastern Australia using stable isotopes of carbon and nitrogen. Can. J. Fish. Aquat. Sci. 2002, 59, 514–530. [Google Scholar] [CrossRef]
- Fry, B.; Mumford, P.L.; Tam, F.; Fox, D.D.; Warren, G.L.; Havens, K.E.; Steinman, A.D. Trophic position and individual feeding histories of fish from Lake Okeechobee, Florida. Can. J. Fish. Aquat. Sci. 1999, 56, 590–600. [Google Scholar] [CrossRef]
- Nahon, S.; Roussel, J.M.; Jaeger, C.; Menniti, C.; Kerherve, P.; Mortillaroj, J.M.; Aubin, J. Characterization of trophic niche partitioning between carp (Cyprinus carpio) and roach (Rutilus rutilus) in experimental polyculture ponds using carbon (delta C-13) and nitrogen (delta N-15) stable isotopes. Aquaculture 2020, 522, 735162. [Google Scholar] [CrossRef]
- Agersted, M.D.; Bode, A.; Nielsen, T.G. Trophic position of coexisting krill species: A stable isotope approach. Mar. Ecol. Prog. Ser. 2014, 516, 139–151. [Google Scholar] [CrossRef]
- Vander Zanden, M.J.; Fetzer, W.W. Global patterns of aquatic food chain length. Oikos 2007, 116, 1378–1388. [Google Scholar] [CrossRef]
- Korotkevich, A.Y.; Potapov, A.M.; Tiunov, A.V.; Kuznetsova, N.A. Collapse of trophic-niche structure in belowground communities under anthropogenic disturbance. Ecosphere 2018, 9, e02528. [Google Scholar] [CrossRef]
- Ru, H.J.; Zhong, L.Q.; Nian, W.; Li, Y.F.; Sheng, Q.; Ni, Z.H. Variations of trophic structure and niche space in fish community along a highly regulated subtropical large river. Ecol. Evol. 2022, 12, e9424. [Google Scholar] [CrossRef]
- Huang, S.L.; Mei, Z.G.; Hao, Y.J.; Zheng, J.S.; Wang, K.X.; Wang, D. Saving the Yangtze finless porpoise: Time is rapidly running out. Biol. Conserv. 2017, 210, 40–46. [Google Scholar] [CrossRef]
- IUCN. The IUCN RED List of Threatened Species. Version 2022-2. 2022. Available online: https://www.iucnredlist.org (accessed on 6 September 2023).
- Mei, Z.G.; Huang, S.L.; Hao, Y.J.; Turvey, S.T.; Gong, W.M.; Wang, D. Accelerating population decline of Yangtze finless porpoise (Neophocaena asiaeorientalis asiaeorientalis). Biol. Conserv. 2012, 153, 192–200. [Google Scholar] [CrossRef]
- Yang, J.W.; Wan, X.L.; Zeng, X.Y.; Zheng, J.S.; Han, Y.; Fan, F.; Hao, Y.J.; Wang, K.X.; Mei, Z.G.; Wang, D. A preliminary study on diet of the Yangtze finless porpoise using next-generation sequencing techniques. Mar. Mammal Sci. 2019, 35, 1579–1586. [Google Scholar] [CrossRef]
- Barros, N.B.; Jefferson, T.A.; Parsons, E.C.M. Food habits of finless porpoises (Neophocaena phocaenoides) in Hong Kong waters. Raffles Bull. Zool. 2002, 50, 115–123. [Google Scholar]
- Chen, B.Y.; Wang, L.; Wang, H.; Li, S.S.; Jefferson, T.A.; Wang, L.; Tang, E.K.; Xu, X.R.; Yang, G. Finless porpoises (Neophocaena asiaeorientalis) in the East China Sea: Insights into feeding habits using morphological, molecular, and stable isotopic techniques. Can. J. Fish. Aquat. Sci. 2017, 74, 1628–1645. [Google Scholar] [CrossRef]
- Lu, Z.C.; Xu, S.Y.; Song, N.; Gao, T.X.; Tian, J.S.; Han, J.B. Analysis of the diet of finless porpoise (Neophocaena asiaeorientalis sunameri) based on prey morphological characters and DNA barcoding. Conserv. Genet. Resour. 2016, 8, 523–531. [Google Scholar] [CrossRef]
- Zhang, X.F.; Wei, Z.; Wang, X.Q.; Yang, J.; Chen, P.X. Studies on the Feasibility of Establishment of a Semi-natural Reserve at Tian-e-Zhou Oxbow for baiji Lipotes vexillifer. Acta Hydrobiol. Sin. 1995, 1995, 110–123. [Google Scholar]
- Gong, J.; Wang, T.; Li, X.; Huang, D.; Shen, J.Z.; Gong, C. Interannual Variation of the Fish Community Structure in the Tian-e-zhou Oxbow of the Yangtze River. J. Hydroecology 2018, 39, 46–53. [Google Scholar] [CrossRef]
- Qiu, L.H.; Ji, F.F.; Qiu, Y.H.; Zhang, S.F.; Shen, J.Z.; Gong, C.; Xu, C.Y. Evolution of fish community structure, cause analysis and implications for ex-situ conservation of Yangtze finless porpoise in the Tian-e-Zhou Oxbow of Yangtze River. J. Lake Sci. 2023, 35, 950–960. [Google Scholar] [CrossRef]
- Zhang, H.S.; Ai, J.S.; Wen, H.J.; Zhang, Y.M.; Li, P.F.; Yang, T.; Zhu, J.Q. The environmental status and protection of the habitat of Shishou Elk. Adv. Meteorol. Sci. Technol. 2018, 8, 109–112. [Google Scholar]
- Yang, C.; Cai, X.B.; Wang, X.L. Remote Sensing of Hydrological Changes in Tian-e-Zhou Oxbow Lake, an Ungauged Area of the Yangtze River Basin. Remote Sens. 2018, 10, 27. [Google Scholar] [CrossRef]
- Huang, D.; Shen, J.Z.; Hu, S.D.; Gong, C.; Zhou, B.; Li, X.; Wang, H.S.; Sun, G.W. Zooplankton Community Structure and Water Quality Assessment in Tian-e-zhou Oxbow of the Yangtze River. Resour. Environ. Yangtze Basin 2014, 23, 328–334. [Google Scholar]
- Ma, X.J.; Shen, J.Z.; Wang, T.; Wang, H.S.; Huang, D.; Sun, G.W.; Gong, C. Macrozoobenthos community structure and water quality evaluation of Tian-e-Zhou Oxbows. Environ. Sci. 2014, 35, 3952–3958. [Google Scholar] [CrossRef]
- Li, Y.T.; Wang, D. Estimation of the carrying capacity of Yangtze finless propoise (Neophocaena asiaeorientalis asiaeorientalis) in Tian-e-Zhou Oxbow based on liner food network model. Appl. Ecol. Environ. Res. 2020, 18, 6981–6994. [Google Scholar] [CrossRef]
- Gao, S.; Gong, S.; Chen, Z.; Zhang, X.; Zhang, S.; Yu, W. Comparing the trophic structure of the Lvsi Fishing Ground as estimated from the Ecopath model and stable isotopic analysis. Estuar. Coast. Shelf Sci. 2021, 262, 107559. [Google Scholar] [CrossRef]
- Wang, T.; Wang, H.S.; Sun, G.W.; Huang, D.; Shen, J.H. Length-weight and length-length relationships for some Yangtze River fishes in Tian-e-zhou Oxbow, China. J. Appl. Ichthyol. 2012, 28, 660–662. [Google Scholar] [CrossRef]
- Huang, D.; Li, X.; Wang, Z.F.; Xiong, J.; Shen, J.Z. Phytoplankton Community Structure and Water Quality Assessment in the Tian-e-zhou Oxbow of the Yangtze River. J. Hydroecology 2016, 37, 8–14. [Google Scholar] [CrossRef]
- Andrades, R.; Jackson, A.L.; Macieira, R.M.; Reis, J.A.; Bernardino, A.F.; Joyeux, J.C.; Giarrizzo, T. Niche-related processes in island intertidal communities inferred from stable isotopes data. Ecol. Indic. 2019, 104, 648–658. [Google Scholar] [CrossRef]
- Ji, F.F.; Ma, X.F.; Qiu, L.H.; Kang, Z.P.; Shen, J.Z. Quantifying the effects of introduced Bighead Carp (Cyprinidae; Aristichthys nobilis) stocking on dominant fish species in the Ulungur Lake, China. Biol. Invasions 2022, 24, 1253–1265. [Google Scholar] [CrossRef]
- Chen, Y.Y. Fauna Sinica Osteichthyes Cypriniformes II; Science Press: Beijing, China, 1998. [Google Scholar]
- Yue, P.Q. Fauna Sinica Osteichthyes Cypriniformes III; Science Press: Beijing, China, 2000. [Google Scholar]
- Zhu, S.Q. Synopsis of Freshwater Fishes of China; Jiangsu Science and Technology Publishing House: Nanjing, China, 1995. [Google Scholar]
- Pinnegar, J.K.; Polunin, N.V.C. Differential fractionation of delta C-13 and delta N-15 among fish tissues: Implications for the study of trophic interactions. Funct. Ecol. 1999, 13, 225–231. [Google Scholar] [CrossRef]
- Vander Zanden, M.J.; Casselman, J.M.; Rasmussen, J.B. Stable isotope evidence for the food web consequences of species invasions in lakes. Nature 1999, 401, 464–467. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Yuan, G.L.; He, X.G.; Xie, P. Mercury bioaccumulation in the food web of Three Gorges Reservoir (China): Tempo-spatial patterns and effect of reservoir management. Sci. Total Environ. 2015, 527, 203–210. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Niu, J.G.; Zhou, Q.; Xie, C.X.; Ke, Z.X.; Li, D.P.; Gao, Y.W. Effects of resource availability and hydrological regime on autochthonous and allochthonous carbon in the food web of a large cross-border river (China). Sci. Total Environ. 2018, 612, 501–512. [Google Scholar] [CrossRef]
- GB 11982; Water Quality—Determination of Permanganate Index. National Standardization Administration of the People’s Republic of China: Beijing, China, 1989.
- Patton, C.J.; Kryskalla, J.R. Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Evaluation of Alkaline Persulfate Digestion as an Alternative to Kjeldahl Digestion for Determination of Total and Dissolved Nitrogen and Phosphorus in Water; United States Geological Survey: Sunrise Valley Drive Reston, VA, USA, 2003.
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Layman, C.A.; Araujo, M.S.; Boucek, R.; Hammerschlag-Peyer, C.M.; Harrison, E.; Jud, Z.R.; Matich, P.; Rosenblatt, A.E.; Vaudo, J.J.; Yeager, L.A.; et al. Applying stable isotopes to examine food-web structure: An overview of analytical tools. Biol. Rev. 2012, 87, 545–562. [Google Scholar] [CrossRef]
- Jackson, M.C.; Ian, D.; Jackson, A.L.; Robert, B.J.; Harper, D.M.; Jonathan, G.; Simon, T. Population-Level Metrics of Trophic Structure Based on Stable Isotopes and Their Application to Invasion Ecology. PLoS ONE 2012, 7, e31757. [Google Scholar] [CrossRef] [PubMed]
- Parnell, A.C.; Inger, R.; Bearhop, S.; Jackson, A.L. Source Partitioning Using Stable Isotopes: Coping with Too Much Variation. PLoS ONE 2010, 5, e9672. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, M.M.; Haffner, G.D.; Legler, N.D.; Rush, S.A.; Fisk, A.T. Fifty years later: Trophic ecology and niche overlap of a native and non-indigenous fish species in the western basin of Lake Erie. Biol. Invasions 2013, 15, 1695–1711. [Google Scholar] [CrossRef]
- Chen, P.X.; Shen, S.J.; Deng, Z.L.; Yu, X.F. The Yangtze River Fishes; Science Press: Beijing, China, 1976. [Google Scholar]
- Power, M.; Swanson, H.K.; Stasko, A.D.; Reist, J.D.; Johnson, J.D. A new probabilistic method for quantifying n-dimensional ecological niches and niche overlap. Ecology 2015, 96, 318–324. [Google Scholar]
- DeNiro, M.J.; Epstein, S. Mechanism of carbon isotope fractionation associated with lipid synthesis. Science 1977, 197, 261–263. [Google Scholar] [CrossRef]
- Haines, E.B.; Montague, C.L. Food Sources of Estuarine Invertebrates Analyzed Using 13C/12C Ratios. Ecology 1979, 60, 48–56. [Google Scholar] [CrossRef]
- Fry, B. Food web structure on Georges Bank from stable C, N, and S isotopic compositions. Limnol. Oceanogr. 1988, 33, 1182–1190. [Google Scholar] [CrossRef]
- Cherel, Y.; Hobson, K.A. Stable isotopes, beaks and predators: A new tool to study the trophic ecology of cephalopods, including giant and colossal squids. Proc. R. Soc. B Biol. Sci. 2005, 272, 1601–1607. [Google Scholar] [CrossRef]
- Varela, J.L.; Sorell, J.M.; Laiz-Carrión, R.; Baro, I.; Uriarte, A.; Macías, D.; Medina, A. Stomach content and stable isotope analyses reveal resource partitioning between juvenile bluefin tuna and Atlantic bonito in Alboran (SW Mediterranean). Fish. Res. 2019, 215, 97–105. [Google Scholar] [CrossRef]
- Cornelissen, I.J.M.; Vijverberg, J.; van den Beld, A.M.; Helmsing, N.R.; Verreth, J.A.J.; Nagelkerke, L.A.J. Heterogeneity in food-web interactions of fish in the Mwanza Gulf, Lake Victoria: A quantitative stable isotope study. Hydrobiologia 2018, 805, 113–130. [Google Scholar] [CrossRef]
- Madurell, T.; Fanelli, E.; Cartes, J.E. Isotopic composition of carbon and nitrogen of suprabenthic fauna in the NW Balearic Islands (western Mediterranean). J. Mar. Syst. 2008, 71, 336–345. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, G.G.; Zhang, H.; Zhang, P.Y.; Xie, P.; Xu, J. Seasonal variations of stable isotopes in fish fauna from east lake Dongting. Acta Hydrobiol. Sin. 2013, 37, 796–798. [Google Scholar]
- Bokhutlo, T.; Keppeler, F.W.; Winemiller, K.O. Seasonal hydrology influences energy channels in food webs of rivers in the lower Okavango Delta. Environ. Biol. Fishes 2021, 104, 1303–1319. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Q.S.; Liu, Q.Y.; Liu, X.J.; Huo, B.; Zhang, M.; Li, D.P. Effect of hydrologic changes on the structure and carbon source of the food web in Lake Basomtso. Freshw. Fish. 2020, 50, 99–107. [Google Scholar]
- Holgerson, M.A.; Post, D.M.; Skelly, D.K. Reconciling the role of terrestrial leaves in pond food webs: A whole-ecosystem experiment. Ecology 2016, 97, 1771–1782. [Google Scholar] [CrossRef]
- Guan, Z.Y.; He, Y.; An, Y.R.; Cai, J.H.; Tong, X.L. Effects of leaf-litter decomposition of exotic plants on benthic macroinvertebrate species diversity and functional feeding groups. Acta Ecol. Sin. 2010, 30, 2828–2835. [Google Scholar]
- Zhou, C.Y.; Fei, Y.J.; Wu, L.; Yang, C.D. Effects of elk grazing on soil physical and chemical properties of grassland on Tiane Island. Acta Prataculturae Sin. 2010, 19, 115–121. [Google Scholar]
- Wantzen, K.M.; de Arruda Machado, F.; Voss, M.; Boriss, H.; Junk, W.J. Seasonal isotopic shifts in fish of the Pantanal wetland, Brazil. Aquat. Sci. 2002, 64, 239–251. [Google Scholar] [CrossRef]
- Lv, H.J.; Yang, L.Y.; Fu, M.; Tang, X.Q.; Deng, H.T.; Zhang, Z.X.; Yao, W.Z. Evaluation on trophic niche characteristics of Coilia nasus from the Three Gorges Reservoir based on stable carbon and nitrogen isotope analysis. Acta Ecol. Sin. 2022, 42, 8739–8750. [Google Scholar]
- Wang, Y.Y.; Yu, X.B.; Zhang, L.; Xu, J. Food web structure of Poyang Lake during the dry season by stable carbon and nitrogen isotopes analysis. Acta Ecol. Sin. 2009, 29, 1181–1188. [Google Scholar]
- Jia, Y.T.; Jiang, Y.H.; Liu, Y.H.; Sui, X.Y.; Feng, X.; Zhu, R.; Chen, Y.F. Understanding trophic structure variation in fish assemblages of subtropical shallow lakes: Combined effects of ecosystem size, productivity, and disturbance. Ecol. Indic. 2021, 129, 107924. [Google Scholar] [CrossRef]
- Layman, C.A.; Arrington, D.A.; Montana, C.G.; Post, D.M. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 2007, 88, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Warry, F.Y.; Reich, P.; Cook, P.L.M.; Mac Nally, R.; Thomson, J.R.; Woodland, R.J. Nitrogen loads influence trophic organization of estuarine fish assemblages. Funct. Ecol. 2016, 30, 1723–1733. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, Y.B.; Wang, B.L.; Mi, W.J.; Song, Q.Y.; Xu, Y.Z.; Bi, Y.H. The analysis of food web structure in the area in front of the Three Gorges Dam using the stable isotope technology. Ecol. Sci. 2020, 39, 82–90. [Google Scholar] [CrossRef]
- Li, B.; Zheng, Y.C.; Xu, D.D.; Tao, M.; Li, H. Seasonal variation in the consumption of food by fish in the Mituo floodplain waters of the upper Yangtze River and implications for food web dynamics. Acta Ecol. Sin. 2023, 43, 1664–1675. [Google Scholar]
- Ye, X.Y.; Ren, L.; Kuang, Z.; Wang, Y.; Xu, D.P. Analysis of the trophic structure of fish populations in Yangcheng Lake based on stable isotope technology. J. Fish. Sci. China 2021, 28, 703–714. [Google Scholar]
- Deng, H.T.; Duan, X.B.; Liu, S.P.; Chen, D.Q. Temporal and spatial variations in the trophic structure of key species in downstream of Daning River. Acta Ecol. Sin. 2014, 34, 7110–7118. [Google Scholar]
- Schoener, T.W. Sizes of feeding territories among birds. Ecology 1968, 49, 123–141. [Google Scholar] [CrossRef]
- Ni, W.J.; Deng, H.T.; He, C.; Pu, Y.; Tian, H.W.; Liu, S.P.; Chen, D.Q.; Duan, X.B. Trohic niche comparison of three species of Culter from the middle and upper reaches of the Yangtze River. J. Fish. Sci. China 2023, 30, 236–246. [Google Scholar]
- Yang, J.; Chen, P.X. Movement and behavior of finless porpoise (Neophocaena asiaeorientalis) at Tian-e-Zhou Oxbow. Acta Hydrobiol. Sin. 1996, 20, 32–40. [Google Scholar]
- Wang, R.L.; Han, Y.; Fan, F.; Molinos, J.G.; Xu, J.; Wang, K.X.; Wang, D.; Mei, Z.G. Need to shift in river-lake connection scheme under the “ten-year fishing ban” in the Yangtze River, China. Ecol. Indic. 2022, 143, 109434. [Google Scholar] [CrossRef]
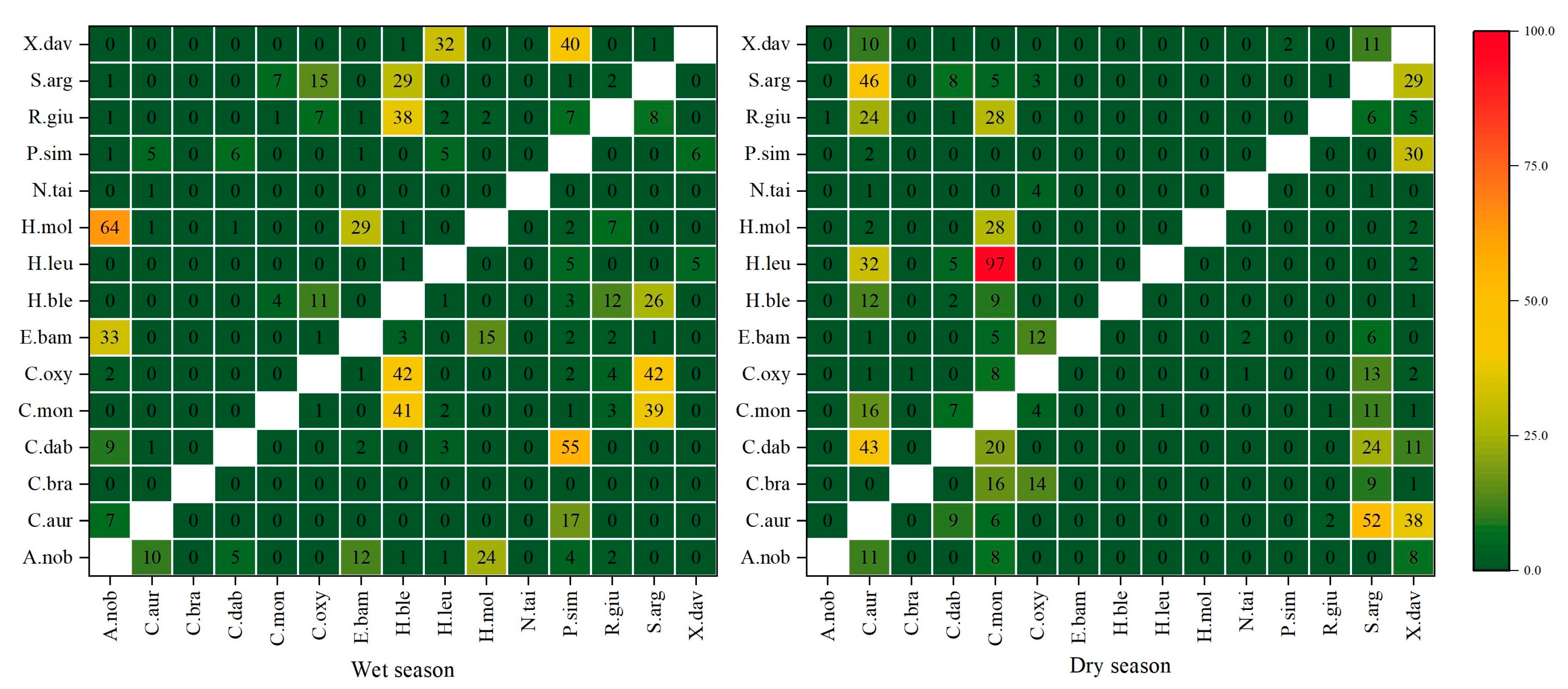
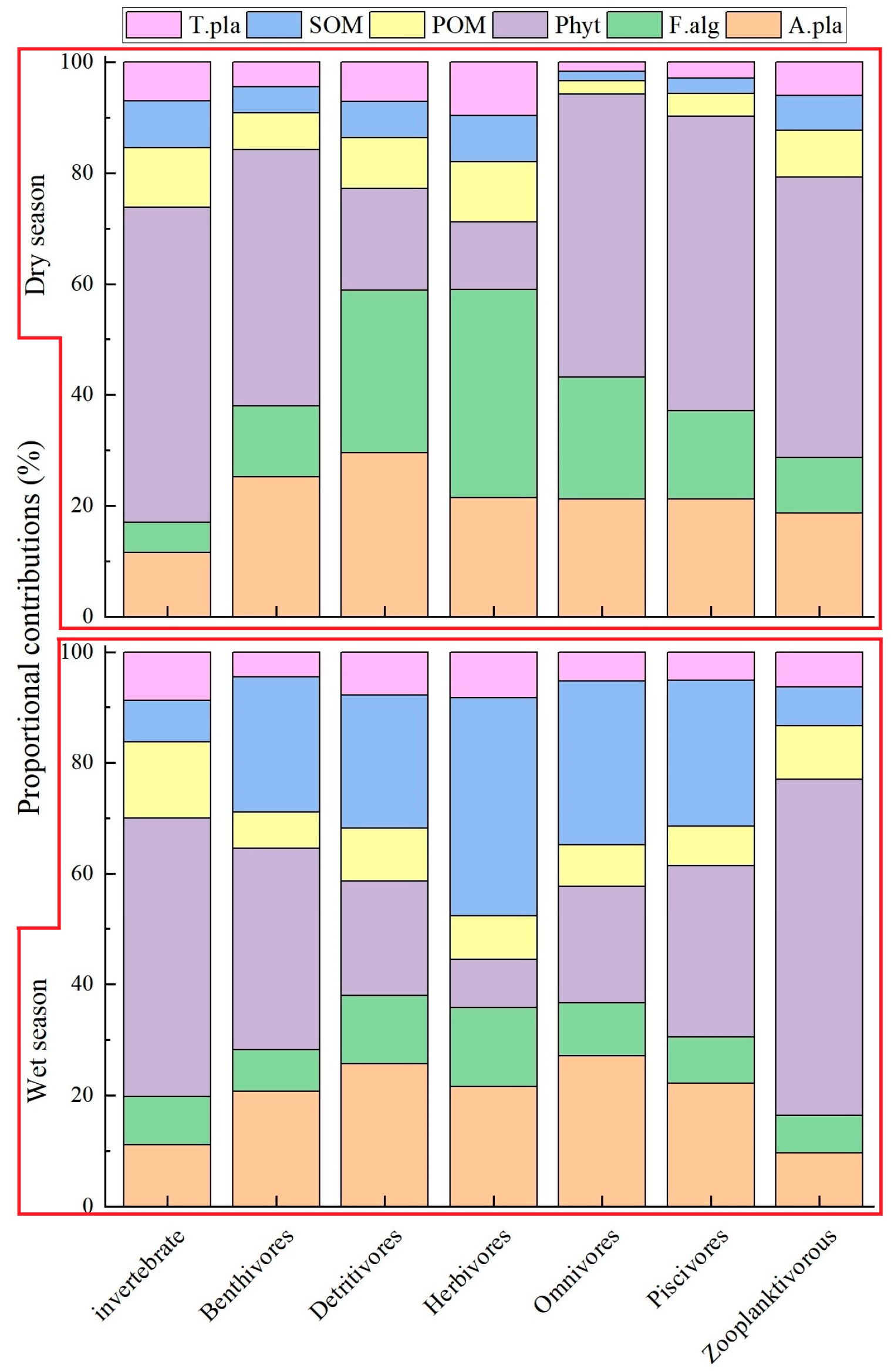
| Parameters | April (n = 5) | July (n = 6) | October (n = 6) | December (n = 6) | One-Way ANOVA |
|---|---|---|---|---|---|
| WT (°C) | 18.0 ± 0.8 b | 28.5 ± 0.2 a | 18.1 ± 0.9 b | 16.1 ± 0.5 c | p = 0.000 |
| DO | 10.35 ± 0.43 a | 9.72 ± 0.53 ab | 8.83 ± 0.74 bc | 8.15 ± 0.69 c | p = 0.000 |
| SD (m) | 76.6 ± 20.2 Aa | 68.7 ± 16.2 a | 63.5 ± 15.5 ab | 41.7 ± 16.7 b | p = 0.033 |
| pH | 8.85 ± 0.05 a | 8.31 ± 0.41 b | 8.33 ± 0.08 b | 8.19 ± 0.16 b | p = 0.002 |
| TDS (mg/L) | 362.6 ± 3.4 b | 333.0 ± 6.4 c | 344.5 ± 7.9 c | 305.7 ± 23.6 a | p = 0.000 |
| CODMn (mg/L) | 2.21 ± 0.44 c | 3.33 ± 0.86 ab | 4.08 ± 0.74 a | 3.03 ± 0.13 cb | p = 0.002 |
| TN (mg/L) | 0.60 ± 0.11 a | 0.65 ± 0.15 a | 0.38 ± 0.08 b | 0.63 ± 0.03 a | p = 0.002 |
| TP (mg/L) | 0.05 ± 0.01 a | 0.08 ± 0.04 a | 0.04 ± 0.01 a | 0.06 ± 0.01 a | p = 0.067 |
| Period | Metrics | Piscivores | Detritivores | Omnivores | Herbivores | Zooplanktivores | Benthivores | Total |
|---|---|---|---|---|---|---|---|---|
| Wet season | TA | 5.62 | 1.47 | 17.94 | 1.23 | 3.15 | 9.14 | 71.58 |
| SEAc | 5.78 | 3.33 | 14.71 | 4.13 | 5.21 | 9.92 | 14.37 | |
| CD | 1.51 | 1.58 | 2.92 | 2.65 | 1.74 | 1.90 | 2.89 | |
| MNND | 1.45 | 2.00 | 1.55 | 3.16 | 2.45 | 2.07 | 0.89 | |
| SDNND | 1.14 | 0.62 | 1.15 | 1.42 | 0.35 | 0.88 | 0.62 | |
| CR | 3.09 | 4.18 | 8.95 | 6.28 | 4.24 | 4.79 | 12.47 | |
| NR | 6.67 | 2.86 | 5.54 | 5.17 | 2.32 | 4.42 | 11.81 | |
| Dry season | TA | 1.96 | —— | 3.60 | 1.08 | 0.45 | 2.37 | 31.49 |
| SEAc | 2.53 | 2.48 | 3.09 | 5.00 | 0.75 | 2.42 | 7.95 | |
| CD | 0.96 | 0.95 | 1.26 | 1.36 | 0.80 | 1.09 | 1.99 | |
| MNND | 0.34 | 1.90 | 0.76 | 1.82 | 0.92 | 0.99 | 0.59 | |
| SDNND | 0.26 | —— | 0.36 | 0.31 | 0.60 | 0.90 | 0.52 | |
| CR | 2.88 | 2.16 | 4.96 | 5.29 | 2.00 | 2.92 | 6.76 | |
| NR | 2.82 | 2.49 | 2.58 | 2.39 | 1.06 | 2.47 | 7.87 |
| Waterbody Name | Times | CR | NR | TA | SEAc | CD | MNND | SDNND | Number of Fish Species |
|---|---|---|---|---|---|---|---|---|---|
| Tian-e-Zhou Oxbow | April (2021) | 12.47 | 11.81 | 71.58 | 14.37 | 2.89 | 0.89 | 0.62 | 27 |
| October (2021) | 6.76 | 7.78 | 31.49 | 7.95 | 1.99 | 0.59 | 0.52 | 28 | |
| Mituo Town of Upper Yangtze River [83] | August (2019) | 11.72 | 9.75 | 60.67 | 12.53 | 2.41 | 0.59 | 0.72 | 23 |
| November (2019) | 8.92 | 7.67 | 38.19 | 8.55 | 1.98 | 0.57 | 0.58 | 13 | |
| Three Gorges Reservoir [82] | 2013 | 8.47 | 4.34 | 13.62 | -- | 3.35 | 1.90 | 0.63 | 19 |
| Yangcheng Lake [84] | April (2018) | 6.84 | 6.64 | 37.07 | 8.37 | 1.94 | 0.56 | 0.52 | 34 |
| October (2017) | 6.70 | 5.72 | 23.20 | 7.83 | 1.96 | 0.51 | 0.50 | 25 | |
| Daning River [85] | May (2011) | 4.93 | 7.45 | 21.41 | -- | 2.37 | 1.62 | 0.88 | 32 |
| October (2011) | 7.45 | 10.40 | 38.28 | -- | 3.28 | 2.22 | 1.45 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, L.; Ji, F.; Qiu, Y.; Xie, H.; Li, G.; Shen, J. Water-Level Fluctuation Control of the Trophic Structure of a Yangtze River Oxbow. Biology 2023, 12, 1359. https://doi.org/10.3390/biology12101359
Qiu L, Ji F, Qiu Y, Xie H, Li G, Shen J. Water-Level Fluctuation Control of the Trophic Structure of a Yangtze River Oxbow. Biology. 2023; 12(10):1359. https://doi.org/10.3390/biology12101359
Chicago/Turabian StyleQiu, Longhui, Fenfen Ji, Yuhui Qiu, Hongyu Xie, Guangyu Li, and Jianzhong Shen. 2023. "Water-Level Fluctuation Control of the Trophic Structure of a Yangtze River Oxbow" Biology 12, no. 10: 1359. https://doi.org/10.3390/biology12101359
APA StyleQiu, L., Ji, F., Qiu, Y., Xie, H., Li, G., & Shen, J. (2023). Water-Level Fluctuation Control of the Trophic Structure of a Yangtze River Oxbow. Biology, 12(10), 1359. https://doi.org/10.3390/biology12101359





