Immune Checkpoints in Solid Organ Transplantation
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Role of Immune Checkpoints and Their Ligands in Solid Organ Transplantation
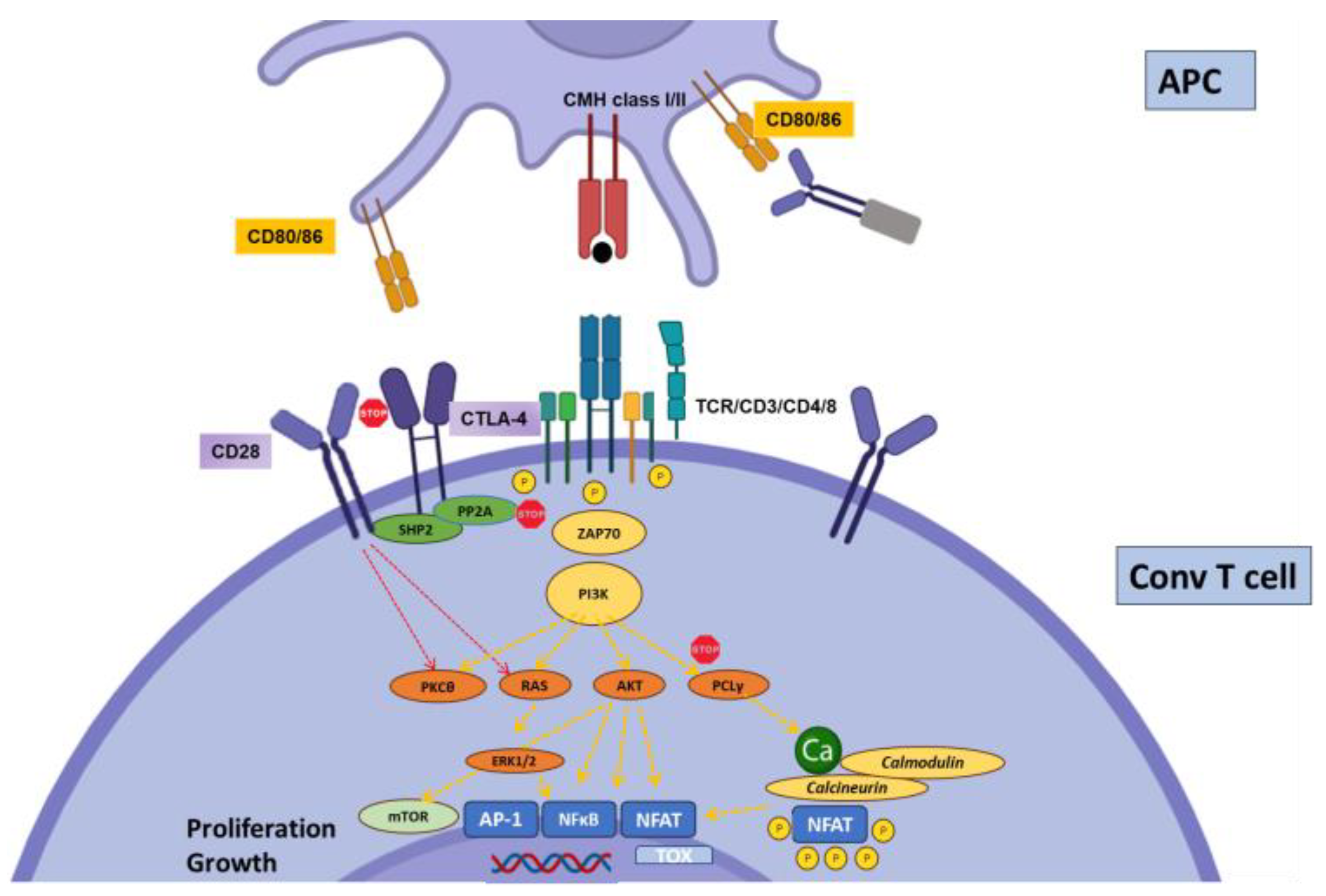
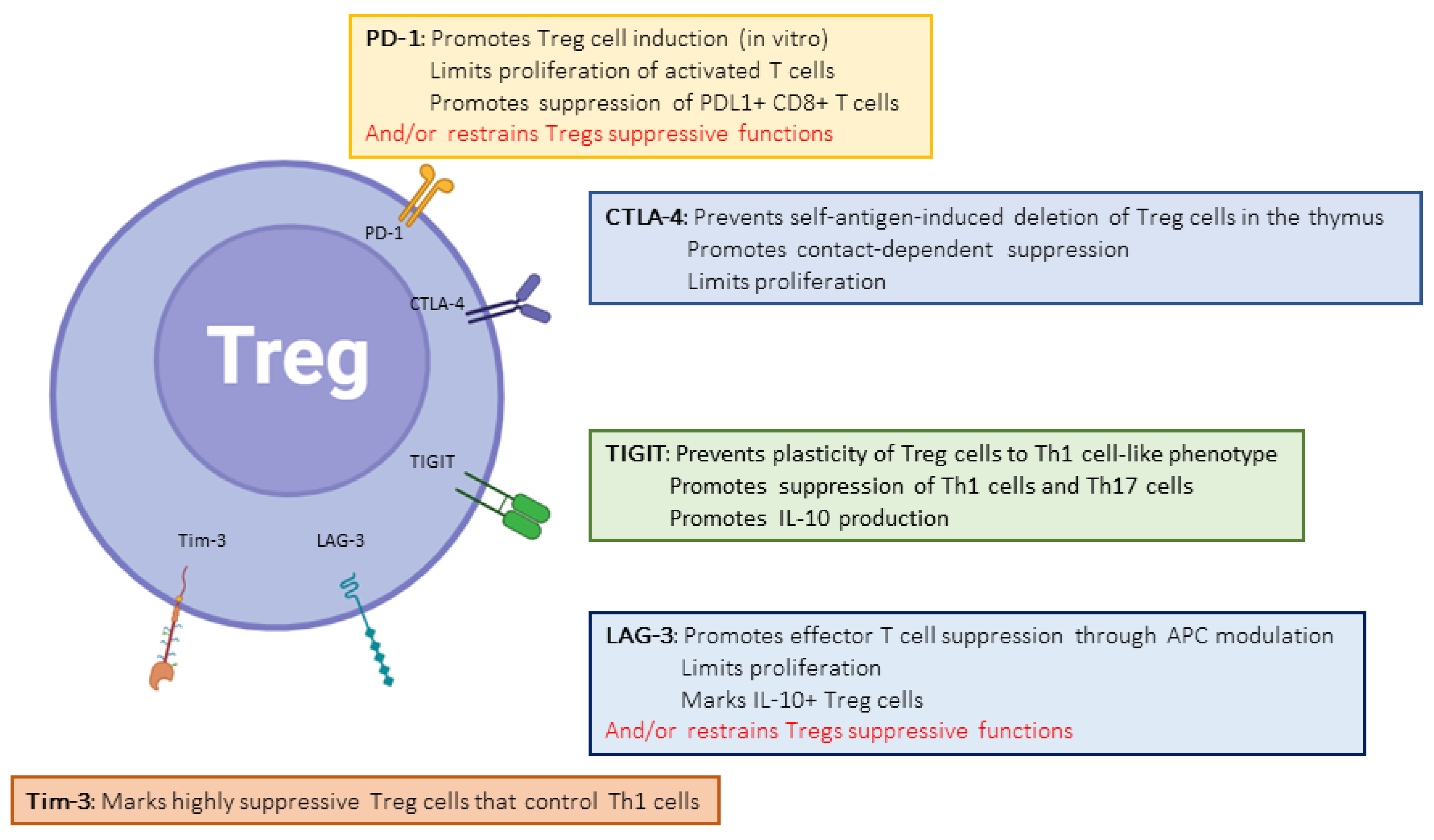
2.1. CTLA-4
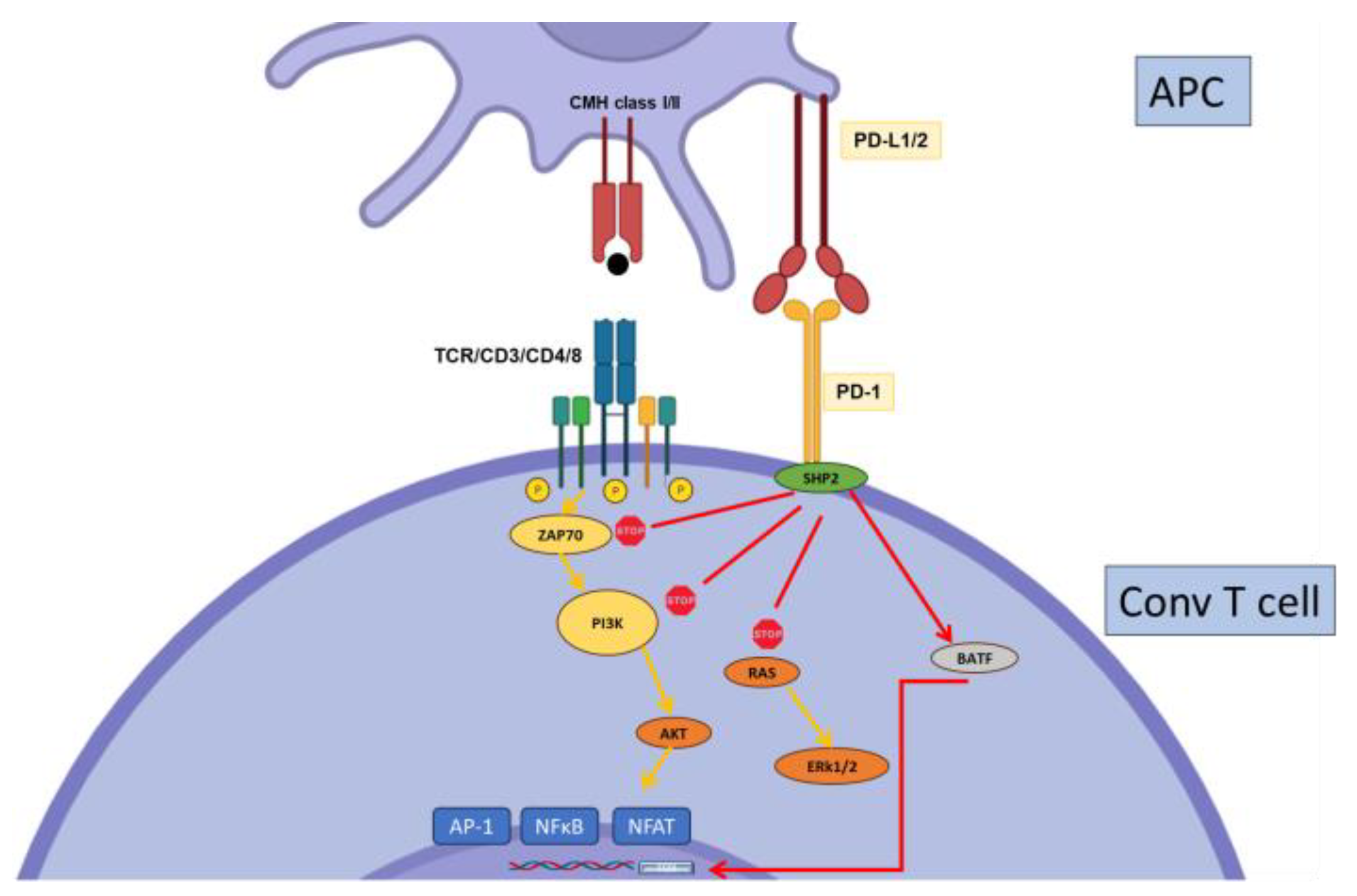
2.2. PD-1
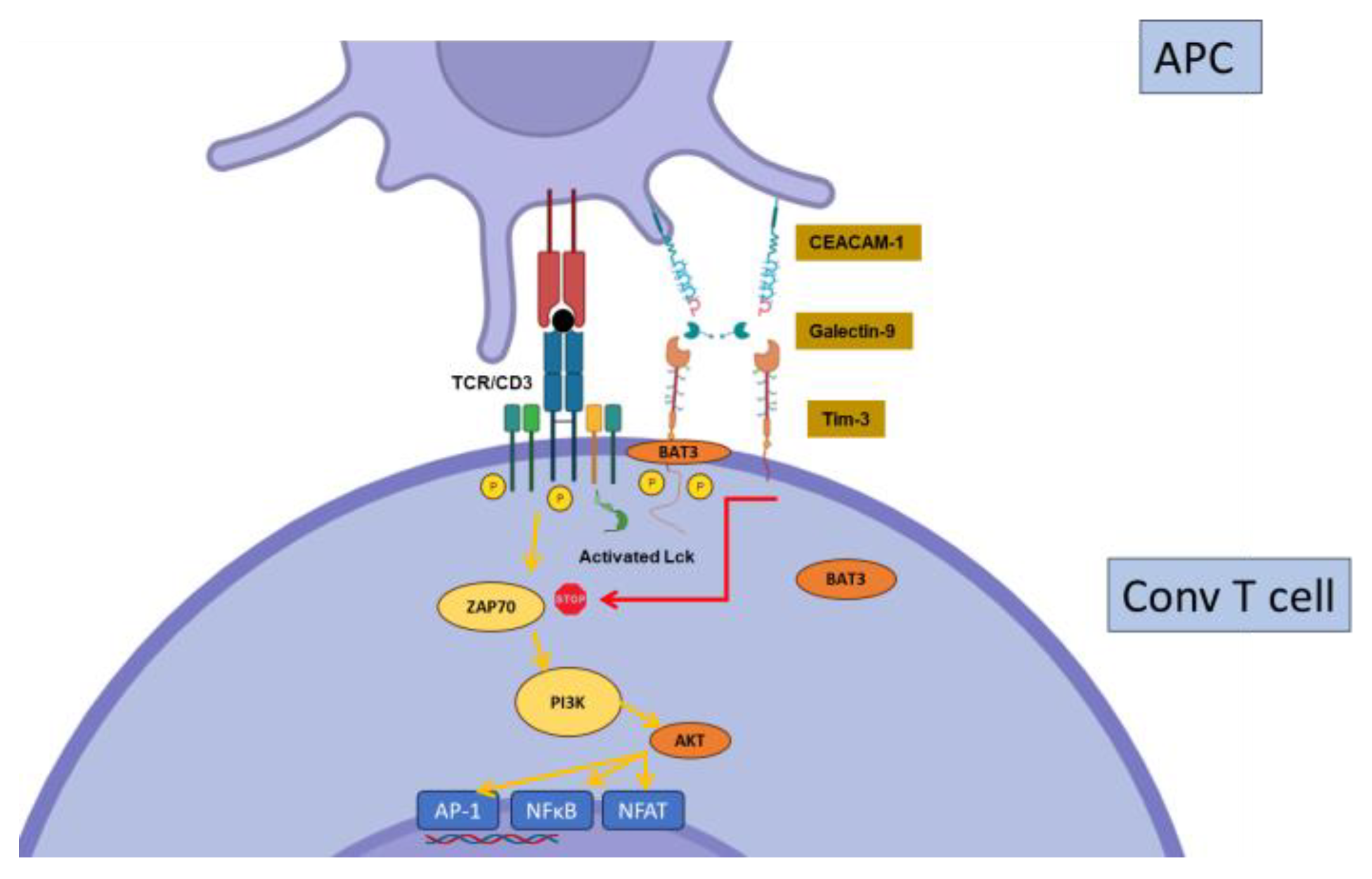
2.3. Tim-3
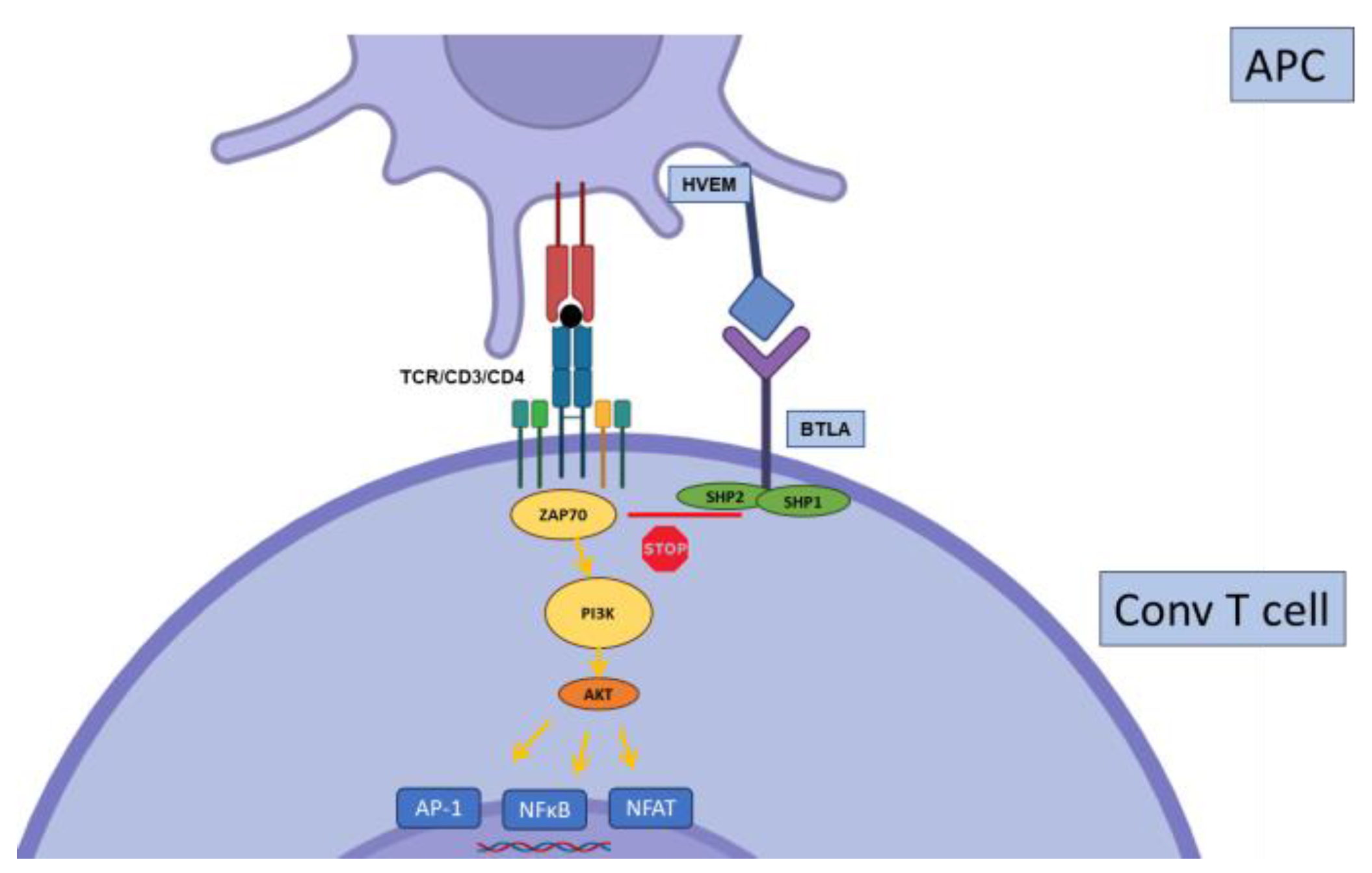
2.4. BTLA
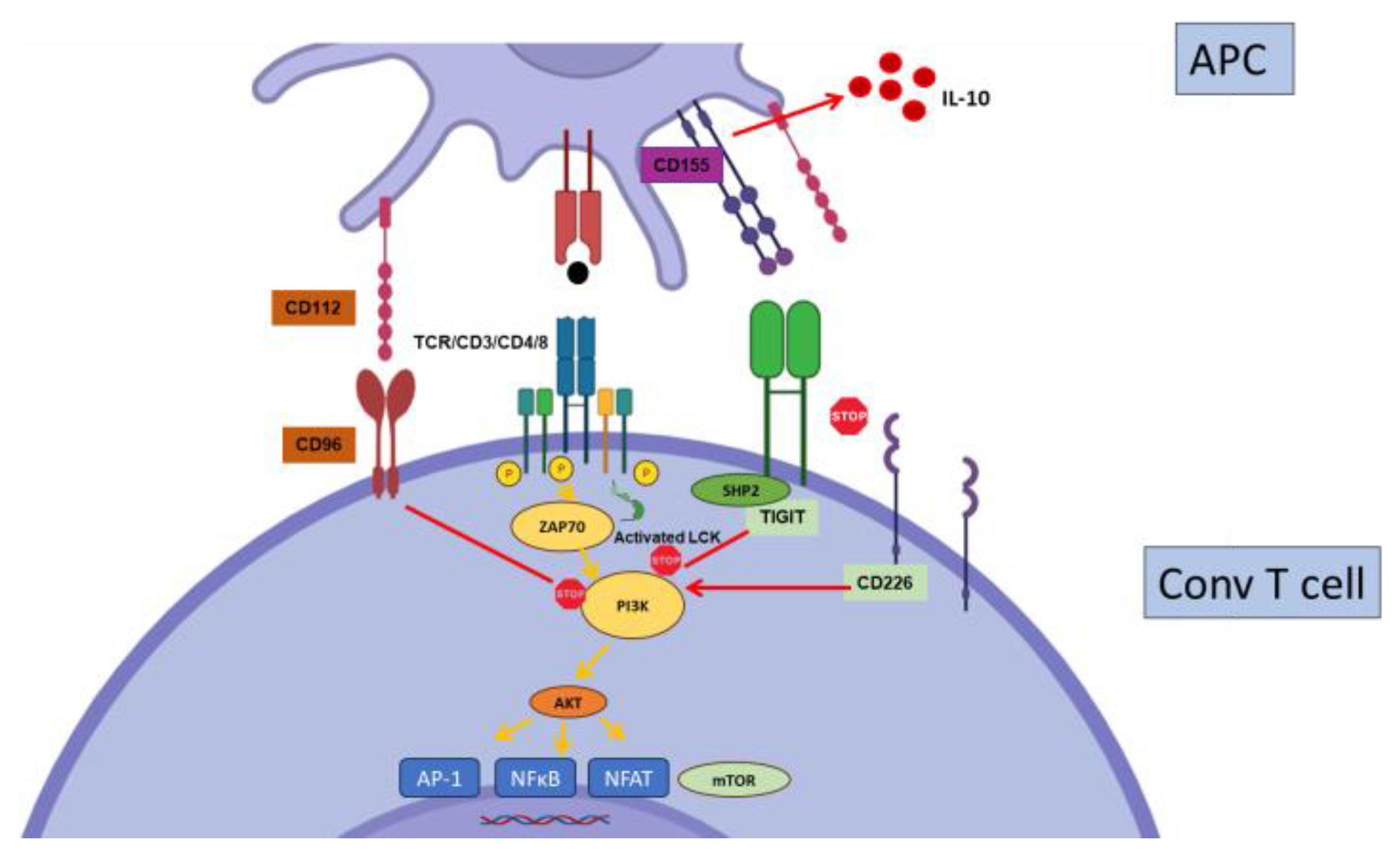
2.5. TIGIT
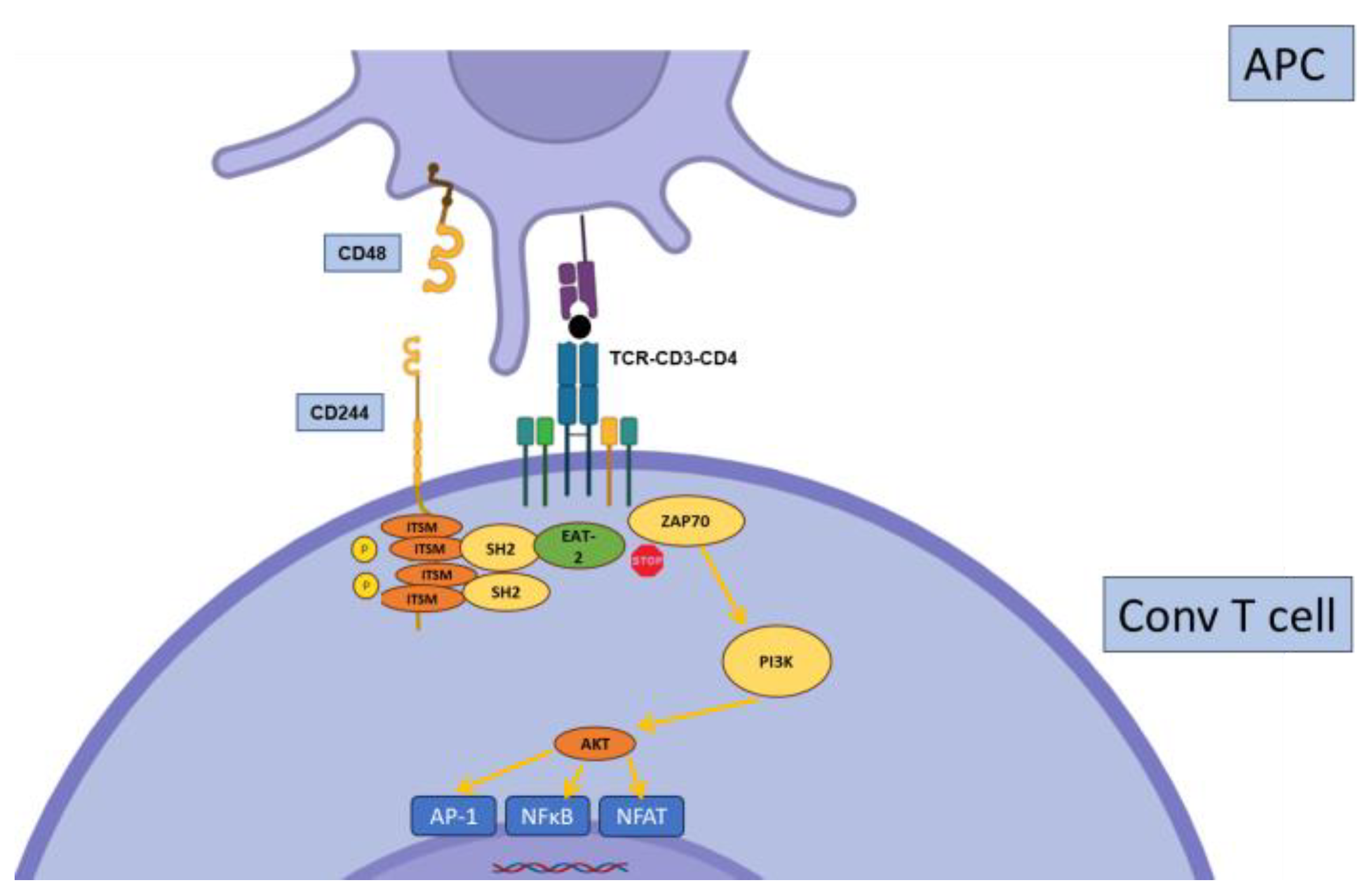
2.6. CD244
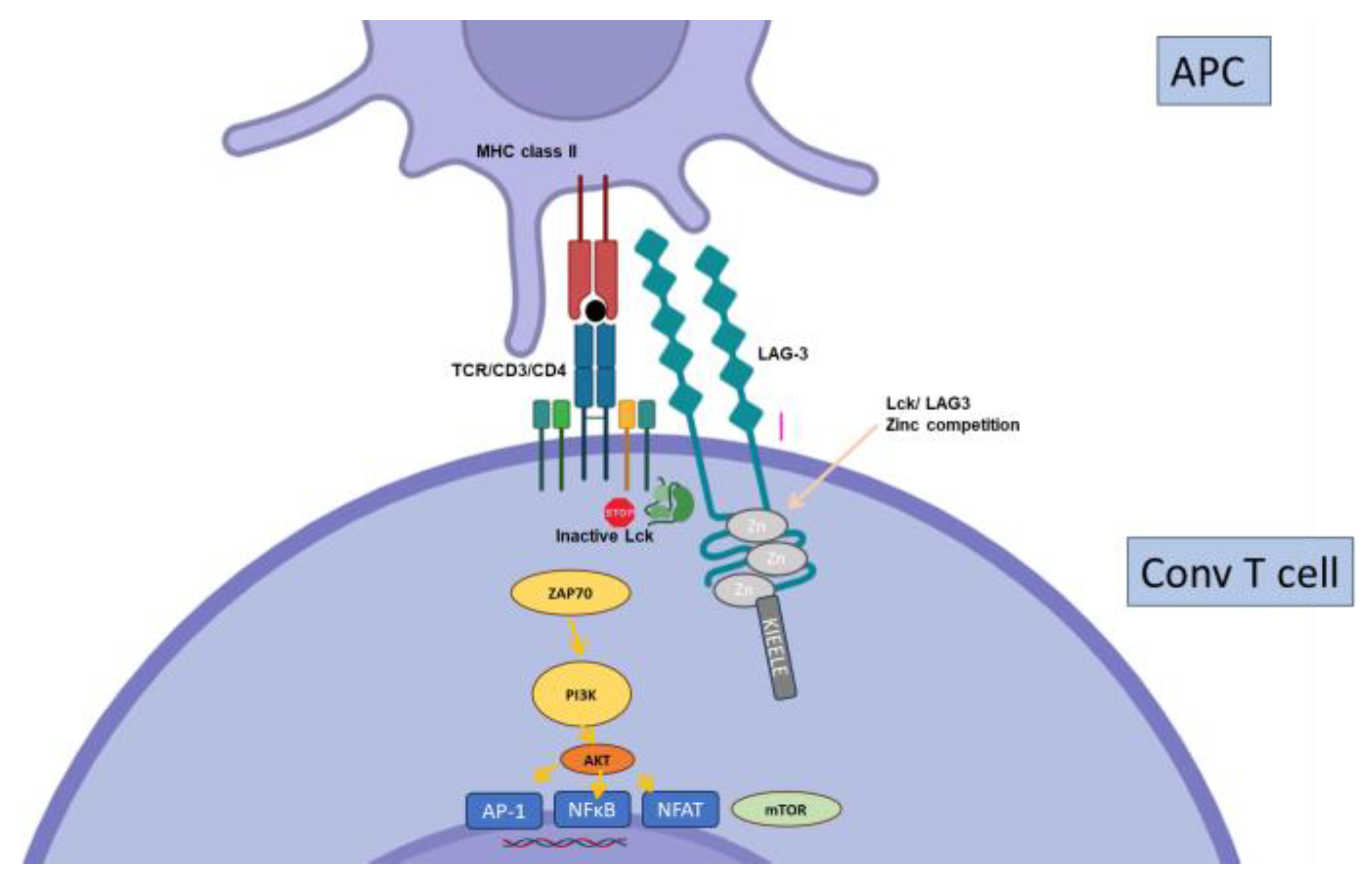
2.7. Other Inhibitory Receptors
3. T Cell Exhaustion in Solid Organ Transplantation
4. Interactions between Immunosuppressive Drugs and IC Expression and Function
5. Harnessing Inhibitory Pathways in Solid Organ Transplantation
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef]
- Le Mercier, I.; Lines, J.L.; Noelle, R.J. Beyond CTLA-4 and PD-1, the Generation Z of Negative Checkpoint Regulators. Front. Immunol. 2015, 6, 418. [Google Scholar] [CrossRef] [PubMed]
- Ziogas, D.C.; Theocharopoulos, C.; Lialios, P.P.; Foteinou, D.; Koumprentziotis, I.A.; Xynos, G.; Gogas, H. Beyond CTLA-4 and PD-1 Inhibition: Novel Immune Checkpoint Molecules for Melanoma Treatment. Cancers 2023, 15, 2718. [Google Scholar] [CrossRef] [PubMed]
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495. [Google Scholar] [CrossRef]
- Franco, F.; Jaccard, A.; Romero, P.; Yu, Y.R.; Ho, P.C. Metabolic and epigenetic regulation of T-cell exhaustion. Nat. Metab. 2020, 2, 1001–1012. [Google Scholar] [CrossRef]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Boumaza, X.; Bonneau, B.; Roos-Weil, D.; Pinnetti, C.; Rauer, S.; Nitsch, L.; Del Bello, A.; Jelcic, I.; Suhs, K.W.; Gasnault, J.; et al. Progressive Multifocal Leukoencephalopathy Treated by Immune Checkpoint Inhibitors. Ann. Neurol. 2023, 93, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Oya, Y.; Watanabe, N.; Owada, T.; Oki, M.; Hirose, K.; Suto, A.; Kagami, S.; Nakajima, H.; Kishimoto, T.; Iwamoto, I.; et al. Development of autoimmune hepatitis-like disease and production of autoantibodies to nuclear antigens in mice lacking B and T lymphocyte attenuator. Arthritis Rheum. 2008, 58, 2498–2510. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef]
- Joller, N.; Hafler, J.P.; Brynedal, B.; Kassam, N.; Spoerl, S.; Levin, S.D.; Sharpe, A.H.; Kuchroo, V.K. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J. Immunol. 2011, 186, 1338–1342. [Google Scholar] [CrossRef] [PubMed]
- Bettini, M.; Szymczak-Workman, A.L.; Forbes, K.; Castellaw, A.H.; Selby, M.; Pan, X.; Drake, C.G.; Korman, A.J.; Vignali, D.A. Cutting edge: Accelerated autoimmune diabetes in the absence of LAG-3. J. Immunol. 2011, 187, 3493–3498. [Google Scholar] [CrossRef] [PubMed]
- Schubert, D.; Bode, C.; Kenefeck, R.; Hou, T.Z.; Wing, J.B.; Kennedy, A.; Bulashevska, A.; Petersen, B.S.; Schaffer, A.A.; Gruning, B.A.; et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat. Med. 2014, 20, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Gough, S.C.; Walker, L.S.; Sansom, D.M. CTLA4 gene polymorphism and autoimmunity. Immunol. Rev. 2005, 204, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Howson, J.M.; Esposito, L.; Heward, J.; Snook, H.; Chamberlain, G.; Rainbow, D.B.; Hunter, K.M.; Smith, A.N.; Di Genova, G.; et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 2003, 423, 506–511. [Google Scholar] [CrossRef]
- Prokunina, L.; Castillejo-Lopez, C.; Oberg, F.; Gunnarsson, I.; Berg, L.; Magnusson, V.; Brookes, A.J.; Tentler, D.; Kristjansdottir, H.; Grondal, G.; et al. A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans. Nat. Genet. 2002, 32, 666–669. [Google Scholar] [CrossRef]
- McKinney, E.F.; Lee, J.C.; Jayne, D.R.; Lyons, P.A.; Smith, K.G. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 2015, 523, 612–616. [Google Scholar] [CrossRef]
- Wiedeman, A.E.; Muir, V.S.; Rosasco, M.G.; DeBerg, H.A.; Presnell, S.; Haas, B.; Dufort, M.J.; Speake, C.; Greenbaum, C.J.; Serti, E.; et al. Autoreactive CD8+ T cell exhaustion distinguishes subjects with slow type 1 diabetes progression. J. Clin. Investig. 2020, 130, 480–490. [Google Scholar] [CrossRef]
- Brunet, J.F.; Denizot, F.; Luciani, M.F.; Roux-Dosseto, M.; Suzan, M.; Mattei, M.G.; Golstein, P. A new member of the immunoglobulin superfamily—CTLA-4. Nature 1987, 328, 267–270. [Google Scholar] [CrossRef]
- Egen, J.G.; Kuhns, M.S.; Allison, J.P. CTLA-4: New insights into its biological function and use in tumor immunotherapy. Nat. Immunol. 2002, 3, 611–618. [Google Scholar] [CrossRef]
- Phan, G.Q.; Yang, J.C.; Sherry, R.M.; Hwu, P.; Topalian, S.L.; Schwartzentruber, D.J.; Restifo, N.P.; Haworth, L.R.; Seipp, C.A.; Freezer, L.J.; et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl. Acad. Sci. USA 2003, 100, 8372–8377. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.S.; Ortuno, S.; Lebrun-Vignes, B.; Johnson, D.B.; Moslehi, J.J.; Hertig, A.; Salem, J.E. Transplant rejections associated with immune checkpoint inhibitors: A pharmacovigilance study and systematic literature review. Eur. J. Cancer 2021, 148, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Tivol, E.A.; Borriello, F.; Schweitzer, A.N.; Lynch, W.P.; Bluestone, J.A.; Sharpe, A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995, 3, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.A.; Kuhns, M.S.; Egen, J.G.; Allison, J.P. CTLA-4-mediated inhibition in regulation of T cell responses: Mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 2001, 19, 565–594. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Nguyen, H.; Chambers, C.; Kang, J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc. Natl. Acad. Sci. USA 2010, 107, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 control over Foxp3+ regulatory T cell function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.M.; Lovitch, S.B.; Sage, P.T.; Juneja, V.R.; Lee, Y.; Trombley, J.D.; Arancibia-Carcamo, C.V.; Sobel, R.A.; Rudensky, A.Y.; Kuchroo, V.K.; et al. Deletion of CTLA-4 on regulatory T cells during adulthood leads to resistance to autoimmunity. J. Exp. Med. 2015, 212, 1603–1621. [Google Scholar] [CrossRef] [PubMed]
- Klocke, K.; Sakaguchi, S.; Holmdahl, R.; Wing, K. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc. Natl. Acad. Sci. USA 2016, 113, E2383–E2392. [Google Scholar] [CrossRef]
- Poirier, N.; Azimzadeh, A.M.; Zhang, T.; Dilek, N.; Mary, C.; Nguyen, B.; Tillou, X.; Wu, G.; Reneaudin, K.; Hervouet, J.; et al. Inducing CTLA-4-dependent immune regulation by selective CD28 blockade promotes regulatory T cells in organ transplantation. Sci. Transl. Med. 2010, 2, 17ra10. [Google Scholar] [CrossRef]
- La Muraglia, G.M., 2nd; Zeng, S.; Crichton, E.S.; Wagener, M.E.; Ford, M.L.; Badell, I.R. Superior inhibition of alloantibody responses with selective CD28 blockade is CTLA-4 dependent and T follicular helper cell specific. Am. J. Transplant. 2021, 21, 73–86. [Google Scholar] [CrossRef]
- Cui, J.; Yu, J.; Xu, H.; Zou, Y.; Zhang, H.; Chen, S.; Le, S.; Zhao, J.; Jiang, L.; Xia, J.; et al. Autophagy-lysosome inhibitor chloroquine prevents CTLA-4 degradation of T cells and attenuates acute rejection in murine skin and heart transplantation. Theranostics 2020, 10, 8051–8060. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, A.; De la Camara, R.; Roman-Gomez, J.; Jimenez-Velasco, A.; Encuentra, M.; Nieto, J.B.; de la Rubia, J.; Urbano-Ispizua, A.; Brunet, S.; Iriondo, A.; et al. CTLA-4 polymorphisms and clinical outcome after allogeneic stem cell transplantation from HLA-identical sibling donors. Blood 2007, 110, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.L.; Sanchez-Perez, L.; Perez-Flores, I.; de la Higuera, M.A.M.; Romero, N.C.; Querol-Garcia, J.; Urcelay, E.; Sanchez-Fructuoso, A.I. Association of Polymorphisms in T-Cell Activation Costimulatory/Inhibitory Signal Genes with Allograft Kidney Rejection Risk. Front. Immunol. 2021, 12, 650979. [Google Scholar] [CrossRef] [PubMed]
- Rosik, J.; Szostak, B.; Machaj, F.; Pawlik, A. The Role of CTLA4 and Its Polymorphisms in Solid Organ and Haematopoietic Stem Cell Transplantation. Int. J. Mol. Sci. 2021, 22, 3081. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Ide, K.; Kashihara, M.; Yamane, H.; Akimoto, S.; Tanimine, N.; Tahara, H.; Ohira, M.; Tanaka, Y.; Ohdan, H. Polymorphisms in CTLA-4 predict de novo donor specific antibody formation after kidney transplantation. Hum. Immunol. 2022, 83, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, S.; Mangus, C.W.; Wang, J.C.; Wei, F.; He, A.; Kapoor, V.; Foley, J.E.; Massey, P.R.; Felizardo, T.C.; Riley, J.L.; et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci. Transl. Med. 2011, 3, 111ra120. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef]
- Tan, C.L.; Kuchroo, J.R.; Sage, P.T.; Liang, D.; Francisco, L.M.; Buck, J.; Thaker, Y.R.; Zhang, Q.; McArdel, S.L.; Juneja, V.R.; et al. PD-1 restraint of regulatory T cell suppressive activity is critical for immune tolerance. J. Exp. Med. 2021, 218, e20182232. [Google Scholar] [CrossRef]
- Chamoto, K.; Yaguchi, T.; Tajima, M.; Honjo, T. Insights from a 30-year journey: Function, regulation and therapeutic modulation of PD1. Nat. Rev. Immunol. 2023, 23, 682–695. [Google Scholar] [CrossRef]
- Shim, Y.J.; Khedraki, R.; Dhar, J.; Fan, R.; Dvorina, N.; Valujskikh, A.; Fairchild, R.L.; Baldwin, W.M., 3rd. Early T cell infiltration is modulated by programed cell death-1 protein and its ligand (PD-1/PD-L1) interactions in murine kidney transplants. Kidney Int. 2020, 98, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Ozkaynak, E.; Wang, L.; Goodearl, A.; McDonald, K.; Qin, S.; O′Keefe, T.; Duong, T.; Smith, T.; Gutierrez-Ramos, J.C.; Rottman, J.B.; et al. Programmed death-1 targeting can promote allograft survival. J. Immunol. 2002, 169, 6546–6553. [Google Scholar] [CrossRef] [PubMed]
- Borges, T.J.; Murakami, N.; Lape, I.T.; Gassen, R.B.; Liu, K.; Cai, S.; Daccache, J.; Safa, K.; Shimizu, T.; Ohori, S.; et al. Overexpression of PD-1 on T cells promotes tolerance in cardiac transplantation via ICOS-dependent mechanisms. JCI Insight 2021, 6, e142909. [Google Scholar] [CrossRef] [PubMed]
- Koehn, B.H.; Ford, M.L.; Ferrer, I.R.; Borom, K.; Gangappa, S.; Kirk, A.D.; Larsen, C.P. PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue. J. Immunol. 2008, 181, 5313–5322. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ueno, T.; Clarkson, M.R.; Yuan, X.; Jurewicz, M.M.; Yagita, H.; Azuma, M.; Sharpe, A.H.; Auchincloss, H., Jr.; Sayegh, M.H.; et al. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J. Immunol. 2005, 174, 6648–6656. [Google Scholar] [CrossRef] [PubMed]
- Riella, L.V.; Watanabe, T.; Sage, P.T.; Yang, J.; Yeung, M.; Azzi, J.; Vanguri, V.; Chandraker, A.; Sharpe, A.H.; Sayegh, M.H.; et al. Essential role of PDL1 expression on nonhematopoietic donor cells in acquired tolerance to vascularized cardiac allografts. Am. J. Transplant. 2011, 11, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Koga, N.; Suzuki, J.; Kosuge, H.; Haraguchi, G.; Onai, Y.; Futamatsu, H.; Maejima, Y.; Gotoh, R.; Saiki, H.; Tsushima, F.; et al. Blockade of the interaction between PD-1 and PD-L1 accelerates graft arterial disease in cardiac allografts. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2057–2062. [Google Scholar] [CrossRef]
- Yang, J.; Popoola, J.; Khandwala, S.; Vadivel, N.; Vanguri, V.; Yuan, X.; Dada, S.; Guleria, I.; Tian, C.; Ansari, M.J.; et al. Critical role of donor tissue expression of programmed death ligand-1 in regulating cardiac allograft rejection and vasculopathy. Circulation 2008, 117, 660–669. [Google Scholar] [CrossRef]
- Luo, Z.; Liao, T.; Zhang, Y.; Zheng, H.; Sun, Q.; Han, F.; Ma, M.; Ye, Y.; Sun, Q. Ex vivo anchored PD-L1 functionally prevent in vivo renal allograft rejection. Bioeng. Transl. Med. 2022, 7, e10316. [Google Scholar] [CrossRef]
- Starke, A.; Lindenmeyer, M.T.; Segerer, S.; Neusser, M.A.; Rusi, B.; Schmid, D.M.; Cohen, C.D.; Wuthrich, R.P.; Fehr, T.; Waeckerle-Men, Y. Renal tubular PD-L1 (CD274) suppresses alloreactive human T-cell responses. Kidney Int. 2010, 78, 38–47. [Google Scholar] [CrossRef]
- Tokita, D.; Mazariegos, G.V.; Zahorchak, A.F.; Chien, N.; Abe, M.; Raimondi, G.; Thomson, A.W. High PD-L1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T-regulatory cells in liver transplant tolerance. Transplantation 2008, 85, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bakthavatsalam, R.; Meng, Z.; Li, Z.; Li, W.; Perkins, J.D.; Reyes, J. PD-L1 signal on liver dendritic cells is critical for Foxp3(+)CD4(+)CD25(+) Treg and liver tolerance induction in mice. Transplant. Proc. 2013, 45, 1853–1855. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Perez-Gutierrez, A.; Nakao, T.; Dai, H.; Camirand, G.; Yoshida, O.; Yokota, S.; Stolz, D.B.; Ross, M.A.; Morelli, A.E.; et al. Graft-infiltrating PD-L1(hi) cross-dressed dendritic cells regulate antidonor T cell responses in mouse liver transplant tolerance. Hepatology 2018, 67, 1499–1515. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.K.; Das, R.; Drow, T.; Nylen, E.A.; de Souza, A.H.; Wang, Z.; Wood, M.W.; Davis, D.B.; Bjorling, D.E.; Galipeau, J. Islet allografts expressing a PD-L1 and IDO fusion protein evade immune rejection and reverse preexisting diabetes in immunocompetent mice without systemic immunosuppression. Am. J. Transplant. 2022, 22, 2571–2585. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Itabashi, Y.; Fleming, T.; Bansal, S.; Bowen, S.; Poulson, C.; Bharat, A.; Bremner, R.; Smith, M.; Mohanakumar, T. Low-dose IL-2 prevents murine chronic cardiac allograft rejection: Role for IL-2-induced T regulatory cells and exosomes with PD-L1 and CD73. Am. J. Transplant. 2022, 22, 2180–2194. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Boenisch, O.; D’Addio, F.; Watanabe, T.; Elyaman, W.; Magee, C.N.; Yeung, M.Y.; Padera, R.F.; Rodig, S.J.; Murayama, T.; Tanaka, K.; et al. TIM-3: A novel regulatory molecule of alloimmune activation. J. Immunol. 2010, 185, 5806–5819. [Google Scholar] [CrossRef] [PubMed]
- Hastings, W.D.; Anderson, D.E.; Kassam, N.; Koguchi, K.; Greenfield, E.A.; Kent, S.C.; Zheng, X.X.; Strom, T.B.; Hafler, D.A.; Kuchroo, V.K. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur. J. Immunol. 2009, 39, 2492–2501. [Google Scholar] [CrossRef]
- Gupta, S.; Thornley, T.B.; Gao, W.; Larocca, R.; Turka, L.A.; Kuchroo, V.K.; Strom, T.B. Allograft rejection is restrained by short-lived TIM-3+PD-1+Foxp3+ Tregs. J. Clin. Investig. 2012, 122, 2395–2404. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, Y.; Zhang, J.P.; Liang, J.; Li, L.; Zheng, L. Tim-3 expression defines regulatory T cells in human tumors. PLoS ONE 2013, 8, e58006. [Google Scholar] [CrossRef]
- Tang, R.; Rangachari, M.; Kuchroo, V.K. Tim-3: A co-receptor with diverse roles in T cell exhaustion and tolerance. Semin. Immunol. 2019, 42, 101302. [Google Scholar] [CrossRef] [PubMed]
- Monney, L.; Sabatos, C.A.; Gaglia, J.L.; Ryu, A.; Waldner, H.; Chernova, T.; Manning, S.; Greenfield, E.A.; Coyle, A.J.; Sobel, R.A.; et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, H.; Nieves-Rosado, H.; Kulkarni, A.; Murter, B.; McGrath, K.V.; Chandran, U.R.; Chang, A.; Szymczak-Workman, A.L.; Vujanovic, L.; Delgoffe, G.M.; et al. Expression of Tim-3 drives phenotypic and functional changes in Treg cells in secondary lymphoid organs and the tumor microenvironment. Cell Rep. 2021, 36, 109699. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.T.; Anderson, A.C.; Tan, W.G.; West, E.E.; Ha, S.J.; Araki, K.; Freeman, G.J.; Kuchroo, V.K.; Ahmed, R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA 2010, 107, 14733–14738. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Munger, M.E.; Veenstra, R.G.; Weigel, B.J.; Hirashima, M.; Munn, D.H.; Murphy, W.J.; Azuma, M.; Anderson, A.C.; Kuchroo, V.K.; et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood 2011, 117, 4501–4510. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cao, J.; Zhao, C.; Li, X.; Zhou, C.; Hirsch, F.R. TIM-3, a promising target for cancer immunotherapy. Onco Targets Ther. 2018, 11, 7005–7009. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Fueyo, A.; Tian, J.; Picarella, D.; Domenig, C.; Zheng, X.X.; Sabatos, C.A.; Manlongat, N.; Bender, O.; Kamradt, T.; Kuchroo, V.K.; et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 2003, 4, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ji, H.; Zhang, Y.; Shen, X.; Gao, F.; He, X.; Li, G.A.; Busuttil, R.W.; Kuchroo, V.K.; Kupiec-Weglinski, J.W. Recipient T cell TIM-3 and hepatocyte galectin-9 signalling protects mouse liver transplants against ischemia-reperfusion injury. J. Hepatol. 2015, 62, 563–572. [Google Scholar] [CrossRef]
- Chou, F.C.; Kuo, C.C.; Wang, Y.L.; Lin, M.H.; Linju Yen, B.; Chang, D.M.; Sytwu, H.K. Overexpression of galectin-9 in islets prolongs grafts survival via downregulation of Th1 responses. Cell Transplant. 2013, 22, 2135–2145. [Google Scholar] [CrossRef]
- Liu, Y.M.; Chen, Y.; Li, J.Z.; Gong, J.P. Up-regulation of Galectin-9 in vivo results in immunosuppressive effects and prolongs survival of liver allograft in rats. Immunol. Lett. 2014, 162, 217–222. [Google Scholar] [CrossRef]
- Wang, F.; He, W.; Zhou, H.; Yuan, J.; Wu, K.; Xu, L.; Chen, Z.K. The Tim-3 ligand galectin-9 negatively regulates CD8+ alloreactive T cell and prolongs survival of skin graft. Cell Immunol. 2007, 250, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zhou, H.; Fang, Z.; Yuan, J.; Niki, T.; Hirashima, M.; He, W.; Chen, Z.K. Galectin-9 in combination with rapamycin induces cardiac allograft tolerance in mice. Transplantation 2013, 96, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Shimmura-Tomita, M.; Wang, M.; Taniguchi, H.; Akiba, H.; Yagita, H.; Hori, J. Galectin-9-mediated protection from allo-specific T cells as a mechanism of immune privilege of corneal allografts. PLoS ONE 2013, 8, e63620. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Liu, K.; Xiong, H. Roles of BTLA in Immunity and Immune Disorders. Front. Immunol. 2021, 12, 654960. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Wang, L.; Han, R.; Wang, T.; Ye, Q.; Honjo, T.; Murphy, T.L.; Murphy, K.M.; Hancock, W.W. Differential effects of B and T lymphocyte attenuator and programmed death-1 on acceptance of partially versus fully MHC-mismatched cardiac allografts. J. Immunol. 2005, 175, 5774–5782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, H.; Wang, Z.; Yang, H.; Chen, H.; Cheng, H.; Zhou, J.; Zheng, M.; Tan, R.; Gu, M. BTLA suppress acute rejection via regulating TCR downstream signals and cytokines production in kidney transplantation and prolonged allografts survival. Sci. Rep. 2019, 9, 12154. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Zhang, J.; Gui, Z.; Han, Z.; Tao, J.; Chen, H.; Sun, L.; Fei, S.; Yang, H.; et al. Combined Immunotherapy With Belatacept and BTLA Overexpression Attenuates Acute Rejection Following Kidney Transplantation. Front. Immunol. 2021, 12, 618737. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, L.; Yin, H.; Feng, X.; Lu, Q. TIGIT: An emerging immune checkpoint target for immunotherapy in autoimmune disease and cancer. Int. Immunopharmacol. 2023, 120, 110358. [Google Scholar] [CrossRef]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef]
- Stanietsky, N.; Simic, H.; Arapovic, J.; Toporik, A.; Levy, O.; Novik, A.; Levine, Z.; Beiman, M.; Dassa, L.; Achdout, H.; et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 17858–17863. [Google Scholar] [CrossRef]
- Lozano, E.; Dominguez-Villar, M.; Kuchroo, V.; Hafler, D.A. The TIGIT/CD226 axis regulates human T cell function. J. Immunol. 2012, 188, 3869–3875. [Google Scholar] [CrossRef]
- Joller, N.; Lozano, E.; Burkett, P.R.; Patel, B.; Xiao, S.; Zhu, C.; Xia, J.; Tan, T.G.; Sefik, E.; Yajnik, V.; et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 2014, 40, 569–581. [Google Scholar] [CrossRef]
- Lucca, L.E.; Axisa, P.P.; Singer, E.R.; Nolan, N.M.; Dominguez-Villar, M.; Hafler, D.A. TIGIT signaling restores suppressor function of Th1 Tregs. JCI Insight 2019, 4, e124427. [Google Scholar] [CrossRef] [PubMed]
- Fribourg, M.; Anderson, L.; Fischman, C.; Cantarelli, C.; Perin, L.; La Manna, G.; Rahman, A.; Burrell, B.E.; Heeger, P.S.; Cravedi, P. T-cell exhaustion correlates with improved outcomes in kidney transplant recipients. Kidney Int. 2019, 96, 436–449. [Google Scholar] [CrossRef]
- Del Bello, A.; Gouin, A.; Chaubet, C.; Kamar, N.; Treiner, E. The CD226/TIGIT axis is involved in T cell hypo-responsiveness appearance in long-term kidney transplant recipients. Sci. Rep. 2022, 12, 11821. [Google Scholar] [CrossRef] [PubMed]
- Del Bello, A.; Kamar, N.; Treiner, E. T cell reconstitution after lymphocyte depletion features a different pattern of inhibitory receptor expression in ABO- versus HLA-incompatible kidney transplant recipients. Clin. Exp. Immunol. 2020, 200, 89–104. [Google Scholar] [CrossRef] [PubMed]
- van der List, A.C.J.; Litjens, N.H.R.; Klepper, M.; Betjes, M.G.H. Expression of Senescence Marker TIGIT Identifies Polyfunctional Donor-Reactive CD4+ T Cells Preferentially Lost After Kidney Transplantation. Front Immunol. 2021, 12, 656846. [Google Scholar] [CrossRef] [PubMed]
- Hartigan, C.R.; Tong, K.P.; Liu, D.; Laurie, S.J.; Ford, M.L. TIGIT agonism alleviates costimulation blockade-resistant rejection in a regulatory T cell-dependent manner. Am. J. Transplant. 2023, 23, 180–189. [Google Scholar] [CrossRef]
- Sun, H.; Hartigan, C.R.; Chen, C.W.; Sun, Y.; Tariq, M.; Robertson, J.M.; Krummey, S.M.; Mehta, A.K.; Ford, M.L. TIGIT regulates apoptosis of risky memory T cell subsets implicated in belatacept-resistant rejection. Am. J. Transplant. 2021, 21, 3256–3267. [Google Scholar] [CrossRef] [PubMed]
- McNerney, M.E.; Lee, K.M.; Kumar, V. 2B4 (CD244) is a non-MHC binding receptor with multiple functions on natural killer cells and CD8+ T cells. Mol. Immunol. 2005, 42, 489–494. [Google Scholar] [CrossRef]
- Vaidya, S.V.; Mathew, P.A. Of mice and men: Different functions of the murine and human 2B4 (CD244) receptor on NK cells. Immunol. Lett. 2006, 105, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Krummey, S.M.; Badell, I.R.; Wagener, M.; Schneeweis, L.A.; Stetsko, D.K.; Suchard, S.J.; Nadler, S.G.; Ford, M.L. 2B4 (CD244) induced by selective CD28 blockade functionally regulates allograft-specific CD8+ T cell responses. J. Exp. Med. 2014, 211, 297–311. [Google Scholar] [CrossRef]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.T.; Workman, C.J.; Flies, D.; Pan, X.; Marson, A.L.; Zhou, G.; Hipkiss, E.L.; Ravi, S.; Kowalski, J.; Levitsky, H.I.; et al. Role of LAG-3 in regulatory T cells. Immunity 2004, 21, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chikina, M.; Szymczak-Workman, A.L.; Horne, W.; Kolls, J.K.; Vignali, K.M.; Normolle, D.; Bettini, M.; Workman, C.J.; Vignali, D.A.A. LAG3 limits regulatory T cell proliferation and function in autoimmune diabetes. Sci. Immunol. 2017, 2, eaah4569. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Workman, C.J.; Vignali, D.A.A. LAG-3 as the third checkpoint inhibitor. Nat. Immunol. 2023, 24, 1415–1422. [Google Scholar] [CrossRef]
- Sega, E.I.; Leveson-Gower, D.B.; Florek, M.; Schneidawind, D.; Luong, R.H.; Negrin, R.S. Role of lymphocyte activation gene-3 (Lag-3) in conventional and regulatory T cell function in allogeneic transplantation. PLoS ONE 2014, 9, e86551. [Google Scholar] [CrossRef]
- Nicosia, M.; Fan, R.; Lee, J.; Gorbacheva, V.; Valenzuela, J.I.; Yamamoto, Y.; Beavers, A.; Dvorina, N.; Chuluyan, E.; Araki, M.; et al. LAG3 regulates antibody responses in a murine model of kidney transplantation. bioRxiv 2022, 31, 478518. [Google Scholar]
- Yamaura, K.; Watanabe, T.; Boenisch, O.; Yeung, M.; Yang, S.; Magee, C.N.; Padera, R.; Datta, S.; Schatton, T.; Kamimura, Y.; et al. In vivo function of immune inhibitory molecule B7-H4 in alloimmune responses. Am. J. Transplant. 2010, 10, 2355–2362. [Google Scholar] [CrossRef]
- Wang, X.; Hao, J.; Metzger, D.L.; Mui, A.; Lee, I.F.; Akhoundsadegh, N.; Chen, C.L.; Ou, D.; Ao, Z.; Verchere, C.B.; et al. Blockade of both B7-H4 and CTLA-4 co-signaling pathways enhances mouse islet allograft survival. Islets 2012, 4, 284–295. [Google Scholar] [CrossRef]
- Wang, X.; Hao, J.; Metzger, D.L.; Mui, A.; Ao, Z.; Verchere, C.B.; Chen, L.; Ou, D.; Warnock, G.L. B7-H4 induces donor-specific tolerance in mouse islet allografts. Cell Transplant. 2012, 21, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Hieu, T.; Malarkannan, S.; Wang, L. The structure, expression, and multifaceted role of immune-checkpoint protein VISTA as a critical regulator of anti-tumor immunity, autoimmunity, and inflammation. Cell Mol. Immunol. 2018, 15, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Park, J.G.; Lee, C.R.; Kim, M.G.; Kim, G.; Shin, H.M.; Jeon, Y.H.; Yang, S.H.; Kim, D.K.; Joo, K.W.; Choi, E.Y.; et al. Kidney residency of VISTA-positive macrophages accelerates repair from ischemic injury. Kidney Int. 2020, 97, 980–994. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhang, Z.; Yu, L.; Wang, Z.; Dong, Y.; Tong, A.; Yang, H. Immune-checkpoint protein VISTA in allergic, autoimmune disease and transplant rejection. Front. Immunol. 2023, 14, 1194421. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006, 439, 682–687. [Google Scholar] [CrossRef]
- Lu, Y.J.; Barreira-Silva, P.; Boyce, S.; Powers, J.; Cavallo, K.; Behar, S.M. CD4 T cell help prevents CD8 T cell exhaustion and promotes control of Mycobacterium tuberculosis infection. Cell Rep. 2021, 36, 109696. [Google Scholar] [CrossRef] [PubMed]
- Matloubian, M.; Concepcion, R.J.; Ahmed, R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 1994, 68, 8056–8063. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.; Angelosanto, J.M.; Kao, C.; Doering, T.A.; Odorizzi, P.M.; Barnett, B.E.; Wherry, E.J. Molecular and transcriptional basis of CD4(+) T cell dysfunction during chronic infection. Immunity 2014, 40, 289–302. [Google Scholar] [CrossRef]
- Miggelbrink, A.M.; Jackson, J.D.; Lorrey, S.J.; Srinivasan, E.S.; Waibl-Polania, J.; Wilkinson, D.S.; Fecci, P.E. CD4 T-Cell Exhaustion: Does It Exist and What Are Its Roles in Cancer? Clin. Cancer Res. 2021, 27, 5742–5752. [Google Scholar] [CrossRef]
- Collier, J.L.; Weiss, S.A.; Pauken, K.E.; Sen, D.R.; Sharpe, A.H. Not-so-opposite ends of the spectrum: CD8(+) T cell dysfunction across chronic infection, cancer and autoimmunity. Nat. Immunol. 2021, 22, 809–819. [Google Scholar] [CrossRef]
- Ghobrial, I.I.; Morris, A.G.; Booth, L.J. Clinical significance of in vitro donor-specific hyporesponsiveness in renal allograft recipients as demonstrated by the MLR. Transpl. Int. 1994, 7, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.D.; Robinson, C.M.; Lechler, R.I. Detection of donor-specific hyporesponsiveness following late failure of human renal allografts. Kidney Int. 1996, 50, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Bestard, O.; Nickel, P.; Cruzado, J.M.; Schoenemann, C.; Boenisch, O.; Sefrin, A.; Grinyo, J.M.; Volk, H.D.; Reinke, P. Circulating alloreactive T cells correlate with graft function in longstanding renal transplant recipients. J. Am. Soc. Nephrol. 2008, 19, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Game, D.S.; Hernandez-Fuentes, M.P.; Chaudhry, A.N.; Lechler, R.I. CD4+CD25+ regulatory T cells do not significantly contribute to direct pathway hyporesponsiveness in stable renal transplant patients. J. Am. Soc. Nephrol. 2003, 14, 1652–1661. [Google Scholar] [CrossRef] [PubMed]
- van der List, A.C.J.; Litjens, N.H.R.; Klepper, M.; Prevoo, F.; Betjes, M.G.H. Progressive Loss of Donor-Reactive CD4(+) Effector Memory T Cells due to Apoptosis Underlies Donor-Specific Hyporesponsiveness in Stable Renal Transplant Recipients. J. Immunol. 2022, 209, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Sarraj, B.; Ye, J.; Akl, A.I.; Chen, G.; Wang, J.J.; Zhang, Z.; Abadja, F.; Abecassis, M.; Miller, S.D.; Kansas, G.S.; et al. Impaired selectin-dependent leukocyte recruitment induces T-cell exhaustion and prevents chronic allograft vasculopathy and rejection. Proc. Natl. Acad. Sci. USA 2014, 111, 12145–12150. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Dai, Y.; Zhang, X.; Wang, G.; Xiao, X.; Jia, P.; Li, X.C.; Guo, Z.; Chen, W. T cell exhaustion is associated with antigen abundance and promotes transplant acceptance. Am. J. Transplant. 2020, 20, 2540–2550. [Google Scholar] [CrossRef]
- Bouvy, A.P.; Klepper, M.; Kho, M.M.; Ijzermans, J.N.; Betjes, M.G.; Weimar, W.; Baan, C.C. T cells Exhibit Reduced Signal Transducer and Activator of Transcription 5 Phosphorylation and Upregulated Coinhibitory Molecule Expression After Kidney Transplantation. Transplantation 2015, 99, 1995–2003. [Google Scholar] [CrossRef]
- Hai Nam, N.; Taura, K.; Koyama, Y.; Nishio, T.; Yamamoto, G.; Uemoto, Y.; Kimura, Y.; Xuefeng, L.; Nakamura, D.; Yoshino, K.; et al. Increased Expressions of Programmed Death Ligand 1 and Galectin 9 in Transplant Recipients Who Achieved Tolerance After Immunosuppression Withdrawal. Liver Transpl. 2022, 28, 647–658. [Google Scholar] [CrossRef]
- Staron, M.M.; Gray, S.M.; Marshall, H.D.; Parish, I.A.; Chen, J.H.; Perry, C.J.; Cui, G.; Li, M.O.; Kaech, S.M. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8(+) T cells during chronic infection. Immunity 2014, 41, 802–814. [Google Scholar] [CrossRef]
- Ando, S.; Perkins, C.M.; Sajiki, Y.; Chastain, C.; Valanparambil, R.M.; Wieland, A.; Hudson, W.H.; Hashimoto, M.; Ramalingam, S.S.; Freeman, G.J.; et al. mTOR regulates T cell exhaustion and PD-1-targeted immunotherapy response during chronic viral infection. J. Clin. Investig. 2023, 133, e160025. [Google Scholar] [CrossRef] [PubMed]
- Hurez, V.; Dao, V.; Liu, A.; Pandeswara, S.; Gelfond, J.; Sun, L.; Bergman, M.; Orihuela, C.J.; Galvan, V.; Padron, A.; et al. Chronic mTOR inhibition in mice with rapamycin alters T, B, myeloid, and innate lymphoid cells and gut flora and prolongs life of immune-deficient mice. Aging Cell 2015, 14, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.J.; Pereira, R.M.; Aijo, T.; Kim, E.Y.; Marangoni, F.; Pipkin, M.E.; Togher, S.; Heissmeyer, V.; Zhang, Y.C.; Crotty, S.; et al. The transcription factor NFAT promotes exhaustion of activated CD8(+) T cells. Immunity 2015, 42, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Senjo, H.; Harada, S.; Kubota, S.I.; Tanaka, Y.; Tateno, T.; Zhang, Z.; Okada, S.; Chen, X.; Kikuchi, R.; Miyashita, N.; et al. Calcineurin inhibitor inhibits tolerance induction by suppressing terminal exhaustion of donor T cells after allo-HCT. Blood 2023, 142, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; An, H.; Shen, C.; Wang, B.; Zhang, T.; Hong, Y.; Jiang, H.; Zhou, P.; Ding, X. Deep phenotyping of T cell populations under long-term treatment of tacrolimus and rapamycin in patients receiving renal transplantations by mass cytometry. Clin. Transl. Med. 2021, 11, e629. [Google Scholar] [CrossRef] [PubMed]
- Shaw, B.I.; Espinosa, J.R.; Stempora, L.; Miller, A.; Adams, B.; Kirk, A.D. Functional Characteristics and Phenotypic Plasticity of CD57(+)PD1(-) CD4 T Cells and Their Relationship with Transplant Immunosuppression. J. Immunol. 2021, 206, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y. Searching for the real function of mTOR signaling in the regulation of PD-L1 expression. Transl. Oncol. 2020, 13, 100847. [Google Scholar] [CrossRef]
- Zeng, Q.; Yuan, X.Y.; Li, W.; Liu, B.W.; Zhao, X.; Ren, G.J.; Wang, Y.; Dou, J.; Wang, G.Y. Effects of tacrolimus (FK506) and mycophenolate mofetil (MMF) on regulatory T cells and co-inhibitory receptors in the peripheral blood of human liver allograft patients. Immunopharmacol. Immunotoxicol. 2019, 41, 380–385. [Google Scholar] [CrossRef]
- Zeng, Q.; Yuan, X.; Cao, J.; Zhao, X.; Wang, Y.; Liu, B.; Liu, W.; Zhu, Z.; Dou, J. Mycophenolate mofetil enhances the effects of tacrolimus on the inhibitory function of regulatory T cells in patients after liver transplantation via PD-1 and TIGIT receptors. Immunopharmacol. Immunotoxicol. 2021, 43, 239–246. [Google Scholar] [CrossRef]
- Aubert, R.D.; Kamphorst, A.O.; Sarkar, S.; Vezys, V.; Ha, S.J.; Barber, D.L.; Ye, L.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc. Natl. Acad. Sci. USA 2011, 108, 21182–21187. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Shi, X.; Xiao, X.; Fan, Y.; Minze, L.J.; Wang, J.; Ghobrial, R.M.; Xia, J.; Sciammas, R.; et al. Ablation of Transcription Factor IRF4 Promotes Transplant Acceptance by Driving Allogenic CD4(+) T Cell Dysfunction. Immunity 2017, 47, 1114–1128 e1116. [Google Scholar] [CrossRef] [PubMed]
- Mondala, P.K.; Vora, A.A.; Zhou, T.; Lazzari, E.; Ladel, L.; Luo, X.; Kim, Y.; Costello, C.; MacLeod, A.R.; Jamieson, C.H.M.; et al. Selective antisense oligonucleotide inhibition of human IRF4 prevents malignant myeloma regeneration via cell cycle disruption. Cell Stem Cell 2021, 28, 623–636 e629. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Tsai, H.I.; Xiao, Y.; Wu, Y.; Su, D.; Yang, M.; Zha, H.; Yan, F.; Liu, X.; Cheng, F.; et al. Engineering Programmed Death Ligand-1/Cytotoxic T-Lymphocyte-Associated Antigen-4 Dual-Targeting Nanovesicles for Immunosuppressive Therapy in Transplantation. ACS Nano 2020, 14, 7959–7969. [Google Scholar] [CrossRef]
- Yang, M.; Xu, Z.; Yan, H.; Tsai, H.I.; Su, D.; Yan, F.; Lu, Q.; Feng, J.; Zeng, W.; Xi, L.; et al. PD-L1 cellular nanovesicles carrying rapamycin inhibit alloimmune responses in transplantation. Biomater. Sci. 2021, 9, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Wolf-van Buerck, L.; Honarpisheh, M.; Zhang, Y.; Schwinzer, R.; Petersen, B.; Seissler, J. Neonatal islets from human PD-L1 transgenic pigs reduce immune cell activation and cellular rejection in humanized nonobese diabetic-scid IL2rgamma(null) mice. Am. J. Transplant. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Moazami, N.; Stern, J.M.; Khalil, K.; Kim, J.I.; Narula, N.; Mangiola, M.; Weldon, E.P.; Kagermazova, L.; James, L.; Lawson, N.; et al. Pig-to-human heart xenotransplantation in two recently deceased human recipients. Nat. Med. 2023, 29, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Y.; Ma, J.; Xu, Z.; Duan, C.; Wang, Y.; Li, X.; Han, J.; Zhuang, R. Competitive binding of CD226/TIGIT with poliovirus receptor regulates macrophage polarization and is involved in vascularized skin graft rejection. Am. J. Transplant. 2023, 23, 920–934. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, J.; Drescher, E.; Simon-Campos, J.A.; Emery, P.; Greenwald, M.; Kivitz, A.; Rha, H.; Yachi, P.; Kiley, C.; Nirula, A. A Phase 2 Trial of Peresolimab for Adults with Rheumatoid Arthritis. N. Engl. J. Med. 2023, 388, 1853–1862. [Google Scholar] [CrossRef]
- Curnock, A.P.; Bossi, G.; Kumaran, J.; Bawden, L.J.; Figueiredo, R.; Tawar, R.; Wiseman, K.; Henderson, E.; Hoong, S.J.; Gonzalez, V.; et al. Cell-targeted PD-1 agonists that mimic PD-L1 are potent T cell inhibitors. JCI Insight 2021, 6, e1524683. [Google Scholar] [CrossRef]
- Grebinoski, S.; Vignali, D.A. Inhibitory receptor agonists: The future of autoimmune disease therapeutics? Curr. Opin. Immunol. 2020, 67, 1–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Bello, A.; Treiner, E. Immune Checkpoints in Solid Organ Transplantation. Biology 2023, 12, 1358. https://doi.org/10.3390/biology12101358
Del Bello A, Treiner E. Immune Checkpoints in Solid Organ Transplantation. Biology. 2023; 12(10):1358. https://doi.org/10.3390/biology12101358
Chicago/Turabian StyleDel Bello, Arnaud, and Emmanuel Treiner. 2023. "Immune Checkpoints in Solid Organ Transplantation" Biology 12, no. 10: 1358. https://doi.org/10.3390/biology12101358
APA StyleDel Bello, A., & Treiner, E. (2023). Immune Checkpoints in Solid Organ Transplantation. Biology, 12(10), 1358. https://doi.org/10.3390/biology12101358





