Effects of Myostatin b Knockout on Offspring Body Length and Skeleton in Yellow Catfish (Pelteobagrus fulvidraco)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
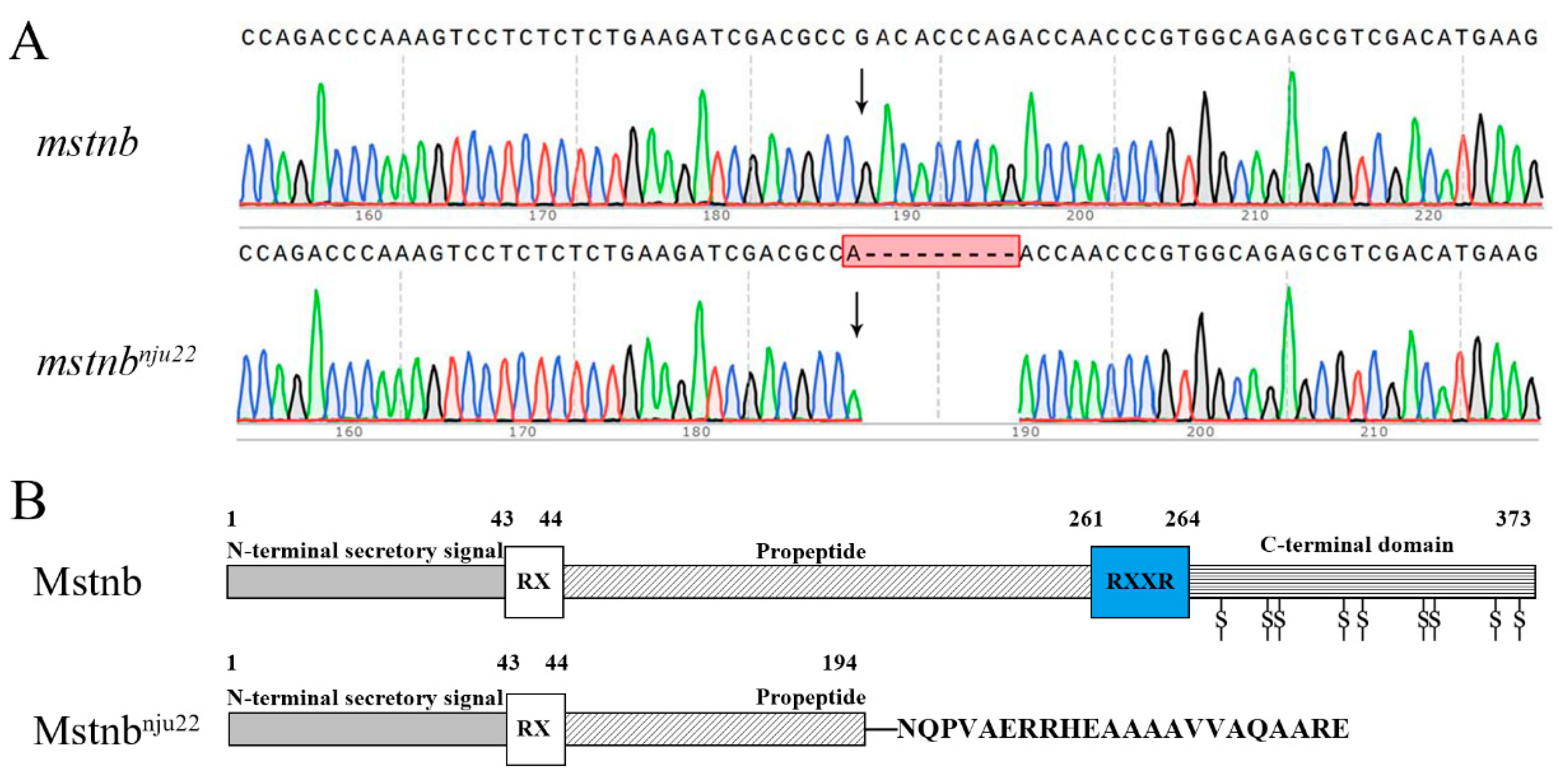
2.1. Artificial Insemination and Breeding of the Heritable Myostatin b Gene (mstnb) Mutation in Yellow Catfish
2.2. Genotyping of Yellow Catfish Carrying Mutated mstnb Allele (nju22)
2.3. Morphological and Growth Analysis of Yellow Catfish
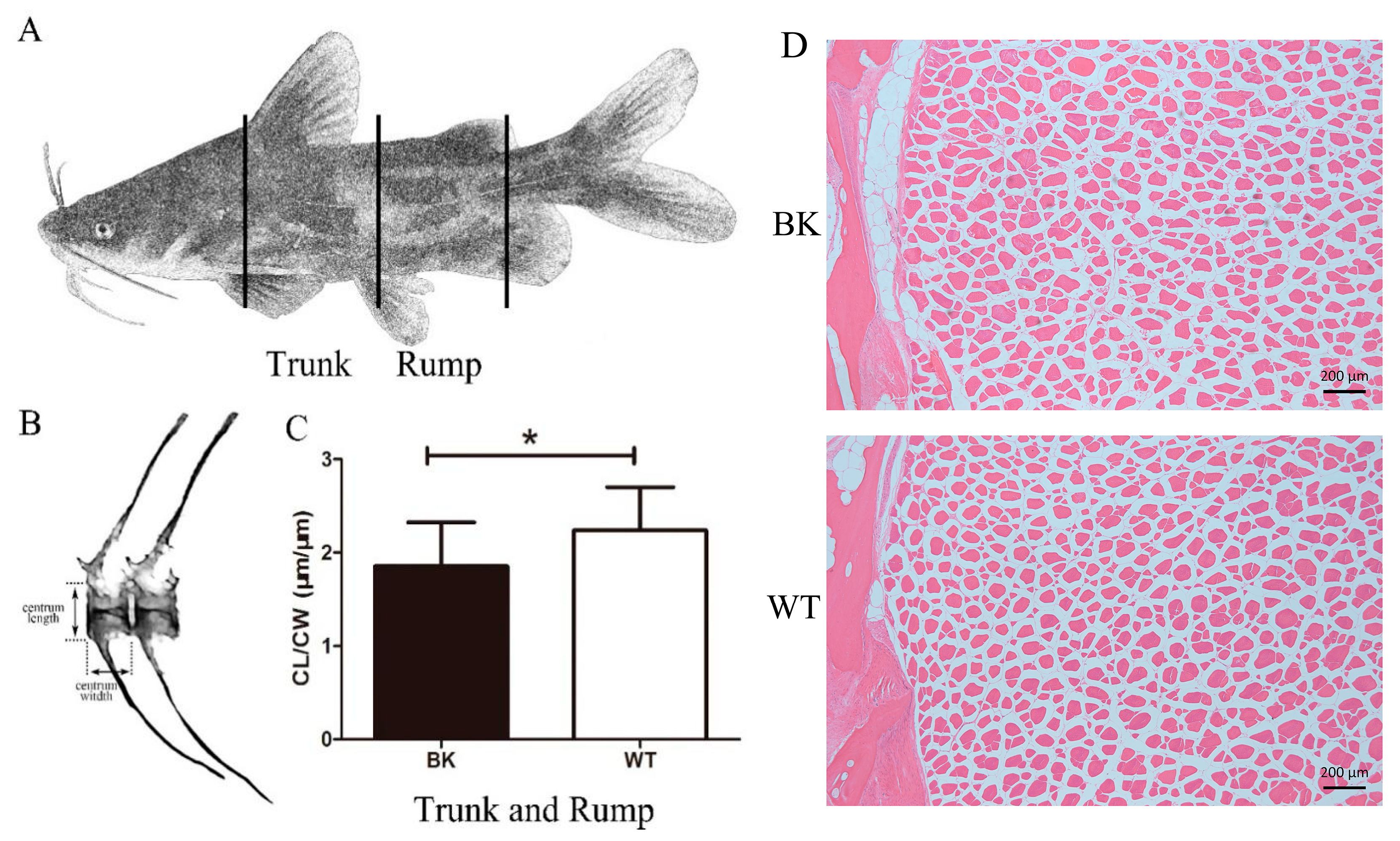
2.4. Skeletal Staining and Vertebral Counts
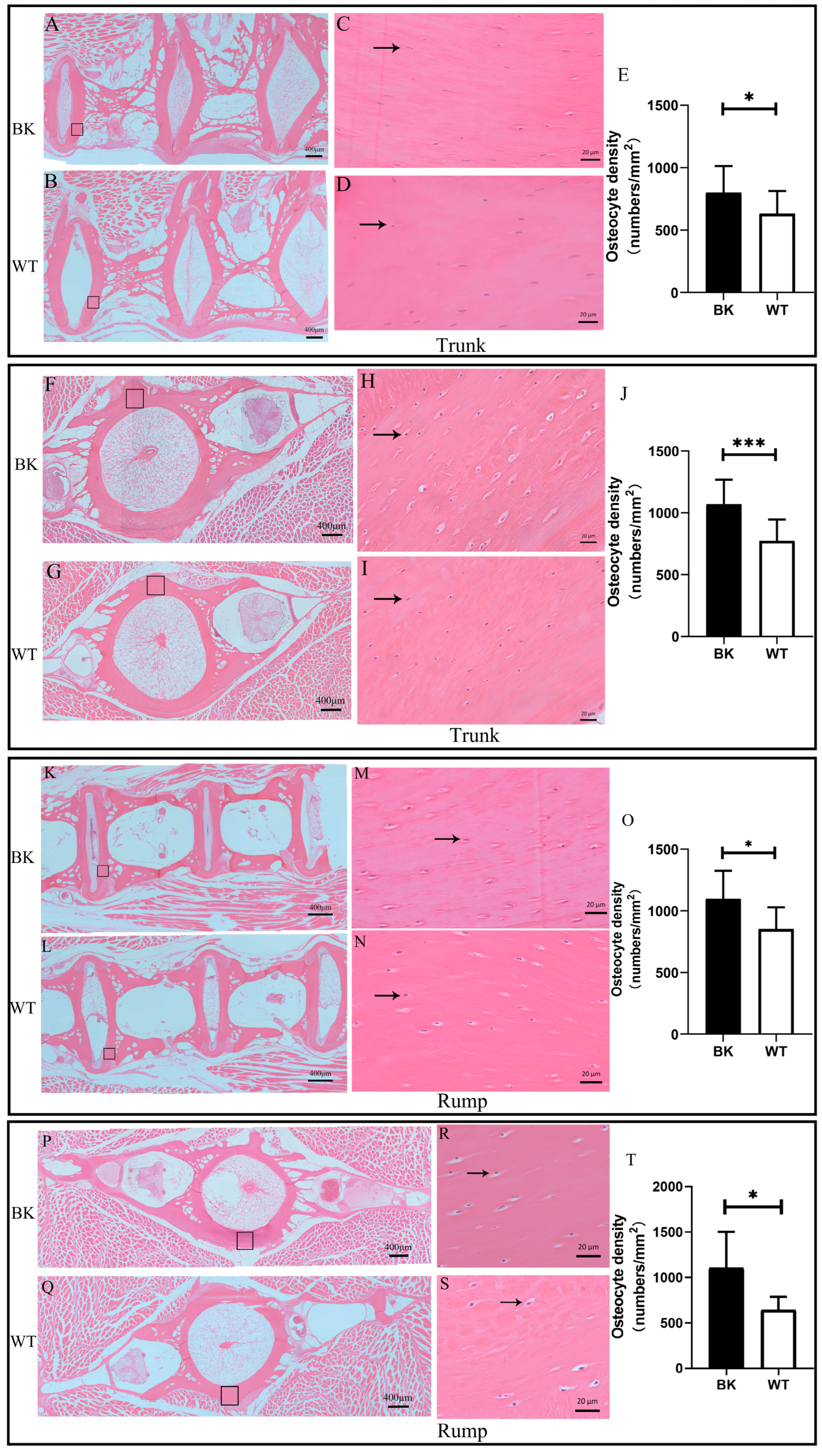
2.5. Histological Analysis on the Vertebral Column of Yellow Catfish
2.6. Quantitative Analysis of Centrum Variations
2.7. Statistical Analysis
3. Results
3.1. Yellow Catfish Carrying Mutated mstnb Have Normal Reproductive Capacity
3.2. Yellow Catfish Carrying mstnb Null Alleles Display Shorter Body Length
3.3. More Vertebral Centra in Wild-Type Yellow Catfish Than mstnb Knockout Yellow Catfish
3.4. Centrum in Wild-Type Displayed Longer Length Than mstnb Knockout Yellow Catfish
3.5. mstnb Knockout Yellow Catfish Have Increased Osteocyte Number in Centrum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rebhan, Y.; Funkenstein, B. Inhibition of fish myostatin activity by recombinant fish follistatin and myostatin prodomain: Potential implications for enhancing muscle growth in farmed fish. Aquaculture 2008, 284, 231–238. [Google Scholar] [CrossRef]
- Lee, S.J.; McPherron, A.C. Myostatin and the control of skeletal muscle mass. Curr. Opin. Genet. Dev. 1999, 9, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Kim, J.H.; Jin, D.H.; Jin, H.J.; Kim, Y.S. Myostatin inhibitory region of fish (Paralichthys olivaceus) myostatin-1 propeptide. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 194–195, 65–70. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lee, S.-J. Double Muscling in Cattle Due to Mutations in the Myostatin Gene. Proc. Natl. Acad. Sci. USA 1997, 94, 12457–12461. [Google Scholar] [CrossRef] [PubMed]
- Clop, A.; Marcq, F.; Takeda, H.; Pirottin, D.; Tordoir, X.; Bibé, B.; Bouix, J.; Caiment, F.; Elsen, J.-M.; Eychenne, F.; et al. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat. Genet. 2006, 38, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Mosher, D.S.; Quignon, P.; Bustamante, C.D.; Sutter, N.B.; Mellersh, C.S.; Parker, H.G.; Ostrander, E.A. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet. 2007, 3, e79. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.-H.; Cho, H.-J.; Han, E.; Hong, T.I.; Ariyasiri, K.; Choi, J.-H.; Hwang, K.-S.; Jeong, Y.-M.; Yang, S.-Y.; Yu, K.; et al. Zebrafish knockout of Down syndrome gene, DYRK1A, shows social impairments relevant to autism. Mol. Autism 2017, 8, 50. [Google Scholar] [CrossRef]
- Zhang, J.; Song, F.; Sun, Y.; Yu, K.; Xiang, J. CRISPR/Cas9-mediated deletion of EcMIH shortens metamorphosis time from mysis larva to postlarva of Exopalaemon carinicauda. Fish Shellfish Immunol. 2018, 77, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.-H.; Wan, S.-M.; Chen, Y.-L.; Huysseune, A.; Wu, Y.-M.; Zhou, J.-J.; Hilsdorf, A.W.S.; Wang, W.-M.; Witten, P.E.; Lin, Q.; et al. Single-cell transcriptomes and runx2b−/− mutants reveal the genetic signatures of intermuscular bone formation in zebrafish. Natl. Sci. Rev. 2022, 9, nwac152. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Ge, J.; Li, K.; Xu, Z.; Liang, D.; Li, J.; Li, J.; Jia, W.; Li, Y.; Dong, X.; et al. Heritable targeted inactivation of myostatin gene in yellow catfish (Pelteobagrus fulvidraco) using engineered zinc finger nucleases. PLoS ONE 2011, 6, e28897. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Ge, J.; Xu, Z.; Dong, X.; Cao, S.; Pan, J.; Zhao, Q. Generation of Myostatin b Knockout Yellow Catfish (Tachysurus fulvidraco) Using Transcription Activator-Like Effector Nucleases. Zebrafish 2014, 11, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, F.; Dong, Z.; Dong, X.; Chi, J.; Chen, H.; Zhao, Q.; Li, K. A new strain of yellow catfish carrying genome edited myostatin alleles exhibits double muscling phenotype with hyperplasia. Aquaculture 2020, 523, 735187. [Google Scholar] [CrossRef]
- Zhong, Z.; Niu, P.; Wang, M.; Huang, G.; Xu, S.; Sun, Y.; Xu, X.; Hou, Y.; Sun, X.; Yan, Y.; et al. Targeted disruption of sp7 and myostatin with CRISPR-Cas9 results in severe bone defects and more muscular cells in common carp. Sci. Rep. 2016, 6, 22953. [Google Scholar] [CrossRef] [PubMed]
- Khalil, K.; Elayat, M.; Khalifa, E.; Daghash, S.; Elaswad, A.; Miller, M.; Abdelrahman, H.; Ye, Z.; Odin, R.; Drescher, D.; et al. Generation of Myostatin Gene-Edited Channel Catfish (Ictalurus punctatus) via Zygote Injection of CRISPR/Cas9 System. Sci. Rep. 2017, 7, 7301. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, Y.L.; Bian, W.P.; Xie, S.L.; Qi, G.L.; Liu, L.; Strauss, P.R.; Zou, J.X.; Pei, D.S. Deletion of mstna and mstnb impairs the immune system and affects growth performance in zebrafish. Fish Shellfish Immunol. 2018, 72, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Kinoshita, M.; Ng, T.H.; Chang, Y.H.; Maekawa, S.; Chiang, Y.A.; Aoki, T.; Wang, H.C. Using CRISPR/Cas9-mediated gene editing to further explore growth and trade-off effects in myostatin-mutated F4 medaka (Oryzias latipes). Sci. Rep. 2017, 7, 11435. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, K.; Washio, Y.; Yoshiura, Y.; Toyoda, A.; Ueno, T.; Fukuyama, H.; Kato, K.; Kinoshita, M. Production of a breed of red sea bream Pagrus major with an increase of skeletal muscle mass and reduced body length by genome editing with CRISPR/Cas9. Aquaculture 2018, 495, 415–427. [Google Scholar] [CrossRef]
- Kim, J.; Cho, J.Y.; Kim, J.-W.; Kim, H.-C.; Noh, J.K.; Kim, Y.-O.; Hwang, H.-K.; Kim, W.-J.; Yeo, S.-Y.; An, C.M.; et al. CRISPR/Cas9-mediated myostatin disruption enhances muscle mass in the olive flounder Paralichthys olivaceus. Aquaculture 2019, 512, 734336. [Google Scholar] [CrossRef]
- Chu, Z.; Guo, W.; Hu, W.; Mei, J. Delayed elimination of paternal mtDNA in the interspecific hybrid of Pelteobagrus fulvidraco and Pelteobagrus vachelli during early embryogenesis. Gene 2019, 704, 1–7. [Google Scholar] [CrossRef]
- Guo, W.; Guo, C.; Wang, Y.; Hu, W.; Mei, J. Population structure and genetic diversity in yellow catfish (Pelteobagrus fulvidraco) assessed with microsatellites. J. Genet. 2019, 98, 26. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, P.D.; Zhang, D.Z.; Zhang, H.B.; Tang, B.P.; Liu, Q.N.; Dai, L.S. Mitochondrial genome of the yellow catfish Pelteobagrus fulvidraco and insights into Bagridae phylogenetics. Genomics 2019, 111, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, L.; Wu, F. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2020. [Google Scholar]
- Hamrick, M.W.; McPherron, A.C.; Lovejoy, C.O. Bone mineral content and density in the humerus of adult myostatin-deficient mice. Calcif. Tissue Int. 2002, 71, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, E.; Pennington, C.; Isales, C.M.; Hamrick, M.W. Muscle-bone interactions in dystrophin-deficient and myostatin-deficient mice. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005, 286, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Dingerkus, G.; Uhler, L.D. Enzyme clearing of alcian blue stained whole small vertebrates for demonstration of cartilage. Stain Technol. 1977, 52, 229. [Google Scholar] [CrossRef]
- Walker, M.; Kimmel, C. A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech. Histochem. 2007, 82, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Rigueur, D.; Lyons, K.M. Whole-Mount Skeletal Staining. In Skeletal Development and Repair: Methods and Protocols; Hilton, M.J., Ed.; Humana Press: Totowa, NJ, USA, 2014; pp. 113–121. [Google Scholar] [CrossRef]
- Bolotovskiy, A.A.; Levin, B.A. Effects of thyroid hormones on vertebral numbers in two cyprinid fish species: Rutilus rutilus (Linnaeus, 1758) and Abramis brama (Linnaeus, 1758). J. Appl. Ichthyol. 2018, 34, 449–454. [Google Scholar] [CrossRef]
- Bird, N.C.; Mabee, P.M. Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae). Dev. Dyn. 2003, 228, 337–357. [Google Scholar] [CrossRef]
- Naseka, A.M. Comparative study on the vertebral column in the Gobioninae (Cyprinidae, Pisces) with special reference to its systematics. Publ. Espec. Inst. Esp. Oceanogr. 1996, 21, 149–167. [Google Scholar]
- Hattori, M.; Sawada, Y.; Takagi, Y.; Suzuki, R.; Okada, T.; Kumai, H. Vertebral deformities in cultured red sea bream, Pagrus major, Temminck and Schlegel. Aquac. Res. 2003, 34, 1129–1137. [Google Scholar] [CrossRef]
- Chisada, S.-i.; Okamoto, H.; Taniguchi, Y.; Kimori, Y.; Toyoda, A.; Sakaki, Y.; Takeda, S.; Yoshiura, Y. Myostatin-deficient medaka exhibit a double-muscling phenotype with hyperplasia and hypertrophy, which occur sequentially during post-hatch development. Dev. Biol. 2011, 359, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Feng, Z.; Yi, X. A general introduction to adjustment for multiple comparisons. J. Thorac. Dis. 2017, 9, 1725–1729. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.R.; Liu, X.S.; Chang, X.L.; Allen, R.E. Muscle regeneration in the prolonged absence of myostatin. Proc. Natl. Acad. Sci. USA 2005, 102, 2519–2524. [Google Scholar] [CrossRef] [PubMed]
- Ohama, M.; Washio, Y.; Kishimoto, K.; Kinoshita, M.; Kato, K. Growth performance of myostatin knockout red sea bream Pagrus major juveniles produced by genome editing with CRISPR/Cas9. Aquaculture 2020, 529, 735672. [Google Scholar] [CrossRef]
- Washio, Y.; Ohama, M.; Kishimoto, K.; Kinoshita, M.; Kato, K. Growth performance and edible ratio of myostatin-knockout young red sea bream Pagrus major produced by genome editing with CRISPR/Cas9. Suisan Zoshoku 2021, 69, 101–112. [Google Scholar] [CrossRef]
- Shahi, N.; Mallik, S.K.; Sarma, D. Muscle growth in targeted knockout common carp (Cyprinus carpio) mstn gene with low off-target effects. Aquaculture 2022, 547, 737423. [Google Scholar] [CrossRef]
- Chiang, Y.A.; Kinoshita, M.; Maekawa, S.; Kulkarni, A.; Lo, C.F.; Yoshiura, Y.; Wang, H.C.; Aoki, T. TALENs-mediated gene disruption of myostatin produces a larger phenotype of medaka with an apparently compromised immune system. Fish Shellfish Immunol. 2016, 48, 212–220. [Google Scholar] [CrossRef]
- Lee, C.Y.; Hu, S.Y.; Gong, H.Y.; Chen, M.H.; Lu, J.K.; Wu, J.L. Suppression of myostatin with vector-based RNA interference causes a double-muscle effect in transgenic zebrafish. Biochem. Biophys. Res. Commun. 2009, 387, 766–771. [Google Scholar] [CrossRef]
- Gao, Y.; Dai, Z.; Shi, C.; Zhai, G.; Jin, X.; He, J.; Lou, Q.; Yin, Z. Depletion of Myostatin b Promotes Somatic Growth and Lipid Metabolism in Zebrafish. Front. Endocrinol. 2016, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Zhang, Q.; Chen, Y.; Sun, Y.; Qi, J.; Wang, Z.; Li, S.; Li, C.; Lan, X. The isolation and characterization of myostatin gene in Japanese flounder (Paralichthys olivaceus): Ubiquitous tissue expression and developmental specific regulation. Aquaculture 2008, 280, 247–255. [Google Scholar] [CrossRef]
- Biga, P.R.; Roberts, S.B.; Iliev, D.B.; McCauley, L.A.R.; Moon, J.S.; Collodi, P.; Goetz, F.W. The isolation, characterization, and expression of a novel GDF11 gene and a second myostatin form in zebrafish, Danio rerio. Comp. Biochem. Physiol. Part B 2005, 141, 218–230. [Google Scholar] [CrossRef]
- Xu, C.; Wu, G.; Zohar, Y.; Du, S.-J. Analysis of myostatin gene structure, expression and function in zebrafish. J. Exp. Biol. 2003, 206, 4067–4079. [Google Scholar] [CrossRef]
- Rescan, P.Y.; Jutel, I.; Rallière, C. Two myostatin genes are differentially expressed in myotomal muscles of the trout (Oncorhynchus mykiss). J. Exp. Biol. 2001, 204, 3523–3529. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.-D.; Sun, C.-F.; Pu, J.-W.; Chen, J.; Jiang, X.-Y.; Zou, S.-M. Two myostatin genes exhibit divergent and conserved functions in grass carp (Ctenopharyngodon idellus). Gen. Comp. Endocrinol. 2015, 214, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Østbye, T.-K.K.; Wetten, O.F.; Tooming-Klunderud, A.; Jakobsen, K.S.; Yafe, A.; Etzioni, S.; Moen, T.; Andersen, Ø. Myostatin (MSTN) gene duplications in Atlantic salmon (Salmo salar): Evidence for different selective pressure on teleost MSTN-1 and -2. Gene 2007, 403, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.W. Increased bone mineral density in the femora of GDF8 knockout mice. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003, 272, 388–391. [Google Scholar] [CrossRef]
- Elkasrawy, M.N.; Hamrick, M.W. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J. Musculoskelet. Neuronal Interact. 2010, 10, 56–63. [Google Scholar] [PubMed]
- Vecchione, L.; Byron, C.; Cooper, G.M.; Barbano, T.; Hamrick, M.W.; Sciote, J.J.; Mooney, M.P. Craniofacial Morphology in Myostatin-deficient Mice. J. Dent. Res. 2007, 86, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yang, X.; Gao, X.; Du, H.; Han, Y.; Zhang, D.; Wang, Z.; Sun, L. Inhibiting myostatin signaling prevents femoral trabecular bone loss and microarchitecture deterioration in diet-induced obese rats. Exp. Biol. Med. 2016, 241, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Gao, X.; Yang, X.; Zhang, D.; Zhang, X.; Du, H.; Han, Y.; Sun, L. Combination of Weight-Bearing Training and Anti-MSTN Polyclonal Antibody Improve Bone Quality In Rats. Int. J. Sport Nutr. Exerc. Metab. 2016, 26, 516–524. [Google Scholar] [CrossRef]
- Kellum, E.; Starr, H.; Arounleut, P.; Immel, D.; Fulzele, S.; Wenger, K.; Hamrick, M.W. Myostatin (GDF-8) deficiency increases fracture callus size, Sox-5 expression, and callus bone volume. Bone 2009, 44, 17–23. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, Y.; Song, S.; Lu, R.; Zhou, M.; He, Z.; Yuan, T.; Yan, K.; Cheng, Y. ‘Double-muscling’ and pelvic tilt phenomena in rabbits with the cystine-knot motif deficiency of myostatin on exon 3. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Tang, M.; Yang, J.; Wang, Q.; Cai, C.; Jiang, S.; Li, H.; Jiang, K.; Gao, P.; Ma, D.; et al. Targeted mutations in myostatin by zinc-finger nucleases result in double-muscled phenotype in Meishan pigs. Sci. Rep. 2015, 5, 14435. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, C.C. Pleomerism, the Widespread Tendency Among Related Fish Species for Vertebral Number to be Correlated with Maximum Body Length. J. Fish. Res. Board Can. 1975, 32, 2453–2469. [Google Scholar] [CrossRef]
- Barriga, J.P.; Milano, D.; Cussac, V.E. Variation in vertebral number and its morphological implication in Galaxias platei. J. Fish. Biol. 2013, 83, 1321–1333. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, J.; Yoshinaga, T.; Tanaka, C.; Ishii, K. Geographic distribution and environmental control of vertebral count in Ammodytes spp. along the northern Pacific coast of Japan. J. Fish. Biol. 2017, 90, 773–785. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, S.J. Regulation of GDF-11 and myostatin activity by GASP-1 and GASP-2. Proc. Natl. Acad. Sci. USA 2013, 110, E3713–E3722. [Google Scholar] [CrossRef]
- Mcdowall, R.M. Variation in Vertebral Number in Galaxiid Fishes, How Fishes Swim and a Possible Reason for Pleomerism. Rev. Fish Biol. Fish. 2003, 13, 247–263. [Google Scholar] [CrossRef]
- Brainerd, E.L.; Patek, S.N. Vertebral Column Morphology, C-Start Curvature, and the Evolution of Mechanical Defenses in Tetraodontiform Fishes. Copeia 1998, 1998, 971–984. [Google Scholar] [CrossRef]
- Ward, A.B.; Azizi, E. Convergent evolution of the head retraction escape response in elongate fishes and amphibians. Zoology 2004, 107, 205–217. [Google Scholar] [CrossRef]
- Hamrick, M.W.; Pennington, C.; Byron, C.D. Bone architecture and disc degeneration in the lumbar spine of mice lacking GDF-8 (myostatin). J. Orthop. Res. 2003, 21, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Amali, A.A.; Lin, C.J.; Chen, Y.H.; Wang, W.L.; Gong, H.Y.; Lee, C.Y.; Ko, Y.L.; Lu, J.K.; Her, G.M.; Chen, T.T.; et al. Up-regulation of muscle-specific transcription factors during embryonic somitogenesis of zebrafish (Danio rerio) by knock-down of myostatin-1. Dev. Dyn. 2004, 229, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, Y.; Xu, Y.; Wang, L.; Ma, X.; Dong, C.; Zhao, X.; Tian, X.; Li, X.; Kong, X. The roles of two myostatins and immune effects after inhibition in Qi river crucian carp (Carassius auratus). Fish Shellfish Immunol. 2020, 98, 710–719. [Google Scholar] [CrossRef] [PubMed]


| Male | Female | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BK Group | WT Group | MeanDiff (95%CI) | t | df | p | BK Group | WT Group | MeanDiff (95%CI) | t | df | p | ||
| 90 | body weight (g) | 10.70 ± 4.08 | 11.60 ± 3.58 | −0.90 (−2.93, 1.13) | −0.886 | 77.000 | 0.379 | 8.05 ± 1.96 | 8.85 ± 1.88 | −0.80 (−1.65, 0.06) | −1.843 | 77.000 | 0.069 |
| full length (cm) | 10.41 ± 1.30 | 10.99 ± 1.27 | −0.58 (−1.27, 0.10) | −1.708 | 77.000 | 0.093 | 9.57 ± 0.77 | 9.88 ± 0.73 | −0.29 (−0.58, 0.00) | −2.023 | 77.000 | 0.047 | |
| body length (cm) | 8.75 ± 1.11 | 9.28 ± 1.12 | −0.53 (−1.13, 0.06) | −1.796 | 77.000 | 0.078 | 8.02 ± 0.658 | 8.32 ± 0.63 | −0.31 (−0.64, 0.03) | −1.818 | 77.000 | 0.073 | |
| body height (mm) | 20.33 ± 2.73 | 20.71 ± 2.23 | −0.38 (−1.70, 0.93) | −0.584 | 77.000 | 0.561 | 18.39 ± 1.75 | 18.94 ± 1.46 | −0.55 (−1.27, 0.17) | −1.516 | 77.000 | 0.134 | |
| body width (mm) | 14.19 ± 1.89 | 14.40 ± 1.80 | −0.21 (−1.19, 0.78) | −0.423 | 77.000 | 0.674 | 12.82 ± 1.41 | 13.38 ± 1.22 | −0.56 (−1.15, 0.03) | −1.896 | 77.000 | 0.062 | |
| 180 | body weight (g) | 32.36 ± 9.17 | 42.40 ± 9.51 | −10.04 (−15.33, −4.75) | −3.799 | 44.000 | 0.000 | 15.16 ± 3.14 | 21.76 ± 5.71 | −6.60 (−9.30, −3.89) | −4.915 | 44 | 0.000 |
| full length (cm) | 14.75 ± 1.48 | 16.34 ± 1.26 | −1.59 (−2.34, −0.85) | −4.279 | 44.000 | 0.001 | 11.55 ± 0.76 | 12.87 ± 1.09 | −1.32 (−1.87, −0.76) | −4.795 | 44 | 0.000 | |
| body length (cm) | 12.44 ± 1.36 | 13.87 ± 1.11 | −1.42 (−2.09, −0.76) | −4.795 | 44.000 | 0.000 | 9.69 ± 0.67 | 10.92 ± 0.96 | −1.23 (−1.72, −0.74) | −5.043 | 44 | 0.000 | |
| body height (mm) | 28.38 ± 2.49 | 31.00 ± 2.56 | −2.62 (−4.06, −1.19) | −3.813 | 35.757 | 0.001 | 21.71 ± 1.93 | 25.32 ± 2.72 | −3.61 (−5.00, −2.22) | −5.2284 | 44 | 0.000 | |
| body width (mm) | 18.83 ± 1.91 | 20.36 ± 1.81 | −1.52 (−2.56, −0.49) | −5.228 | 44.000 | 0.000 | 14.58 ± 1.43 | 16.88 ± 1.87 | −2.30 (−3.28, −1.31) | −4.7056 | 44 | 0.000 | |
| head length (cm) | 3.26 ± 0.34 | 3.51 ± 0.26 | −0.24 (−0.41, −0.08) | −4.706 | 44.000 | 0.001 | 2.54 ± 0.15 | 2.76 ± 0.23 | −0.22 (−0.34, −0.10) | −3.81334 | 35.75654 | 0.001 | |
| 250 | body weight (g) | 57.19 ± 23.55 | 58.54 ± 18.89 | −1.35 (−17.23, 14.53) | −0.174 | 28.000 | 0.863 | 23.46 ± 7.58 | 26.98 ± 6.23 | −6.60 (−9.30, −3.89) | −4.915 | 36 | 0.127 |
| full length (cm) | 17.46 ± 2.45 | 18.04 ± 2.06 | −0.58 (−2.27, 1.10) | −0.710 | 28.000 | 0.483 | 12.81 ± 1.48 | 13.59 ± 0.94 | −1.32 (−1.87, −0.76) | −4.79503 | 31.065 | 0.040 | |
| body length (cm) | 14.87 ± 2.17 | 15.51 ± 1.83 | −0.64 (−2.14, 0.86) | −0.867 | 28.000 | 0.388 | 10.83 ± 1.24 | 11.56 ± 0.81 | −1.23 (−1.72, −0.74) | −5.04327 | 30.528 | 0.127 | |
| body height (mm) | 33.87 ± 4.61 | 34.98 ± 4.25 | −1.11 (−4.45, 2.22) | −0.688 | 28.000 | 0.497 | 26.09 ± 3.44 | 28.29 ± 3.15 | −3.61 (−5.00, −2.22) | −5.2284 | 36 | 0.060 | |
| body width (mm) | 23.95 ± 4.31 | 22.80 ± 3.09 | 1.16 (−1.62, 3.94) | 0.835 | 23.244 | 0.412 | 18.17 ± 2.82 | 18.81 ± 1.95 | −2.30 (−3.28, −1.31) | −4.7056 | 36 | 0.061 | |
| head length (cm) | 3.86 ± 0.54 | 3.87 ± 0.43 | −0.01 (−0.37, 0.35) | −0.064 | 28.000 | 0.950 | 2.86 ± 0.33 | 2.88 ± 0.20 | −0.22 (−0.34, −0.10) | −3.81334 | 36 | 0.001 | |
| 360 | body weight (g) | 94.36 ± 41.07 | 104.98 ± 41.95 | −10.62 (−32.39, 11.15) | −0.977 | 57.000 | 0.333 | 36.04 ± 10.32 | 37.34 ± 12.00 | −1.30 (−7.35, 4.75) | −0.431 | 53.000 | 0.668 |
| full length (cm) | 20.49 ± 3.32 | 21.38 ± 3.38 | −0.90 (−2.66, 0.86) | −1.021 | 57.000 | 0.311 | 14.64 ± 1.48 | 14.84 ± 1.42 | −0.21 (−0.99, 0.58) | −0.523 | 53.000 | 0.603 | |
| body length (cm) | 17.22 ± 2.89 | 17.92 ± 2.93 | −0.69 (−2.22, 0.83) | −0.911 | 57.000 | 0.366 | 12.18 ± 1.32 | 12.42 ± 1.28 | −0.24 (−0.95, 0.47) | −0.682 | 53.000 | 0.498 | |
| body height (mm) | 40.27 ± 6.63 | 42.20 ± 7.49 | −1.92 (−5.67, 1.83) | −1.044 | 57.000 | 0.301 | 30.54 ± 3.36 | 30.90 ± 3.69 | −0.36 (−2.27, 1.55) | −0.378 | 53.000 | 0.707 | |
| body width (mm) | 28.72 ± 4.27 | 30.07 ± 4.75 | −1.35 (−3.71, 1.00) | −1.149 | 57.000 | 0.255 | 22.10 ± 2.48 | 22.25 ± 2.63 | −0.15 (−1.53, 1.23) | −0.222 | 53.000 | 0.825 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, F.; Ou, M.; Liu, H.; Luo, Q.; Fei, S.; Zhao, J.; Chen, K.; Zhao, Q.; Li, K. Effects of Myostatin b Knockout on Offspring Body Length and Skeleton in Yellow Catfish (Pelteobagrus fulvidraco). Biology 2023, 12, 1331. https://doi.org/10.3390/biology12101331
Zhang X, Wang F, Ou M, Liu H, Luo Q, Fei S, Zhao J, Chen K, Zhao Q, Li K. Effects of Myostatin b Knockout on Offspring Body Length and Skeleton in Yellow Catfish (Pelteobagrus fulvidraco). Biology. 2023; 12(10):1331. https://doi.org/10.3390/biology12101331
Chicago/Turabian StyleZhang, Xincheng, Fang Wang, Mi Ou, Haiyang Liu, Qing Luo, Shuzhan Fei, Jian Zhao, Kunci Chen, Qingshun Zhao, and Kaibin Li. 2023. "Effects of Myostatin b Knockout on Offspring Body Length and Skeleton in Yellow Catfish (Pelteobagrus fulvidraco)" Biology 12, no. 10: 1331. https://doi.org/10.3390/biology12101331
APA StyleZhang, X., Wang, F., Ou, M., Liu, H., Luo, Q., Fei, S., Zhao, J., Chen, K., Zhao, Q., & Li, K. (2023). Effects of Myostatin b Knockout on Offspring Body Length and Skeleton in Yellow Catfish (Pelteobagrus fulvidraco). Biology, 12(10), 1331. https://doi.org/10.3390/biology12101331









