Immune Thrombosis: Exploring the Significance of Immune Complexes and NETosis
Abstract
Simple Summary
Abstract
1. Introduction
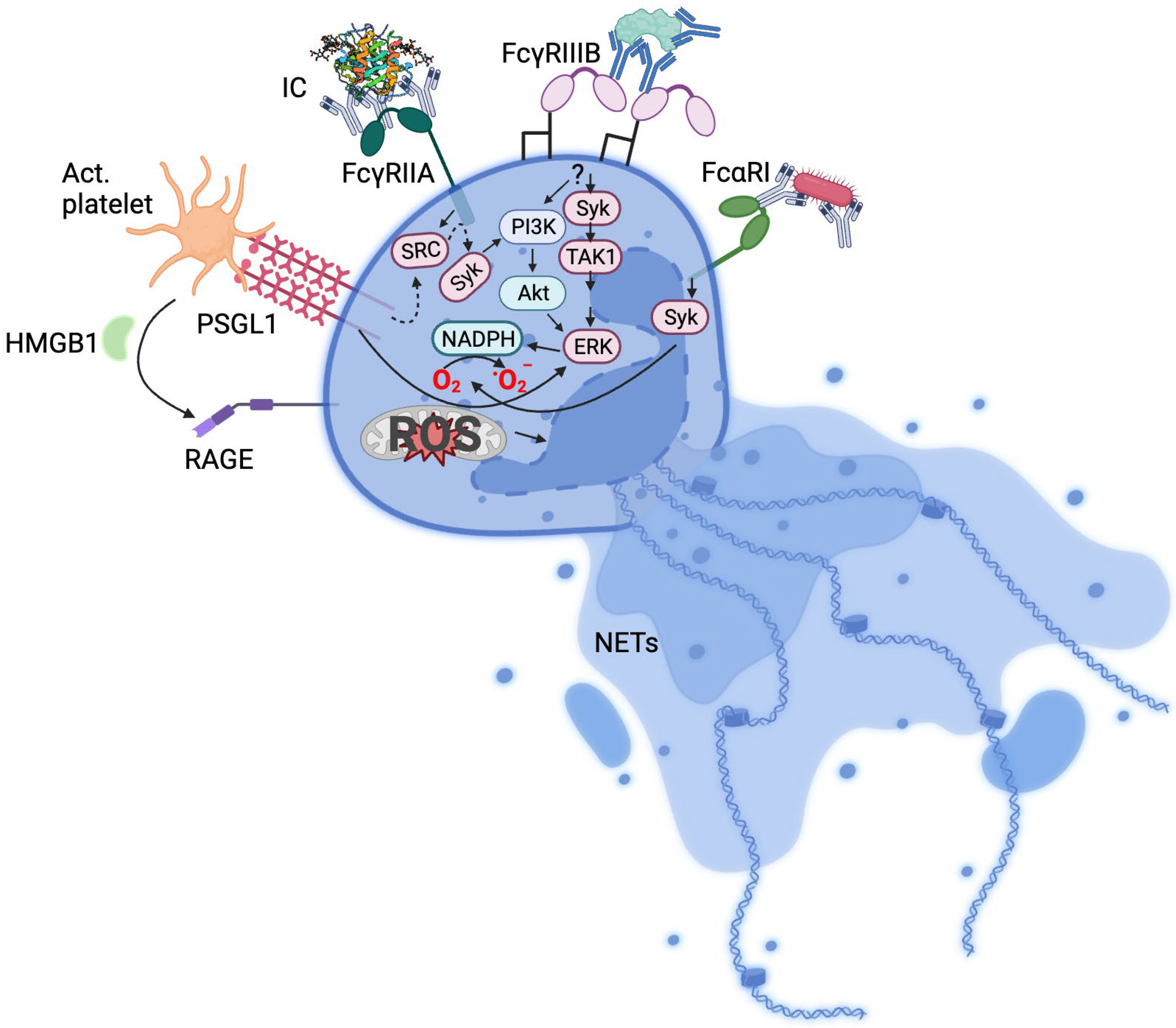
2. Immune Complexes and Fc Receptors
| Condition | Fc Receptor Involved | Receptor Binding Specificity | Antigen-Antibody Complex | Reference |
|---|---|---|---|---|
| Influenza A | FcαR1 (CD89) | IgA | Influenza—IgA | Stacey et al. [21] |
| Human immunodeficiency virus (HIV) | FcαR1 (CD89) | IgA | HIV—IgA | Stacey et al. [21] |
| SARS-CoV-2 | FcαR1 (CD89) FcγRIIA (CD32a) | IgA IgG | SARS-CoV2—IgA Spike protein—IgG | Stacey et al. [21] Bye et al. [65] |
| Staphylococcus aureus | FcαR1 (CD89) | IgA | S. aureus—IgA | Aleyd et al. [58] |
| Vasculitis | FcαR1 (CD89) | IgA | Aggregated vasculitis—IgA | Mayer-Hain et al. [46] |
| Rheumatoid arthritis | FcαR1 (CD89), FcγRI (CD64), FcγRIIA (CD32a) | IgA, IgG | Rheumatoid factor—IgA/IgG, citrullinated protein—anticitrullinated protein antibody (ACPA) IgG, cyclic citrullinated peptide—IgA/IgG, antineutrophil cytoplasmic antibodies (ANCA)—IgA/IgG | Mathsson et al. [41]; Aleyd et al. [47]; Kempers et al. [66] |
| Granulomatosis with polyangiitis (Wegener’s granulomatosis) | FcαR1 (CD89), FcγRIIIB (CD16b) | IgA, IgG | Antineutrophil cytoplasmic antibodies (ANCA)—IgA/IgG | Kelley et al. [39] |
| Heparin-induced thrombocytopenia (HIT) | FcγRIIA (CD32a) | IgG | Heparin—PF4 -HIT IgG | Kelton et al. [67]; Chong et al. [68] |
| Vaccine-induced thrombotic thrombocytopenia (VITT) | FcγRIIA (CD32a) | IgG | PF4—VITT IgG | Greinacher et al. [69] |
| Autoimmune inflammatory disorder | FcγRIIA (CD32a), FcγRIIIB (CD16b) | IgG | Bovine serum albumin (BSA)—IgG, human serum albumin (HAS)—IgG, cross linking—FcγRIIIB | Aleman et al. [29]; Behnen et al. [22] |
| Systemic lupus erythematosus (SLE) | FcγRIIA (CD32a) | IgG | DNA—IgG | Bonegio et al. [35]; Bruneau et al. [70]; Patiño-Trives et al. [71]; Dema and Charles [34]. |
3. Mechanisms of Neutrophil Activation
4. Immune Complexes, NETs, and Immune Thrombosis
| Condition | Comments | References |
|---|---|---|
| Cardiovascular | AMI patients. Neutrophils are activated in acute coronary syndrome. NETs are formed at culprit lesion site. Stroke patients. Neutrophils and NETs markers detected in retrieved stroke thrombi. NETs are associated with severity and mortality. DVT patients. Elevated levels of activated neutrophils and circulating nucleosomes increase risk of DVT. DVT mouse model. NETs promote thrombus formation. | [19,78,94,104,105,106,107,108,109,110] |
| Diabetes | Type I diabetes patients and mice. Type II diabetes patients. Platelet/neutrophil aggregates present. Neutrophils are primed to undergo NETosis. Homocysteine elevated in diabetic patients and correlates with NETosis. | [111,112,113,114] |
| Autoimmunity | APS patients. APS antibodies promote NETs and thrombosis. HIT patients and mouse model. NETs are essential for thrombosis. VITT patients and mouse model. NETs mediate thrombosis. Rheumatoid arthritis patients. NETs mediate cartilage damage. Psoriasis patients. NETs promote inflammation. Lupus erythematosus. Anti-DNA antibodies promote NETosis. Association with endothelial disfunction and cardiovascular disease. | [15,16,47,54,71,115,116,117] |
| Cancer | Pancreatic adenocarcinoma. NETs contribute to cell migration and invasion. Gastric cancer patients. NETs are involved in metastasis. Breast cancer patients and mouse model. NETs contribute to cancer-associated thrombosis. Chronic myelogenous leukemia mouse model. Neutrophils in CML mice more susceptible to NET formation. | [118,119,120,121,122,123] |
| Infection | Sepsis patients and mouse model. NETs and NETs components promote coagulation and death. Influenza A patients. Virus-IgA complexes stimulate NETosis and inactivate viruses. NETs induce inflammation. HIV-1 patients. Virus-IgA complexes stimulate NETosis and inactivate viruses. NETs induce inflammation. SARS-CoV2. NETs mediate severe COVID-19 pathology. CHIKV patients. NETs limit viral load. Hantaan virus. Strong NETs stimulation. May contribute to kidney and lung damage. Dengue virus. Viral exosomes induce NETs and promote proinflammatory cytokine release. S. aureus. NETs promote intravascular coagulation. A. fumigatus C. albicans. NETs mediate fungal killing. E. coli. NETs promote intravascular coagulation. | [2,21,99,100,101,102,124,125,126,127,128,129,130,131,132,133] |
5. NETs Targeting in Immune Thrombosis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Takei, H.; Araki, A.; Watanabe, H.; Ichinose, A.; Sendo, F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukoc. Biol. 1996, 59, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Pilsczek, F.H.; Salina, D.; Poon, K.K.H.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.Y.; Surette, M.G.; Sugai, M.; et al. A Novel Mechanism of Rapid Nuclear Neutrophil Extracellular Trap Formation in Response to Staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef] [PubMed]
- Malawista, S.E.; Van Blaricom, G.; Breitenstein, M.G. Cryopreservable neutrophil surrogates. Stored cytoplasts from human polymorphonuclear leukocytes retain chemotactic, phagocytic, and microbicidal function. J. Clin. Investig. 1989, 83, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood 2013, 122, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
- Burgener, S.S.; Schroder, K. Neutrophil Extracellular Traps in Host Defense. Cold Spring Harb. Perspect. Biol. 2020, 12, a037028. [Google Scholar] [CrossRef]
- Chen, K.W.; Monteleone, M.; Boucher, D.; Sollberger, G.; Ramnath, D.; Condon, N.D.; von Pein, J.B.; Broz, P.; Sweet, M.J.; Schroder, K. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci. Immunol. 2018, 3, eaar6676. [Google Scholar] [CrossRef]
- Csomós, K.; Kristóf, E.; Jakob, B.; Csomós, I.; Kovács, G.; Rotem, O.; Hodrea, J.; Bagoly, Z.; Muszbek, L.; Balajthy, Z.; et al. Protein cross-linking by chlorinated polyamines and transglutamylation stabilizes neutrophil extracellular traps. Cell Death Dis. 2016, 7, e2332. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, X.; Liu, X. NETosis and Neutrophil Extracellular Traps in COVID-19: Immunothrombosis and Beyond. Front. Immunol. 2022, 13, 838011. [Google Scholar] [CrossRef]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef]
- Jung, C.J.; Yeh, C.Y.; Hsu, R.B.; Lee, C.M.; Shun, C.T.; Chia, J.S. Endocarditis pathogen promotes vegetation formation by inducing intravascular neutrophil extracellular traps through activated platelets. Circulation 2015, 131, 571–581. [Google Scholar] [CrossRef]
- Carestia, A.; Kaufman, T.; Rivadeneyra, L.; Landoni, V.I.; Pozner, R.G.; Negrotto, S.; D’Atri, L.P.; Gómez, R.M.; Schattner, M. Mediators and molecular pathways involved in the regulation of neutrophil extracellular trap formation mediated by activated platelets. J. Leukoc. Biol. 2016, 99, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Yalcinkaya, M.; Fotakis, P.; Liu, W.; Endo-Umeda, K.; Dou, H.; Abramowicz, S.; Xiao, T.; Libby, P.; Wang, N.; Tall, A.R.; et al. Cholesterol accumulation in macrophages drives NETosis in atherosclerotic plaques via IL-1β secretion. Cardiovasc. Res. 2023, 119, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Romo, G.S.; Caielli, S.; Vega, B.; Connolly, J.; Allantaz, F.; Xu, Z.; Punaro, M.; Baisch, J.; Guiducci, C.; Coffman, R.L.; et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra20. [Google Scholar] [CrossRef] [PubMed]
- Perdomo, J.; Leung, H.H.L.; Ahmadi, Z.; Yan, F.; Chong, J.J.H.; Passam, F.H.; Chong, B.H. Neutrophil activation and NETosis are the major drivers of thrombosis in heparin-induced thrombocytopenia. Nat. Commun. 2019, 10, 1322. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.H.L.; Perdomo, J.; Ahmadi, Z.; Zheng, S.S.; Rashid, F.N.; Enjeti, A.; Ting, S.B.; Chong, J.J.H.; Chong, B.H. NETosis and thrombosis in vaccine-induced immune thrombotic thrombocytopenia. Nat. Commun. 2022, 13, 5206. [Google Scholar] [CrossRef]
- Holm, S.; Kared, H.; Michelsen, A.E.; Kong, X.Y.; Dahl, T.B.; Schultz, N.H.; Nyman, T.A.; Fladeby, C.; Seljeflot, I.; Ueland, T.; et al. Immune complexes, innate immunity, and NETosis in ChAdOx1 vaccine-induced thrombocytopenia. Eur. Heart J. 2021, 42, 4064–4072. [Google Scholar] [CrossRef]
- Etulain, J.; Martinod, K.; Wong, S.L.; Cifuni, S.M.; Schattner, M.; Wagner, D.D. P-selectin promotes neutrophil extracellular trap formation in mice. Blood 2015, 126, 242–246. [Google Scholar] [CrossRef]
- Maugeri, N.; Campana, L.; Gavina, M.; Covino, C.; De Metrio, M.; Panciroli, C.; Maiuri, L.; Maseri, A.; D’Angelo, A.; Bianchi, M.E.; et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 2014, 12, 2074–2088. [Google Scholar] [CrossRef]
- Chen, T.; Li, Y.; Sun, R.; Hu, H.; Liu, Y.; Herrmann, M.; Zhao, Y.; Muñoz, L.E. Receptor-Mediated NETosis on Neutrophils. Front. Immunol. 2021, 12, 775267. [Google Scholar] [CrossRef]
- Stacey, H.D.; Golubeva, D.; Posca, A.; Ang, J.C.; Novakowski, K.E.; Zahoor, M.A.; Kaushic, C.; Cairns, E.; Bowdish, D.M.E.; Mullarkey, C.E.; et al. IgA potentiates NETosis in response to viral infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2101497118. [Google Scholar] [CrossRef] [PubMed]
- Behnen, M.; Leschczyk, C.; Möller, S.; Batel, T.; Klinger, M.; Solbach, W.; Laskay, T. Immobilized immune complexes induce neutrophil extracellular trap release by human neutrophil granulocytes via FcγRIIIB and Mac-1. J. Immunol. 2014, 193, 1954–1965. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, C.G.; Koffler, D. Immune complex disease in experimental animals and man. Adv. Immunol. 1973, 16, 185–264. [Google Scholar] [CrossRef] [PubMed]
- Otten, M.A.; van Egmond, M. The Fc receptor for IgA (FcalphaRI, CD89). Immunol. Lett. 2004, 92, 23–31. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Voice, J.K.; Lachmann, P.J. Neutrophil Fc gamma and complement receptors involved in binding soluble IgG immune complexes and in specific granule release induced by soluble IgG immune complexes. Eur. J. Immunol. 1997, 27, 2514–2523. [Google Scholar] [CrossRef]
- Mkaddem, S.B.; Murua, A.; Flament, H.; Titeca-Beauport, D.; Bounaix, C.; Danelli, L.; Launay, P.; Benhamou, M.; Blank, U.; Daugas, E.; et al. Lyn and Fyn function as molecular switches that control immunoreceptors to direct homeostasis or inflammation. Nat. Commun. 2017, 8, 246. [Google Scholar] [CrossRef]
- Chen, K.; Nishi, H.; Travers, R.; Tsuboi, N.; Martinod, K.; Wagner, D.D.; Stan, R.; Croce, K.; Mayadas, T.N. Endocytosis of soluble immune complexes leads to their clearance by FcγRIIIB but induces neutrophil extracellular traps via FcγRIIA in vivo. Blood 2012, 120, 4421–4431. [Google Scholar] [CrossRef]
- Alemán, O.R.; Mora, N.; Cortes-Vieyra, R.; Uribe-Querol, E.; Rosales, C. Transforming Growth Factor-β-Activated Kinase 1 Is Required for Human FcγRIIIb-Induced Neutrophil Extracellular Trap Formation. Front. Immunol. 2016, 7, 277. [Google Scholar] [CrossRef]
- Humphrey, J.H.; Jaques, R. The release of histamine and 5-hydroxytryptamine (serotonin) from platelets by antigen-antibody reactions (in vitro). J. Physiol. 1955, 128, 9–27. [Google Scholar] [CrossRef]
- Granger, V.; Faille, D.; Marani, V.; Noël, B.; Gallais, Y.; Szely, N.; Flament, H.; Pallardy, M.; Chollet-Martin, S.; de Chaisemartin, L. Human blood monocytes are able to form extracellular traps. J. Leukoc. Biol. 2017, 102, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Liu, Q.; Li, H.; Zhang, M.; You, L.; Lin, Y.; Wang, K.; Gou, Q.; Wang, Z.; Zhou, S.; et al. The role of monocytes in thrombotic diseases: A review. Front. Cardiovasc. Med. 2023, 10, 1113827. [Google Scholar] [CrossRef]
- Pertiwi, K.R.; de Boer, O.J.; Mackaaij, C.; Pabittei, D.R.; de Winter, R.J.; Li, X.; van der Wal, A.C. Extracellular traps derived from macrophages, mast cells, eosinophils and neutrophils are generated in a time-dependent manner during atherothrombosis. J. Pathol. 2019, 247, 505–512. [Google Scholar] [CrossRef]
- Dema, B.; Charles, N. Autoantibodies in SLE: Specificities, Isotypes and Receptors. Antibodies 2016, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Bonegio, R.G.; Lin, J.D.; Beaudette-Zlatanova, B.; York, M.R.; Menn-Josephy, H.; Yasuda, K. Lupus-Associated Immune Complexes Activate Human Neutrophils in an FcγRIIA-Dependent but TLR-Independent Response. J. Immunol. 2019, 202, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.H.L.; Perdomo, J.; Ahmadi, Z.; Yan, F.; McKenzie, S.E.; Chong, B.H. Inhibition of NADPH oxidase blocks NETosis and reduces thrombosis in heparin-induced thrombocytopenia. Blood Adv. 2021, 5, 5439–5451. [Google Scholar] [CrossRef]
- Mancini, I.; Ferrari, B.; Valsecchi, C.; Pontiggia, S.; Fornili, M.; Biganzoli, E.; Peyvandi, F. ADAMTS13-specific circulating immune complexes as potential predictors of relapse in patients with acquired thrombotic thrombocytopenic purpura. Eur. J. Intern. Med. 2017, 39, 79–83. [Google Scholar] [CrossRef]
- Sui, J.; Lu, R.; Halkidis, K.; Kocher, N.K.; Cao, W.; Marques, M.B.; Zheng, X.L. Plasma levels of S100A8/A9, histone/DNA complexes, and cell-free DNA predict adverse outcomes of immune thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2021, 19, 370–379. [Google Scholar] [CrossRef]
- Kelley, J.M.; Monach, P.A.; Ji, C.; Zhou, Y.; Wu, J.; Tanaka, S.; Mahr, A.D.; Johnson, S.; McAlear, C.; Cuthbertson, D.; et al. IgA and IgG antineutrophil cytoplasmic antibody engagement of Fc receptor genetic variants influences granulomatosis with polyangiitis. Proc. Natl. Acad. Sci. USA 2011, 108, 20736–20741. [Google Scholar] [CrossRef]
- Mazzitelli, I.; Bleichmar, L.; Ludueña, M.G.; Pisarevsky, A.; Labato, M.; Chiaradia, V.; Finocchieto, P.; Paulin, F.; Hormanstorfer, M.; Baretto, M.C.; et al. Immunoglobulin G Immune Complexes May Contribute to Neutrophil Activation in the Course of Severe Coronavirus Disease 2019. J. Infect. Dis. 2021, 224, 575–585. [Google Scholar] [CrossRef]
- Mathsson, L.; Lampa, J.; Mullazehi, M.; Rönnelid, J. Immune complexes from rheumatoid arthritis synovial fluid induce FcγRIIa dependent and rheumatoid factor correlated production of tumour necrosis factor-α by peripheral blood mononuclear cells. Arthritis Res. Ther. 2006, 8, R64. [Google Scholar] [CrossRef] [PubMed]
- Bruhns, P.; Iannascoli, B.; England, P.; Mancardi, D.A.; Fernandez, N.; Jorieux, S.; Daeron, M. Specificity and affinity of human Fc gamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009, 113, 3716–3725. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Lang, M.L.; Wade, W.F. Protein kinase C-alpha and -delta are required for FcalphaR (CD89) trafficking to MHC class II compartments and FcalphaR-mediated antigen presentation. Traffic 2004, 5, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Ji, C.Y.; Xie, F.L.; Langefeld, C.D.; Qian, K.; Gibson, A.W.; Edberg, J.C.; Kimberly, R.P. Fc alpha RI (CD89) Alleles determine the proinflammatory potential of serum IgA. J. Immunol. 2007, 178, 3973–3982. [Google Scholar] [CrossRef]
- Lang, M.L.; Kerr, M.A. Characterization of Fc alpha R-triggered Ca2+ signals: Role in neutrophil NADPH oxidase activation. Biochem. Biophys. Res. Commun. 2000, 276, 749–755. [Google Scholar] [CrossRef]
- Mayer-Hain, S.; Gebhardt, K.; Neufeld, M.; Ehrchen, J.M.; Molyneux, K.; Barratt, J.; Nattkemper, E.; Gerloff, D.; Roth, J.; Vogl, T.; et al. Systemic Activation of Neutrophils by Immune Complexes Is Critical to IgA Vasculitis. J. Immunol. 2022, 209, 1048–1058. [Google Scholar] [CrossRef]
- Aleyd, E.; Al, M.; Tuk, C.W.; van der Laken, C.J.; van Egmond, M. IgA Complexes in Plasma and Synovial Fluid of Patients with Rheumatoid Arthritis Induce Neutrophil Extracellular Traps via FcαRI. J. Immunol. 2016, 197, 4552–4559. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Rivera, C.; Carlucci, P.M.; Goel, R.R.; James, E.; Brooks, S.R.; Rims, C.; Hoffmann, V.; Fox, D.A.; Buckner, J.H.; Kaplan, M.J. Neutrophil extracellular traps mediate articular cartilage damage and enhance cartilage component immunogenicity in rheumatoid arthritis. JCI Insight 2020, 5, 139388. [Google Scholar] [CrossRef] [PubMed]
- Mosalem, O.; Garcilazo, N.H.; Saleh, Y.; Abu Rous, F. Pulmonary embolism as the primary presentation of IgA vasculitis. BMJ Case Rep. 2020, 13, e235884. [Google Scholar] [CrossRef]
- He, Y.T.; Zha, Q.L.; Liu, D.Y.; Lu, A.P. Relations between serum IgA level and cartilage erosion in 436 cases of rheumatoid arthritis. Immunol. Investig. 2007, 36, 285–291. [Google Scholar] [CrossRef]
- Teitsson, I.; Withrington, R.H.; Seifert, M.H.; Valdimarsson, H. Prospective-Study of Early Rheumatoid-Arthritis.1. Prognostic Value of Iga Rheumatoid-Factor. Ann. Rheum. Dis. 1984, 43, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Karimifar, M.; Moussavi, H.; Babaei, M.; Akbari, M. The association of immunoglobulin A, immunoglobulin G and anti-cyclic citrullinated peptide antibodies with disease activity in seronegative rheumatoid arthritis patients. J. Res. Med. Sci. 2014, 19, 823–826. [Google Scholar]
- Serrano, M.; Martínez-Flores, J.A.; Pérez, D.; García, F.; Cabrera-Marante, O.; Pleguezuelo, D.; Paz-Artal, E.; Morales, J.M.; González, E.; Serrano, A. β(2)-Glycoprotein I/IgA Immune Complexes: A Marker to Predict Thrombosis after Renal Transplantation in Patients with Antiphospholipid Antibodies. Circulation 2017, 135, 1922–1934. [Google Scholar] [CrossRef]
- Yalavarthi, S.; Gould, T.J.; Rao, A.N.; Mazza, L.F.; Morris, A.E.; Núñez-Álvarez, C.; Hernández-Ramírez, D.; Bockenstedt, P.L.; Liaw, P.C.; Cabral, A.R.; et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: A newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol. 2015, 67, 2990–3003. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef]
- Remijsen, Q.; Kuijpers, T.W.; Wirawan, E.; Lippens, S.; Vandenabeele, P.; Vanden Berghe, T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011, 18, 581–588. [Google Scholar] [CrossRef]
- Bakema, J.E.; van Egmond, M. The human immunoglobulin A Fc receptor FcalphaRI: A multifaceted regulator of mucosal immunity. Mucosal Immunol. 2011, 4, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Aleyd, E.; van Hout, M.W.; Ganzevles, S.H.; Hoeben, K.A.; Everts, V.; Bakema, J.E.; van Egmond, M. IgA enhances NETosis and release of neutrophil extracellular traps by polymorphonuclear cells via Fcα receptor I. J. Immunol. 2014, 192, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Cortjens, B.; de Boer, O.J.; de Jong, R.; Antonis, A.F.; Sabogal Pineros, Y.S.; Lutter, R.; van Woensel, J.B.; Bem, R.A. Neutrophil extracellular traps cause airway obstruction during respiratory syncytial virus disease. J. Pathol. 2016, 238, 401–411. [Google Scholar] [CrossRef]
- Toussaint, M.; Jackson, D.J.; Swieboda, D.; Guedan, A.; Tsourouktsoglou, T.D.; Ching, Y.M.; Radermecker, C.; Makrinioti, H.; Aniscenko, J.; Bartlett, N.W.; et al. Host DNA released by NETosis promotes rhinovirus-induced type-2 allergic asthma exacerbation. Nat. Med. 2017, 23, 681–691. [Google Scholar] [CrossRef]
- Narasaraju, T.; Yang, E.; Samy, R.P.; Ng, H.H.; Poh, W.P.; Liew, A.A.; Phoon, M.C.; van Rooijen, N.; Chow, V.T. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am. J. Pathol. 2011, 179, 199–210. [Google Scholar] [CrossRef]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Dassler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.A.; He, X.Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Radermecker, C.; Detrembleur, N.; Guiot, J.; Cavalier, E.; Henket, M.; d’Emal, C.; Vanwinge, C.; Cataldo, D.; Oury, C.; Delvenne, P.; et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J. Exp. Med. 2020, 217, e20201012. [Google Scholar] [CrossRef]
- Bye, A.P.; Hoepel, W.; Mitchell, J.L.; Jégouic, S.; Loureiro, S.; Sage, T.; Vidarsson, G.; Nouta, J.; Wuhrer, M.; de Taeye, S.; et al. Aberrant glycosylation of anti-SARS-CoV-2 spike IgG is a prothrombotic stimulus for platelets. Blood 2021, 138, 1481–1489. [Google Scholar] [CrossRef]
- Kempers, A.C.; Nejadnik, M.R.; Rombouts, Y.; Ioan-Facsinay, A.; van Oosterhout, M.; Jiskoot, W.; Huizinga, T.W.J.; Toes, R.E.M.; Scherer, H.U. Fc gamma receptor binding profile of anti-citrullinated protein antibodies in immune complexes suggests a role for FcγRI in the pathogenesis of synovial inflammation. Clin. Exp. Rheumatol. 2018, 36, 284–293. [Google Scholar]
- Kelton, J.G.; Sheridan, D.; Santos, A.; Smith, J.; Steeves, K.; Smith, C.; Brown, C.; Murphy, W.G. Heparin-induced thrombocytopenia: Laboratory studies. Blood 1988, 72, 925–930. [Google Scholar] [CrossRef]
- Chong, B.H.; Fawaz, I.; Chesterman, C.N.; Berndt, M.C. Heparin-induced thrombocytopenia: Mechanism of interaction of the heparin-dependent antibody with platelets. Br. J. Haematol. 1989, 73, 235–240. [Google Scholar] [CrossRef]
- Greinacher, A.; Selleng, K.; Mayerle, J.; Palankar, R.; Wesche, J.; Reiche, S.; Aebischer, A.; Warkentin, T.E.; Muenchhoff, M.; Hellmuth, J.C.; et al. Anti-platelet factor 4 antibodies causing VITT do not cross-react with SARS-CoV-2 spike protein. Blood 2021, 138, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, C.D.; Edmonds, J.P.; Hughes, G.R.; Aarden, L. Detection and characterization of DNA-anti-DNA complexes in a patient with systemic lupus erythematosus. Clin. Exp. Immunol. 1977, 28, 433–436. [Google Scholar] [PubMed]
- Patiño-Trives, A.M.; Pérez-Sánchez, C.; Pérez-Sánchez, L.; Luque-Tévar, M.; Ábalos-Aguilera, M.C.; Alcaide-Ruggiero, L.; Arias-de la Rosa, I.; Román-Rodríguez, C.; Seguí, P.; Espinosa, M.; et al. Anti-dsDNA Antibodies Increase the Cardiovascular Risk in Systemic Lupus Erythematosus Promoting a Distinctive Immune and Vascular Activation. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2417–2430. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Visentin, G.P.; Dayananda, K.M.; Neelamegham, S. Immune complexes formed following the binding of anti-platelet factor 4 (CXCL4) antibodies to CXCL4 stimulate human neutrophil activation and cell adhesion. Blood 2008, 112, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Y.; Wang, X.; Yang, L.; Li, H.; Wang, Y.; Liu, M.; Zhao, X.; Xie, Y.; Yang, Y.; et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef]
- Mereweather, L.J.; Constantinescu-Bercu, A.; Crawley, J.T.B.; Salles, C., II. Platelet-Neutrophil Crosstalk in Thrombosis. Int. J. Mol. Sci. 2023, 24, 1266. [Google Scholar] [CrossRef]
- Hirsch, J.; Uzun, G.; Zlamal, J.; Singh, A.; Bakchoul, T. Platelet-neutrophil interaction in COVID-19 and vaccine-induced thrombotic thrombocytopenia. Front. Immunol. 2023, 14, 1186000. [Google Scholar] [CrossRef]
- von Brühl, M.L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- Brill, A.; Fuchs, T.A.; Savchenko, A.S.; Thomas, G.M.; Martinod, K.; De Meyer, S.F.; Bhandari, A.A.; Wagner, D.D. Neutrophil extracellular traps promote deep vein thrombosis in mice. J. Thromb. Haemost. 2012, 10, 136–144. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D., Jr.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef]
- Page, C.; Pitchford, S. Neutrophil and platelet complexes and their relevance to neutrophil recruitment and activation. Int. Immunopharmacol. 2013, 17, 1176–1184. [Google Scholar] [CrossRef]
- Kirschenbaum, L.A.; Adler, D.; Astiz, M.E.; Barua, R.S.; Saha, D.; Rackow, E.C. Mechanisms of platelet-neutrophil interactions and effects on cell filtration in septic shock. Shock 2002, 17, 508–512. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stark, K.; Philippi, V.; Stockhausen, S.; Busse, J.; Antonelli, A.; Miller, M.; Schubert, I.; Hoseinpour, P.; Chandraratne, S.; von Brühl, M.L.; et al. Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood 2016, 128, 2435–2449. [Google Scholar] [CrossRef] [PubMed]
- Vogel, S.; Bodenstein, R.; Chen, Q.; Feil, S.; Feil, R.; Rheinlaender, J.; Schäffer, T.E.; Bohn, E.; Frick, J.S.; Borst, O.; et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. J. Clin. Investig. 2015, 125, 4638–4654. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, T.; Panitch, A. Endothelial cells, neutrophils and platelets: Getting to the bottom of an inflammatory triangle. Open Biol. 2020, 10, 200161. [Google Scholar] [CrossRef]
- Dupuy, A.; Aponte-Santamaría, C.; Yeheskel, A.; Hortle, E.; Oehlers, S.H.; Gräter, F.; Hogg, P.J.; Passam, F.H.; Chiu, J. Mechano-Redox Control of Mac-1 De-Adhesion by PDI Promotes Directional Movement under Flow. Circ. Res. 2023, 132, e151–e168. [Google Scholar] [CrossRef]
- Gaul, D.S.; Stein, S.; Matter, C.M. Neutrophils in cardiovascular disease. Eur. Heart J. 2017, 38, 1702–1704. [Google Scholar] [CrossRef]
- Folco, E.J.; Mawson, T.L.; Vromman, A.; Bernardes-Souza, B.; Franck, G.; Persson, O.; Nakamura, M.; Newton, G.; Luscinskas, F.W.; Libby, P. Neutrophil Extracellular Traps Induce Endothelial Cell Activation and Tissue Factor Production through Interleukin-1α and Cathepsin G. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1901–1912. [Google Scholar] [CrossRef]
- Franck, G.; Mawson, T.L.; Folco, E.J.; Molinaro, R.; Ruvkun, V.; Engelbertsen, D.; Liu, X.; Tesmenitsky, Y.; Shvartz, E.; Sukhova, G.K.; et al. Roles of PAD4 and NETosis in Experimental Atherosclerosis and Arterial Injury: Implications for Superficial Erosion. Circ. Res. 2018, 123, 33–42. [Google Scholar] [CrossRef]
- Sylvestre, D.; Clynes, R.; Ma, M.; Warren, H.; Carroll, M.C.; Ravetch, J.V. Immunoglobulin G-mediated inflammatory responses develop normally in complement-deficient mice. J. Exp. Med. 1996, 184, 2385–2392. [Google Scholar] [CrossRef]
- Leppkes, M.; Knopf, J.; Naschberger, E.; Lindemann, A.; Singh, J.; Herrmann, I.; Stürzl, M.; Staats, L.; Mahajan, A.; Schauer, C.; et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. eBioMedicine 2020, 58, 102925. [Google Scholar] [CrossRef]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Stakos, D.A.; Kambas, K.; Konstantinidis, T.; Mitroulis, I.; Apostolidou, E.; Arelaki, S.; Tsironidou, V.; Giatromanolaki, A.; Skendros, P.; Konstantinides, S.; et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur. Heart J. 2015, 36, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Chilingaryan, Z.; Deshmukh, T.; Leung, H.H.L.; Perdomo, J.; Emerson, P.; Kurup, R.; Chong, B.H.; Chong, J.J.H. Erythrocyte interaction with neutrophil extracellular traps in coronary artery thrombosis following myocardial infarction. Pathology 2022, 54, 87–94. [Google Scholar] [CrossRef]
- Laridan, E.; Denorme, F.; Desender, L.; François, O.; Andersson, T.; Deckmyn, H.; Vanhoorelbeke, K.; De Meyer, S.F. Neutrophil extracellular traps in ischemic stroke thrombi. Ann. Neurol. 2017, 82, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Krumbholz, M.; Schönermarck, U.; Back, W.; Gross, W.L.; Werb, Z.; Gröne, H.J.; Brinkmann, V.; Jenne, D.E. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009, 15, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Borowiec, A.; Hadzik-Błaszczyk, M.; Kowalik, I.; Rusinowicz, T.; Krupa, R.; Jankowski, J.; Kandyba, P.; Józefik, E.; Gawałkiewicz, A.; Życińska, K. High incidence of venous thromboembolism but not of coronary artery disease in granulomatosis with polyangiitis in first years after diagnosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2019, 36, 202–208. [Google Scholar] [CrossRef]
- Misra, D.P.; Thomas, K.N.; Gasparyan, A.Y.; Zimba, O. Mechanisms of thrombosis in ANCA-associated vasculitis. Clin. Rheumatol. 2021, 40, 4807–4815. [Google Scholar] [CrossRef]
- Meng, H.; Yalavarthi, S.; Kanthi, Y.; Mazza, L.F.; Elfline, M.A.; Luke, C.E.; Pinsky, D.J.; Henke, P.K.; Knight, J.S. In Vivo Role of Neutrophil Extracellular Traps in Antiphospholipid Antibody–Mediated Venous Thrombosis. Arthritis Rheumatol. 2017, 69, 655–667. [Google Scholar] [CrossRef]
- Janiuk, K.; Jablonska, E.; Garley, M. Significance of NETs Formation in COVID-19. Cells 2021, 10, 151. [Google Scholar] [CrossRef]
- Veras, F.P.; Pontelli, M.C.; Silva, C.M.; Toller-Kawahisa, J.E.; de Lima, M.; Nascimento, D.C.; Schneider, A.H.; Caetite, D.; Tavares, L.A.; Paiva, I.M.; et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020, 217, e20201129. [Google Scholar] [CrossRef]
- Arcanjo, A.; Logullo, J.; Menezes, C.C.B.; de Souza Carvalho Giangiarulo, T.C.; Dos Reis, M.C.; de Castro, G.M.M.; da Silva Fontes, Y.; Todeschini, A.R.; Freire-de-Lima, L.; Decote-Ricardo, D.; et al. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19). Sci. Rep. 2020, 10, 19630. [Google Scholar] [CrossRef] [PubMed]
- Narasaraju, T.; Tang, B.M.; Herrmann, M.; Muller, S.; Chow, V.T.K.; Radic, M. Neutrophilia and NETopathy as Key Pathologic Drivers of Progressive Lung Impairment in Patients With COVID-19. Front. Pharmacol. 2020, 11, 870. [Google Scholar] [CrossRef] [PubMed]
- Kolb, P.; Giese, S.; Voll, R.E.; Hengel, H.; Falcone, V. Immune complexes as culprits of immunopathology in severe COVID-19. Med. Microbiol. Immunol. 2023, 212, 185–191. [Google Scholar] [CrossRef]
- Conteduca, V.; Scarpi, E.; Wetterskog, D.; Brighi, N.; Ferroni, F.; Rossi, A.; Romanel, A.; Gurioli, G.; Bleve, S.; Gianni, C.; et al. Plasma tumor DNA is associated with increased risk of venous thromboembolism in metastatic castration-resistant cancer patients. Int. J. Cancer 2022, 150, 1166–1173. [Google Scholar] [CrossRef]
- de Boer, O.J.; Li, X.; Teeling, P.; Mackaay, C.; Ploegmakers, H.J.; van der Loos, C.M.; Daemen, M.J.; de Winter, R.J.; van der Wal, A.C. Neutrophils, neutrophil extracellular traps and interleukin-17 associate with the organisation of thrombi in acute myocardial infarction. Thromb. Haemost. 2013, 109, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Mangold, A.; Alias, S.; Scherz, T.; Hofbauer, M.; Jakowitsch, J.; Panzenbock, A.; Simon, D.; Laimer, D.; Bangert, C.; Kammerlander, A.; et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ. Res. 2015, 116, 1182–1192. [Google Scholar] [CrossRef]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allergy Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef]
- Valles, J.; Lago, A.; Santos, M.T.; Latorre, A.M.; Tembl, J.I.; Salom, J.B.; Nieves, C.; Moscardo, A. Neutrophil extracellular traps are increased in patients with acute ischemic stroke: Prognostic significance. Thromb. Haemost. 2017, 117, 1919–1929. [Google Scholar] [CrossRef]
- van Montfoort, M.L.; Stephan, F.; Lauw, M.N.; Hutten, B.A.; Van Mierlo, G.J.; Solati, S.; Middeldorp, S.; Meijers, J.C.; Zeerleder, S. Circulating nucleosomes and neutrophil activation as risk factors for deep vein thrombosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 147–151. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, L.; Braun, O.O.; Westman, J.; Madhi, R.; Herwald, H.; Morgelin, M.; Thorlacius, H. Neutrophil extracellular trap-microparticle complexes enhance thrombin generation via the intrinsic pathway of coagulation in mice. Sci. Rep. 2018, 8, 4020. [Google Scholar] [CrossRef]
- Carestia, A.; Frechtel, G.; Cerrone, G.; Linari, M.A.; Gonzalez, C.D.; Casais, P.; Schattner, M. NETosis before and after Hyperglycemic Control in Type 2 Diabetes Mellitus Patients. PLoS ONE 2016, 11, e0168647. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.B.; Baipadithaya, G.; Balakrishnan, A.; Hegde, M.; Vohra, M.; Ahamed, R.; Nagri, S.K.; Ramachandra, L.; Satyamoorthy, K. Elevated homocysteine levels in type 2 diabetes induce constitutive neutrophil extracellular traps. Sci. Rep. 2016, 6, 36362. [Google Scholar] [CrossRef] [PubMed]
- Popp, S.K.; Vecchio, F.; Brown, D.J.; Fukuda, R.; Suzuki, Y.; Takeda, Y.; Wakamatsu, R.; Sarma, M.A.; Garrett, J.; Giovenzana, A.; et al. Circulating platelet-neutrophil aggregates characterize the development of type 1 diabetes in humans and NOD mice. JCI Insight 2022, 7, e153993. [Google Scholar] [CrossRef]
- Wong, L.S.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 2015, 21, 815–819. [Google Scholar] [CrossRef]
- Gollomp, K.; Kim, M.; Johnston, I.; Hayes, V.; Welsh, J.; Arepally, G.M.; Kahn, M.; Lambert, M.P.; Cuker, A.; Cines, D.B.; et al. Neutrophil accumulation and NET release contribute to thrombosis in HIT. JCI Insight 2018, 3, 99445. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.C.; Yu, H.; Yen, F.; Lin, C.; Chen, G.; Lan, C. Neutrophil extracellular trap formation is increased in psoriasis and induces human β-defensin-2 production in epidermal keratinocytes. Sci. Rep. 2016, 6, 31119. [Google Scholar] [CrossRef]
- Shao, S.; Fang, H.; Dang, E.; Xue, K.; Zhang, J.; Li, B.; Qiao, H.; Cao, T.; Zhuang, Y.; Shen, S.; et al. Neutrophil Extracellular Traps Promote Inflammatory Responses in Psoriasis via Activating Epidermal TLR4/IL-36R Crosstalk. Front. Immunol. 2019, 10, 746. [Google Scholar] [CrossRef]
- Demers, M.; Krause, D.S.; Schatzberg, D.; Martinod, K.; Voorhees, J.R.; Fuchs, T.A.; Scadden, D.T.; Wagner, D.D. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 13076–13081. [Google Scholar] [CrossRef]
- Jin, W.; Yin, H.; Li, H.; Yu, X.J.; Xu, H.X.; Liu, L. Neutrophil extracellular DNA traps promote pancreatic cancer cells migration and invasion by activating EGFR/ERK pathway. J. Cell Mol. Med. 2021, 25, 5443–5456. [Google Scholar] [CrossRef]
- Leal, A.C.; Mizurini, D.M.; Gomes, T.; Rochael, N.C.; Saraiva, E.M.; Dias, M.S.; Werneck, C.C.; Sielski, M.S.; Vicente, C.P.; Monteiro, R.Q. Tumor-Derived Exosomes Induce the Formation of Neutrophil Extracellular Traps: Implications For The Establishment of Cancer-Associated Thrombosis. Sci. Rep. 2017, 7, 6438. [Google Scholar] [CrossRef]
- Lecot, P.; Sarabi, M.; Pereira Abrantes, M.; Mussard, J.; Koenderman, L.; Caux, C.; Bendriss-Vermare, N.; Michallet, M.C. Neutrophil Heterogeneity in Cancer: From Biology to Therapies. Front. Immunol. 2019, 10, 2155. [Google Scholar] [CrossRef]
- Snoderly, H.T.; Boone, B.A.; Bennewitz, M.F. Neutrophil extracellular traps in breast cancer and beyond: Current perspectives on NET stimuli, thrombosis and metastasis, and clinical utility for diagnosis and treatment. Breast Cancer Res. 2019, 21, 145. [Google Scholar] [CrossRef]
- Zhu, T.; Zou, X.; Yang, C.; Li, L.; Wang, B.; Li, R.; Li, H.; Xu, Z.; Huang, D.; Wu, Q. Neutrophil extracellular traps promote gastric cancer metastasis by inducing epithelial-mesenchymal transition. Int. J. Mol. Med. 2021, 48, 127. [Google Scholar] [CrossRef]
- Ellett, F.; Jorgensen, J.; Frydman, G.H.; Jones, C.N.; Irimia, D. Neutrophil Interactions Stimulate Evasive Hyphal Branching by Aspergillus fumigatus. PLoS Pathog. 2017, 13, e1006154. [Google Scholar] [CrossRef] [PubMed]
- Hiroki, C.H.; Toller-Kawahisa, J.E.; Fumagalli, M.J.; Colon, D.F.; Figueiredo, L.T.M.; Fonseca, B.; Franca, R.F.O.; Cunha, F.Q. Neutrophil Extracellular Traps Effectively Control Acute Chikungunya Virus Infection. Front. Immunol. 2019, 10, 3108. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.; Davis, R.P.; Kim, S.J.; Tse, M.; Esmon, C.T.; Kolaczkowska, E.; Jenne, C.N. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 2017, 129, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Raftery, M.J.; Lalwani, P.; Krautkrämer, E.; Peters, T.; Scharffetter-Kochanek, K.; Kruger, R.; Hofmann, J.; Seeger, K.; Kruger, D.H.; Schonrich, G. β2 integrin mediates hantavirus-induced release of neutrophil extracellular traps. J. Exp. Med. 2014, 211, 1485–1497. [Google Scholar] [CrossRef]
- Sung, P.S.; Huang, T.F.; Hsieh, S.L. Extracellular vesicles from CLEC2-activated platelets enhance dengue virus-induced lethality via CLEC5A/TLR2. Nat. Commun. 2019, 10, 2402. [Google Scholar] [CrossRef]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef]
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, X.; Pelayo, R.; Monestier, M.; Ammollo, C.T.; Semeraro, F.; Taylor, F.B.; Esmon, N.L.; Lupu, F.; Esmon, C.T. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009, 15, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Qi, H.; Kan, K.; Chen, J.; Xie, H.; Guo, X.; Zhang, L. Neutrophil Extracellular Traps Promote Hypercoagulability in Patients with Sepsis. Shock 2017, 47, 132–139. [Google Scholar] [CrossRef]
- Chamardani, T.M.; Amiritavassoli, S. Inhibition of NETosis for treatment purposes: Friend or foe? Mol. Cell. Biochem. 2022, 477, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Ngo, A.T.P.; Gollomp, K. Building a better NET: Neutrophil extracellular trap targeted therapeutics in the treatment of infectious and inflammatory disorders. Res. Pract. Thromb. Haemost. 2022, 6, e12808. [Google Scholar] [CrossRef]
- Schwab, I.; Nimmerjahn, F. Intravenous immunoglobulin therapy: How does IgG modulate the immune system? Nat. Rev. Immunol. 2013, 13, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Debré, M.; Bonnet, M.C.; Fridman, W.H.; Carosella, E.; Philippe, N.; Reinert, P.; Vilmer, E.; Kaplan, C.; Teillaud, J.L.; Griscelli, C. Infusion of Fc gamma fragments for treatment of children with acute immune thrombocytopenic purpura. Lancet 1993, 342, 945–949. [Google Scholar] [CrossRef]
- Yu, X.; Lazarus, A.H. Targeting FcγRs to treat antibody-dependent autoimmunity. Autoimmun. Rev. 2016, 15, 510–512. [Google Scholar] [CrossRef]
- Heo, Y.A. Efgartigimod: First Approval. Drugs 2022, 82, 341–348. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perdomo, J.; Leung, H.H.L. Immune Thrombosis: Exploring the Significance of Immune Complexes and NETosis. Biology 2023, 12, 1332. https://doi.org/10.3390/biology12101332
Perdomo J, Leung HHL. Immune Thrombosis: Exploring the Significance of Immune Complexes and NETosis. Biology. 2023; 12(10):1332. https://doi.org/10.3390/biology12101332
Chicago/Turabian StylePerdomo, José, and Halina H. L. Leung. 2023. "Immune Thrombosis: Exploring the Significance of Immune Complexes and NETosis" Biology 12, no. 10: 1332. https://doi.org/10.3390/biology12101332
APA StylePerdomo, J., & Leung, H. H. L. (2023). Immune Thrombosis: Exploring the Significance of Immune Complexes and NETosis. Biology, 12(10), 1332. https://doi.org/10.3390/biology12101332






