Life-History and Ecological Correlates of Egg and Clutch Mass Variation in Sympatric Bird Species at High Altitude
Abstract
:Simple Summary
Abstract
1. Introduction
2. Study Site and Methods
3. Statistical Analyses
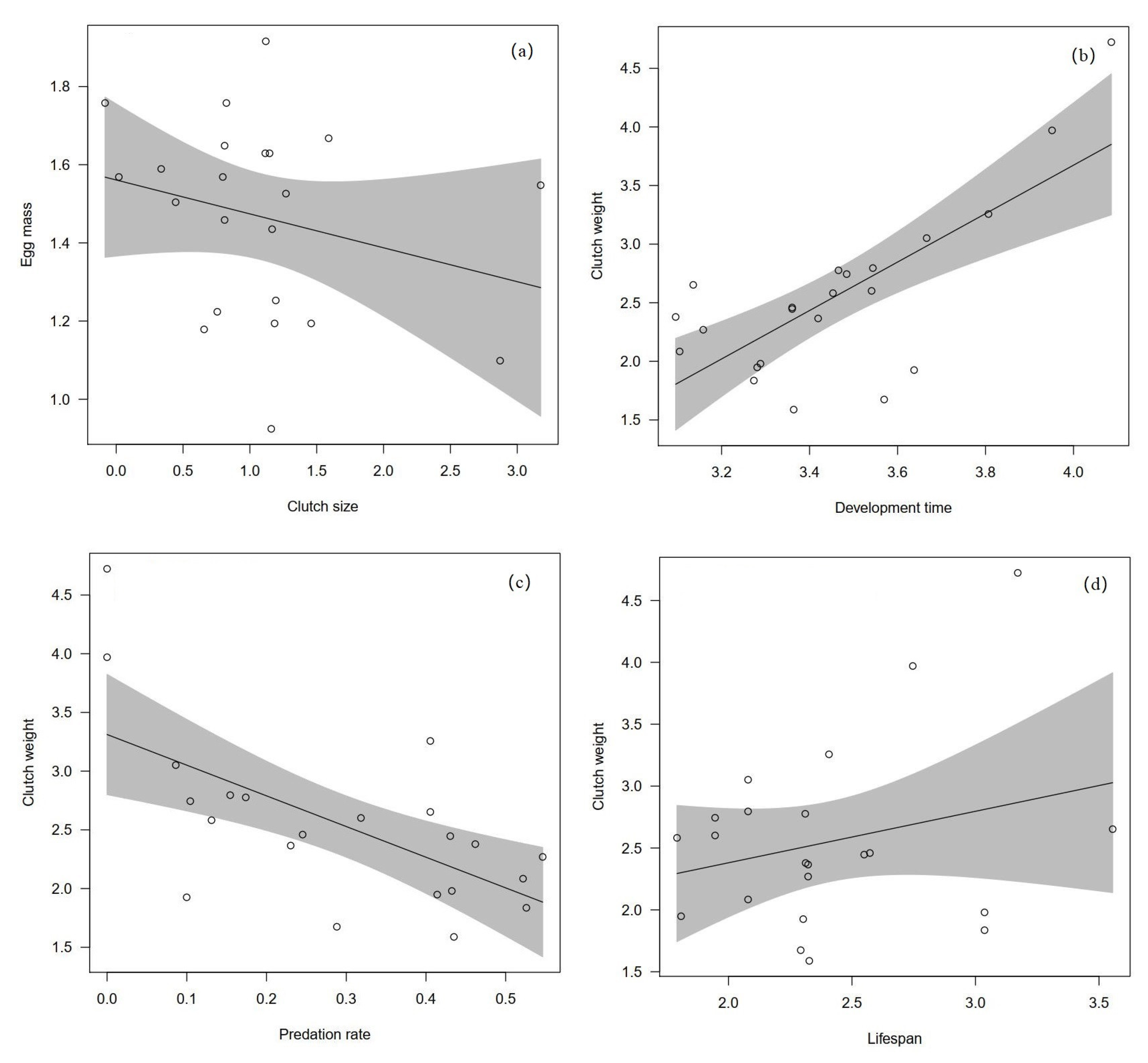
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simpson, M.R. Covariation of Spider Egg and Clutch Size: The Influence of Foraging and Parental Care. Ecology 1995, 76, 795–800. [Google Scholar] [CrossRef]
- Christians, J.K. Avian Egg Size: Variation within Species and Inflexibility within Individuals. Biol. Rev. 2002, 77, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.Y.; Chen, P.Y.; Yang, D.C.; Chen, Y.; Huang, Y.C.; Yeh, J.Y.; Lin, Y.H.; Yang, J.T.; Wu, P.C. The Avian Egg Exhibits General Allometric Invariances in Mechanical Design. Sci. Rep. 2017, 7, 14205. [Google Scholar] [CrossRef] [PubMed]
- Roff, D.A. Life History Evolution; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Huang, J.; Liu, J.; Li, G.; Yan, H.; Li, S. Breeding Biology and Mating System of Brown Accentors Prunella fulvescens on the Tibet Plateau. Bird Study 2020, 67, 232–238. [Google Scholar] [CrossRef]
- Li, S.; Gao, H.; Liu, J.; Li, C.; Li, G.; Li, D. Life History Variation between Two Eurasian Tree Sparrow Passer montanus Populations at Different Altitudes. Anim. Biol. 2022, 72, 385–394. [Google Scholar] [CrossRef]
- Bernardo, J. The Particular Maternal Effect of Propagule Size, Especially Egg Size: Patterns, Models, Quality of Evidence and Interpretations. Am. Zool. 1996, 36, 216–236. [Google Scholar] [CrossRef]
- Liao, W.B.; Lou, S.L.; Zeng, Y.; Kotrschal, A. Large Brains, Small Guts: The Expensive Tissue Hypothesis Supported in Anurans. Am. Nat. 2016, 188, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Du, X.; Li, G.; Liao, W. Nest Complexity Correlates with Larger Brain Size but Smaller Body Mass across Bird Species. Integr. Zool. 2023, 18, 1–7. [Google Scholar] [CrossRef]
- Czesak, M.E.; Fox, C.W. Evolutionary Ecology of Egg Size and Number in a Seed Beetle: Genetic Trade-Off Differs between Environments. Evolution 2003, 57, 1121–1132. [Google Scholar]
- Jiménez-Ortega, D.; Kolm, N.; Immler, S.; Maklakov, A.A.; Gonzalez-Voyer, A. Long Life Evolves in Large-Brained Bird Lineages. Evolution 2020, 74, 2617–2628. [Google Scholar] [CrossRef]
- Blackburn, T.M. An Interspecific Relationship between Egg Size and Clutch Size in Birds. Auk 1991, 108, 973–977. [Google Scholar]
- Poiani, A.; Jermiin, L.S. A Comparative Analysis of Some Life-History Traits between Cooperatively and Non-Cooperatively Breeding Australian Passerines. Evol. Ecol. 1994, 8, 471–488. [Google Scholar] [CrossRef]
- Martin, T.E.; Bassar, R.D.; Bassar, S.K.; Fontaine, J.J.; Lloyd, P.; Mathewson, H.A.; Niklison, A.M.; Chalfoun, A. Life-History and Ecological Correlates of Geographic Variation in Egg and Clutch Mass among Passerine Species. Evolution 2006, 60, 390–398. [Google Scholar] [PubMed]
- Schwarzkopf, L.; Blows, M.W.; Caley, M.J. Life-History Consequences of Divergent Selection on Egg Size in Drosophila melanogaster. Am. Nat. 1999, 154, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.J.; Schwarzkopf, L.; Shine, R. Does Total Reproductive Effort Evolve Independently of Offspring Size? Evolution 2001, 55, 1245–1248. [Google Scholar] [PubMed]
- Koenig, W.; Dickinson, J. Cooperative Breeding in Vertebrates; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Ruber, L.; Britz, R.; Tan, H.H.; Ng, P.K.L.; Zardoya, R. Evolution of Mouthbrooding and Life-History Correlates in the Fighting Fish Genus Betta. Evolution 2004, 58, 799–813. [Google Scholar] [PubMed]
- Martin, T.E. A New View of Avian Life-History Evolution Tested on an Incubation Paradox. Proc. R. Soc. B Biol. Sci. 2002, 269, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Ricklefs, R.E. An Analysis of Nesting Mortality in Birds. Smithson. Contrib. Zool. 1969, 9, 1–48. [Google Scholar] [CrossRef]
- Martin, T.E. Avian Life-History Evolution in Relation to Nest Sites, Nest Predation, and Food. Ecol. Monogr. 1995, 65, 101–127. [Google Scholar] [CrossRef]
- Martin, T.E.; Scott, J.; Menge, C. Nest Predation Increases with Parental Activity: Separating Nest Site and Parental Activity Effects. Proc. R. Soc. B Biol. Sci. 2000, 267, 2287–2293. [Google Scholar] [CrossRef]
- Fontaine, J.J.; Martin, T.E. Reproductive Responses to Experimentally Reduced Nest Predation Risk among Coexisting Bird Species. Ecol. Lett. 2006, 9, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, B. Evolution in Age Structured Populations; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Ghalambor, C.K.; Martin, T.E. Fecundity-Survival Trade-Offs and Parental Risk-Taking in Birds. Science 2001, 292, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yan, H.; Li, G.; Li, S. Nest Concealment Is Associated with Reproductive Traits across Sympatric Bird Species. Ecol. Evol. 2021, 11, 14079–14087. [Google Scholar] [CrossRef] [PubMed]
- de Zwaan, D.R.; Scridel, D.; Altamirano, T.A.; Gokhale, P.; Kumar, R.S.; Sevillano-Ríos, S.; Barras, A.G.; Arredondo-Amezcua, L.; Asefa, A.; Carrillo, R.A.; et al. GABB: A Global Dataset of Alpine Breeding Birds and Their Ecological Traits. Sci. Data 2022, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Peng, W.; Guo, C.; Lu, X. Factors Affecting Nest Success of the Oriental Skylark on the Tibetan Plateau. Ornithol. Sci. 2015, 47, 19–25. [Google Scholar] [CrossRef]
- Li, S.; Shi, R.; Li, W.; Li, G. Grazing Pressure Affects Offspring Sex Ratio in a Socially Monogamous Passerine on the Tibet Plateau. J. Avian Biol. 2018, 49, e01660. [Google Scholar] [CrossRef]
- Wang, C.C.; Lu, X. Hamilton’s inclusive fitness maintains heritable altruism polymorphism through rb = c. Proc. Natl. Acad. Sci. USA 2018, 115, 1860–1864. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Che, X.; Ning, T.; Zou, F. Distribution of birds in the high-altitude area of Mount Everest. Integr. Zool. 2023, 18, 199–204. [Google Scholar] [CrossRef]
- Lu, X.; Yu, T.; Ke, D. Helped Ground Tit Parents in Poor Foraging Environments Reduce Provisioning Effort Despite Nestling Starvation. Anim. Behav. 2011, 82, 861–867. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Guo, C.; Zhang, G.; Zhou, Y.; Lu, X. Nest Helpers Improve Parental Survival but Not Offspring Production in a High-Elevation Passerine, the Ground Tit Pseudopodoces humilis. Ibis 2015, 157, 567–574. [Google Scholar] [CrossRef]
- Li, S.; Lu, X. Breeding Biology of Rock Sparrows Petronia petronia in the Tibetan Plateau, with Special Reference to Life History Variation across Altitudes. Acta Ornithol. 2012, 47, 19–25. [Google Scholar]
- Myhrvold, N.P.; Baldridge, E.; Chan, B.; Sivam, D.; Freeman, D.L.; Ernest, S.K.M. An Amniote Life-History Database to Perform Comparative Analyses with Birds, Mammals, and Reptiles. Ecology 2015, 96, 3109. [Google Scholar] [CrossRef]
- Tacutu, R.; Craig, T.; Budovsky, A.; Wuttke, D.; Lehmann, G.; Taranukha, D.; Costa, J.; Fraifeld, V.E.; de Magalhaes, J.P. Human Ageing Genomic Resources: Integrated Databases and Tools for the Biology and Genetics of Ageing. Nucleic Acids Res. 2013, 41, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Green, J.P.; Freckleton, R.P.; Hatchwell, B.J. Variation in Helper Effort among Cooperatively Breeding Bird Species Is Consistent with Hamilton’s Rule. Nat. Commun. 2016, 7, 12663. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.H.; Pagel, M.D. The Comparative Method in Evolutionary Biology; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Freckleton, R.P.; Harvey, P.H.; Pagel, M. Phylogenetic Analysis and Comparative Data: A Test and Review of Evidence. Am. Nat. 2002, 160, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Revell, L.J. Phylogenetic Signal and Linear Regression on Species Data. Methods Ecol. Evol. 2010, 1, 319–329. [Google Scholar] [CrossRef]
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Hartmann, K.; Mooers, A.O. The Global Diversity of Birds in Space and Time. Nature 2012, 491, 444–448. [Google Scholar] [CrossRef]
- Hackett, S.J.; Kimball, R.T.; Reddy, S.; Bowie, R.C.; Braun, E.L.; Braun, M.J.; Chojnowski, J.L.; Cox, W.A.; Han, K.-L.; Yuri, T.; et al. A Phylogenomic Study of Birds Reveals Their Evolutionary History. Science 2008, 320, 1763–1768. [Google Scholar] [CrossRef]
- Schliep, K.P. phangorn: Phylogenetic analysis in R. Bioinformatics 2011, 27, 592–593. [Google Scholar] [CrossRef]
- Sayol, F.; Downing, P.A.; Iwaniuk, A.N.; Maspons, J.; Sol, D. Predictable Evolution towards Larger Brains in Birds Colonizing Oceanic Islands. Nat. Commun. 2018, 9, 2820. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R Language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; Fretwell, S.D. The Optimal Balance between the Size and Number of Offspring. Am. Nat. 1974, 108, 499–506. [Google Scholar] [CrossRef]
- Saether, B.E. The Influence of Body Weight on the Covariation between Reproductive Traits in European Birds. Oikos 1987, 48, 79–88. [Google Scholar] [CrossRef]
- Williams, T.D. Experimental Manipulation of Female Reproduction Reveals an Intraspecific Egg Size-Clutch Size Trade-Off. Proc. R. Soc. B 2001, 268, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, X.; Li, G.; Du, X. Large-Brained Birds Lay Smaller but Heavier Clutches. Avian Res. 2023, 14, 100116. [Google Scholar] [CrossRef]
- Iwaniuk, A.N.; Nelson, J.E. Developmental Differences Are Correlated with Relative Brain Size in Birds: A Comparative Analysis. Can. J. Zool. 2003, 81, 1913–1928. [Google Scholar] [CrossRef]
- Martin, T.E. Interaction of Nest Predation and Food Limitation in Reproductive Strategies. Curr. Ornithol. 1991, 9, 163–197. [Google Scholar]
- Li, S.; Peng, W.; Guo, C.; Lu, X. Breeding Biology of the Small Snowfinch Pyrgilauda davidiana on the Tibetan Plateau. Forktail 2013, 29, 155–157. [Google Scholar]
- Vaupel, J.W. Post-Darwinian Longevity. Popul. Dev. Rev. 2003, 29, 258–269. [Google Scholar]
- Smeele, S.Q.; Conde, D.A.; Baudisch, A.; Bruslund, S.; Iwaniuk, A.; Staerk, J.; Wright, T.F.; Young, A.M.; McElreath, M.B.; Aplin, L. Coevolution of Relative Brain Size and Life Expectancy in Parrots. Proc. R. Soc. B 2022, 289, 20212397. [Google Scholar] [CrossRef]
- Götmark, F. The effects of investigator disturbance on nesting birds. In Current Ornithology; Power, D.M., Ed.; Springer: Boston, MA, USA, 1992; pp. 63–104. [Google Scholar]
- Carey, M.J. The effects of investigator disturbance on procellariiform seabirds: A review. N. Z. J. Zool. 2009, 36, 367–377. [Google Scholar] [CrossRef]
- Hu, Q.; Wen, Y.; Yu, G.; Yin, J.; Guan, H.; Lv, L.; Wang, P.; Xu, J.; Wang, Y.; Zhang, Z.; et al. Research activity does not affect nest predation rates of the silver-throated tit, a passerine bird building domed nests. Avian Res. 2020, 11, 326–335. [Google Scholar] [CrossRef]
- Li, S.; Cheng, G.; Peng, W. Breeding Patterns of Asian Horned Larks (Eremophila alpestris nigrifrons) on the Tibet Plateau. Wilson J. Ornithol. 2016, 128, 174–179. [Google Scholar] [CrossRef]
- Li, S.; Guo, C.; Zhang, G. Nesting Ecology of Tibetan Sand Martins Riparia riparia with Special Reference to Cooperative Breeding. Ornithol. Sci. 2016, 15, 227–233. [Google Scholar] [CrossRef]
- Li, S.; Lu, X. Reproductive Ecology of Isabelline Wheatears at the Extreme of Their Altitude Distribution. Ardeola 2012, 59, 301–307. [Google Scholar] [CrossRef]
- Li, S.; Qin, J.; Jin, Z.; Li, W.; Yan, H. An Experimental Test of the Concealment Hypothesis Using Oriental Skylark (Alauda gulgula) Nests on the Tibet Plateau. Russ. J. Ecol. 2018, 49, 588–590. [Google Scholar]
| Relationship | Slope ± SE | t | p |
|---|---|---|---|
| Intercept | −1.632 ± 0.309 | −5.278 | <0.001 |
| Clutch size | −0.123 ± 0.145 | −0.845 | 0.408 |
| Body mass | 0.856 ± 0.040 | 21.261 | <0.001 |
| Relationship | Slope ± SE | t | p |
|---|---|---|---|
| Intercept | −3.037 ± 0.546 | −5.559 | <0.001 |
| Development period | 0.953 ± 0.179 | 5.304 | <0.001 |
| Body mass | 0.675 ± 0.052 | 12.887 | <0.001 |
| Intercept | 0.503 ± 0.258 | 1.949 | 0.066 |
| Predation rate | −1.204 ± 0.319 | −3.774 | <0.001 |
| Body mass | 0.721 ± 0.058 | 12.372 | <0.001 |
| Intercept | −0.057 ± 0.258 | −0.223 | 0.826 |
| Lifespan | −0.219 ± 0.147 | −1.495 | 0.151 |
| Body mass | 0.873 ± 0.071 | 12.197 | <0.001 |
| Relationship | Slope ± SE | t | p |
|---|---|---|---|
| Response: Development time | |||
| Intercept | 2.900 ± 0.536 | 5.410 | <0.001 |
| Clutch size | 0.655 ± 0.155 | 4.232 | 0.001 |
| Egg mass | 0.556 ± 0.203 | 2.745 | 0.013 |
| Body mass | −0.278 ± 0.195 | −1.427 | 0.041 |
| Response: Predation rate | |||
| Intercept | 0.280 ± 0.377 | 0.743 | 0.467 |
| Clutch size | −0.366 ± 0.121 | −3.027 | 0.007 |
| Egg mass | −0.181 ± 0.159 | −1.132 | 0.273 |
| Body mass | 0.016 ± 0.155 | 0.103 | 0.619 |
| Response: Lifespan | |||
| Intercept | 2.469 ± 1.496 | 1.649 | 0.116 |
| Clutch size | −0.667 ± 0.440 | −1.515 | 0.039 |
| Egg mass | −0.196 ± 0.553 | −0.355 | 0.626 |
| Body mass | 0.375 ± 0.535 | 0.702 | 0.491 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Du, X.; Li, G.; Liu, Y.; Li, S. Life-History and Ecological Correlates of Egg and Clutch Mass Variation in Sympatric Bird Species at High Altitude. Biology 2023, 12, 1303. https://doi.org/10.3390/biology12101303
Liu Y, Du X, Li G, Liu Y, Li S. Life-History and Ecological Correlates of Egg and Clutch Mass Variation in Sympatric Bird Species at High Altitude. Biology. 2023; 12(10):1303. https://doi.org/10.3390/biology12101303
Chicago/Turabian StyleLiu, Yuxin, Xiaolong Du, Guopan Li, Yingbao Liu, and Shaobin Li. 2023. "Life-History and Ecological Correlates of Egg and Clutch Mass Variation in Sympatric Bird Species at High Altitude" Biology 12, no. 10: 1303. https://doi.org/10.3390/biology12101303
APA StyleLiu, Y., Du, X., Li, G., Liu, Y., & Li, S. (2023). Life-History and Ecological Correlates of Egg and Clutch Mass Variation in Sympatric Bird Species at High Altitude. Biology, 12(10), 1303. https://doi.org/10.3390/biology12101303





