An Evaluation of Suitable Habitats for Amur Tigers (Panthera tigris altaica) in Northeastern China Based on the Random Forest Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Study Areas and Methods
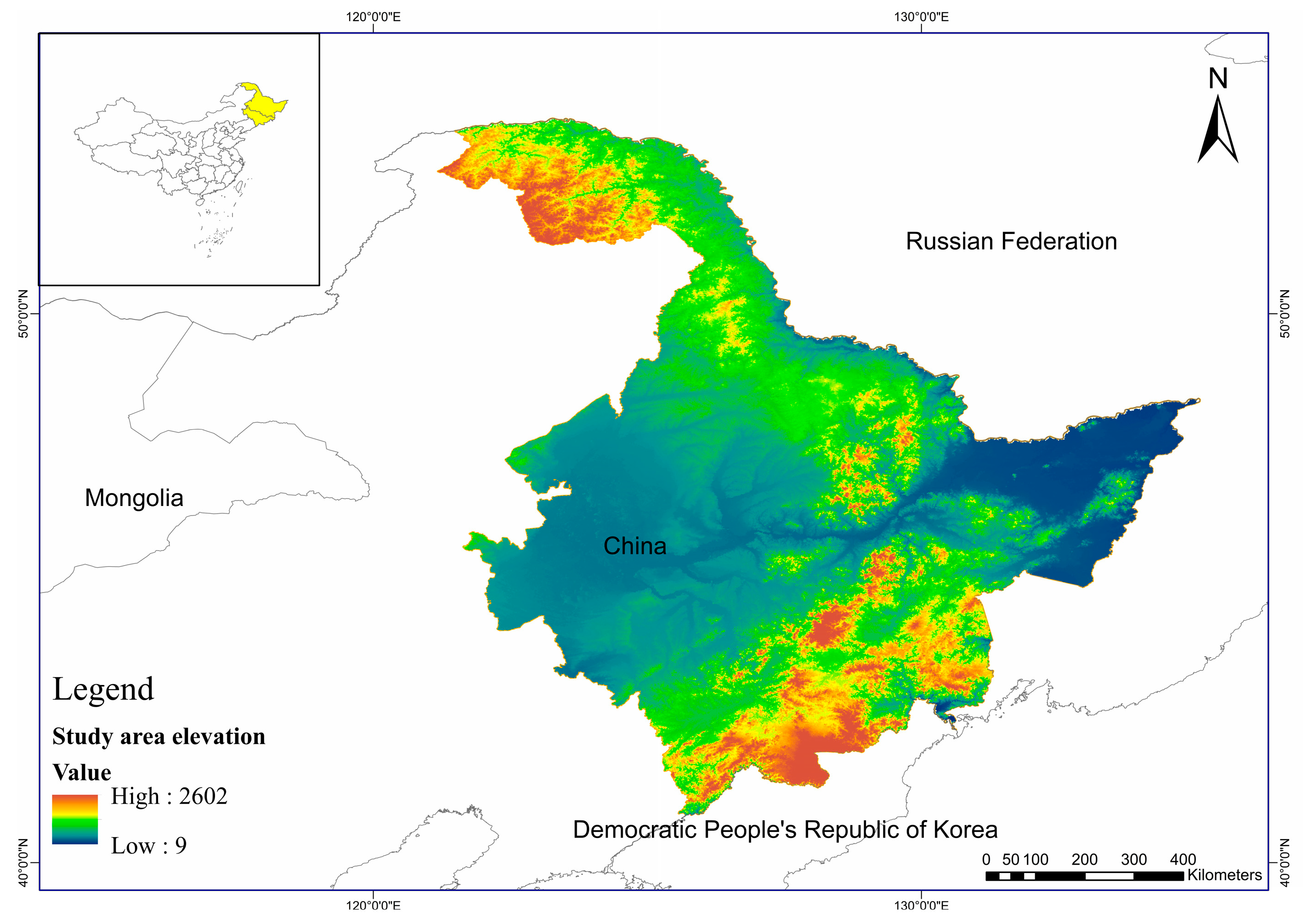
2.1. Study Areas
2.2. Methods
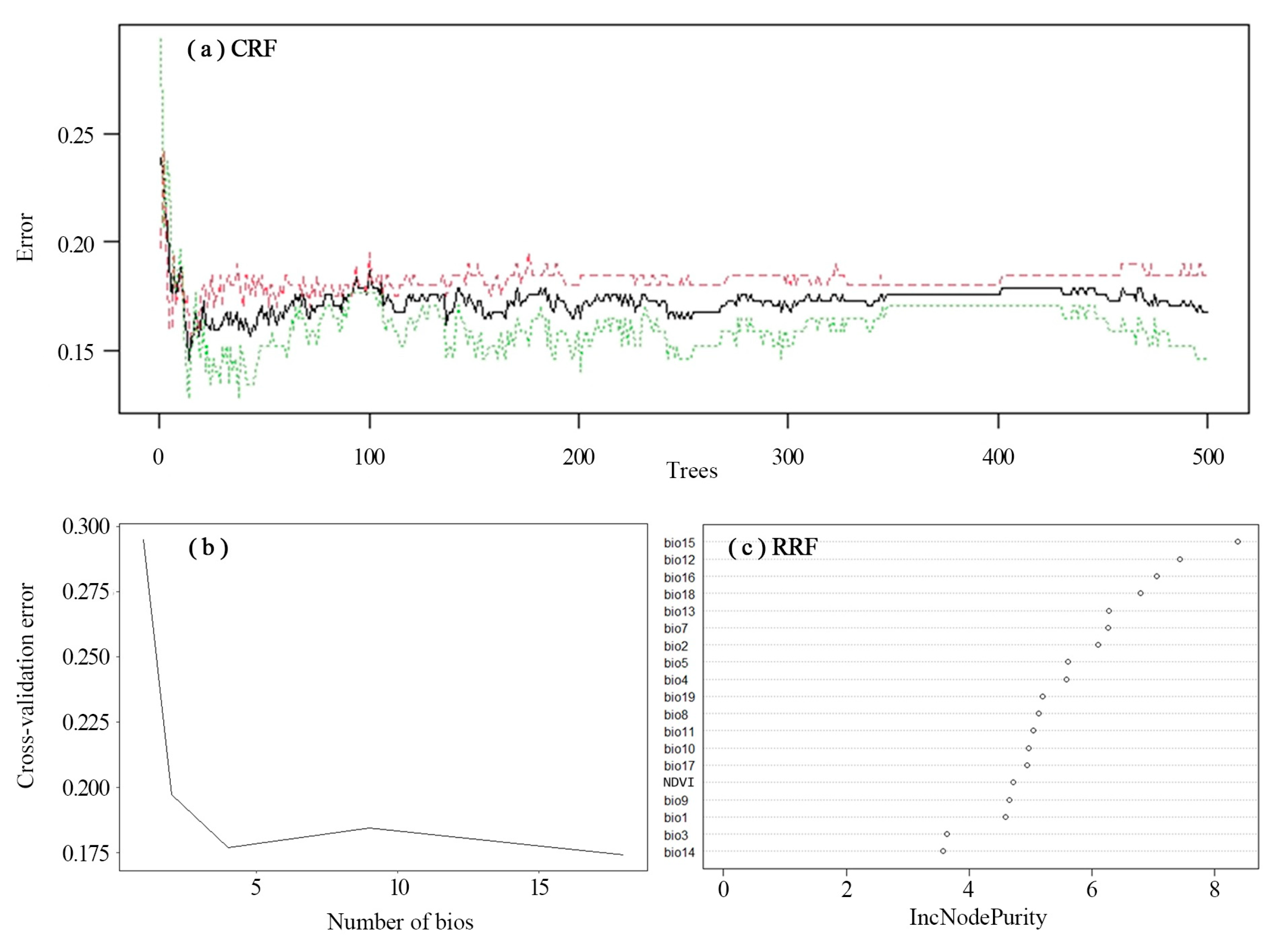
2.2.1. Prediction of Prey Potential Richness in Amur Tigers
2.2.2. Prediction of Suitable Habitats for the Amur Tigers
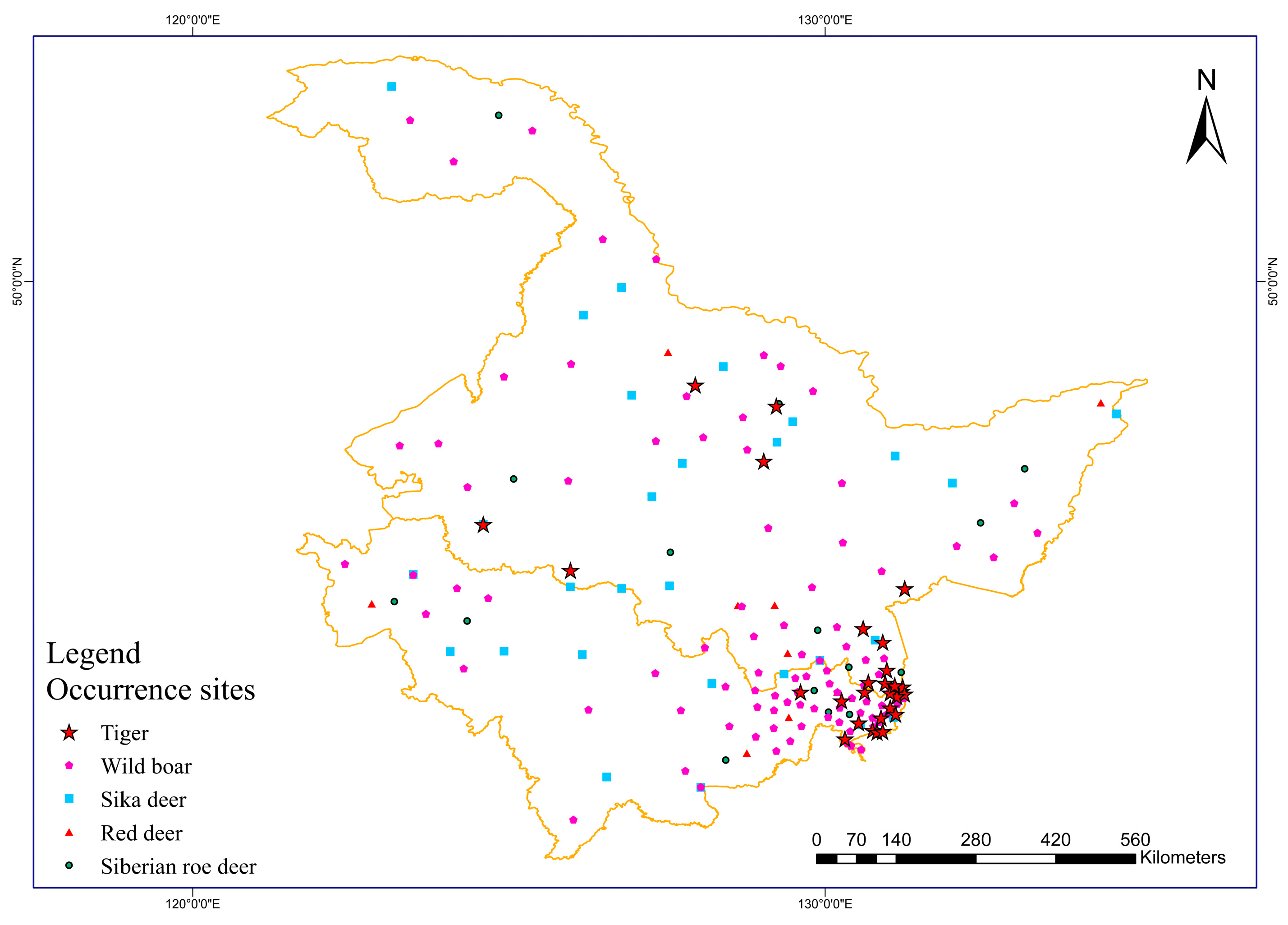
Obtaining Distribution Data for Amur Tigers
Environmental Variables
Model Fitting and Testing
3. Results
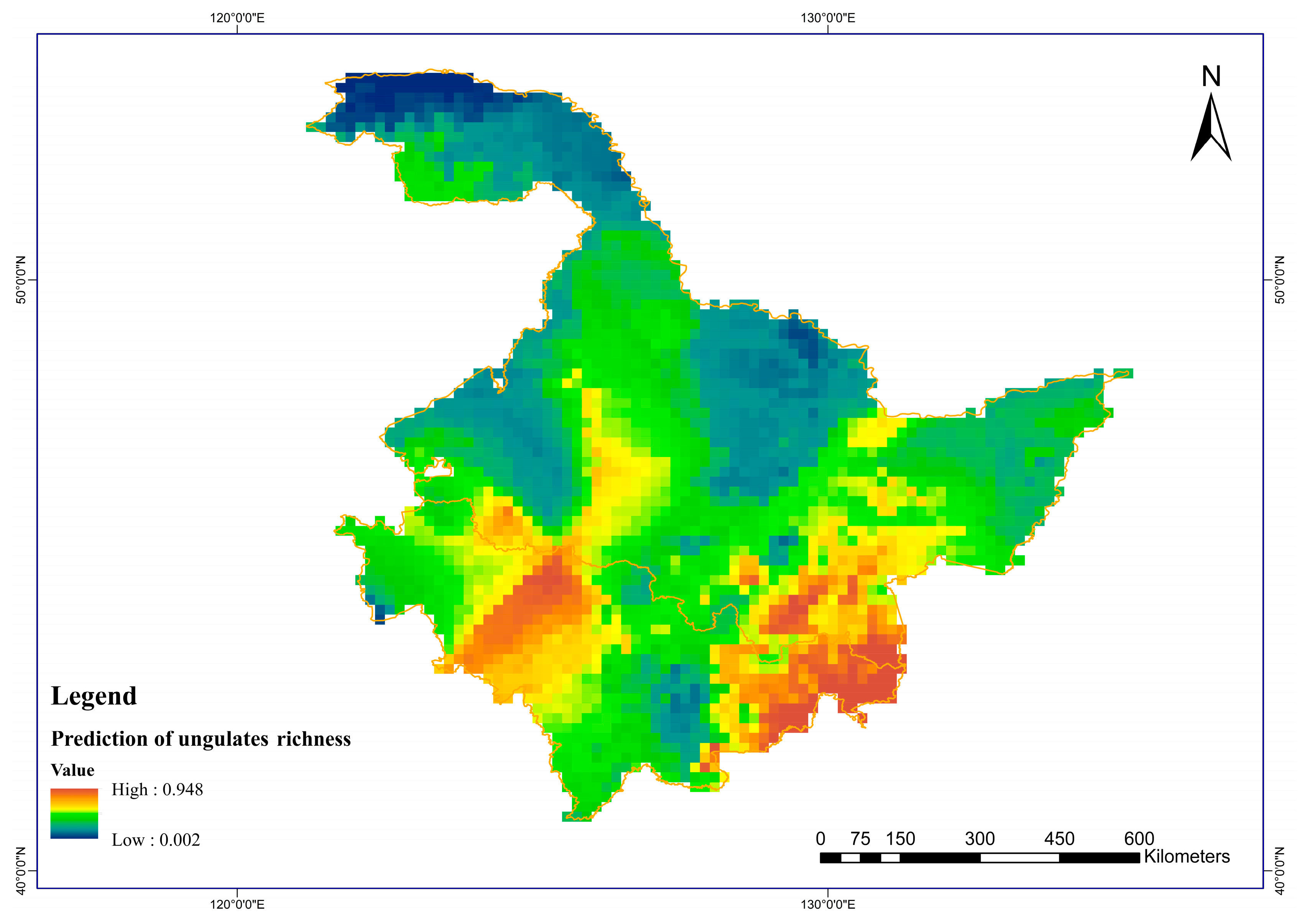
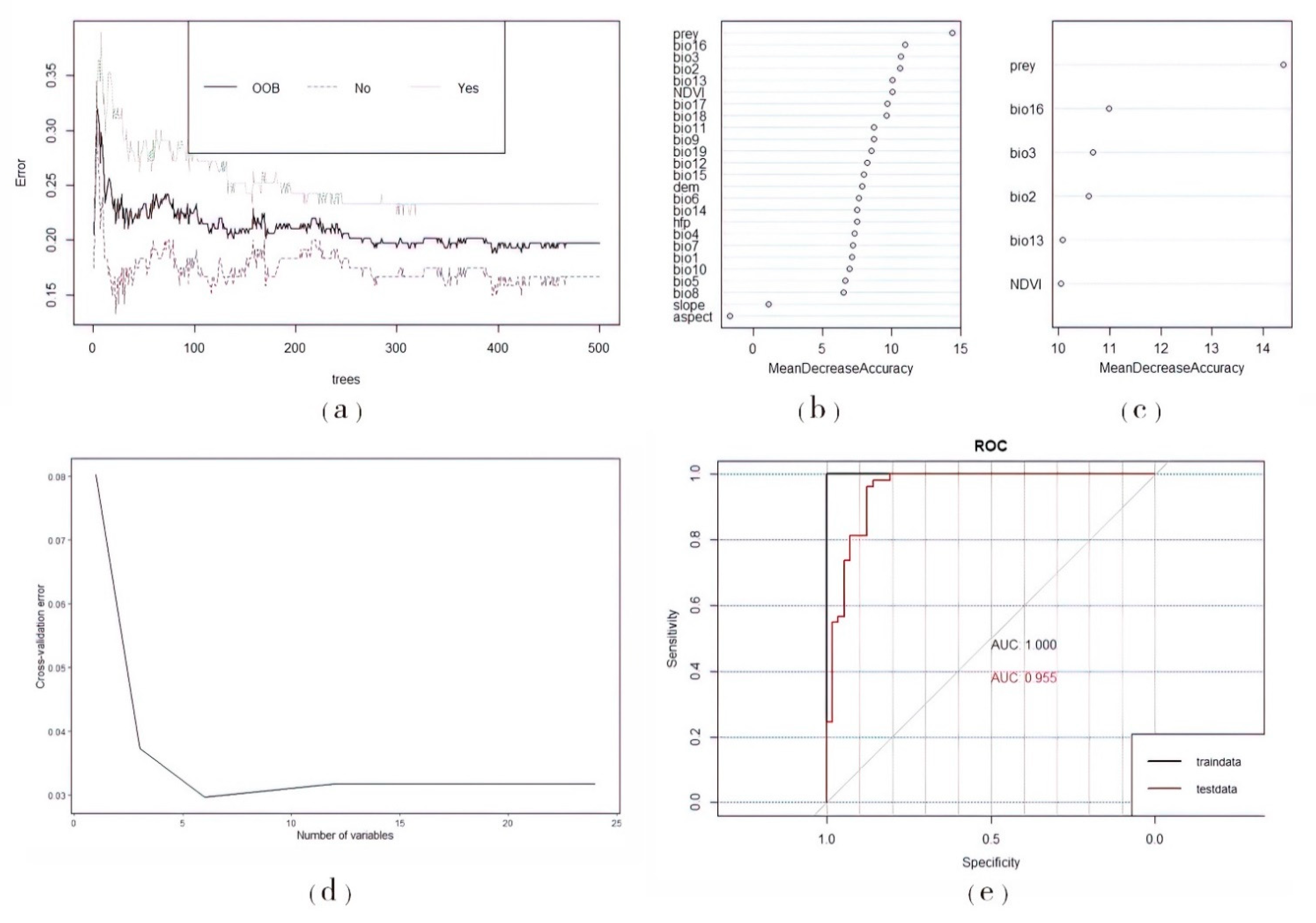
3.1. Prediction of Prey Potential Richness in Amur Tigers
3.2. Prediction of Suitable Habitats for Amur Tigers
3.3. Comparison of Potential Habitat Characteristics between Amur Tigers and Their Prey
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delibes, M.; Gaona, P.; Ferreras, P.; Doak, D.F. Effects of an Attractive Sink Leading into Maladaptive Habitat Selection. Am. Nat. 2001, 158, 277. [Google Scholar] [CrossRef] [PubMed]
- Zexu, L. Research on Habitat Change, Core Habitat and Corridor Identification of Amur Tiger; Northeast Forestry University: Harbin, China, 2021. (In Chinese) [Google Scholar]
- Miquelle, D.G.; Merrill, W.T.; Dunishenko, Y.M.; Smirnov, E.N.; Quigley, H.B.; Pikunov, D.G.; Hornocker, M.G. A habitat protection plan for the Amur tiger: Developing political and ecological criteria for a viable land use plan. In Riding the Tiger: Tiger Conservation in Human Dominated Landscapes; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Zhang, C.; Zhang, M.; Stott, P. Does prey density limit Amur tiger Panthera tigris altaica recovery in northeastern China? Wildl. Biol. 2013, 19, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Hayward, M.W.; Jedrzejewski, W.; Jedrzewska, B. Prey preferences of the tiger Panthera tigris. J. Zool. 2012, 286, 221–231. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, J.; Kou, X.; Li, Z.; Wang, T.; Mou, P.; Ge, J. Spatiotemporal pattern and major causes of the Amur tiger population dynamics. Biodivers. Sci. 2009, 17, 211–225. [Google Scholar]
- Miquelle, D.G.; Smirnov, E.N.; Quigley, H.G.; Hornocker, M.G.; Nikolaev, I.G.; Matyushkin, E.N. Food habits of Amur tigers in Sikhote-Alin Zapovednik and the Russian Far East, and implications for conservation. J. Wildl. Res. 1996, 1, 138–147. [Google Scholar]
- Zhang, C.; Zhang, M.; Jiang, G. Effectiveness evaluation of monitoring methods for Wild Amur tigers in China. Acta Ecol. Sin. 2012, 32, 5943–5952. (In Chinese) [Google Scholar] [CrossRef][Green Version]
- Zhou, S.; Liang, Z.; Jin, G.; Zhang, Z. Population size and distribution of sika deer in Heilongjiang Laoyeling Amur tiger National Nature Reserve. For. Sci. Technol. 2018, 43, 1–3, 64. (In Chinese) [Google Scholar]
- Li, Z.; Kang, A.; Lang, J.; Xuan, Y.; Ren, Y.; Zhu, Z.; Ma, J.; Liu, P.; Jiang, G. Discuss the population assessment method of large cats and their prey based on infrared camera technology. Biodiversity 2014, 22, 725–732. (In Chinese) [Google Scholar]
- Kolipaka, S.S.; Tamis WL, M.; Van ‘t Zelfde, M.; Persoon, G.A.; De Iongh, H.H. Wild versus domestic prey in the diet of reintroduced tigers (Panthera tigris) in the livestock-dominated multiple-use forests of Panna Tiger Reserve, India. PLoS ONE 2017, 12, e0174844. [Google Scholar] [CrossRef]
- Ivan, J.S.; White, G.C.; Shenk, T.M. Using simulation to compare models for estimating density from capture-recapture data. Ecology 2013, 94, 817–826. [Google Scholar] [CrossRef]
- Luskin, M.S.; Albert, W.R.; Tobler, M.W. Sumatran tiger survival threatened by deforestation despite increasing densities in parks. Nat. Commun. 2017, 8, 1783. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F.l.; Franklin, J. Bioclim: The first species distribution modelling package, its early applications and relevance to most current MaxEnt studies. Divers. Distrib. 2014, 20, 12144. [Google Scholar] [CrossRef]
- Austin, M. Species distribution models and ecological theory: A critical assessment and some possible new approaches. Ecol. Model. 2017, 200, 1–19. [Google Scholar] [CrossRef]
- Elith, J.; HGraham, C.; PAnderson, R.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2010, 29, 129–151. [Google Scholar] [CrossRef]
- Rahman, D.A.; Santosa, Y.; Purnamasari, I.; Condro, A.A. Drivers of Three Most Charismatic Mammalian Species Distribution across a Multiple-Use Tropical Forest Landscape of Sumatra, Indonesia. Animals 2022, 12, 2722. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.S.; Murphy, M.A.; Holden, Z.A.; Cushman, S.A. Modeling Species Distribution and Change Using Random Forest. In Predictive Species and Habitat Modeling in Landscape Ecology; Drew, C., Wiersma, Y., Huettmann, F., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Testing whether ensemble modeling is advantageous for maximizing predictive performance of species distribution models. Ecography 2020, 43, 549–558. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Zhao, S.; Lu, M.; Zhang, Y.; Wang, C.; Li, C. Application progress of machine learning in mosquito and mosquito borne infectious diseases. Chin. J. Vector Biol. Control. 2021, 32, 503–508. (In Chinese) [Google Scholar]
- Zhang, Y.; Yu, Z.; Luan, J.; Wang, Y.; Ye, X.; Liu, S. Spatio temporal variation characteristics of vegetation greenness in the northeast forest belt from 1982 to 2020. Acta Ecol. Sin. 2023, 1–12. (In Chinese) [Google Scholar]
- Støen, O.G.; Wegge, P. Prey selection and prey removal by tiger (Panthera tigris) during the dry season in lowland Nepal. Mammalia 1996, 60, 363–374. [Google Scholar] [CrossRef]
- Ge, D.; Wen, Z.; Cheng, J.; Xia, L.; Yang, Q. Using R software to download and screen species distribution records on GBIF website and extract climate data for analysis. Bio-Protoc. 2021, 1010609. [Google Scholar] [CrossRef]
- Zizka, A.; Carvalho, F.A.; Calvente, A.; Baez-Lizarazo, M.R.; Cabral, A.; Coelho, J.F.R.; Colli-Silva, M.; Fantinati, M.R.; Fernandes, M.F.; Ferreira-Araújo, T.; et al. No one-size-fits-all solution to clean GBIF. Cold Spring Harb. Lab. 2020, 8, e9916. [Google Scholar] [CrossRef]
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Duarte Ritter, C.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R.; et al. Coordinate Cleaner: Standardized cleaning of occurrence records from biological collection databases. Methods Ecol. Evol. 2019, 10, 744–751. [Google Scholar] [CrossRef]
- Breiman, L. Random forests, machine learning 45. J. Clin. Microbiol. 2001, 2, 199–228. [Google Scholar]
- Lozier, J.D.; Hickerson, P.A.J. Predicting the distribution of sasquatch in western north america: Anything goes with ecological niche modelling. J. Biogeogr. 2010, 36, 1623–1627. [Google Scholar] [CrossRef]
- Mu, H.; Li, X.; Wen, Y.; Huang, J.; Du, P.; Su, W.; Miao, S.; Geng, M. A global record of annual terrestrial Human Footprint dataset from 2000 to 2018. Sci. Data 2022, 9, 176. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S. Review of classifier evaluation and design based on AUC. Pattern Recognit. Artif. Intell. 2011, 24, 64–71. (In Chinese) [Google Scholar]
- Swets, J. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2010, 43, 1223–1232. [Google Scholar] [CrossRef]
- Kampichler, C.; Wieland, R.; Calmé, S.; Weissenberger, H.; Arriaga-Weiss, S. Classification in conservation biology: A comparison of five machine-learning methods. Ecol. Inform. 2010, 5, 441–450. (In Chinese) [Google Scholar] [CrossRef]
- Sun, H.; Lu, X.; Wu, J.; Yi, Y.; Li, L.; Zhou, S.; Yu, H.; Lu, Y. Investigation of the ecological corridor of Amur tiger and its protection and restoration planning. For. Sci. Technol. 2015, 6, 46–51. [Google Scholar]
- Liu, Y.; Yu, H.; Liu, D.; Zhu, L. Spatial differentiation mechanism of development intensity pattern evolution of construction land in Northeast China. Acta Geogr. Sin. 2018, 73, 818–831. (In Chinese) [Google Scholar]
- Guo, S. Stand structure and restoration of broad-leaved Korean pine forest in Xiaoxing’an Mountains. For. Eng. 2014, 30, 8–12. (In Chinese) [Google Scholar]
- Liu, S.; Duan, W.; Feng, J.; Han, S. Effects of forest gaps on tree species regeneration and species diversity of broad-leaved Korean pine forest in Xiaoxing’an Mountains. J. Appl. Ecol. 2011, 22, 8. (In Chinese) [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Hong, Y.; Sun, S.; Zhang, N.; Liu, X.; Wang, Z.; Zhou, S.; Zhang, M. An Evaluation of Suitable Habitats for Amur Tigers (Panthera tigris altaica) in Northeastern China Based on the Random Forest Model. Biology 2023, 12, 1444. https://doi.org/10.3390/biology12111444
Gao C, Hong Y, Sun S, Zhang N, Liu X, Wang Z, Zhou S, Zhang M. An Evaluation of Suitable Habitats for Amur Tigers (Panthera tigris altaica) in Northeastern China Based on the Random Forest Model. Biology. 2023; 12(11):1444. https://doi.org/10.3390/biology12111444
Chicago/Turabian StyleGao, Chunyu, Yang Hong, Shiquan Sun, Ning Zhang, Xinxin Liu, Zheyu Wang, Shaochun Zhou, and Minghai Zhang. 2023. "An Evaluation of Suitable Habitats for Amur Tigers (Panthera tigris altaica) in Northeastern China Based on the Random Forest Model" Biology 12, no. 11: 1444. https://doi.org/10.3390/biology12111444
APA StyleGao, C., Hong, Y., Sun, S., Zhang, N., Liu, X., Wang, Z., Zhou, S., & Zhang, M. (2023). An Evaluation of Suitable Habitats for Amur Tigers (Panthera tigris altaica) in Northeastern China Based on the Random Forest Model. Biology, 12(11), 1444. https://doi.org/10.3390/biology12111444




