Comparative Effect of Three Different Exercise Intensities in Combination with Diazoxide on Contraction Capacity and Oxidative Stress of Skeletal Muscle in Obese Rats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Groups
2.2. Monitoring of Body Weight and Blood Glucose Levels
2.3. Muscles Dissection
2.4. Isometric Tension Measurements
2.5. Reactive Oxygen Species Analysis
2.6. Lipid Peroxidation Measurement
2.7. Determination of Glutathione Status Redox
2.8. Data Analysis
3. Results
3.1. Effect of Different Exercise Intensities and Diazoxide on Weight, Glucose, and Visceral and Perigonadal Fat in Obese Rats
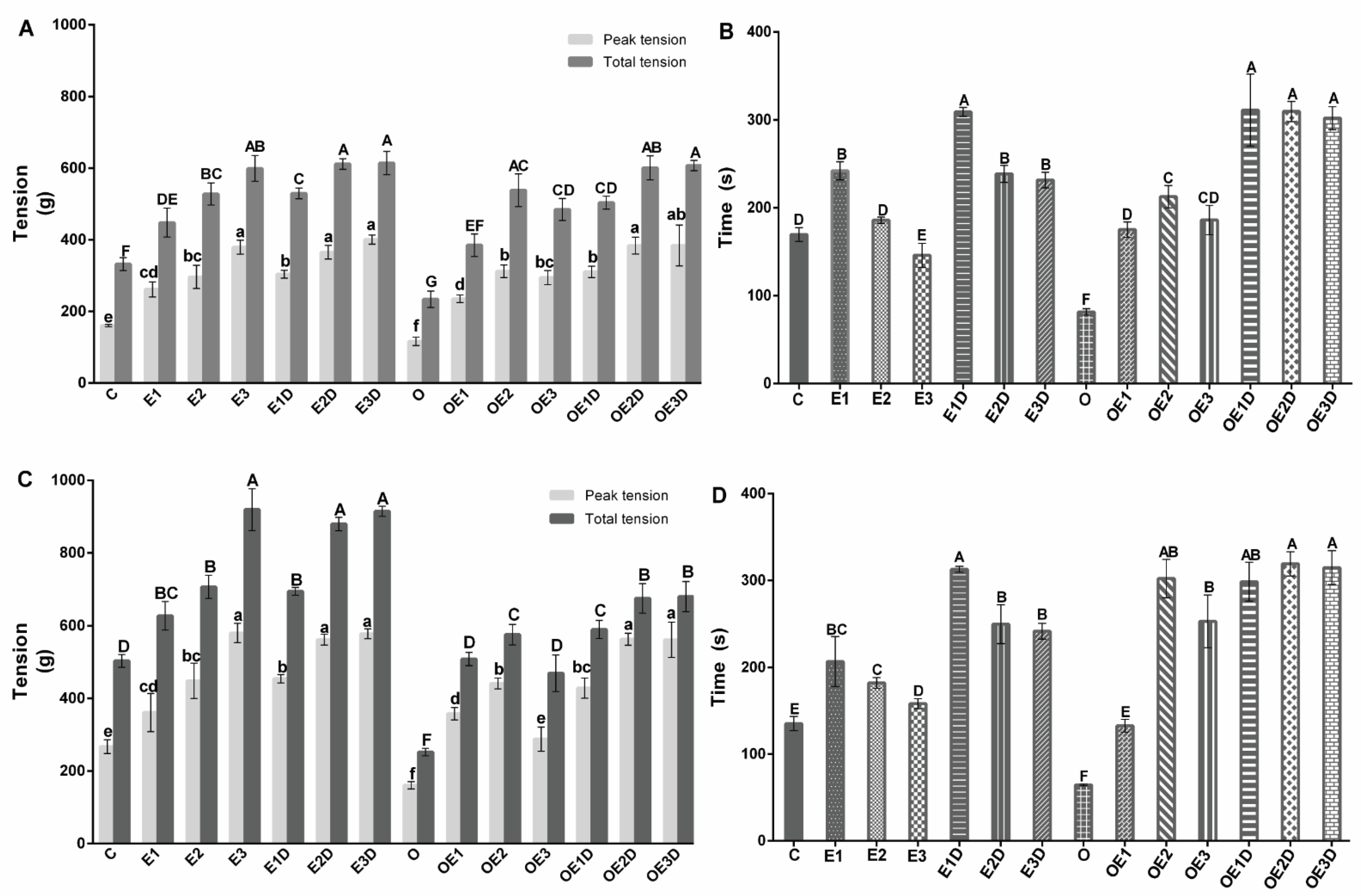
3.2. Different Exercise Intensities and Diazoxide Enhance Contraction and Promote Fatigue Resistance of Skeletal Muscle in Obese Rats
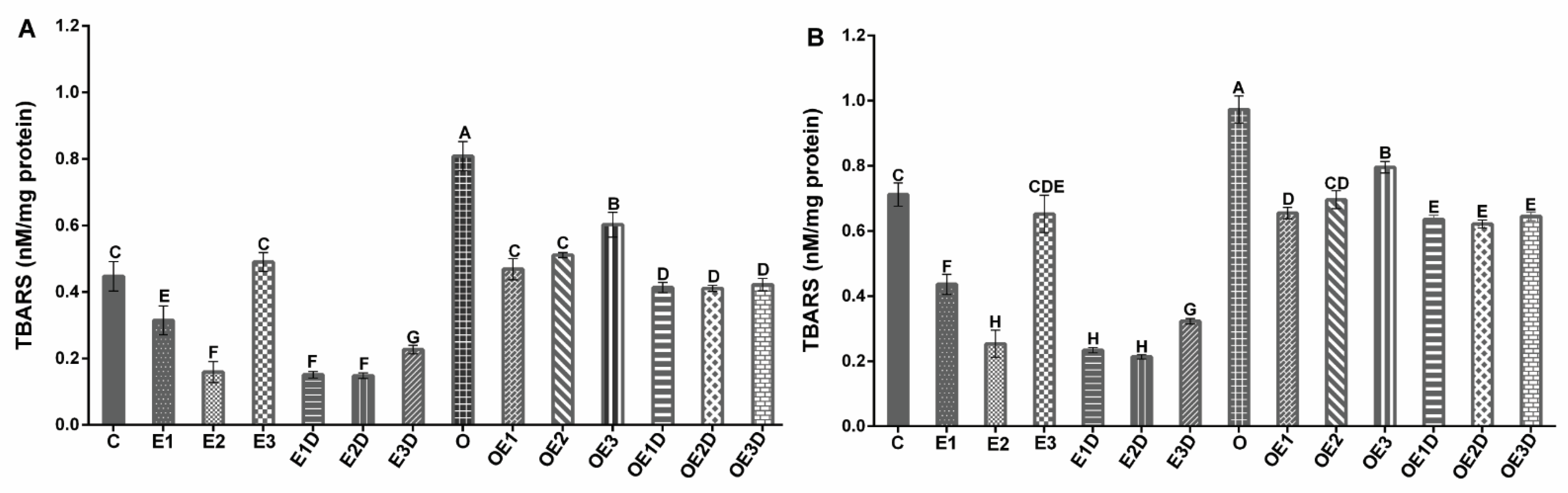
3.3. Different Intensities of Exercise and Diazoxide Decrease Oxidative Stress and Improve Antioxidant Defense in Skeletal Muscle of Obese Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erskine, R.M.; Tomlinson, D.J.; Morse, C.I.; Winwood, K.; Hampson, P.; Lord, J.M.; Onambélé, G.L. The individual and combined effects of obesity- and aging-induced systemic inflammation on human skeletal muscle properties. Int. J. Obes. 2017, 41, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.W.; Yoo, S.Z.; No, M.H.; Park, D.H.; Kang, J.H.; Kim, T.W.; Kim, C.J.; Seo, D.Y.; Han, J.; Yoon, J.H.; et al. Exercise Training Attenuates Obesity-Induced Skeletal Muscle Remodeling and Mitochondria-Mediated Apoptosis in the Skeletal Muscle. Int. J. Environ. Res. Public Health 2018, 15, 2301. [Google Scholar] [CrossRef]
- Bae, J.Y. Resistance Exercise Regulates Hepatic Lipolytic Factors as Effective as Aerobic Exercise in Obese Mice. Int. J. Environ. Res. Public Health 2020, 17, 8307. [Google Scholar] [CrossRef]
- Abrigo, J.; Rivera, J.C.; Aravena, J.; Cabrera, D.; Simon, F.; Ezquer, F.; Ezquer, M.; Cabello-Verrugio, C. High Fat Diet-Induced Skeletal Muscle Wasting Is Decreased by Mesenchymal Stem Cells Administration: Implications on Oxidative Stress, Ubiquitin Proteasome Pathway Activation, and Myonuclear Apoptosis. Oxid. Med. Cell Longev. 2016, 2016, 9047821. [Google Scholar] [CrossRef]
- Martinez-Huenchullan, S.F.; Ban, L.A.; Olaya-Agudo, L.F.; Maharjan, B.R.; Williams, P.F.; Tam, C.S.; Mclennan, S.V.; Twigg, S.M. Constant-Moderate and High-Intensity Interval Training Have Differential Benefits on Insulin Sensitive Tissues in High-Fat Fed Mice. Front Physiol. 2019, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Faßhauer, M.; Stumvoll, M.; et al. Inflammatory cytokines in general and central obesity and modulating ef-fects of physical activity. PLoS ONE 2015, 10, e0121971. [Google Scholar] [CrossRef]
- Small, L.; Brandon, A.E.; Parker, B.L.; Deshpande, V.; Samsudeen, A.F.; Kowalski, G.M.; Reznick, J.; Wilks, D.L.; Preston, E.; Bruce, C.R.; et al. Reduced insulin action in muscle of high fat diet rats over the diurnal cycle is not associated with defective insulin signaling. Mol Metab. 2019, 25, 107–118. [Google Scholar] [CrossRef]
- Espinosa, A.; Henríquez-Olguín, C.; Jaimovich, E. Reactive oxygen species and calcium signals in skeletal muscle: A crosstalk involved in both normal signaling and disease. Cell Calcium 2016, 60, 172–179. [Google Scholar] [CrossRef]
- Choi, S.J.; Files, D.C.; Zhang, T.; Wang, Z.M.; Messi, M.L.; Gregory, H.; Stone, J.; Lyles, M.F.; Dhar, S.; Marsh, A.P.; et al. Intramyocellular Lipid and Impaired Myofiber Contraction in Normal Weight and Obese Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 557–564. [Google Scholar] [CrossRef]
- Heo, J.W.; No, M.H.; Park, D.H.; Kang, J.H.; Seo, D.Y.; Han, J.; Neufer, P.D.; Kwak, H.B. Effects of exercise on obesity-induced mitochondrial dysfunction in skeletal muscle. Korean J. Physiol. Pharmacol. 2017, 21, 567–577. [Google Scholar] [CrossRef]
- Lipina, C.; Hundal, H.S. Lipid modulation of skeletal muscle mass and function. J. Cachexia Sarcopenia Muscle 2017, 8, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Barroso, M.; Moreno-Calderón, K.M.; Sánchez-Duarte, E.; Cortés-Rojo, C.; Saavedra-Molina, A.; Rodríguez-Orozco, A.R.; Montoya-Pérez, R. Diazoxide and Exercise Enhance Muscle Contraction during Obesity by Decreasing ROS Levels, Lipid Peroxidation, and Improving Glutathione Redox Status. Antioxidants 2020, 9, 1232. [Google Scholar] [CrossRef]
- Alemzadeh, R.; Karlstad, M.D.; Tushaus, K.; Buchholz, M. Diazoxide enhances basal metabolic rate and fat oxidation in obese Zucker rats. Metabolism 2008, 57, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Bischof, J.M.; Wevrick, R. Chronic diazoxide treatment decreases fat mass and improves endurance capacity in an obese mouse model of Prader-Willi syndrome. Mol. Genet. Metab. 2018, 123, 511–517. [Google Scholar] [CrossRef]
- Loves, S.; van Groningen, L.; Filius, M.; Mekking, M.; Brandon, T.; Tack, C.J.; Hermus, A.; de Boer, H. Effects of Diazoxide-Mediated Insulin Suppression on Glucose and Lipid Metabolism in Nondiabetic Obese Men. J. Clin. Endocrinol. Metab. 2018, 103, 2346–2353. [Google Scholar] [CrossRef]
- Martinez-Huenchullan, S.F.; Maharjan, B.R.; Williams, P.F.; Tam, C.S.; Mclennan, S.V.; Twigg, S.M. Differential metabolic effects of constant moderate versus high intensity interval training in high-fat fed mice: Possible role of muscle adiponectin. Physiol. Rep. 2018, 6, e13599. [Google Scholar] [CrossRef]
- Botezelli, J.D.; Cambri, L.T.; Ghezzi, A.C.; Dalia, R.A.; MScariot, P.P.; Ribeiro, C.; Voltarelli, F.A.; Mello, M.A. Different exercise protocols improve metabolic syndrome markers, tissue triglycerides content and antioxidant status in rats. Diabetol. Metab. Syndr. 2011, 3, 35. [Google Scholar] [CrossRef]
- Cartee, G.D.; Arias, E.B.; Yu, C.S.; Pataky, M.W. Novel single skeletal muscle fiber analysis reveals a fiber type-selective effect of acute exercise on glucose uptake. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E818–E824. [Google Scholar] [CrossRef]
- Effting, P.S.; Brescianini, S.M.S.; Sorato, H.R.; Fernandes, B.B.; Fidelis, G.D.S.P.; Silva, P.R.L.D.; Silveira, P.C.L.; Nesi, R.T.; Ceddia, R.B.; Pinho, R.A. Resistance Exercise Modulates Oxidative Stress Parameters and TNF-α Content in the Heart of Mice with Diet-Induced Obesity. Arq. Bras. Cardiol. 2019, 112, 545–552. [Google Scholar] [CrossRef]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef]
- Botezelli, J.D.; Coope, A.; Ghezzi, A.C.; Cambri, L.T.; Moura, L.P.; Scariot, P.P.; Gaspar, R.S.; Mekary, R.A.; Ropelle, E.R.; Pauli, J.R. Strength Training Prevents Hyperinsulinemia, Insulin Resistance, and Inflammation Independent of Weight Loss in Fructose-Fed Animals. Sci. Rep. 2016, 6, 31106. [Google Scholar] [CrossRef]
- Hannan, A.L.; Hing, W.; Simas, V.; Climstein, M.; Coombes, J.S.; Jayasinghe, R.; Byrnes, J.; Furness, J. High-intensity interval training versus moderate-intensity continuous training within cardiac rehabilitation: A systematic review and meta-analysis. Open Access J. Sports Med. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Wang, L.; Lavier, J.; Hua, W.; Wang, Y.; Gong, L.; Wei, H.; Wang, J.; Pellegrin, M.; Millet, G.P.; Zhang, Y. High-Intensity Interval Training and Moderate-Intensity Continuous Training Attenuate Oxidative Damage and Promote Myokine Response in the Skeletal Muscle of ApoE KO Mice on High-Fat Diet. Antioxidants 2021, 10, 992. [Google Scholar] [CrossRef] [PubMed]
- Moghtadaei, M.; Habibey, R.; Ajami, M.; Soleimani, M.; Ebrahimi, S.A.; Pazoki-Toroudi, H. Skeletal muscle post-conditioning by diazoxide, anti-oxidative and anti-apoptotic mechanisms. Mol. Biol. Rep. 2012, 39, 11093–11103. [Google Scholar] [CrossRef] [PubMed]
- Gornall, A.G.; Baradawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751. [Google Scholar] [CrossRef]
- Ortiz-Avila, O.; Sámano-García, C.A.; Calderón-Cortés, E.; Pérez-Hernández, I.H.; Mejía-Zepeda, R.; Rodríguez-Orozco, A.R.; Saa-vedra-Molina, A.; Cortés-Rojo, C. Dietary avocado oil supplementation attenuates the alterations induced by type I diabetes and oxidative stress in electron transfer at the complex II-complex III segment of the electron transport chain in rat kidney mitochondria. J. Bioenerg. Biomembr. 2013, 45, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Meth. Enzymol. 1978, 52, 302–310. [Google Scholar]
- Peña-Montes, D.J.; Huerta-Cervantes, M.; Ríos-Silva, M.; Trujillo, X.; Cortés-Rojo, C.; Huerta, M.; Saavedra-Molina, A. Effects of dietary iron restriction on kidney mitochondria function and oxidative stress in streptozotocin-diabetic rats. Mitochondrion 2020, 54, 41–48. [Google Scholar] [CrossRef]
- Pompeani, N.; Rybalka, E.; Latchman, H.; Murphy, R.M.; Croft, K.; Hayes, A. Skeletal muscle atrophy in sedentary Zucker obese rats is not caused by calpain-mediated muscle damage or lipid peroxidation induced by oxidative stress. J. Negat. Results Biomed. 2014, 13, 19. [Google Scholar] [CrossRef][Green Version]
- Lu, Y.; Li, H.; Shen, S.W.; Shen, Z.H.; Xu, M.; Yang, C.J.; Li, F.; Feng, Y.B.; Yun, J.T.; Wang, L.; et al. Swimming exercise increases serum irisin level and reduces body fat mass in high-fat-diet fed Wistar rats. Lipids Health Dis. 2016, 15, 93. [Google Scholar] [CrossRef]
- Hong, S.H.; Choi, K.M. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int. J. Mol. Sci. 2020, 21, 494. [Google Scholar] [CrossRef]
- Liubaoerjijin, Y.; Terada, T.; Fletcher, K.; Boulé, N.G. Effect of aerobic exercise intensity on glycemic control in type 2 diabetes: A meta-analysis of head-to-head randomized trials. Acta Diabetol. 2016, 53, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Chicco, A.J.; Jew, K.N.; Johnson, M.S.; Lynch, J.M.; Watson, P.A.; Moore, R.L. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J. Physiol. 2005, 569 Pt 3, 913–924. [Google Scholar] [CrossRef]
- Andrukhiv, A.; Costa, A.D.; West, I.C.; Garlid, K.D. Opening mitoKATP increases superoxide generation from complex I of the electron transport chain. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2067–H2074. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.D.; Quinlan, C.L.; Andrukhiv, A.; West, I.C.; Jabůrek, M.; Garlid, K.D. The direct physiological effects of mitoKATP opening on heart mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H406–H415. [Google Scholar] [CrossRef] [PubMed]
- García, M.C.; Hernández, A.; Sánchez, J.A. Role of mitochondrial ATP-sensitive potassium channels on fatigue in mouse muscle fibers. Biochem. Biophys. Res. Commun. 2009, 385, 28–32. [Google Scholar] [CrossRef]
- Sánchez-Duarte, E.; Trujillo, X.; Huerta, M.; Ortiz-Mesina, M.; Cortés-Rojo, C.; Manzo-Ávalos, S.; Saavedra-Molina, A.; Mon-toya-Pérez, R. Mitochondrial KATP channels in skeletal muscle: Are protein kinases C and G, and nitric oxide synthase in-volved in the fatigue process? Open Access Animal Physiol. 2012, 4, 21–28. [Google Scholar]
- Powers, S.K.; Bomkamp, M.; Ozdemir, M.; Hyatt, H. Mechanisms of exercise-induced preconditioning in skeletal muscles. Redox Biol. 2020, 35, 101462. [Google Scholar] [CrossRef]
- Persson, M.; Steinz, M.M.; Westerblad, H.; Lanner, J.T.; Rassier, D.E. Force generated by myosin cross-bridges is reduced in myofibrils exposed to ROS/RNS. Am. J. Physiol. Cell Physiol. 2019, 317, C1304–C1312. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B. Free radicals and muscle fatigue: Of ROS, canaries, and the IOC. Free Radic. Biol. Med. 2008, 44, 169–179. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar] [CrossRef] [PubMed]
- Westerblad, H.; Bruton, J.D.; Katz, A. Skeletal muscle: Energy metabolism, fiber types, fatigue and adaptability. Exp. Cell Res. 2010, 316, 3093–3099. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.M.; Da Silva-Grigoletto, M.E.; Túnez-Fiñana, I. Estrés oxidativo inducido por el ejercicio. Rev. Andal Med. Deporte 2009, 2, 19–34. [Google Scholar]
- Ji, L.L. Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free Radic. Biol. Med. 2008, 44, 142–152. [Google Scholar] [CrossRef]
- Lambertucci, R.H.; Levada-Pires, A.C.; Rossoni, L.V.; Curi, R.; Pithon-Curi, T.C. Effects of aerobic exercise training on antioxidant enzyme activities and mRNA levels in soleus muscle from young and aged rats. Mech. Ageing Dev. 2007, 128, 267–275. [Google Scholar] [CrossRef]
- Beckendorf, L.; Linke, W.A. Emerging importance of oxidative stress in regulating striated muscle elasticity. J. Muscle Res. Cell Motil. 2015, 36, 25–36. [Google Scholar] [CrossRef]
- Nonaka, K.; Une, S.; Tatsuta, N.; Ito, K.; Akiyama, J. Changes in antioxidant enzymes and lipid peroxidation in extensor digitorum longus muscles of streptozotocin-diabetic rats may contribute to muscle atrophy. Acta Physiol. Hung. 2014, 101, 421–428. [Google Scholar] [CrossRef]
- Estepa, V.; Ródenas, S.; Martín, M.C. Optimización de un método para la determinación de la peroxidación lipídica en suero humano. Anal. Real Acad. Farm. 2001, 67, 1–17. [Google Scholar]
- Kim, H.K.; Ando, K.; Tabata, H.; Konishi, M.; Takahashi, M.; Nishimaki, M.; Xiang, M.; Sakamoto, S. Effects of Different Intensities of Endurance Exercise in Morning and Evening on the Lipid Metabolism Response. J. Sports Sci. Med. 2016, 15, 467–476. [Google Scholar]
- Marotte, C.; Zeni, S.N. Reactive oxygen species on bone cells activity. Acta Bioquím. Clín. Latinoam. 2013, 47, 661–674. [Google Scholar]
- Theofilidis, G.; Bogdanis, G.C.; Koutedakis, Y.; Karatzaferi, C. Monitoring Exercise-Induced Muscle Fatigue and Adaptations: Making Sense of Popular or Emerging Indices and Biomarkers. Sports 2018, 6, 153. [Google Scholar] [CrossRef] [PubMed]


| Groups | Diet | Diazoxide 35 mg/kg | Exercise |
|---|---|---|---|
| Control (C) | Standard rodent chow® | no | no |
| Diazoxide (D) | Standard rodent chow® | yes | no |
| Low-intensity exercise (E1) | Standard rodent chow® | no | yes |
| medium intensity exercise (E2) | Standard rodent chow® | no | yes |
| High-intensity exercise (E3) | Standard rodent chow® | no | yes |
| Low-intensity exercise diazoxide (E1D) | Standard rodent chow® | yes | yes |
| Medium intensity exercise diazoxide (E2D) | Standard rodent chow® | yes | yes |
| High-intensity exercise diazoxide (E3D) | Standard rodent chow® | yes | yes |
| Obese (O) | High-fat diet | no | no |
| Obese diazoxide (OD) | High-fat diet | yes | no |
| Obese low-intensity exercise (OE1) | High-fat diet | no | yes |
| Obese medium intensity exercise di (OE2) | High-fat diet | no | yes |
| Obese low-intensity exercise diazoxide (OE3) | High-fat diet | no | yes |
| Obese low-intensity exercise diazoxide (OE1D) | High-fat diet | yes | yes |
| Obese medium-intensity exercise diazoxide (OE2D) | High-fat diet | yes | yes |
| Obese low-intensity exercise diazoxide (OE3D) | High-fat diet | yes | yes |
| Groups | E1 | E2 | E3 |
|---|---|---|---|
| Week 1 | 10 m/min/10 min | 10 m/min/10 min | 10 m/min/15 min |
| Week 2 | 10 m/min/10 min | 10 m/min/15 min | 10 m/min/20 min |
| Week 3 | 10 m/min/10 min | 10 m/min/15 min 17 m/min/5 min | 17 m/min/15 min |
| Week 4 | 10 m/min/15 min | 10 m/min/15 min 17 m/min/10 min | 17 m/min/20 min |
| Week 5 | 10 m/min/15 min | 10 m/min/15 min 17 m/min/10 min 22 m/min/5 min | 17 m/min/10 min 22 m/min/10 min |
| Week 6 | 10 m/min/20 min | 10 m/min/15 min 17 m/min/10 min 22 m/min/5 min | 17 m/min/10 min 22 m/min/10 min |
| Week 7 | 10 m/min/15 min 17 m/min/5 min | 10 m/min/20 min 17 m/min/15 min 22 m/min/5 min | 17 m/min/5 min 22 m/min/20 min |
| Week 8 | 10 m/min/15 min 17 m/min/10 min | 10 m/min/20 min 17 m/min/15 min 22 m/min/5 min | 17 m/min/10 min 22 m/min/25 min |
| Groups | Bodyweight (g) | Glucose (mg/dL) | Viceral and Perigonadal Fat (g) |
|---|---|---|---|
| C | 408.245 ± 18.18 D | 73.83 ± 6.04 E | 11.07 ± 6.04 D |
| D | 413.5 ± 15.23 D | 86.66 ± 3.66 C | 9.84 ± 2.84 D |
| E1 | 425.33 ± 39.62 CD | 77 ± 5.09 EF | 6.2 ± 2.24 E |
| E2 | 435.8 ± 15.75 CD | 69.2 ± 4.08 G | 6.28 ± 3.43 E |
| E3 | 417.5 ± 11.18 D | 69 ± 2.73 G | 5.8 ± 2.49 E |
| E1D | 588.83 ± 32.86 A | 111 ± 4.09 A | 43.32 ± 4.38 A |
| E2D | 483.8 ± 23.92 B | 73.83 ± 2.22 E | 18.47 ± 3.33 C |
| E3D | 497 ± 16.68 B | 82.85 ± 6.25 CD | 21.39 ± 4.60 C |
| O | 455.14 ± 40.71 C | 85.71 ± 4.75 C | 21.61 ± 3.79 C |
| OD | 515 ± 67.32 B | 105.16 ± 6.55 B | 30.88 ± 8.14 B |
| OE1 | 441.16 ± 30.43 C | 74.5 ± 2.88 E | 19.16 ± 4.84 C |
| OE2 | 441.75 ± 19.55 C | 68.83 ± 3.54 G | 20.11 ± 5.94 C |
| OE3 | 461.13 ± 17.03 C | 79 ± 2.58D F | 11.25 ± 3.61 D |
| OE1D | 406.4 ± 10.23 D | 81.8 ± 2.38 D | 11.85 ± 2.17 D |
| OE2D | 404.25 ± 7.45 D | 70.5 ± 4.66 G | 10.30 ± 3.12 D |
| OE3D | 402.75 ± 10.56 D | 73.6 ± 3.43 EG | 6.62 ± 1.23 E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Barroso, M.; Vargas-Vargas, M.A.; Peña-Montes, D.J.; Cortés-Rojo, C.; Saavedra-Molina, A.; Sánchez-Duarte, E.; Rodríguez-Orozco, A.R.; Montoya-Pérez, R. Comparative Effect of Three Different Exercise Intensities in Combination with Diazoxide on Contraction Capacity and Oxidative Stress of Skeletal Muscle in Obese Rats. Biology 2022, 11, 1367. https://doi.org/10.3390/biology11091367
Gómez-Barroso M, Vargas-Vargas MA, Peña-Montes DJ, Cortés-Rojo C, Saavedra-Molina A, Sánchez-Duarte E, Rodríguez-Orozco AR, Montoya-Pérez R. Comparative Effect of Three Different Exercise Intensities in Combination with Diazoxide on Contraction Capacity and Oxidative Stress of Skeletal Muscle in Obese Rats. Biology. 2022; 11(9):1367. https://doi.org/10.3390/biology11091367
Chicago/Turabian StyleGómez-Barroso, Mariana, Manuel A. Vargas-Vargas, Donovan J. Peña-Montes, Christian Cortés-Rojo, Alfredo Saavedra-Molina, Elizabeth Sánchez-Duarte, Alain R. Rodríguez-Orozco, and Rocío Montoya-Pérez. 2022. "Comparative Effect of Three Different Exercise Intensities in Combination with Diazoxide on Contraction Capacity and Oxidative Stress of Skeletal Muscle in Obese Rats" Biology 11, no. 9: 1367. https://doi.org/10.3390/biology11091367
APA StyleGómez-Barroso, M., Vargas-Vargas, M. A., Peña-Montes, D. J., Cortés-Rojo, C., Saavedra-Molina, A., Sánchez-Duarte, E., Rodríguez-Orozco, A. R., & Montoya-Pérez, R. (2022). Comparative Effect of Three Different Exercise Intensities in Combination with Diazoxide on Contraction Capacity and Oxidative Stress of Skeletal Muscle in Obese Rats. Biology, 11(9), 1367. https://doi.org/10.3390/biology11091367







