Simple Summary
We used carbon and nitrogen stable isotopes as ecological tracers to investigate isotopic niche overlap between 21 odontocete (toothed whale) species inhabiting neritic, mesopelagic, and bathypelagic waters. Results showed a clear niche separation for the bathypelagic Gray’s beaked whales (Mesoplodon grayi) and sperm whales (Physeter macrocephalus), but high isotopic niche overlap and potential interspecific competition for neritic and mesopelagic species. This study represents the first insights into the coexistence of odontocetes in a biodiverse hotspot and provides a critical baseline for a system already undergoing ecosystem changes via ocean warming and its subsequent effect on prey abundance and distribution.
Abstract
Species occurring in sympatry and relying on similar and limited resources may partition resource use to avoid overlap and interspecific competition. Aotearoa, New Zealand hosts an extraordinarily rich marine megafauna, including 50% of the world’s cetacean species. In this study, we used carbon and nitrogen stable isotopes as ecological tracers to investigate isotopic niche overlap between 21 odontocete (toothed whale) species inhabiting neritic, mesopelagic, and bathypelagic waters. Results showed a clear niche separation for the bathypelagic Gray’s beaked whales (Mesoplodon grayi) and sperm whales (Physeter macrocephalus), but high isotopic niche overlap and potential interspecific competition for neritic and mesopelagic species. For these species, competition could be reduced via temporal or finer-scale spatial segregation or differences in foraging behaviour. This study represents the first insights into the coexistence of odontocetes in a biodiverse hotspot. The data presented here provide a critical baseline to a system already ongoing ecosystem change via ocean warming and subsequent effects on prey abundance and distributions.
Keywords:
diet; dolphins; stable isotopes; nitrogen; carbon; feeding ecology; trophic relationships; SGD14 1. Introduction
An ecological niche is defined as a region in a multi-dimensional space of biotic and abiotic conditions that affect the welfare and viability of a species [1]. These dimensions can generally be divided into three main groups: temporal (i.e., diel foraging patterns or annual migrations), spatial (both horizontal and vertical), and trophic (i.e., trophic level, diet composition). The competitive exclusion principle, also known as the Gause principle or Gause’s law [2], states that two species cannot exist at constant population numbers if they are competing for the same limited resource and thus occupy the same ecological niche. To avoid competitive exclusion, co-occurring species can attain niche differentiation through resource partitioning via any of the three dimensions (temporal, spatial, or trophic) [1,2].
Knowledge of an animal’s diet is fundamental to understanding its habitat requirements, movement, and functional position in the food web [3,4,5]. Such data inform the degree of interactions between different taxa, as well as the grade of dietary specialisation and hence foraging plasticity of a species [6,7,8]. Stable isotope analysis enables an animal’s niche to be quantified using the concept of “isotopic niche” [9], with bulk carbon heavy-to-light isotopic ratios (δ13C) providing information on likely carbon sources relating to feeding habitat [10,11,12], and bulk nitrogen isotopic ratios (δ15N) informing trophic level [13,14] and foraging ecology [15,16,17,18].
Aotearoa, New Zealand is home to an extraordinarily rich marine fauna, including 50% of the world’s cetacean species [19,20]. Of these, 35 are odontocetes (toothed whales), which are apex- and mesopredators that play a crucial part in maintaining ecosystem health [20]. Due to New Zealand’s latitudinal spread and isolated geographical location, these species occupy a range of different habitats, ranging from shallow coastal and shelf areas over deep ocean trenches to subantarctic waters [21,22]. While a degree of inherent spatial segregation between taxa is expected given their different habitat requirements and foraging behaviour, many species generally overlap in their distribution [19]. To minimise interspecific competition, predators occupying the same habitat usually practise resource partitioning by exploiting available prey differently [23,24]. Alternatively, or in some cases, additionally, predators segregate spatially or temporally to avoid competition [25,26]. Besides being home to a diverse cetacean community, New Zealand is also a hotspot for cetacean stranding events, including live single and mass stranding events, as well as dead ‘beachcast’ specimens that wash ashore [27,28,29]. While many of these stranding events involve species that regularly occur in New Zealand’s Economic Exclusion Zone, occasionally even vagrant Antarctic species are recorded on New Zealand shores (New Zealand National Strandings Database, Department of Conservation).
In this study, we investigated the isotopic niche of 21 odontocete species occurring in New Zealand waters using skin samples collected from live-stranded and beach-cast individuals. We hypothesised that species with similar habitat requirements would occupy their own respective isotopic niche, thus avoiding interspecific resource competition.
2. Materials and Methods
2.1. Sample Collection
We collected skin samples originating from live strandings or fresh to mild beach-cast events (herein collectively referred to as ‘stranded’) around the New Zealand coast (41°18′ S, 174°47′ E; Figure 1) between 2010 and 2021. Any carcass believed to be less than 24 h old, as determined by the presence of rigour mortis, the condition of the skin, and the turgor, clarity, and moisture of the eye, was defined as ‘fresh’ (codes 1 & 2 from IJsseldijk, et al. [30]). Carcasses with early stages of visible decomposition, including eyes and skin degradation, were considered ‘mild autolysis/decomposition’ (code 3) [30,31]. Animals were sexed anatomically or genetically (see Appendix A.1 for details). Sampling of both sexes occurred across independent animals only (i.e., individuals deemed to be maternally independent based on species-specific total body length as outlined by Jefferson, et al. [32]). As ontogenetic diet shifts affect isotopic values, we conservatively excluded any animals whose total body length did not fall clearly within the expected range of an independent animal from this study. We systematically sampled skin tissue comprising the complete epidermal layer from stranded carcasses and stored them in 95% ethanol or frozen at −20 °C upon collection [31].
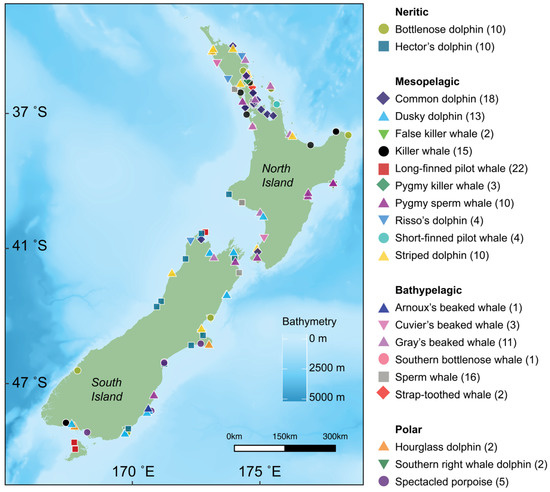
Figure 1.
Locations for 21 species of cetaceans (denoted by shape and colour) sampled in New Zealand between 2010 and 2021. Sample size for each species is denoted in parentheses. Bathymetry is depicted with darker shades of blue representing deeper waters (data from the National Institute of Water and Atmospheric Research (NIWA) under a CC BY license, with permission from NIWA original copyright [33]).
We analysed samples from a total of 21 species, representing a wide diversity of habitats [19], represented by five families (Ziphiidae, Delphinidae, Physeteridae, Kogiidae, and Phocoenidae, Table 1).

Table 1.
Suess-corrected carbon (δ13C) and nitrogen (δ15N) isotope values and sample sizes (n) for independent odontocete species sampled in Aotearoa, New Zealand, 2010–2021. Values are given as mean ± 1 SD. See Figure 2 for an illustration of habitat zones.
2.2. Stable Isotope Analysis
We used δ13C and δ15N data as proxies for habitat and trophic position, respectively. We analysed skin samples from 164 individuals across 21 odontocete species (Table 1, Figure 1). Skin samples stored in ethanol were placed under a fume hood or a stream of nitrogen gas until the ethanol had fully evaporated. Frozen samples were slowly defrosted at room temperature. Using a stainless-steel scalpel, we cut each sample into about 0.2 mm fine slices, equivalent to approximately 10 mg of skin, and then oven dried them for at least 48 h.
We sealed 0.5–1.0 mg of dried homogenised sample into tin capsules, which were subsequently analysed using a DELTA V Plus continuous flow isotope ratio mass spectrometer linked to a Flash 2000 elemental analyser with a MAS 200 R autosampler (Thermo Fisher Scientific, Bremen, Germany), as detailed in Peters, et al. [55]. Repeat analysis of international NIST standards produced data accurate to within or better than 0.15‰ for both δ13C and δ15N, and a precision of better than 0.24‰ for δ13C and 0.22‰ for δ15N.
Isotopic ratios were calculated as:
where Χ is 13C or 15N, and Rsample and Rstandard are the 13C/12C and 15N/14N ratios in the sample and standard, respectively. See Peters, et al. [55] for more details on the analytical protocol.
2.3. Lipid Extraction
Cetacean skin is known to have a high lipid content [16,56,57], which can lead to decreased δ13C values due to the 12C enrichment in the lipids [58]. Several of our bulk isotope sample analyses had a mass ratio of carbon and nitrogen (C:N) > 3.5, indicating lipid content of the tissue and thus lipid “contamination” of the carbon isotope value [59,60,61]. To account for the effect of lipid content on δ13C values, tissue samples need to either be lipid-extracted chemically a priori, or results need to be mathematically corrected a posteriori (normalisation). A combination of these two methods can also be applied by chemically analysing a subset of the samples to then develop a species-specific mathematical correction formula, which has cost-benefits compared to chemical lipid extraction of all samples.
To develop a mathematical normalisation formula specific to our study species, we lipid-extracted a subset of 74 samples across species, selected to cover the range of measured C:N mass ratio values (from 3.0 to 8.6). Freeze-dried material was sub-sampled and wrapped in GF/C filters prior to lipid extraction on a DIONEX 200 accelerated solvent extraction system (ASE). Samples were transferred to 22 mL s/s ASE cells and extracted three times with dichloromethane at 70 °C and 1500 psi for a static hold time of five minutes. All samples were heated to 40 °C in an oven overnight following extraction, to evaporate off any traces of solvent prior to isotope analysis.
We calculated a δ13C lipid-correction factor using averaged bootstrapped linear regression analysis of the δ13C values of the original bulk (non-lipid-extracted) and lipid-extracted samples (see Appendix A.2 for details and correction formulae). Using this factor, we mathematically corrected non-lipid extracted δ13C values for samples with C:N mass ratios > 3.5. For samples with C:N mass ratios < 3.5, we used the original bulk non-lipid corrected δ13C values. As lipid extraction can affect δ15N values [62,63,64], we used the non-lipid extracted δ15N values for all samples.
Since the samples were collected over a span of twelve years (2010–2021), we applied a correction of –0.022‰ year−1 [65] referenced to the year 2021 to carbon isotope values of all samples to account for the “Suess effect” (changes in the δ13C values of atmospheric carbon dioxide (CO2) due to the burning of fossil fuels) [65,66].
2.4. Analysis
We assigned all species to one of four groups based on their most common known distribution and habitat in New Zealand waters see Table 1 in [19]: neritic habitat (bottlenose dolphin Tursiops truncatus, Hector’s dolphin Cephalorhyncus hectori hectori); mesopelagic habitat (common dolphin Delphinus delphis, dusky dolphin Lagenorhynchus obscurus, false killer whale Pseudorca crassidens, killer whale Orcinus orca, long-finned pilot whale Globicephala melas edwardii, pygmy killer whale Feresa attenuata, pygmy sperm whale Kogia breviceps, Risso’s dolphin Grampus griseus, short-finned pilot whale G. macrorhynchus, striped dolphin Stenella coeruleoalba); bathypelagic habitat (Arnoux’s beaked whale Berardius arnuxii, Cuvier’s beaked whale Ziphius cavirostris, Gray’s beaked whale Mesoplodon grayi, southern bottlenose whale Hyperoodon planifrons, sperm whale Physeter macrocephalus, strap-toothed whale Mesoplodon layardii); and polar habitat (hourglass dolphin L. cruciger, southern right whale dolphin Lissodelphis peronii, spectacled porpoise Phocoena dioptrica) (Table 1, and see Figure 2 for illustration of habitat zones). We calculated the mean and standard deviation of δ15N and δ13C values for all species except for Arnoux’s beaked and southern bottlenose whales, for which only one specimen per species was available.
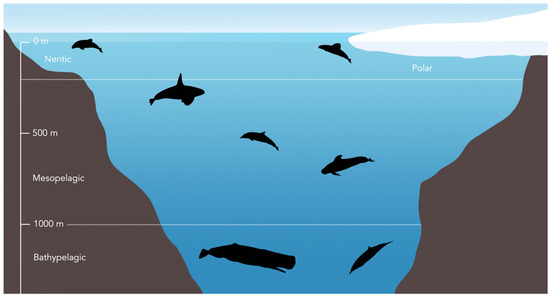
Figure 2.
Habitat zones in which exemplar odontocete species are grouped (see Table 1). Note that not all species included in the study are shown here.
Ten species had n ≥ 10 (Table 1), allowing for statistical analyses. We compared mean δ13C and δ15N values between these species using a two-sample randomisation test with 10,000 permutations at a 0.05 level. This test compares the difference of the mean isotopic values between two species with the difference obtained by randomly allocating the values among the two species [67]. Randomisation tests do not assume normal distribution or homogeneity of variances and hence are an excellent test to use for small sample sizes and biological data [67].
While the remaining 11 species had sample sizes <10, we have included their data in a descriptive sense as, due to the rarity of these samples for most of these cetaceans, their results certainly hold value. For these species, our data were often biased towards males. For most species with n ≥ 10, data were reasonably balanced between sexes, except for sperm whales, which were all male. However, overall sample sizes were too low to investigate sex-specific differences within species.
We used six different Layman metrics (δ13C range, δ15N range, total area (TA), mean distance to centroid (CD), mean nearest neighbour distance (MNND), and standard deviation of nearest neighbour distance (SDNND) to compare isotopic niches between species (see Appendix A.3 for metric definitions). All Layman metrics were bootstrapped with replacement (n = 10,000, indicated with a subscript ‘boot’) based on the smallest sample size in the data set (neritic: n = 10; pelagic: n = 10; bathypelagic: n = 11) to enable statistical comparison between species [67,68]. To further assess niche widths and isotopic niche overlap for each species, we followed a Bayesian approach using multivariate ellipse-based metrics [69]. This method is particularly useful when comparing groups with small sample sizes, as it corrects for the influence of outliers. We calculated standard ellipse areas (SEA), which are the bivariate equivalent to standard deviation in univariate analyses, and further calculated SEA corrected (SEAC) to minimise bias introduced by small sample sizes. In addition, we calculated the Bayesian SEA (SEAB) using 1000 posterior draws to statistically compare niche width between species. The SEAB was used to calculate the niche overlap between species of the same habitat group, which was calculated as the proportion of the total SEAB for each species, respectively. All statistical analyses were done using R version 4.1.0 [70]. We calculated Layman metrics and SEAs with the R package SIBER (Stable Isotope Bayesian Ellipses in R [69]).
3. Results
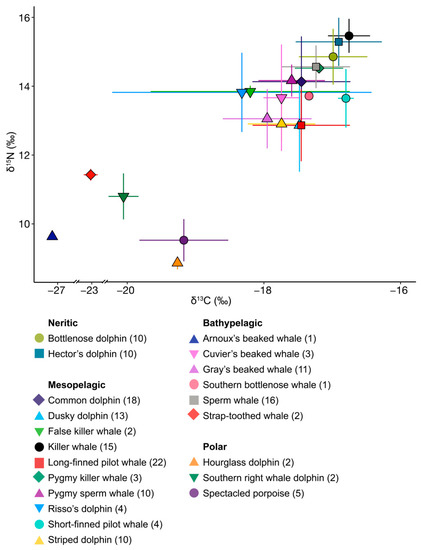
Mean isotopic values for both δ13C and δ15N differed between the 10 species with n ≥ 10 (randomisation test, Table 2 and Table A1, Figure 3), with all species showing differences in either δ13C or δ15N values or both compared to at least seven of the remaining nine species. Of all the pairings, 52.5% (21/40) differed in both δ13C and δ15N values, 7.5% of pairings (3/40) differed only in δ13C values, and 25.0% (10/40) differed only in δ15N values.

Table 2.
Pairwise comparisons (randomisation test) of δ13C and δ15N isotopic values between odontocete species with n ≥ 10 within each habitat group. Colour denotes the level of difference (at 0.05 significance level): dark blue = difference in both δ13C and δ15N isotopic values, light blue: difference only in δ15N isotopic values, white = no difference. Neritic group (yellow): BD = bottlenose dolphin, HD = Hector’s dolphin, mesopelagic group (orange): CD = common dolphin, DD = dusky dolphin, KW = killer whale, LPW = long-finned pilot whale, PSW = pygmy sperm whale, SD = striped dolphin, bathypelagic group (green): GW = Gray’s beaked whale, SBW = southern bottlenose whale, SW = sperm whale. Dark grey indicates matrix diagonal, light grey fields refer to species not in the same habitat. See Table A1 for p-values.
3.1. Neritic Group
Bottlenose and Hector’s dolphins did not differ in their mean isotopic values for either δ13C or δ15N (randomisation test: δ13C p = 0.373, δ15N p = 0.106, Table 2 and Table A1, Figure 3 and Figure A2a). Hector’s dolphins exhibited a larger SEAB compared to bottlenose dolphins. However, the probability for Hector’s dolphins to have higher values for the isotopic metrics considered was low (35.3–68.0%) except for the δ13C range (76.1%, Table 3a). Their SEABs overlapped substantially (bottlenose dolphin = 41.0%, Hector’s dolphin = 34.0%, Table 4, Figure 4a and Figure 5a).

Table 3.
Isotopic niche metrics (including the six Layman metrics) for adult odontocetes with n ≥ 10. SEA = standard ellipse area, SEAC = standard ellipse area corrected for small sample size, SEAB = Bayesian SEA, TA = total area, CD = mean distance to centroid, MNND = mean nearest neighbour distance, SDNND = standard deviation of nearest neighbour distance, see supplementary material for metric definitions. The subscript ‘boot’ indicates that the value (mean) has been generated via bootstrapping. Probability refers to the probability of the respective bootstrapped metric being larger in one species over another across all draws.

Table 4.
Bayesian niche overlap (40%) for each species (left column) with each other species (top row) in their habitat group for species with n ≥ 10. Species abbreviations: Neritic group (yellow): BD = bottlenose dolphin, HD = Hector’s dolphin, mesopelagic group (orange): CD = common dolphin, DD = dusky dolphin, KW = killer whale, LPW = long-finned pilot whale, PSW = pygmy sperm whale, SD = striped dolphin, bathypelagic group (green): GW = Gray’s beaked whale, SW = sperm whale. Dark grey indicates matrix diagonal, light grey fields refer to species not in the same habitat.
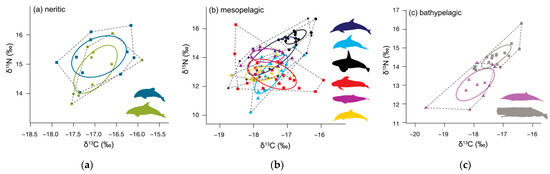
Figure 4.
Standard ellipse area corrected for small sample size (SEAc, solid lines) and convex hull area (TA, dotted line) for adults. (a) neritic odontocetes: bottlenose dolphins (Tursiops truncatus, olive green), Hector’s dolphins (Lagenorhynchus obscurus, teal), (b) mesopelagic odontocetes: common dolphins (Delphinus delphis, dark blue), dusky dolphins (Lagenorhynchus obscurus, light blue), killer whales (Orcinus orca, black), pygmy sperm whales (Kogia breviceps, magenta), long-finned pilot whales (Globicephala melas, red), and striped dolphins (Stenella coeruleoalba, yellow), and (c) bathypelagic odontocetes: Gray’s beaked whales (Mesoplodon grayi, purple) and sperm whales (Physeter macrocephalus, grey). Ellipse areas hold 40% of the data.
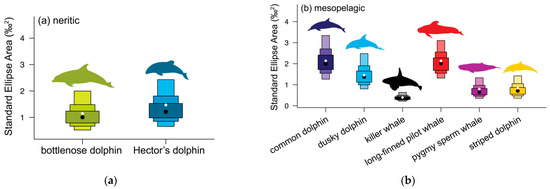
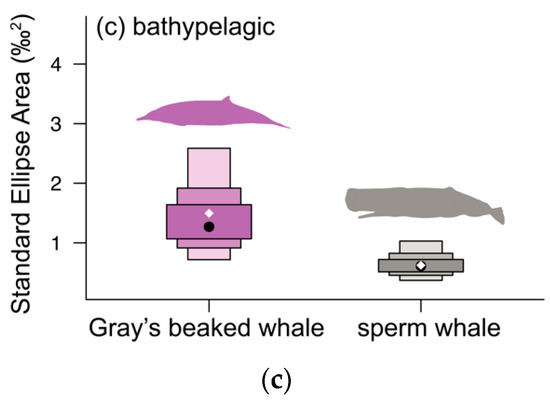
Figure 5.
Density plot showing the 95, 75, and 50% credible intervals of standard ellipses area using Bayesian techniques for (a) neritic odontocetes: bottlenose dolphins (Tursiops truncatus), Hector’s dolphins (Lagenorhynchus obscurus), (b) mesopelagic odontocetes: common dolphins (Delphinus delphis), dusky dolphins (Lagenorhynchus obscurus), killer whales (Orcinus orca), pygmy sperm whales (Kogia breviceps), long-finned pilot whales (Globicephala melas edwardii), and striped dolphins (Stenella coeruleoalba), and (c) bathypelagic odontocetes: Gray’s beaked whales (Mesoplodon grayi) and sperm whales (Physeter macrocephalus). Black dots represent the mean standard ellipses area (SEA) for each species; white diamonds indicate the corrected standard ellipses area (SEAC).
3.2. Mesopelagic Group
Common dolphins had lower δ13C and δ15N mean isotopic values than killer whales, and higher δ15N values than long-finned pilot whales and striped dolphins (randomisation test, p = ≤ 0.007, Table 2 and Table A1). Of the six mesopelagic species analysed, common dolphins had the largest SEAC and SEAB (Table 3b), and they were more likely to have higher bootstrapped values in most Layman metrics than killer whales and striped dolphins, and in all Layman metrics compared to pygmy sperm whales (Table 5). SEAB overlap for common dolphins was highest with killer whales (50.0%), smallest with striped dolphins (11.0%) and intermediate with the remaining pelagic species (19.0–27.0%) (Table 4).

Table 5.
The probability of each respective bootstrapped metric being larger in one species over another across all draws for mesopelagic species with n ≥ 10. Species abbreviations: CD = common dolphin, DD = dusky dolphin, KW = killer whale, LPW = long-finned pilot whale, PSW = pygmy sperm whale, SD = striped dolphin. Probabilities ≥ 90% are highlighted in bold.
Dusky dolphins, together with long-finned pilot whales, had the lowest mean δ15N values of all mesopelagic species (12.84 ± 1.34‰, Table 1, Figure 3 and Figure A2c). They differed from common dolphins in both δ13C and δ15N mean isotopic values (randomisation test, p = 0.001, Table 2 and Table A1), but not compared to long-finned pilot whales and striped dolphins. For all Layman metrics except SDNND, dusky dolphins were more likely to have higher bootstrapped values compared to killer whales, pygmy sperm whales, and striped dolphins (Table 5). They showed high SEAB overlap with long-finned pilot whales (54%) and moderate overlap with common dolphins, striped dolphins, and pygmy sperm whales (22.0–26.0%) (Table 4).
Killer whales had lower mean δ13C values (−16.75 ± 0.31‰) and higher mean δ15N values (15.43 ± 0.49‰, Table 1, Figure 3 and Figure A2c) than the other six mesopelagic species analysed (randomisation test, all p ≤ 0.001 Table 2 and Table A1). Killer whales were also likely to have smaller values for almost all Layman metrics compared to the other mesopelagic species (Table 5). Their SEAC was the smallest compared to the other mesopelagic species, but this was not reflected in their SEAB. Killer whales overlapped in SEAB only with common dolphins (30.0%, Table 4).
Long-finned pilot whales, together with dusky dolphins, had the lowest δ15N values of the six mesopelagic species analysed (12.84 ± 1.04‰, Table 1, Figure 3 and Figure A2c). This difference was significant when compared to killer whales (for both δ13C and δ15N values), and to common dolphins and pygmy sperm whales (for δ15N, randomisation test, p ≤ 0.001, Table 2 and Table A1). Long-finned pilot whales had the second highest SEAC and were likely to have higher bootstrapped values in most Layman metrics compared to killer whales, pygmy sperm whales, and striped dolphins (Table 5). They overlapped in SEAB with dusky dolphins (39.0%), common dolphins (28.0%), and striped dolphins (24.0%) (Table 4).
Pygmy sperm whales had the second lowest δ13C value (−17.59 ± 0.49‰) and the second highest mean δ15N value (14.14 ± 0.46‰) of the six mesopelagic species analysed (Table 1, Figure 3 and Figure A2c). They had lower mean isotopic values for both δ13C and δ15N compared to killer whales, but higher mean δ15N values than dusky dolphins, long-finned pilot whales, and striped dolphins (randomisation test, p ≤ 0.007, Table 2 and Table A1). They were likely to have lower values for most Layman metrics than common and dusky dolphins, as well as long-finned pilot whales (Table 5). Although pygmy sperm whales had the second smallest SEA and SEAC, this was not reflected in their SEAB (Figure 5b). They showed high SEAB overlap with common dolphins (both 64%) and moderate overlap with long-finned pilot whales (18%) and dusky dolphins (15%, Table 4).
Striped dolphins had lower mean δ13C and δ15N values compared to killer whales’ mean isotopic values (randomisation test, p < 0.001, Table 2 and Table A1) and lower mean δ15N values than common dolphins and pygmy sperm whales (randomisation test, p ≤ 0.003, Table 2 and Table A1). They were likely to have lower values for most Layman metrics than long-finned pilot whales, common, and dusky dolphins (Table 5). Striped dolphins had the second smallest SEAC and SEAB (Table 3b, Figure 5b), overlapping in SEAB with long-finned pilot whales (63.0%), dusky dolphins (42.0%), and common dolphins (31.0%) (Table 4).
The mean isotopic values of false killer whales, pygmy killer whales, Risso’s dolphins, and short-finned pilot whales fell within the δ13C and δ15N ranges of the other mesopelagic species. Notably, Risso’s dolphins (n = 4) had a large SD for δ13C (1.9‰).
3.3. Bathypelagic Group
Gray’s beaked whales had lower mean δ13C and δ15N values than sperm whales (Gray’s beaked whales δ13C −17.95 ± 0.65‰, δ15N 13.03 ± 0.86‰, sperm whales δ13C−17.24 ± 0.5‰, δ15N 14.54 ± 0.62‰, randomisation test: p ≤ 0.001, Table 1, Table 2 and Table A1, Figure 3 and Figure A2e). Gray’s beaked whales had higher values than sperm whales for all Layman metrics except bootstrapped MNND (Table 3c). The probability of Gray’s beaked whales having higher values was high for SEAB (98.0%) and CD (86.6%), and low to moderate for all other values (64.1–78.8%, Table 3c, Figure 5c). Both species barely overlapped in their respective SEABs (Gray’s beaked whales = 4.0%, sperm whales = 9.0%, Table 4, Figure 4c and Figure 5c).
Mean values for Cuvier’s beaked whales and the single values for southern bottlenose whales aligned with Gray’s beaked whales. Arnoux’s beaked whale had the lowest δ15N value of all species in the bathypelagic group (9.6‰). Furthermore, of all the samples analysed here, Arnoux’s beaked whale had by far the lowest δ13C value (−27.16‰), followed by the two samples of strap-toothed whales (mean δ13C = −23.03 ± 0.16‰, Table 1, Figure A1).
3.4. Polar Group
Of all the species in this study, hourglass dolphins and spectacled porpoises had the lowest mean δ15N value, (8.85 ± 0.18 and 9.51 ± 0.61‰, respectively). Of the three odontocetes in the polar group, the two southern right whale dolphins had the lowest mean δ13C value (−20.06 ± 0.22‰) and the highest mean δ15N (10.78 ± 0.66‰, Table 1, Figure 3 and Figure A2g).
4. Discussion
Understanding how species navigate interspecific competition is a challenge across practically all ecological spheres. However, when dealing with some of the most poorly understood cryptic bathypelagic species, such as beaked whales, for which almost nothing is known about their ecology, this challenge is even greater. In this study, we show differing levels of isotopic niche partitioning among 21 odontocete species inhabiting three different habitats (neritic, meso-, and bathypelagic) within New Zealand waters. Our findings demonstrate the considerable overlap of stable isotopic niches among species, highlighting the need for extended assessment of foraging ecology between key overlapping species using multiple dietary markers to ascertain the full extent of overlap using complimentary methods of diet assessment. Furthermore, we use the most common feeding habitat for each species to compare species within each habitat. It is possible that a portion of feeding behaviour takes place in other habitats as well.
4.1. Neritic Group
Bottlenose and Hector’s dolphins, which both inhabit New Zealand’s neritic waters, demonstrated high isotopic overlap and little evidence for trophic segregation. For Hector’s dolphins, stomach content analyses indicate a diet of predominantly small and often juvenile fish and squid from throughout the water column [71]. Like many small delphinids, Hector’s dolphins appear to be relatively opportunistic feeders. Typically, their diets reflect prey species availability, which differs between regions [71], and may also be affected by opportunistic feeding, such as observed feeding behind trawlers [72].
Bottlenose dolphins (Tursiops truncatus) are known to occur in New Zealand as two morphologically different ecotypes, coastal and oceanic [73,74]. In this study, we only included individuals of the coastal ecotype. The diet of bottlenose dolphins globally varies between locations and populations, with a variety of fish and squid species described [34]. Isotopic values for bottlenose dolphin skin collected from free-ranging individuals in Doubtful Sound, South Island, New Zealand, yielded similar isotopic values to those presented here (δ13C = –16.5 ± 0.38‰, δ15N = 15.1 ± 0.36‰, n = 11, [75]). Results suggested that the local dolphin population mainly feeds on reef-associated and demersal fishes [75].
Given their shared use of coastal waters and similar foraging ecology as opportunistic generalists, we expected Hector’s and bottlenose dolphins to differ in their respective isotopic niches as a method of avoiding resource competition, which does not seem to be the case. As it is possible for different food resources to show similar isotopic composition [76,77], we cannot completely exclude the possibility of trophic segregation based on stable isotope data alone. Two coastal delphinid species in Queensland, Australia, the Australian humpback (Sousa sahulensis) and the snubfin dolphin (Orcaella heinsohni), also showed high isotopic niche overlap [78]. In this case, their co-existence was attributed to subtle differences in habitat use and prey selection [78]. However, those two species are similar in size and can exploit the same prey species. In the case of Hector’s and bottlenose dolphins, the smaller mouth gape makes it highly unlikely for Hector’s dolphins to be able to feed on adults of fish species such as mullet and snapper, which have been documented as prey species of bottlenose dolphins (Massey University unpublished data). However, it is possible that Hector’s dolphins feed on juveniles of the same prey species as bottlenose dolphins. Furthermore, there may be spatial or temporal segregation between the species, which reduces direct competition and thus enables their coexistence.
4.2. Mesopelagic Group
Odontocetes in the mesopelagic group varied widely in niche space and Layman metrics, although we noted considerable overlap between species. For example, common dolphins exhibited the largest isotopic niche of all mesopelagic species, which reflects their generalist status, mainly feeding opportunistically on locally abundant small schooling fish or cephalopods [79,80,81]. Furthermore, rather than exclusively foraging offshore, in New Zealand the species is abundant within inshore coastal waters, such as the Hauraki Gulf [82]. Such a shallow coastal habitat likely widens the foraging niche of the population, as it includes a range of benthic species [55,79]. Indeed, common dolphins overlapped most (50%) in the Bayesian niche with killer whales, and least (11%) with striped dolphins, which is considerably smaller compared to an overlap of 45% observed with striped dolphins in the Mediterranean Sea [7].
Long-finned pilot whales demonstrated the second largest isotopic niche (Figure 4b), which overlapped with common, dusky, and striped dolphins. The large δ13C and δ15N ranges reported for this species indicate that long-finned pilot whales in New Zealand waters forage over a range of different habitats and prey of different trophic levels [83]. Based on stomach content studies, the diet of long-finned pilot whales in the Southern Ocean appears to be dominated by adult cephalopods, with local variation in the number of different species consumed [84,85,86]. Cephalopods are generally an important part of the diets of mesopelagic dolphins [87,88], which may explain the niche overlap of long-finned pilot whales with these species.
Striped dolphins demonstrated a small niche which overlapped substantially with long-finned pilot whales (63%), dusky (42%), and common dolphins (31%). Like long-finned pilot whales, striped dolphins are typically observed further offshore [19], and they likely forage in deep waters [89]. In other locations, the species is known to feed on cephalopods, fish, and secondarily on crustaceans [90,91,92]. Compared to the Mediterranean Sea, striped dolphins in New Zealand waters exhibited a larger isotopic niche [7]. While dietary similarities exist between regions, no detailed dietary information exists for New Zealand waters. A deep-water cephalopod-heavy diet would explain the high niche overlap with long-finned pilot whales.
Like common dolphins, dusky dolphins displayed a large δ15N range, indicating high trophic variety throughout the population, although the mean δ15N value was lower than in common dolphins. Dusky dolphins feed on small schooling fishes, targeting some of the same species as common dolphins, such as anchovy (Engraulis australis), garfish (Hyporhamphus ihi), and pilchard (Sardinops neopilchardus) [79,93,94]. However, although generally using the same mesopelagic habitat type throughout their range in New Zealand waters, dusky and common dolphins rarely occur in the same location [95], which may allow overlap in prey species. Niche overlap between dusky dolphins and common dolphins was not as high as between dusky dolphins and long-finned pilot whales (26% vs. 63%).
Pygmy sperm whales signalled a small niche area and δ15N range, and their Layman values were likely to be lower compared to the other mesopelagic species, except killer whales. Despite the samples having been collected in five different years between 2009 and 2021, pygmy sperm whales had a small isotopic niche, indicating little diversity in basal resources. Similarly, to long-finned pilot whales, the diet of pygmy sperm whales consists mainly of oceanic cephalopods caught in depths of up to 1100 m, with smaller proportions of fish and crustaceans [96,97,98,99]. However, niche overlap was three times as high with common dolphins (64%) than with long-finned pilot whales (18%), suggesting more competition with common dolphins. Given the greater potential foraging depth of pygmy sperm whales compared to common dolphins [79,100,101], it is likely that vertical niche stratification alleviates competition between these two species.
As expected, based on what is known about their foraging ecology as apex predators, [39], killer whales had the highest mean δ15N values of all species analysed here. Killer whales are generalist predators with a diet that includes both fish and marine mammals [39]. However, local populations can be highly specialised in foraging and hunting strategies, with some populations primarily feeding on fish [102,103,104] and others almost exclusively on marine mammals [102,105]. In New Zealand, killer whales are documented to regularly feed on stingrays [106] and sharks [107,108], with marine mammal predations also recorded [109]. In locations where prey specialisation occurs, stable isotope analysis has been able to quantify inter-individual dietary variation, showing higher δ15N values for individuals feeding on marine mammals compared to those feeding on fish [104,110,111,112]. Interestingly, the isotopic niche of killer whales in this study was the smallest of all mesopelagic species, suggesting little isotopic variety in their diet and limited inter-individual differences, which is further confirmed by the low Layman metrics values. Our killer whale data comprise samples from six stranding events (two mass strandings and four single strandings). It is possible that the eleven animals deriving from the two mass strandings (five and six animals, respectively) skew the data somewhat, particularly if the mass stranded animals comprised part of the same group and foraged in the same area and on similar prey prior to stranding.
Risso’s dolphins and false killer whales had the lowest mean δ13C values (−18.32 ± 1.90‰ and −18.20 ± 1.46‰, respectively) of the mesopelagic species, indicating that these animals likely foraged further offshore than the other species. While nothing is known about the diet of Risso’s dolphin in New Zealand, false killer whales are known to feed cooperatively with oceanic bottlenose dolphins on Kahawai (Arrips trutta) off North Island, New Zealand [113] and travel large distances across north-eastern New Zealand [114]. Contrastingly, mean δ13C values of pygmy killer whales (−17.19 ± 0.35‰) and short-finned pilot whales (−16.8 ± 0.11‰) suggested feeding in waters closer to shore, similar to killer whales. However, sample sizes for these four species were low, preventing isotopic niche analyses.
Overall, species in the mesopelagic group showed less niche differentiation than similar species conglomerates in other locations [5,115,116]. This suggests that competition between species is reduced through other mechanisms, such as fine-scale spatial or temporal segregation. Furthermore, the samples analysed here originate from various locations around New Zealand, which itself spans a large latitudinal range (S 34.5–47). It is possible that local populations of some species differ in their foraging ecology, which could change their isotopic overlap with local competitors. For most of the mesopelagic species analysed here, there is little information about either their diet or fine-scale distribution in New Zealand waters. Consequently, they may be feeding on abundant prey, allowing coexistence, or alternatively consuming different prey with similar isotopic compositions, which would remain undetected through bulk stable isotope analysis. Furthermore, vertical niche segregation via differences in foraging depths could also be at play.
4.3. Bathypelagic Group
Although sperm whales and Gray’s beaked whales are both species that forage in the deep sea, they have almost no overlap in their core isotopic niche. Due to their deep-water habitat and generally elusive nature, little is known about the foraging ecology and diet of beaked whales [51]. Prey items for species of the genus Mesoplodon are thought to include small mesopelagic squid, fish, and also crustaceans [117]. However, the stomachs of three Gray’s beaked whales from Brazil, South Africa, and the Indian Ocean did not contain any squid, indicating this species may feed primarily on fish [117]. While very little information is available on the diet of beaked whales in New Zealand waters, identifiable hard part remains from the stomachs of five Gray’s beaked whales included lanternfish (Myctophidae), as well as other mid-water fish and squid remains [118].
Although the New Zealand sperm whale diet is dominated by oceanic cephalopods, it also includes demersal fish [119]. As males tend to eat more demersal fish than females [50], this likely reflects the distributional bias towards males below 42° latitude [120], which is echoed in our data as all 16 animal samples were males. Sperm whales had higher δ15N values than Gray’s beaked whales, suggesting that they feed on larger prey. The considerable size difference between the two species (total body length range of individuals analysed here: sperm whales = 1105–1677 cm, Gray’s beaked whales = 305–490 cm) and the resulting difference in energy requirements is consistent with this assumption. Although sperm whales are known to be capable of hunting very large prey such as giant squid (Architeuthis spp.) [50], they predominantly feed on smaller squid (<1 m length) in New Zealand [119,121], which may still be larger than the prey consumed by the comparatively smaller Gray’s beaked whales.
While the Gray’s beaked whale samples analysed here were collected across five different years and at least six different stranding events, the sperm whale samples originated from three stranding events (two mass strandings and one single stranding) across two years. The two groups of three and twelve animals had likely been foraging in similar habitats, respectively. Therefore, it is not surprising that the Layman metrics values were generally higher for Gray’s beaked whales, reflecting higher inter-individual differences and larger niche width (Figure 5b). However, these differences could also reflect a genuine broader variety in prey consumed by Gray’s beaked whales compared to sperm whales.
Sperm whales in New Zealand exhibit subtle seasonal differences in their foraging patterns [122] and can also display different foraging strategies depending on their location [123]. Our data were most similar to the mean isotopic values observed in Kaikōura, New Zealand, in the winter months [122], which matches the temporal distribution (May–July) of 13 out of the 16 sperm whale samples included in this study. The remaining group of three sperm whales had lower δ15N and δ13C values, suggesting that they likely foraged elsewhere. Interestingly, their isotopic values were closer to those of the Gray’s beaked whales, suggesting that niche overlap between these two species could be higher in some locations than observed here.
Gray’s beaked whales stranded along the coast of Tierra del Fuego, Argentina, showed 15% niche overlap with Cuvier’s beaked whales [124]. The three Cuvier’s beaked whales included here had similar isotopic values to Gray’s beaked whales as well as the southern bottlenose whale. While little is known about the southern bottlenose whale diet, stomach contents of stranded individuals suggest that they mainly feed on cephalopods [125], similar to northern bottlenose whales (H. ampullatus) [126,127,128]. While we did not have a sufficient sample size to allow for isotopic niche comparison for all beaked whale species presented here, mean isotopic values of δ15N and δ13C did indicate some degree of niche overlap between Gray’s and Cuvier’s beaked whales, as well as southern bottlenose whales too.
Conversely, the two strap-toothed beaked whales demonstrated lower mean δ15N values compared to the other beaked whales, except for the Arnoux’s beaked whale, indicating they likely feed on small prey. Most cephalopods found in the stomachs of strap-toothed beaked whales in New Zealand ranged between 20–100 g and were approximately 15 cm in length, matching the species’ small gape caused by fully erupted teeth in adults, and thus their adaptation to relatively small prey [129]. Prey items to date identified include bathypelagic squid including Chiroteuthidae, Cranchiidae, and Histioteuthidae [118]. The low δ15N values observed for the Arnoux’s beaked whale and the two strap-toothed beaked whales included here indicate some level of resource partitioning among species, which could alleviate interspecific competition. However, the low δ15N values also suggest that these animals have been feeding in Antarctic or subantarctic waters, which may have exposed them to different prey than the other bathypelagic species.
4.4. Polar Group
Hourglass dolphins have been observed feeding in surface waters along the Antarctic convergence [52], and their diet likely includes fish and squid [130]. Similarly, southern right whale dolphins mainly occur between the subtropical and the Antarctic convergences where they feed on fish and squid [53]. Due to their remote oceanic habitat, next to nothing is known about the diet and behaviour of spectacled porpoises, although they are suspected to also forage on fish and squid in cold waters near the Antarctic convergence [54].
From the three species in the polar group, southern right whale dolphins had the highest δ15N and the lowest δ13C values, while spectacled porpoises and hourglass dolphins had similar isotopic values. Mean δ13C values for all three species ranged between −20.06 and −19.17‰, which suggests that these individuals were likely not feeding at the polar front, which would have resulted in lower δ13C values [131]. Based on the isotopic values of the individuals analysed here, spectacled porpoises and hourglass dolphins likely overlap in prey and foraging habitat. Stable isotopes from bone collagen collected from strandings in Tierra del Fuego, Argentina, indicated a similar pattern for the three polar species included here, with high isotopic similarity for spectacled porpoises and hourglass dolphins, and higher δ15N values for southern right whale dolphins [132].
To date, no consensus exists on the turnover time of cetacean skin, and estimates range from several days [133,134] to several months [135]. Given that the samples analysed here originate from animals that were stranded in New Zealand, it is possible that these individuals had already been feeding outside of their usual polar habitat for a considerable time, which means that their isotopic values may not reflect these species’ usual foraging ecology.
5. Conclusions
We present the first comparative isotopic niche assessment of New Zealand’s odontocetes. Aside from Gray’s beaked whales and sperm whales, which show clear niche separation, all species overlapped substantially in their isotopic niche with at least one other species in the same habitat. Our findings suggest other mechanisms must be at play to reduce interspecific competition and thus enable coexistence in New Zealand’s biodiverse waters. Many of the species included here are unlikely to be directly observed while foraging owing to their elusive nature, offshore/deep-water habitat and/or foraging behaviour. Accordingly, future studies would benefit from a combined approach that extends bulk stable isotope analyses and includes fatty-acid and compound-specific stable isotopes alongside to provide a deeper understanding of the diet of these species. Where relevant, these analyses would support stomach content studies, which could further be assisted using metabarcoding of prey. Furthermore, bulk stable isotopes of hydrogen, sulphur, and oxygen may provide increased resolution on habitat use and resource pathways [136,137,138]. Since interspecific competition only occurs when the resource that the species compete for is limited [23,24], studies are required to examine the extent of species competition by assessing prey abundance, fine-scale habitat use, foraging depths, and differences between local populations for odontocete species in New Zealand. While this study clearly highlights the many remaining unknowns in foraging ecology and potential interspecific competition of New Zealand’s odontocete community, it presents an important foundational step, particularly considering current and future ecosystem changes such as ocean warming and fishery pressures causing alterations to prey abundance and distributions.
Author Contributions
Conceptualization, K.J.P. and K.A.S.; formal analysis, K.J.P. and S.J.B.; data curation, K.A.S. and E.L.B.; writing—original draft preparation, K.J.P.; writing—review and editing, all authors; visualization, K.J.P. and K.A.S.; project administration, K.J.P. and K.A.S.; funding acquisition, K.A.S., K.J.P. and G.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this project was provided by the PADI Foundation (K.J.P.) and Massey University Research Fund (K.A.S.). During part of this study, K.J.P. was supported by an Australia Awards Endeavour Research Fellowship, and a Postdoc Grant from the University of Zurich. K.A.S. was supported by a Royal Society Te Aparangi Rutherford Discovery Fellowship (2019–2024). B.H. was supported by a Massey University Doctoral Scholarship and a Wildbase Trust research grant.
Institutional Review Board Statement
This project was completed under Department of Conservation permits 39239-MAR and Rnw/NO/2009/06 issued to Massey University.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available at https://github.com/kjopeters/Peters-et-al-2022-Biology-Stable-Isotopes.
Acknowledgments
We are grateful to Julie Brown, Josette Delgado, Anna Kilimnik, Greg Olsen, and Rahul Peethambaran at NIWA for conducting the stable isotope analyses, and all Department of Conservation staff and Tangata Whenua who facilitated the collection of tissue samples used in this study. We also thank Théo Pinheíro, Rebecca M. Boys, Emily Palmer, and Odette Howarth for their dedicated assistance with lab work.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A. Extended Methods
Appendix A.1. Molecular Sexing
We extracted DNA from the skin using the Qiagen DNeasy Blood and Tissue Kits according to standard protocol. Molecular sexing was undertaken by targeting the SRY and ZFX gene loci [139]. We carried out PCR in 25 μL volumes using the MyTaq™ DNA polymerase kit (Bioline, Eveleigh, Australia), including: 0.3 μM of the primers PMSRYF [140], ZFX0582F, and ZFX0923R [141]; 0.6 μM of the primer TtSRYR [139]; and 50–75 ng of template DNA. Thermocycling entailed an initial denaturation at 92 °C for 30 s followed by 35 cycles of 94 °C for 30 seconds, 41 °C for 45 s, and 72 °C for 45 s, following the protocol as described in Rosel [139]. We ran the PCR products plus a negative control on a 2.8% agarose gel at 80 V for 1 h, to observe the separation of amplicons indicating male (two bands) or female (one band).
Appendix A.2. Mathematical Lipid Correction
For the remaining species, we used a bootstrapping approach to derive a sample correction formula that is robust against outliers. We randomly selected a subset of 80% of all lipid-corrected samples (n = 74). We then used a linear regression analysis of the δ13C values of the original whole (non-lipid-extracted) and lipid-extracted samples in this subset. We repeated this process 1000 times and used the average coefficients from these 1000 replicates to derive our final correction formula:
where δ13C is the non-lipid extracted value and δ13CLEC is the mathematically corrected value. For common dolphins, we used a previously published species-specific correction formula [55] as this one performed slightly better for this species.
Appendix A.3. Details on Layman Metrics
We used six different Layman metrics [69,142] to measure niche variation between different odontocete species:
- (1)
- δ15N range: distance between the highest and lowest δ15N values (i.e., max δ15N—min δ15N). Measure of trophic length of the community.
- (2)
- δ13C range: distance between the highest and lowest δ13C values (i.e., max δ13C—min δ13C). Estimates the diversity of basal resources.
- (3)
- Total area (TA): total area of the convex hull comprising all data points. Measure of the total amount of niche space occupied and an indication of niche width.
- (4)
- Mean distance to centroid (CD): average Euclidean distance of each sample to the centroid. Measure of niche width and sample spacing.
- (5)
- Mean nearest neighbour distance (MNND): mean of the Euclidean distances to each sample’s nearest neighbour. Measure of the density and clustering of individuals.
- (6)
- Standard deviation of nearest neighbour distance (SDNND): measure of the evenness of spatial density and the packing of individuals. Low SDNND values indicate a more even distribution of trophic niches.

Table A1.
Results of pairwise comparisons (randomisation test) of δ15N and δ13C isotopic values between odontocete species with n ≥ 7. Significant values (at 0.05 level) denoted in bold. Neritic group (yellow): BD = bottlenose dolphin, HD = Hector’s dolphin, mesopelagic group (orange): CD = common dolphin, DD = dusky dolphin, KW = killer whale, LPW = long-finned pilot whale, PSW = pygmy sperm whale, SD = striped dolphin, bathypelagic group (green): GW = Gray’s beaked whale, SW = sperm whale. Dark grey indicates matrix diagonal, light grey fields refer to species not in the same habitat.
Table A1.
Results of pairwise comparisons (randomisation test) of δ15N and δ13C isotopic values between odontocete species with n ≥ 7. Significant values (at 0.05 level) denoted in bold. Neritic group (yellow): BD = bottlenose dolphin, HD = Hector’s dolphin, mesopelagic group (orange): CD = common dolphin, DD = dusky dolphin, KW = killer whale, LPW = long-finned pilot whale, PSW = pygmy sperm whale, SD = striped dolphin, bathypelagic group (green): GW = Gray’s beaked whale, SW = sperm whale. Dark grey indicates matrix diagonal, light grey fields refer to species not in the same habitat.
| δ15N | BD | HD | CD | DD | KW | LPW | PSW | SD | GW | SW |
|---|---|---|---|---|---|---|---|---|---|---|
| δ13C | ||||||||||
| BD | 0.106 | |||||||||
| HD | 0.373 | |||||||||
| CD | 0.007 | 0.001 | 0.001 | 0.473 | 0.003 | |||||
| DD | 0.451 | <0.001 | 0.494 | 0.005 | 0.463 | |||||
| KW | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| LPW | 0.496 | 0.446 | 0.001 | 0.001 | 0.485 | |||||
| PSW | 0.286 | 0.294 | <0.001 | 0.288 | <0.001 | |||||
| SD | 0.134 | 0.104 | <0.001 | 0.132 | 0.256 | |||||
| GW | <0.001 | |||||||||
| SW | 0.001 |
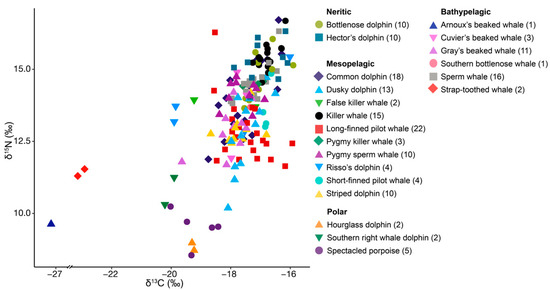
Figure A1.
Isotopic values for individual odontocetes (species denoted by shape and colour) stranded in New Zealand between 2010 and 2021 (Table 1). Note that the x-axis is split for display purposes.
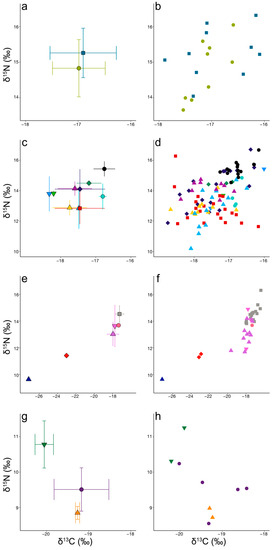
Figure A2.
Isotopic values for individual odontocetes stranded in New Zealand between 2010 and 2021 displayed by habitat (Table 1) for (a,b) neritic odontocetes, (c,d) mesopelagic odontocetes, (e,f) bathypelagic odontocetes, and (g,h) polar odontocetes. Species denoted by shape and colour, (for legend see Figure A1).
References
- Hutchinson, G.E. A Treatise on Limnology; Wiley: New York, NY, USA, 1957; Volume 1, p. 243. [Google Scholar]
- Gause, G.F. The Struggle for Existence: A Classic of Mathematical Biology and Ecology; Williams & Wilkins Company: Baltimore, MD, USA, 1934. [Google Scholar]
- Arrizabalaga-Escudero, A.; Garin, I.; García-Mudarra, J.L.; Alberdi, A.; Aihartza, J.; Goiti, U. Trophic requirements beyond foraging habitats: The importance of prey source habitats in bat conservation. Biol. Conserv. 2015, 191, 512–519. [Google Scholar] [CrossRef]
- Tucker, A.D.; Fitzsimmons, N.N.; Gibbons, J.W. Resource partitioning by the estuarine turtle Malaclemys terrapin: Trophic, spatial, and temporal foraging constraints. Herpetologica 1995, 51, 167–181. [Google Scholar]
- Mèndez-Fernandez, P.; Bustamante, P.; Bode, A.; Chouvelon, T.; Ferreira, M.; Lopez, A.; Pierce, G.J.; Santos, M.B.; Spitz, J.; Vingada, J.V. Foraging ecology of five toothed whale species in the Northwest Iberian Peninsula, inferred using carbon and nitrogen isotope ratios. J. Exp. Mar. Biol. Ecol. 2012, 413, 150–158. [Google Scholar] [CrossRef]
- Teixeira, C.R.; Botta, S.; Daura-Jorge, F.G.; Pereira, L.B.; Newsome, S.D.; Simões-Lopes, P.C. Niche overlap and diet composition of three sympatric coastal dolphin species in the southwest Atlantic Ocean. Mar. Mammal Sci. 2020, 37, 111–126. [Google Scholar] [CrossRef]
- Giménez, J.; Cañadas, A.; Ramírez, F.; Afán, I.; García-Tiscar, S.; Fernández-Maldonado, C.; Castillo, J.J.; de Stephanis, R. Intra-and interspecific niche partitioning in striped and common dolphins inhabiting the southwestern Mediterranean Sea. Mar. Ecol. Prog. Ser. 2017, 567, 199–210. [Google Scholar] [CrossRef]
- Méndez-Fernandez, P.; Pierce, G.J.; Bustamante, P.; Chouvelon, T.; Ferreira, M.; González, A.F.; López, A.; Read, F.L.; Santos, M.B.; Spitz, J.; et al. Ecological niche segregation among five toothed whale species off the NW Iberian Peninsula using ecological tracers as multi-approach. Mar. Biol. 2013, 160, 2825–2840. [Google Scholar] [CrossRef]
- Newsome, S.D.; del Rio, C.M.; Bearhop, S.; Phillips, D.L. A niche for isotopic ecology. Front. Ecol. Environ. 2007, 5, 429–436. [Google Scholar] [CrossRef]
- Fry, B.; Sherr, E. 13C measurements as indicators of carbon flow in marine food webs. Contrib. Mar. Sci. 1984, 27, 15–47. [Google Scholar]
- Rounick, J.; Winterbourn, M. Stable carbon isotopes and carbon flow in ecosystems. Bioscience 1986, 36, 171–177. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Minagawa, M.; Wada, E. Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ15N and animal age. Geochim. Cosmochim. Acta 1984, 48, 1135–1140. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Browning, N.E.; McCulloch, S.D.; Bossart, G.D.; Worthy, G.A.J. Fine-scale population structure of estuarine bottlenose dolphins (Tursiops truncatus) assessed using stable isotope ratios and fatty acid signature analyses. Mar. Biol. 2014, 161, 1307–1317. [Google Scholar] [CrossRef]
- Giménez, J.; Ramírez, F.; Forero, M.G.; Almunia, J.; de Stephanis, R.; Navarro, J. Lipid effects on isotopic values in bottlenose dolphins (Tursiops truncatus) and their prey with implications for diet assessment. Mar. Biol. 2017, 164, 122. [Google Scholar] [CrossRef]
- Loizaga de Castro, R.; Saporiti, F.; Vales, D.G.; García, N.A.; Cardona, L.; Crespo, E.A. What are you eating? A stable isotope insight into the trophic ecology of short-beaked common dolphins in the Southwestern Atlantic Ocean. Mamm. Biol. 2016, 81, 571–578. [Google Scholar] [CrossRef]
- Giménez, J.; Marçalo, A.; Ramírez, F.; Verborgh, P.; Gauffier, P.; Esteban, R.; Nicolau, L.; González-Ortegón, E.; Baldó, F.; Vilas, C. Diet of bottlenose dolphins (Tursiops truncatus) from the Gulf of Cadiz: Insights from stomach content and stable isotope analyses. PLoS ONE 2017, 12, e0184673. [Google Scholar] [CrossRef]
- Stephenson, F.; Goetz, K.; Sharp, B.R.; Mouton, T.L.; Beets, F.L.; Roberts, J.; MacDiarmid, A.B.; Constantine, R.; Lundquist, C.J.; Sarmento Cabral, J. Modelling the spatial distribution of cetaceans in New Zealand waters. Divers. Distrib. 2020, 26, 495–516. [Google Scholar] [CrossRef]
- Baker, C.; Boren, L.; Childerhouse, S.; Constantine, R.; van Helden, A.; Lundquist, D.; Rolfe, J. Conservation Status of New Zealand Marine Mammals, 2019; New Zealand Threat Classification Series 29; Department of Conservation: Wellington, New Zealand, 2019.
- Bradford-Grieve, J.M.; Lewis, K.B.; Stanton, B.R. Advances in New Zealand oceanography, 1967–1991. N. Z. J. Mar. Freshw. Res. 1991, 25, 429–441. [Google Scholar] [CrossRef]
- Stevens, C.L.; O’Callaghan, J.M.; Chiswell, S.M.; Hadfield, M.G. Physical oceanography of New Zealand/Aotearoa shelf seas—A review. N. Z. J. Mar. Freshw. Res. 2019, 5, 6–45. [Google Scholar] [CrossRef]
- Schoener, T.W. Resource partitioning in ecological communities. Science 1974, 185, 27–39. [Google Scholar] [CrossRef]
- Pianka, E.R. Guild structure in desert lizards. Oikos 1980, 35, 194–201. [Google Scholar] [CrossRef]
- Arlettaz, R. Habitat selection as a major resource partitioning mechanism between the two sympatric sibling bat species Myotis myotis and Myotis blythii. J. Anim. Ecol. 1999, 68, 460–471. [Google Scholar] [CrossRef]
- Friedlaender, A.S.; Lawson, G.L.; Halpin, P.N. Evidence of resource partitioning between humpback and minke whales around the western Antarctic Peninsula. Mar. Mammal Sci. 2009, 25, 402–415. [Google Scholar] [CrossRef]
- Betty, E.L.; Bollard, B.; Murphy, S.; Ogle, M.; Hendriks, H.; Orams, M.B.; Stockin, K.A. Using emerging hot spot analysis of stranding records to inform conservation management of a data-poor cetacean species. Biodivers. Conserv. 2020, 29, 643–665. [Google Scholar] [CrossRef]
- Stockin, K.A.; Duignan, P.J.; Roe, W.D.; Meynier, L.; Alley, M.; Fettermann, T. Causes of mortality in stranded common dolphins (Delphinus sp.) from New Zealand waters between 1998 and 2008. Pac. Conserv. Biol. 2009, 15, 217–227. [Google Scholar] [CrossRef]
- Brabyn, M.W.; McLean, I.G. Oceanography and coastal topography of herd-stranding sites for whales in New Zealand. J. Mammal. 1992, 73, 469–476. [Google Scholar] [CrossRef]
- IJsseldijk, L.L.; Brownlow, A.C.; Mazzariol, S. Best Practice on Cetacean Post Mortem Investigation and Tissue Sampling. 2019. Available online: https://scholar.google.co.jp/scholar?q=Best+practice+on+cetacean+post+mortem+investigation+and+tissue+sampling&hl=ja&as_sdt=0&as_vis=1&oi=scholart (accessed on 1 August 2022).
- Geraci, J.R.; Lounsbury, V.J. Marine Mammals Ashore: A Field Guide for Strandings; National Aquarium in Baltimore: Baltimore, MD, USA, 2005. [Google Scholar]
- Jefferson, T.A.; Webber, M.A.; Pitman, R. Marine Mammals of the World: A Comprehensive Guide to Their Identification; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Charting Around New Zaland (CANZ). New Zealand Region Bathymetry, 1:4,000,000, 2nd ed.; NIWA Chart Miscellaneous Series No. 85; National Institute of Water and Atmospheric Research (NIWA): Wellington, New Zealand, 2008. [Google Scholar]
- Wells, R.S.; Scott, M.D. Bottlenose dolphin, Tursiops truncatus, common bottlenose dolphin. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: London, UK, 2018; pp. 118–125. [Google Scholar]
- Dawson, S.M. Cephalorhynchus dolphins: C. heavisidii, C. eutropia, C. hectori, and C. commersonii. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: London, UK, 2018; pp. 166–172. [Google Scholar]
- Stockin, K.A.; Orams, M. The status of common dolphins (Delphinus delphis) within New Zealand waters. J. Cetacean Res. Manag. 2009, SC/61/SM20, 1–13. [Google Scholar]
- Van Waerebeek, K.; Würsig, B. Dusky dolphin: Lagenorhynchus obscurus. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: London, UK, 2018; pp. 277–280. [Google Scholar]
- Baird, R.W. False killer whale: Pseudorca crassidens. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J., Kovacs, K.M., Eds.; Academic Press: London, UK, 2018; pp. 347–349. [Google Scholar]
- Ford, J.K.B. Killer whale: Orcinus orca. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: London, UK, 2018; pp. 531–537. [Google Scholar]
- Olson, P.A. Pilot whales: Globicephala melas and G. macrorhynchus. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: London, UK, 2018; pp. 701–705. [Google Scholar]
- Baird, R.W. Pygmy killer whale: Feresa attenuata. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: London, UK, 2018; pp. 788–790. [Google Scholar]
- McAlpine, D.F. Pygmy and dwarf sperm whales: Kogia breviceps and K. sima. In Encyclopedia of Marine Mammals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 786–788. [Google Scholar]
- Baird, R.W. Risso’s dolphin: Grampus griseus. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: London, UK, 2018; pp. 824–827. [Google Scholar]
- Archer, F.I. Striped dolphin: Stenella coeruleoalba. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: London, UK, 2018; pp. 954–956. [Google Scholar]
- Thewissen, J. Berardius beaked whales: Berardius bairdii and B. arnuxii. In Encyclopedia of Marine Mammals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 97–99. [Google Scholar]
- Baird, R.W. Cuvier’s beaked whale: Ziphius cavirostris. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: London, UK, 2018; pp. 234–237. [Google Scholar]
- Patel, S.; Thompson, K.F.; Santure, A.W.; Constantine, R.; Millar, C.D. Genetic Kinship analyses reveal that Gray’s beaked whales strand in unrelated groups. J. Hered. 2017, 108, 456–461. [Google Scholar] [CrossRef]
- Thompson, K.F.; Ruggiero, K.; Millar, C.D.; Constantine, R.; van Helden, A.L. Large-scale multivariate analysis reveals sexual dimorphism and geographic differences in the Gray’s beaked whale. J. Zool. 2014, 294, 13–21. [Google Scholar] [CrossRef]
- Moors-Murphy, H.B. Bottlenose Whales: Hyperoodon ampullatus and H. planifrons. In Encyclopedia of Marine Mammals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 130–132. [Google Scholar]
- Whitehead, H. Sperm whale: Physeter macrocephalus. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: London, UK, 2018; pp. 919–925. [Google Scholar]
- Pitman, R. Mesoplodon beaked whales: Mesoplodon spp. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: London, UK, 2018; pp. 788–790. [Google Scholar]
- Cipriano, F. Hourglass dolphin: Lagenorhynchus cruciger. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: London, UK, 2018; pp. 480–483. [Google Scholar]
- Lipsky, J.D.; Brownell Jr, R.L. Right Whale Dolphins: Lissodelphis borealis and L. peronii. In Encyclopedia of Marine Mammals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 813–817. [Google Scholar]
- Goodall, R.N.P.; Brownell, R.L., Jr. Spectacled porpoise: Phocoena dioptrica. In Encyclopedia of Marine Mammals; Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: London, UK, 2018; pp. 912–916. [Google Scholar]
- Peters, K.J.; Bury, S.J.; Betty, E.L.; Parra, G.J.; Tezanos-Pinto, G.; Stockin, K.A. Foraging ecology of the common dolphin Delphinus delphis revealed by stable isotope analysis. Mar. Ecol. Prog. Ser. 2020, 652, 173–186. [Google Scholar] [CrossRef]
- Ryan, C.; McHugh, B.; Trueman, C.N.; Harrod, C.; Berrow, S.D.; O’Connor, I. Accounting for the effects of lipids in stable isotope (δ13C and δ15N values) analysis of skin and blubber of balaenopterid whales. Rapid Commun. Mass Spectrom. 2012, 26, 2745–2754. [Google Scholar] [CrossRef] [PubMed]
- Lesage, V.; Morin, Y.; Rioux, È.; Pomerleau, C.; Ferguson, S.H.; Pelletier, É. Stable isotopes and trace elements as indicators of diet and habitat use in cetaceans: Predicting errors related to preservation, lipid extraction, and lipid normalization. Mar. Ecol. Prog. Ser. 2010, 419, 249–265. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- Skinner, M.M.; Martin, A.A.; Moore, B.C. Is lipid correction necessary in the stable isotope analysis of fish tissues? Rapid Commun. Mass Spectrom. 2016, 30, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Post, D.; Layman, C.; Arrington, D.A.; Takimoto, G.; Quattrochi, J.; Montaña, C. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef]
- Yurkowski, D.J.; Hussey, N.E.; Semeniuk, C.; Ferguson, S.H.; Fisk, A.T. Effects of lipid extraction and the utility of lipid normalization models on δ13C and δ15N values in Arctic marine mammal tissues. Polar Biol. 2015, 38, 131–143. [Google Scholar] [CrossRef]
- Groß, J.; Fry, B.; Burford, M.A.; Bengtson Nash, S. Assessing the effects of lipid extraction and lipid correction on stable isotope values (δ13C and δ15N) of blubber and skin from southern hemisphere humpback whales. Rapid Commun. Mass Spectrom. 2021, 35, e9140. [Google Scholar] [CrossRef]
- Ricca, M.; Miles, A.; Anthony, R.; Deng, X.; Hung, S. Effect of lipid extraction on analyses of stable carbon and stable nitrogen isotopes in coastal organisms of the Aleutian archipelago. Can. J. Zool. 2007, 85, 40–48. [Google Scholar] [CrossRef]
- Mintenbeck, K.; Brey, T.; Jacob, U.; Knust, R.; Struck, U. How to account for the lipid effect on carbon stable-isotope ratio (δ13C): Sample treatment effects and model bias. J. Fish. Biol. 2008, 72, 815–830. [Google Scholar] [CrossRef]
- Quay, P.; Sonnerup, R.; Westby, T.; Stutsman, J.; McNichol, A. Changes in the 13C/12C of dissolved inorganic carbon in the ocean as a tracer of anthropogenic CO2 uptake. Glob. Biogeochem. Cycles 2003, 17, 4-1–4-20. [Google Scholar] [CrossRef]
- Keeling, C.D.; Mook, W.G.; Tans, P.P. Recent trends in the 13C/12C ratio of atmospheric carbon dioxide. Nature 1979, 277, 121. [Google Scholar] [CrossRef]
- Manly, B.F.J. Randomization, Bootstrap and Monte Carlo Methods in Biology, 2nd ed.; Chapman and Hall: London, UK, 1997. [Google Scholar]
- Jackson, M.C.; Donohue, I.; Jackson, A.L.; Britton, J.R.; Harper, D.M.; Grey, J. Population-level metrics of trophic structure based on stable isotopes and their application to invasion ecology. PLoS ONE 2012, 7, e31757. [Google Scholar] [CrossRef]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/:2022 (accessed on 1 August 2022).
- Miller, E.; Lalas, C.; Dawson, S.; Ratz, H.; Slooten, E. Hector’s dolphin diet: The species, sizes and relative importance of prey eaten by Cephalorhynchus hectori, investigated using stomach content analysis. Mar.Mammal Sci. 2013, 29, 606–628. [Google Scholar] [CrossRef]
- Rayment, W.; Webster, T. Observations of Hector’s dolphins (Cephalorhynchus hectori) associating with inshore fishing trawlers at Banks Peninsula, New Zealand. N. Z. J. Mar. Freshw. Res. 2009, 43, 911–916. [Google Scholar] [CrossRef]
- Baker, C.; Chilvers, B.; Constantine, R.; DuFresne, S.; Mattlin, R.; Van Helden, A.; Hitchmough, R. Conservation status of New Zealand marine mammals (suborders Cetacea and Pinnipedia), 2009. N. Z. J. Mar. Freshw. Res. 2010, 44, 101–115. [Google Scholar] [CrossRef][Green Version]
- Zaeschmar, J.R.; Tezanos-Pinto, G.; Dwyer, S.L.; Peters, C.H.; Berghan, J.; Donnelly, D.; Meissner, A.M.; Visser, I.N.; Weir, J.S.; Judkins, A.G. Occurrence, site fidelity, and associations of oceanic common bottlenose dolphins (Tursiops truncatus) off northeastern New Zealand. Mar. Mammal Sci. 2020, 36, 1180–1195. [Google Scholar] [CrossRef]
- Lusseau, S.M.; Wing, S.R. Importance of local production versus pelagic subsidies in the diet of an isolated population of bottlenose dolphins Tursiops sp. Mar. Ecol. Prog. Ser. 2006, 321, 283–293. [Google Scholar] [CrossRef][Green Version]
- Moreno, R.; Jover, L.; Munilla, I.; Velando, A.; Sanpera, C. A three-isotope approach to disentangling the diet of a generalist consumer: The yellow-legged gull in northwest Spain. Mar. Biol. 2010, 157, 545–553. [Google Scholar] [CrossRef]
- Ramírez, F.; Abdennadher, A.; Sanpera, C.; Jover, L.; Wassenaar, L.I.; Hobson, K.A. Assessing waterbird habitat use in coastal evaporative systems using stable isotopes (δ13C, δ15N and δD) as environmental tracers. Estuar. Coast. Shelf Sci. 2011, 92, 217–222. [Google Scholar] [CrossRef]
- Parra, G.J.; Wojtkowiak, Z.; Peters, K.J.; Cagnazzi, D. Isotopic niche overlap between sympatric Australian snubfin and humpback dolphins. Ecol. Evol. 2022, 12, e8937. [Google Scholar] [CrossRef] [PubMed]
- Meynier, L.; Stockin, K.; Bando, M.; Duignan, P. Stomach contents of common dolphin (Delphinus sp.) from New Zealand waters. N. Z. J. Mar. Freshw. Res. 2008, 42, 257–268. [Google Scholar] [CrossRef]
- Pusineri, C.; Magnin, V.; Meynier, L.; Spitz, J.; Hassani, S.; Ridoux, V. Food and feeding ecology of the common dolphin (Delphinus delphis) in the oceanic northeast atlantic and comparison with its diet in neritic areas. Mar. Mammal Sci. 2007, 23, 30–47. [Google Scholar] [CrossRef]
- Silva, M. Diet of common dolphins, Delphinus delphis, off the Portuguese continental coast. J. Mar. Biol. Assoc. UK 1999, 79, 531–540. [Google Scholar] [CrossRef]
- Stockin, K.A.; Pierce, G.J.; Binedell, V.; Wiseman, N.; Orams, M.B. Factors affecting the occurrence and demographics of common dolphins (Delphinus sp.) in the Hauraki Gulf, New Zealand. Aquat. Mamm. 2008, 34, 200–211. [Google Scholar] [CrossRef]
- Hinton, B.; Stockin, K.A.; Bury, S.J.; Peters, K.J.; Betty, E.l. Trophic niche analysis of long-finned pilot whales (Globicephala melas edwardii) in New Zealand waters. Biology, 2022; accepted. [Google Scholar]
- Beasley, I.; Cherel, Y.; Robinson, S.; Betty, E.; Hagihara, R.; Gales, R. Stomach contents of long-finned pilot whales, Globicephala melas mass-stranded in Tasmania. PLoS ONE 2019, 14, e0206747. [Google Scholar] [CrossRef]
- Beatson, E.; O’Shea, S.; Ogle, M. First report on the stomach contents of long-finned pilot whales, Globicephala melas, stranded in New Zealand. N. Z. J. Zool. 2007, 34, 51–56. [Google Scholar] [CrossRef]
- Hinton, B.; Stockin, K.A.; Betty, E.L. Intraspecific dietary variation of long-finned pilot whales (Globicephala melas edwardii) stranded on the Aotearoa, New Zealand coast. Mar. Biol. 2022; accepted. [Google Scholar]
- Luna, A.; Sánchez, P.; Chicote, C.; Gazo, M. Cephalopods in the diet of Risso’s dolphin (Grampus griseus) from the Mediterranean Sea: A review. Mar. Mammal Sci. 2021, 38, 725–741. [Google Scholar] [CrossRef]
- Stockin, K.A.; Amiot, C.; Meynier, L.M.; Purvin, C.; Machovsky-Capuska, G.E. A duplex lens: Understanding common dolphin and Australasian gannet feeding associations from an ethological and nutritional perspective. ICES J. Mar. 2022. [Google Scholar] [CrossRef]
- Meissner, A.M.; MacLeod, C.D.; Richard, P.; Ridoux, V.; Pierce, G. Feeding ecology of striped dolphins, Stenella coeruleoalba, in the north-western Mediterranean Sea based on stable isotope analyses. J. Mar. Biol. Assoc. UK 2012, 92, 1677–1687. [Google Scholar] [CrossRef]
- Spitz, J.; Richard, E.; Meynier, L.; Pusineri, C.; Ridoux, V. Dietary plasticity of the oceanic striped dolphin, Stenella coeruleoalba, in the neritic waters of the Bay of Biscay. J. Sea Res. 2006, 55, 309–320. [Google Scholar] [CrossRef]
- Ringelstein, J.; Pusineri, C.; Hassani, S.; Meynier, L.; Nicolas, R.; Ridoux, V. Food and feeding ecology of the striped dolphin, Stenella coeruleoalba, in the oceanic waters of the north-east Atlantic. J. Mar. Biol. Assoc. UK 2006, 86, 909–918. [Google Scholar] [CrossRef]
- Würtz, M.; Marrale, D. Food of striped dolphin, Stenella coeruleoalba, in the Ligurian Sea. J. Mar. Biol. Assoc. UK 1993, 73, 571–578. [Google Scholar] [CrossRef]
- McFadden, C.J. Behavioral Flexibility of Feeding Dusky Dolphins (Lagenorhynchus obscurus) in Admiralty Bay, New Zealand; Texas A&M University: College Station, TX, USA, 2004. [Google Scholar]
- Vaughn, R.L.; Shelton, D.E.; Timm, L.L.; Watson, L.A.; Würsig, B. Dusky dolphin (Lagenorhynchus obscurus) feeding tactics and multi-species associations. N. Z. J. Mar. Freshw. Res. 2007, 41, 391–400. [Google Scholar] [CrossRef]
- Markowitz, T.M. Social Organization of the New Zealand Dusky Dolphin; Texas A&M University: College Station, TX, USA, 2004. [Google Scholar]
- Beatson, E. The diet of pygmy sperm whales, Kogia breviceps, stranded in New Zealand: Implications for conservation. Rev. Fish. Biol. Fish. 2007, 17, 295–303. [Google Scholar] [CrossRef]
- West, K.; Walker, W.; Baird, R.; White, W.; Levine, G.; Brown, E.; Schofield, D. Diet of pygmy sperm whales (Kogia breviceps) in the Hawaiian Archipelago. Mar. Mammal Sci. 2009, 25, 931–943. [Google Scholar] [CrossRef]
- Spitz, J.; Cherel, Y.; Bertin, S.; Kiszka, J.; Dewez, A.; Ridoux, V. Prey preferences among the community of deep-diving odontocetes from the Bay of Biscay, Northeast Atlantic. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2011, 58, 273–282. [Google Scholar] [CrossRef]
- Staudinger, M.D.; McAlarney, R.J.; McLellan, W.A.; Ann Pabst, D. Foraging ecology and niche overlap in pygmy (Kogia breviceps) and dwarf (Kogia sima) sperm whales from waters of the US mid-Atlantic coast. Mar. Mammal Sci. 2014, 30, 626–655. [Google Scholar] [CrossRef]
- Meynier, L.; Pusineri, C.; Spitz, J.; Santos, M.B.; Pierce, G.J.; Ridoux, V. Intraspecific dietary variation in the short-beaked common dolphin Delphinus delphis in the Bay of Biscay: Importance of fat fish. Mar. Ecol. Prog. Ser. 2008, 354, 277–287. [Google Scholar] [CrossRef]
- Plön, S. The Status and Natural History of Pygmy (Kogia breviceps) and Dwarf (K. sima) Sperm Whales off Southern Africa; Rhodes University: Grahamstown, South Africa, 2004. [Google Scholar]
- Ford, J.K.; Ellis, G.M.; Barrett-Lennard, L.G.; Morton, A.B.; Palm, R.S.; Balcomb III, K.C. Dietary specialization in two sympatric populations of killer whales (Orcinus orca) in coastal British Columbia and adjacent waters. Can. J. Zool. 1998, 76, 1456–1471. [Google Scholar] [CrossRef]
- Ford, J.K.; Ellis, G.M. Selective foraging by fish-eating killer whales Orcinus orca in British Columbia. Mar. Ecol. Prog. Ser. 2006, 316, 185–199. [Google Scholar] [CrossRef]
- Andvik, C.; Jourdain, E.; Lyche, J.L.; Karoliussen, R.; Borga, K. High Levels of Legacy and Emerging Contaminants in Killer Whales (Orcinus orca) from Norway, 2015 to 2017. Environ. Toxicol. Chem. 2021, 40, 1848–1858. [Google Scholar] [CrossRef] [PubMed]
- Saulitis, E.; Matkin, C.; Barrett-Lennard, L.; Heise, K.; Ellis, G. Foraging strategies of sympatric killer whale (Orcinus orca) populations in Prince William Sound, Alaska. Mar. Mammal Sci. 2000, 16, 94–109. [Google Scholar] [CrossRef]
- Visser, I. Benthic foraging on stingrays by killer whales (Orcinus orca) in New Zealand waters. Mar. Mammal Sci. 1999, 15, 220–227. [Google Scholar] [CrossRef]
- Visser, I.N. First observations of feeding on thresher (Alopias vulpinus) and hammerhead (Sphyrna zygaena) sharks by killer whales (Orcinus orca) specialising on elasmobranch prey. Aquat. Mamm. 2005, 31, 83–88. [Google Scholar] [CrossRef]
- Visser, I.N.; Berghan, J.; van Meurs, R.; Fertl, D. Killer whale (Orcinus orca) predation on a shortfin mako shark (Isurus oxyrinchus) in New Zealand waters. Aquat. Mamm. 2000, 26, 229–231. [Google Scholar]
- Visser, I.N. A summary of interactions between orca (Orcinus orca) and other cetaceans in New Zealand waters. Ingrid N Viss. 1999, 24, 101–112. [Google Scholar]
- Jourdain, E.; Andvik, C.; Karoliussen, R.; Ruus, A.; Vongraven, D.; Borgå, K. Isotopic niche differs between seal and fish-eating killer whales (Orcinus orca) in northern Norway. Ecol. Evol. 2020, 10, 4115–4127. [Google Scholar] [CrossRef]
- Borisova, E.A.; Filatova, O.A.; Fedutin, I.D.; Tiunov, A.V.; Shpak, O.V.; Hoyt, E. Ecotype and geographical variation in carbon and nitrogen stable isotope values in western North Pacific killer whales (Orcinus orca). Mar. Mammal Sci. 2020, 36, 925–938. [Google Scholar] [CrossRef]
- Herman, D.; Burrows, D.; Wade, P.; Durban, J.; Matkin, C.; LeDuc, R.; Barrett-Lennard, L.; Krahn, M.M. Feeding ecology of eastern North Pacific killer whales Orcinus orca from fatty acid, stable isotope, and organochlorine analyses of blubber biopsies. Mar. Ecol. Prog. Ser. 2005, 302, 275–291. [Google Scholar] [CrossRef]
- Zaeschmar, J.R.; Dwyer, S.L.; Stockin, K.A. Rare observations of false killer whales (Pseudorca crassidens) cooperatively feeding with common bottlenose dolphins (Tursiops truncatus) in the Hauraki Gulf, New Zealand. Mar. Mammal Sci. 2013, 29, 555–562. [Google Scholar] [CrossRef]
- Zaeschmar, J.R.; Visser, I.N.; Fertl, D.; Dwyer, S.L.; Meissner, A.M.; Halliday, J.; Berghan, J.; Donnelly, D.; Stockin, K.A. Occurrence of false killer whales (Pseudorca crassidens) and their association with common bottlenose dolphins (Tursiops truncatus) off northeastern New Zealand. Mar. Mammal Sci. 2014, 30, 594–608. [Google Scholar] [CrossRef]
- Giménez, J.; Cañadas, A.; Ramírez, F.; Afán, I.; García-Tiscar, S.; Fernández-Maldonado, C.; Castillo, J.J.; de Stephanis, R. Living apart together: Niche partitioning among Alboran Sea cetaceans. Ecol. Indic. 2018, 95, 32–40. [Google Scholar] [CrossRef]
- MacKenzie, K.M.; Lydersen, C.; Haug, T.; Routti, H.; Aars, J.; Andvik, C.M.; Borgå, K.; Fisk, A.T.; Meier, S.; Biuw, M.; et al. Niches of marine mammals in the European Arctic. Ecol. Indic. 2022, 136, 108661. [Google Scholar] [CrossRef]
- MacLeod, C.D.; Santos, M.; Pierce, G.J. Review of data on diets of beaked whales: Evidence of niche separation and geographic segregation. J. Mar. Biol. Assoc. UK 2003, 83, 651–665. [Google Scholar] [CrossRef]
- Beatson, E. Stomach contents of beaked whales (Ziphiidae) stranded on the New Zealand coast. In Proceedings of the New Zealand Marine Sciences Society Conference, Auckland, New Zealand, 24—26 November 2009. [Google Scholar]
- Gómez-Villota, F. Sperm Whale Diet in New Zealand; Auckland University of Technology: Auckland, New Zealand, 2007. [Google Scholar]
- Palmer, E.; Alexander, A.; Liggins, L.; Guerra, M.; Bury, S.J.; Hendriks, H.; Stockin, K.A.; Peters, K.J. A piece of the puzzle: Analyses of recent strandings and historical records reveal new genetic and ecological insights on New Zealand sperm whales Mar. Ecol. Prog. Ser. 2022, 690, 201–217. [Google Scholar] [CrossRef]
- Clarke, M.; Roper, C. Cephalopods represented by beaks in the stomach of a sperm whale stranded at Paekakariki, North Island, New Zealand. Afr. J. Mar. Sci. 1998, 20, 1814–2338. [Google Scholar] [CrossRef]
- Guerra, M.; Wing, L.; Dawson, S.; Rayment, W. Stable isotope analyses reveal seasonal and inter-individual variation in the foraging ecology of sperm whales. Mar. Ecol. Prog. Ser. 2020, 638, 207–219. [Google Scholar] [CrossRef]
- Guerra, M.; Hickmott, L.; van der Hoop, J.; Rayment, W.; Leunissen, E.; Slooten, E.; Moore, M. Diverse foraging strategies by a marine top predator: Sperm whales exploit pelagic and demersal habitats in the Kaikōura submarine canyon. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2017, 128, 98–108. [Google Scholar] [CrossRef]
- Riccialdelli, L.; Viola, M.N.P.; Panarello, H.O.; Goodall, R.N.P. Evaluating the isotopic niche of beaked whales from the southwestern South Atlantic and Southern Oceans. Mar. Ecol. Prog. Ser. 2017, 581, 183–198. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Klages, N.; Findlay, K. Feeding habits and possible movements of southern bottlenose whales (Hyperoodon planifrons). Proc. NIPR Symp. Polar Biol. 1993, 6, 84–97. [Google Scholar]
- Fernández, R.; Pierce, G.J.; MacLeod, C.D.; Brownlow, A.; Reid, R.J.; Rogan, E.; Addink, M.; Deaville, R.; Jepson, P.D.; Santos, M.B. Strandings of northern bottlenose whales, Hyperoodon ampullatus, in the north-east Atlantic: Seasonality and diet. J. Mar. Biol. Assoc. UK 2014, 94, 1109–1116. [Google Scholar] [CrossRef]
- Santos, M.; Pierce, G.J.; Smeenk, C.; Addink, M.; Kinze, C.; Tougaard, S.; Herman, J. Stomach contents of northern bottlenose whales Hyperoodon ampullatus stranded in the North Sea. J. Mar. Biol. Assoc. UK 2001, 81, 143–150. [Google Scholar] [CrossRef]
- Hooker, S.K.; Iverson, S.J.; Ostrom, P.; Smith, S.C. Diet of northern bottlenose whales inferred from fatty-acid and stable-isotope analyses of biopsy samples. Can. J. Zool. 2001, 79, 1442–1454. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Klages, N.; Best, P. The diet of strap-toothed whales (Mesoplodon layardii). J. Zool. 1996, 239, 453–463. [Google Scholar] [CrossRef]
- Goodall, R.; Baker, A.; Best, P.; Meyer, M.; Miyazaki, N. On the biology of the hourglass dolphin, Lagenorhynchus cruciger (Quoy and Gaimard, 1824). Rep. Int. Whal. Comm. 1997, 47, 985–999. [Google Scholar]
- Cherel, Y.; Hobson, K.A. Geographical variation in carbon stable isotope signatures of marine predators: A tool to investigate their foraging areas in the Southern Ocean. Mar. Ecol. Prog. Ser. 2007, 329, 281–287. [Google Scholar] [CrossRef]
- Riccialdelli, L.; Newsome, S.D.; Fogel, M.L.; Goodall, R.N.P. Isotopic assessment of prey and habitat preferences of a cetacean community in the southwestern South Atlantic Ocean. Mar. Ecol. Prog. Ser. 2010, 418, 235–248. [Google Scholar] [CrossRef]
- Browning, N.E.; Dold, C.J.I.F.; Worthy, G.A. Isotope turnover rates and diet-tissue discrimination in skin of ex situ bottlenose dolphins (Tursiops truncatus). J. Exp. Biol. 2014, 217, 214–221. [Google Scholar] [CrossRef]
- Giménez, J.; Ramírez, F.; Almunia, J.G.; Forero, M.; de Stephanis, R. From the pool to the sea: Applicable isotope turnover rates and diet to skin discrimination factors for bottlenose dolphins (Tursiops truncatus). J. Exp. Mar. Biol. Ecol. 2016, 475, 54–61. [Google Scholar] [CrossRef]
- Busquets-Vass, G.; Newsome, S.D.; Calambokidis, J.; Serra-Valente, G.; Jacobsen, J.K.; Aguíñiga-García, S.; Gendron, D. Estimating blue whale skin isotopic incorporation rates and baleen growth rates: Implications for assessing diet and movement patterns in mysticetes. PLoS ONE 2017, 12, e0177880. [Google Scholar] [CrossRef] [PubMed]
- Connolly, R.M.; Guest, M.A.; Melville, A.J.; Oakes, J.M. Sulfur stable isotopes separate producers in marine food-web analysis. Oecologia 2004, 138, 161–167. [Google Scholar] [CrossRef]
- Clementz, M.T.; Koch, P.L. Differentiating aquatic mammal habitat and foraging ecology with stable isotopes in tooth enamel. Oecologia 2001, 129, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Shipley, O.N.; Matich, P. Studying animal niches using bulk stable isotope ratios: An updated synthesis. Oecologia 2020, 193, 27–51. [Google Scholar] [CrossRef] [PubMed]
- Rosel, P.E. PCR-based sex determination in Odontocete cetaceans. Conserv. Genet. 2003, 4, 647–649. [Google Scholar] [CrossRef]
- Richard, K.R.; McCarrey, S.W.; Wright, J.M. DNA sequence from the SRY gene of the sperm whale (Physeter macrocephalus) for use in molecular sexing. Can. J. Zool. 1994, 72, 873–877. [Google Scholar] [CrossRef]
- Bérubé, M.; Palsbøll, P. Identification of sex in cetaceans by multiplexing with three ZFX and ZFY specific primers. Mol. Ecol. 1996, 5, 283–287. [Google Scholar] [CrossRef]
- Layman, C.A.; Arrington, D.A.; Montaña, C.G.; Post, D.M. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 2007, 88, 42–48. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).