Ecological Traits and Trophic Plasticity in The Greater Pipefish Syngnathus acus in the NW Iberian Peninsula
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
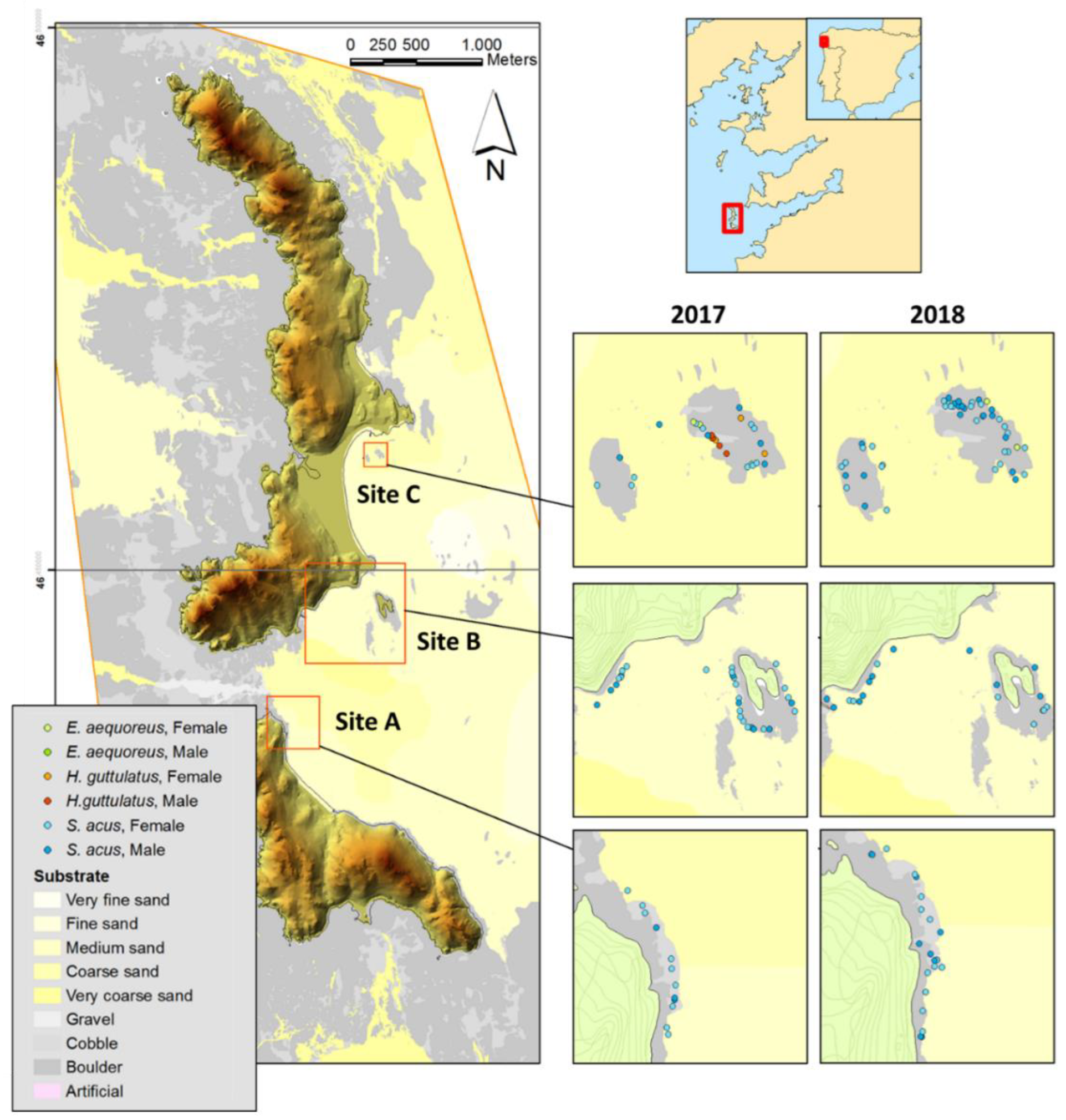
2.1. Study Sites
2.2. Fish Collection and Sampling
2.3. Stable Isotopes Analysis (SIA)
2.4. Data Analysis
2.5. Ethics
3. Results
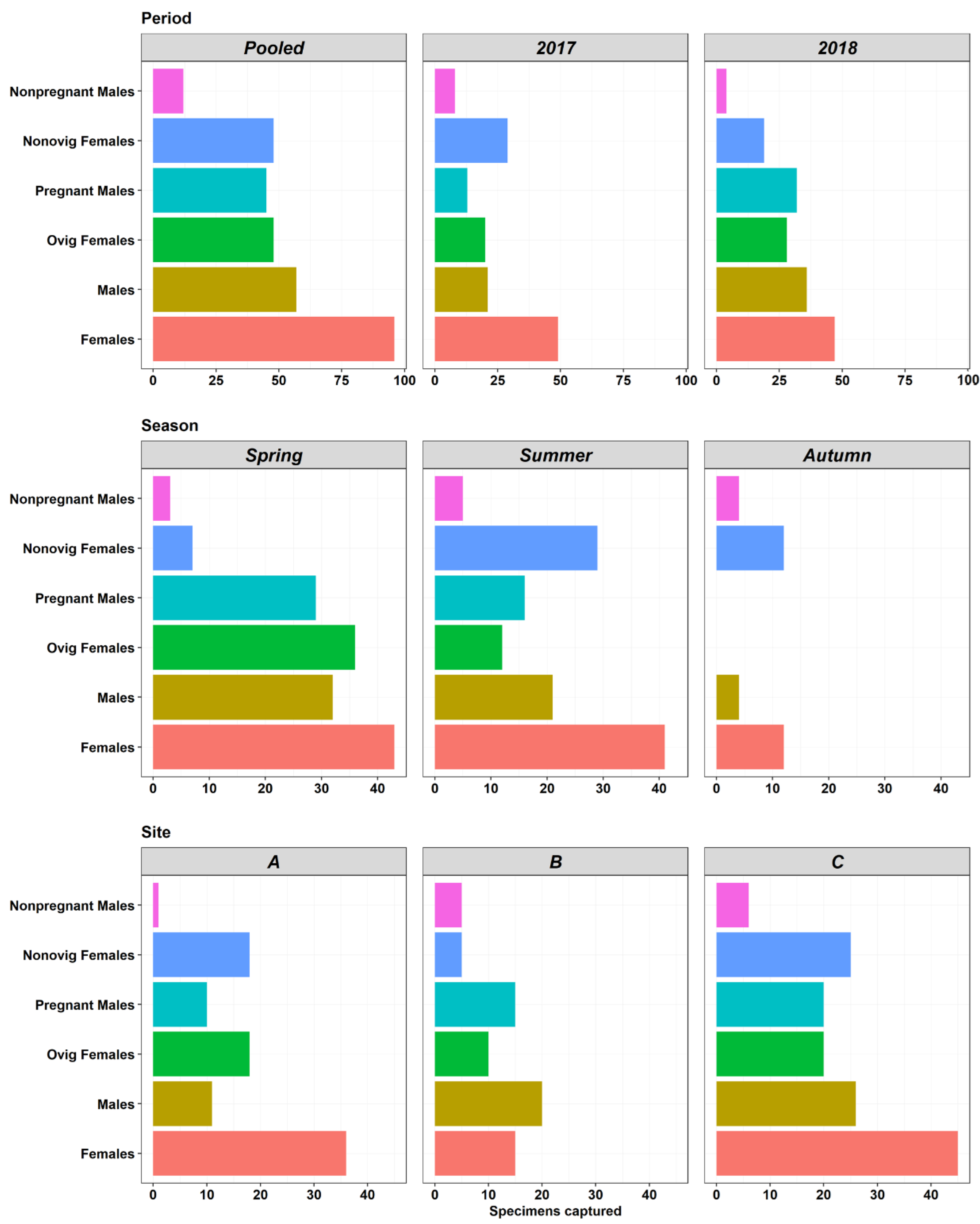
3.1. Abundances and Population Characteristics
3.2. Reproduction Traits
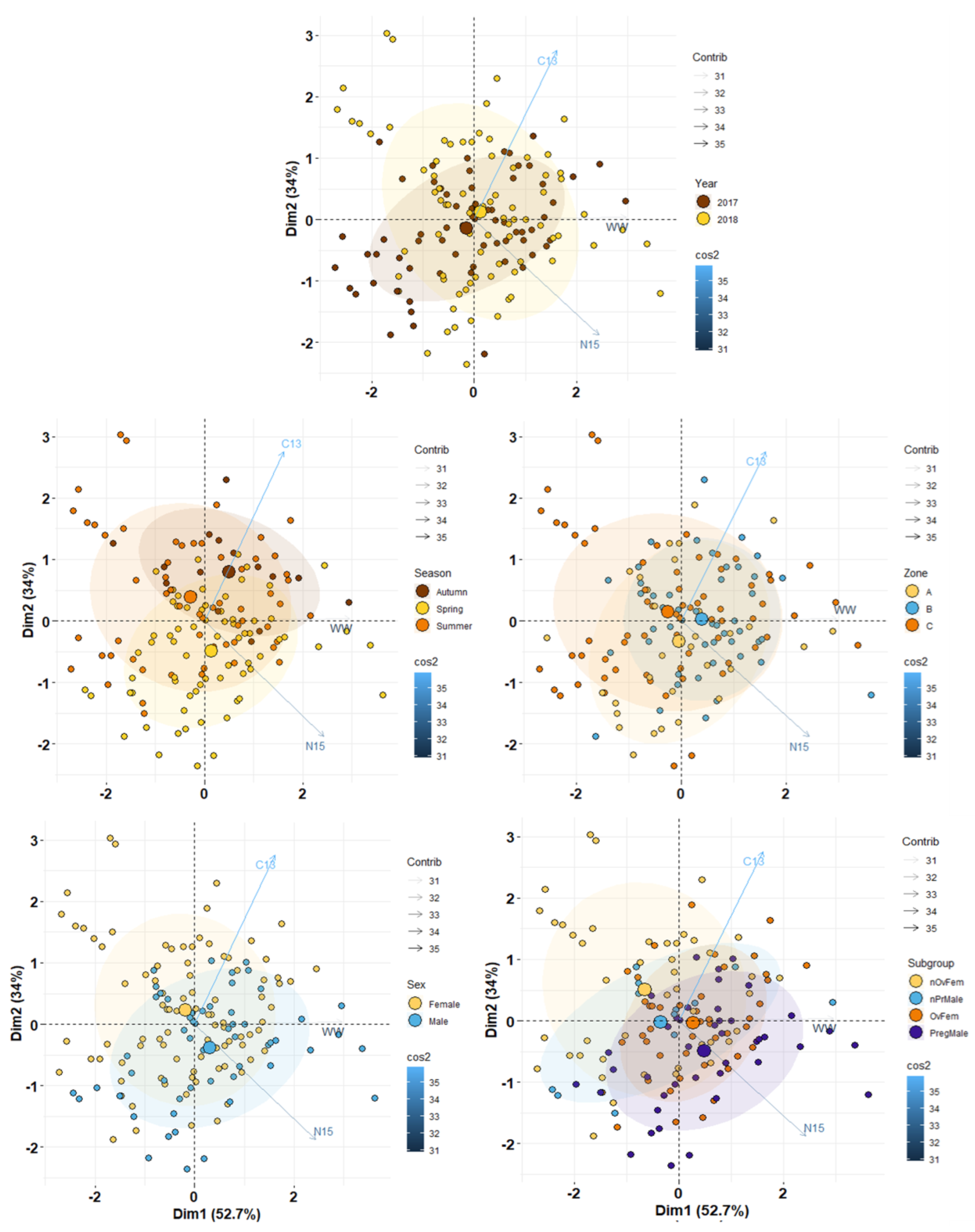
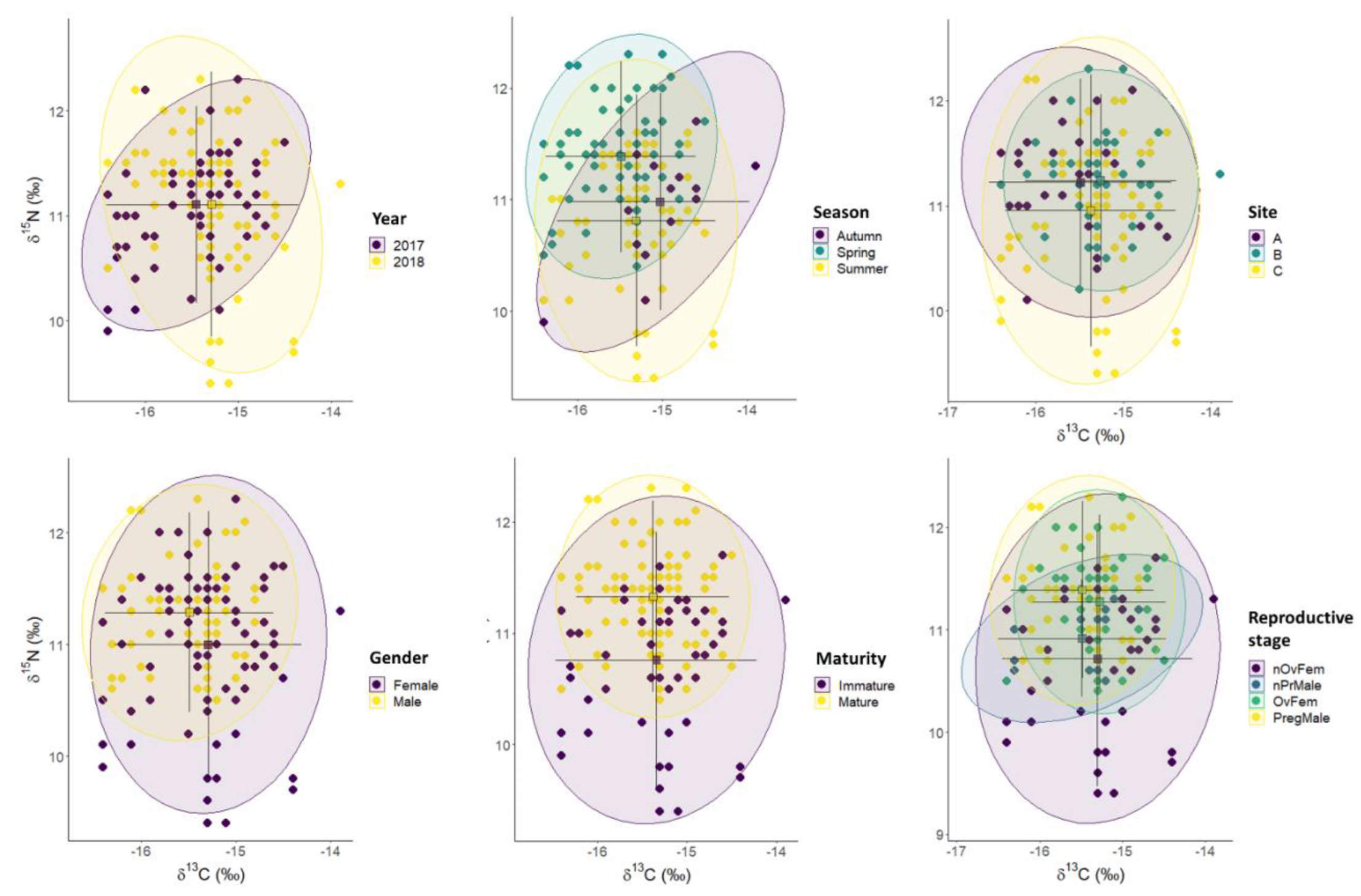
3.3. Isotopic Profiles
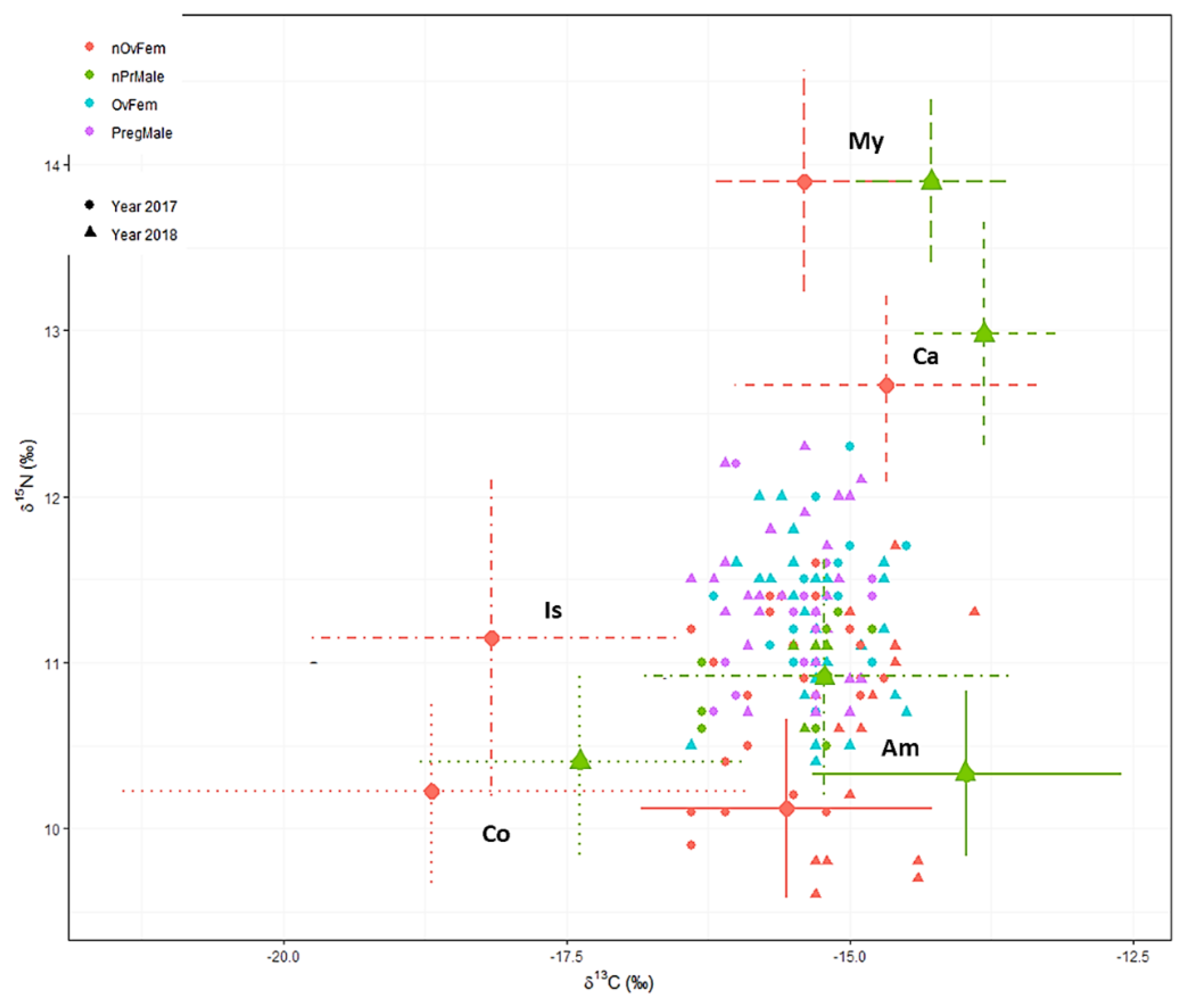
3.4. Isotopic Inheritance
3.5. Trophic Position
3.6. Trophic Niche
3.7. Feeding Regimes
4. Discussion
4.1. Population Characteristics
4.2. Early Development
4.3. Trophic Features
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FishBase. Syngnathus acus . 2022. Available online: https://www.fishbase.de/summary/Syngnathus-acus.html (accessed on 11 January 2022).
- Gurkan, Ş.; Taşkavak, E.; Hossucu, B. The reproductive biology of the Great Pipefish Syngnathus acus (Family: Syngnathidae) in the Aegean Sea. North-West J. Zool. 2009, 5, 179–190. [Google Scholar]
- Smith-Vaniz, W.F. Syngnathus acus. The IUCN Red List of Threatened Species 2015: E.T198765A44933898. Available online: 10.2305/ıucn.uk.20154 (accessed on 13 January 2022).
- Vincent, A.C.J.; Berglund, A.; Ahnesjö, I. Reproductive ecology of five pipefish species in one eelgrass meadow. Environ. Biol. Fish. 1995, 44, 347–361. [Google Scholar] [CrossRef]
- Gurkan, Ş.; Taşkavak, E. Length-weight relationships for syngnathid fishes of the Aegean Sea. Turkey. Belg. J. Zool. 2007, 137, 219–222. [Google Scholar]
- Vieira, R.P.; Monteiro, P.; Ribeiro, J.; Bentes, L.; Oliveira, F.; Erzini, K.; Gonçalves, J.M.S. Length-weight relationships of six syngnathid species from Ria Formosa, SW Iberian coast. Cah. Biol Mar. 2014, 55, 9–12. [Google Scholar]
- Yildiz, T.; Uzer, U.; Karakulak, F.S. Preliminary report of a biometric analysis of greater pipefish Syngnathus acus Linnaeus, 1758 for the western Black Sea. Turk. J. Zool. 2015, 39, 917–924. [Google Scholar] [CrossRef]
- Gurkan, Ş.; Innal, D. Some morphometric features of congeneric pipefish species (Syngnathus abaster Risso 1826, Syngnathus acus Linnaeus, 1758) distributed in Lake Bafa (Turkey). Oceanol. Hydrobiol. Stud. 2018, 47, 239–247. [Google Scholar] [CrossRef]
- Taşkavak, E.; Gürkan, Ş.; Severa, T.M.; Akalına, S.; Özaydına, O. Gut contents and feeding habits of the Great Pipefish, Syngnathus acus Linnaeus, 1758, in Izmir Bay (Aegean Sea, Turkey). Zool. Middle East 2010, 50, 75–82. [Google Scholar] [CrossRef]
- Gurkan, Ş.; Taşkavak, E. The relationships between gut length and prey preference of three pipefish (Syngnathus acus, Syngnathus typhle, Nerophis ophidion Linnaeus, 1758) species distributed in Aegean Sea, Turkey. Iran. J. Fish. Sci. 2019, 18, 1093–1100. [Google Scholar] [CrossRef]
- Gurkan, Ş.; Innal, D.; Gulle, I. Monitoring of the trophic ecology of pipefish species (Syngnathus abaster, Syngnathus acus) in an alluvial lake habitat (Lake Bafa, Turkey). Oceanol. Hydrobiol. Stud. 2021, 50, 24–32. [Google Scholar] [CrossRef]
- Jennings, S.; van der Molen, J. Trophic levels of marine consumers from nitrogen stable isotope analysis: Estimation and uncertainty. ICES J. Mar. Sci. 2015, 72, 2289–2300. [Google Scholar] [CrossRef]
- Silva, K.; Monteiro, N.M.; Almada, V.C.; Vieira, M.N. Development and early life history behaviour of aquarium reared Syngnathus acus (Pisces: Syngnathidae). J Mar Biol Ass UK 2006, 86, 1469–1472. [Google Scholar] [CrossRef]
- Piñeiro-Corbeira, C.; Iglesias, L.; Nogueira, R.; Campos, S.; Jiménez, A.; Regueira, M.; Barreiro, R.; Planas, M. Structure and trophic niches in mobile epifauna assemblages associated with seaweeds and habitats of syngnathid fishes in Cíes Archipelago (Atlantic Islands Marine National Park, NW Iberia). Front. Mar. Sci. 2021, 8, 773367. [Google Scholar] [CrossRef]
- Planas, M.; Piñeiro-Corbeira, C.; Bouza, C.; Castejón-Silvo, I.; Vera, M.; Regueira, M.; Ochoa, V.; Bárbara, I.; Terrados, J.; Chamorro, A.; et al. A multidisciplinary approach to identify priority areas for the monitoring of a vulnerable family of fishes in Spanish Marine National Parks. BMC Ecol. Evol. 2021, 21, 4. [Google Scholar] [CrossRef]
- Hernández-Urcera, J.; Murillo, F.J.; Regueira, M.; Cabanellas-Reboredo, M.; Planas, M. Preferential habitats prediction in syngnathids using species distribution models. Mar. Environ. Res. 2021, 172, 105488. [Google Scholar] [CrossRef] [PubMed]
- Manning, C.G.; Foster, S.J.; Vincent, A.C.J. A review of the diets and feeding behaviours of a family of biologically diverse marine fishes (Family Syngnathidae). Rev. Fish Biol. Fisheries 2019, 29, 197–221. [Google Scholar] [CrossRef]
- Ryer, C.H. Pipefish foraging: Effects of fish size, prey size and altered habitat complexity. Mar. Ecol. Prog. Ser. 1988, 48, 37–45. [Google Scholar] [CrossRef]
- Ape, F.; Corriero, G.; Mirto, S.; Pierri, C.; Lazic, T.; Gristina, M. Trophic flexibility and prey selection of the wild long-snouted seahorse Hippocampus guttulatus Cuvier, 1829 in three coastal habitats. Estuar. Coast. Shelf. Sea 2019, 224, 1–10. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochi. Cosmochi. Act. 1978, 42, 495–506. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochi. Cosmochi. Act. 1981, 45, 341–351. [Google Scholar] [CrossRef]
- Hesslein, R.H.; Hallard, K.A.; Ramlal, P. Replacement of sulphur, carbon and nitrogen in tissue of growing broad whitefish (Coregonus nasus) in response to a change in diet traced by δ 34S, δ 13C and δ 15N. Can. J. Fish. Aquat. Sci. 1993, 50, 2071–2076. [Google Scholar] [CrossRef]
- Vander Zanden, M.J.; Rasmussen, J.B. Variation in δ15N and δ13C trophic fractionation: Implications for aquatic food web studies. Limnol. Oceanogr. 2001, 46, 2061–2066. [Google Scholar] [CrossRef]
- Gamboa-Delgado, J.; Cañavate, J.P.; Zerolo, R.; Le Vay, L. Natural carbon stable isotope ratios as indicators of the relative contribution of live and inert diets to growth in larval Senegalese sole (Solea senegalensis). Aquaculture 2008, 280, 190–197. [Google Scholar] [CrossRef]
- Caut, S.; Angulo, E.; Courchamp, F. Variation in discrimination factors (∆15N and ∆13C): The effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 2009, 46, 443–453. [Google Scholar] [CrossRef]
- Soto, D.X.; Wassenaar, L.I.; Hobson, K.A. Stable hydrogen and oxygen isotopes in aquatic food webs are tracers of diet and provenance. Funct. Ecol. 2013, 27, 535–543. [Google Scholar] [CrossRef]
- Hobson, K.A.; Barnett-Johnson, R.; Cerling, T. Using isoscapes to track animal migration. In Isoscapes: Understanding Movement, Pattern, and Process on Earth through Isotope Mapping; West, J.B., Bowen, G.J., Dawson, T.E., Tu, K.P., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 273–298. [Google Scholar]
- Zbinden, J.A.; Bearhop, S.; Bradshaw, P.; Gill, B.; Margaritoulis, D.; Newton, J.; Godley, B.J. Migratory dichotomy and associated phenotypic variation in marine turtles revealed by satellite tracking and stable isotope analysis. Mar. Ecol. Prog. Ser. 2011, 421, 291–302. [Google Scholar] [CrossRef]
- Doucett, R.R.; Hooper, W.; Power, G. Identification of anadromous and nonanadromous brook trout and their progeny in the Tabusintac River, New Brunswick, by means of multiple-stable-isotope analysis. Trans. Am. Fish. Soc. 1999, 128, 278–288. [Google Scholar] [CrossRef]
- Sare, D.T.J.; Miller, J.S.; Longstaffe, F.J. Nitrogen- and carbon-isotope fractionation between mothers and offspring in red-backed voles (Clethrionomys gapperi). Can. J. Zool. 2005, 83, 712–716. [Google Scholar] [CrossRef]
- Jardine, T.D.; Chernoff, E.; Curry, R.A. Maternal transfer of carbon and nitrogen to progeny of sea-run and resident brook trout (Salvelinus fontinalis). Can. J. Fish. Aquat. Sci. 2008, 65, 2201–2210. [Google Scholar] [CrossRef][Green Version]
- Vaudo, J.J.; Matich, P.; Heithaus, M.R. Mother-offspring isotope fractionation in two species of placentatrophic sharks. J. Fish Biol 2010, 77, 1724–1727. [Google Scholar] [CrossRef]
- Frankel, N.S.; Vander Zanden, A.B.; Reich, K.J.; Williams, K.L.; Bjorndal, K.A. Mother−offspring stable isotope discrimination in loggerhead sea turtles Caretta caretta. Endanger. Species Res. 2012, 17, 133–138. [Google Scholar] [CrossRef]
- UNESCO. World Heritage candidate. Available online: https://whc.unesco.org/en/tentativelists/6286/ (accessed on 13 December 2019).
- Fernández, E.; Barañano, C.; Alejo, I.; Barreiro, R.; Bellas, J.; Besada, V.; Calviño-Cancela, M.; Cordero-Rivera, A.; González, A.; Méndez, G.; et al. Islas Cíes: Un Ecosistema en la Frontera; Concello de Vigo: Vigo, Spain, 2020; p. 240. [Google Scholar]
- Planas, M.; Chamorro, A.; Quintas, P.; Vilar, A. Establishment and maintenance of threatened long-snouted seahorse, Hippocampus guttulatus, broodstock in captivity. Aquaculture 2008, 283, 19–28. [Google Scholar] [CrossRef]
- Valladares, S.; Planas, M. Non-lethal dorsal fin sampling for stable isotope analysis in seahorses. Aquat. Ecol. 2012, 46, 363–370. [Google Scholar] [CrossRef]
- Bouza, C.; Vera, M.; Pardo, B.G.; Planas, M.; Castejón-Silvo, I. Caracterización genética de signátidos en los Parques Nacionales de las Islas Atlánticas y del Archipiélago de Cabrera. In Proyectos de Investigación en Parques Nacionales: 2015–2018; Amengual, J., Ed.; Organismo Autónomo de Parques Nacionales, Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain. (in press)
- Wilson, A.B.; Vincent, A.C.J.; Ahnesjö, I.; Meyer, A. Male pregnancy in seahorses and pipefishes (Family Syngnathidae): Rapid diversification of paternal brood pouch morphology inferred from a molecular phylogeny. J. Hered. 2001, 92, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Woodall, L.C.; Koldewey, H.J.; Boehm, J.T.; Shaw, W. Past and present drivers of population structure in a small coastal fish, the European long snouted seahorse Hippocampus guttulatus. Conserv. Genet. 2015, 16, 1139–1153. [Google Scholar] [CrossRef]
- Emlen, S.T.; Oring, L.W. Ecology, sexual selection, and the evolution of mating systems. Science 1977, 197, 215–223. [Google Scholar] [CrossRef]
- Monteiro, N.M.; Almada, V.C.; Vieira, M.N. Early life history of the pipefish Nerophis lumbriciformis (Pisces: Syngnathidae). J. Mar. Biol. Ass. UK 2003, 83, 1179–1182. [Google Scholar] [CrossRef]
- Sommer, S.; Whittington, C.M.; Wilson, A.B. Standardised classification of pre-release development in male-brooding pipefish, seahorses, and seadragons (Family Syngnathidae). BMC Dev. Biol. 2012, 12, 12–39. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Post, D.M.; Layman, C.A.; Arrington, G.; Takimoto, J.; Quatrocchi, J.; Montaña, C.G. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef]
- Planas, M.; Paltrinieri, A.; Dias-Carneiro, M.D.; Hernández-Urcera, J. Effects of tissue preservation on carbon and nitrogen stable isotope signatures in Syngnathid fishes and prey. Animals 2020, 10, 2301. [Google Scholar] [CrossRef]
- Jaschinski, S.; Hansen, T.; Sommer, U. Effects of acidification in multiple stable isotope analyses. Limnol. Oceanogr. Methods 2008, 6, 12–15. [Google Scholar] [CrossRef]
- Vafeiadou, A.M.; Adão, H.; De Troch, M.; Moens, T. Sample acidification effects on carbon and nitrogen stable isotope ratios of macrofauna from a Zostera noltii bed. Mar. Freshw. Res. 2013, 64, 741–745. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org (accessed on 13 December 2020).
- Kassambara, A. Ggpubr: ‘Ggplot2′ Based Publication Ready Plots. 2020. Available online: https://CRAN.R-project.org/package=clinfun (accessed on 23 February 2020).
- Giraudoux, P.; Antonietti, J.P.; Beale, C.; Pleydell, D.; Treglia, M. Spatial Analysis and Data Mining for Field Ecologists v 1.6.9. 2018. Available online: https://cran.r-project.org/web/packages/pgirmess/index.html (accessed on 16 January 2018).
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Oksanen, J.; Guillaume Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 13 February 2020).
- Husson, F.; Josse, J.; Le, S.; Mazet, J. FactoMineR: Multivariate Exploratory Data Analysis and Data Mining. 2020. Available online: https://CRAN.R-project.org/package=FactoMineR (accessed on 20 March 2020).
- Kassambara, A. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 13 February 2020).
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. corrplot: Visualization of a Correlation Matrix. WWW Document. 2017. Available online: https://CRAN.R-project.org/package=corrplot (accessed on 13 December 2020).
- Quezada-Romegialli, C.; Jackson, A.L.; Hayden, B.; Kahilainen, K.K.; Lopes, C.; Harrod, C. tRophicPosition, an R package for the Bayesian estimation of trophic position from consumer stable isotope ratios. Methods Ecol. Evol. 2018, 9, 1592–1599. [Google Scholar] [CrossRef]
- Planas, M.; Chamorro, A.; Paltrinieri, A.; Campos, S.; Nedelec, K.; Hernández-Urcera, J. Effect of diet on breeders and inheritance in Syngnathids: Application of isotopic experimentally derived data to field studies. Mar. Ecol. Prog. Ser. 2020, 650, 107–123. [Google Scholar] [CrossRef]
- Swanson, H.K.; Lysy, M.; Power, M.; Stasko, A.D.; Johnson, J.D.; Reist, J.D. A new probabilistic method for quantifying n-dimensional ecological niches and niche overlap. Ecology 2015, 96, 318–324. [Google Scholar] [CrossRef]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER-Stable isotope Bayesian ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef]
- Lysy, M.; Stasko, A.D.; Swanson, H.K. Nicherover: (Niche) (R)egion and Niche(Over)Lap Metrics for Multidimensional Ecological Niches. R Package Version 1.1.0. 2014. Available online: https://CRAN.R-project.org/package=nicheROVER (accessed on 13 December 2020).
- Stock, B.; Semmens, B. MixSIAR GUI us.er manu.al. 2016. Version 3. 2016. Available online: https://github.com/brianstock/MixSIAR (accessed on 13 December 2020). [CrossRef]
- Stock, B.C.; Jackson, A.L.; Ward, E.J.; Parnell, A.C.; Phillips, D.L.; Semmens, B.X. Analyzing mixing systems using a new generation of Bayesian tracer mixing models. Peer. J. 2018, 6, e5096. [Google Scholar] [CrossRef]
- Phillips, D.L.; Gregg, J.W. Source partitioning using stable isotopes: Coping with too many sources. Oecologia 2003, 136, 261–269. [Google Scholar] [CrossRef]
- Smith, J.A.; Mazumder, D.; Suthers, I.M.; Taylor, M.D. To fit or not to fit: Evaluating stable isotope mixing models using simulated mixing polygons. Methods Ecol. Evol. 2013, 4, 612–618. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. 2020. Available online: https://CRAN.R-project.org/package=ggplot2. (accessed on 17 September 2019).
- Sarkar, D.; Andrews, F.; Wright, K.; Klepeis, N.; Murrell, P. Lattice: Trellis Graphics for R. 2020. Available online: https://cran.r-project.org/web/packages/lattice/index.html (accessed on 17 September 2019).
- Lazzari, K.; Able, K.W. Northern pipefish, Syngnathus fuscus, occurrences over the Mid-Atlantic Bight continental shelf: Evidence of seasonal migration. Environ. Biol. Fishes 1990, 27, 177–185. [Google Scholar] [CrossRef]
- Franzoi, P.; Maccagnani, R.; Rossi, R.; Ceccherelli, V.U. Life cycles and feeding habits of Syngnathus taenionotus and S. abaster (Pisces, Syngnathidae) in of the PO River Delta (Adriatic Sea). Mar. Ecol. Prog. Ser. 1993, 97, 71–81. [Google Scholar] [CrossRef]
- Monteiro, N.M.; Almada, V.C.; Santos, A.M.; Vieira, M.N. The breeding ecology of the pipefish Nerophis lumbriciformis and its relation to latitude and water temperature. J. Mar. Biol. Ass. UK 2001, 81, 1031–1033. [Google Scholar] [CrossRef]
- Masonjones, H.D.; Rose, E.; McRae, L.B.; Dixson, D.L. An examination of the population dynamics of syngnathid fishes within Tampa Bay, Florida, USA. Curr. Zool. 2010, 56, 118–133. [Google Scholar] [CrossRef]
- Valle, C.; Bayle, J.T.; Ramos, A.A. Weight-length relationships for selected fish species of the western Mediterranean Sea. J. Appl. Ichthyol. 2003, 19, 261–262. [Google Scholar] [CrossRef]
- Day, J.H.; Blaber, S.J.M.; Wallace, H. Estuarine fishes. In Estuarine Ecology with Particular Reference to Southern Africa; Day, J.H., Ed.; AA Balkema: Cape Town, South Africa, 1981; pp. 197–221. [Google Scholar]
- Harrison, T.D. Length-weight relationships of fishes from South African estuaries. J. Appl. Ichthyol. 2001, 17, 46–48. [Google Scholar] [CrossRef]
- Dawson, C.E. Syngnathidae. In Fishes of the North-eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hereau, J.C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; pp. 628–639. [Google Scholar]
- Veiga, P.; Machado, D.; Almeida, C.; Bentes, L.; Monteiro, P.; Oliveira, F.; Ruano, M.; Erzini, K.; Gonçalves, J.M.S. Weight-length relationships for 54 species of the Arade estuary, southern Portugal. J. Appl. Ichthyol. 2009, 25, 493–496. [Google Scholar] [CrossRef]
- Koutrakis, E.T.; Tsikliras, A.C. Length-weight relationships of fishes from three northern Aegean estuarine systems (Greece). J. Appl. Ichthyol. 2003, 19, 258–260. [Google Scholar] [CrossRef]
- Watanabe, S.; Watanabe, Y. Brooding season, sex ratio, and brood pouch development in the seaweed pipefish, Syngnathus schlegeli, in Otsuchi Bay, Japan. Ichthyol. Res. 2001, 48, 155–160. [Google Scholar] [CrossRef]
- Ahnesjö, I. Consequences of male brood care: Weight and number of newborn in a sex-role reversed pipefish. Funct.Ecol. 1992, 6, 274–281. [Google Scholar] [CrossRef]
- Berglund, A.; Rosenqvist, G. Selective males and ardent females in pipefishes. Behav. Ecol. Sociobiol. 1993, 32, 331–336. [Google Scholar] [CrossRef]
- Ripley, J.L.; Foran, C.M. Differential parental nutrient allocation in two congeneric pipefish species (Syngnathidae: Syngnathus spp.). J. Exp. Biol. 2006, 209, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.B.; Ahnesjö, I.; Vincent, A.C.J.; Meyer, A. The dynamics of male brooding, mating patterns, and sex roles in pipefishes and seahorses (family Syngnathidae). Evolution 2003, 57, 1374–1386. [Google Scholar] [CrossRef] [PubMed]
- Ahnesjö, I.; Forsgren, E.; Kvarnemo, C. Variation in sexual selection in fishes. In Fish Behaviour; Magnhagen, C., Braithwaite, V.A., Forsgren, E., Kapoor, B.G., Eds.; Science Publishers Inc.: Boca Raton, FL, USA, 2008; pp. 303–336. [Google Scholar]
- Steffe, A.S.; Westoby, M.; Bell, J.D. Habitat selection and diet in two species of pipefish from seagrass: Sex differences. Mar. Ecol. Prog. Ser. 1989, 55, 23–30. [Google Scholar] [CrossRef]
- Rosenqvist, G. Sex role reversal in a pipefish. Mar. Behav. Mar. Behav. Physiol. 1993, 23, 219–230. [Google Scholar] [CrossRef]
- Svensson, I. Reproductive costs in two sex-role reversed pipefish species (Syngnathidae). J. Anim. Ecol. 1988, 57, 929–942. [Google Scholar] [CrossRef]
- Vincent, A.; Ahnesjö, I.; Berglund, A.; Rosenqvist, G. Pipefishes and seahorses: Are they all sex role reversed? Trends. Ecol. Evol. 1992, 7, 237–241. [Google Scholar] [CrossRef]
- Berglund, A.; Rosenqvist, G. Sex role reversal in pipefish. Adv. Study Behav. 2003, 32, 131–167. [Google Scholar] [CrossRef]
- Rosenqvist, G.; Berglund, A. Sexual signals and mating patterns in Syngnathidae. J. Fish Biol. 2011, 78, 1647–1661. [Google Scholar] [CrossRef]
- Jones, A.G.; Avise, J.C. Mating systems and sexual selection in male-pregnant pipefishes and seahorses: Insights from microsatellite-based studies of maternity. J. Hered. 2001, 92, 150–158. [Google Scholar] [CrossRef]
- Finn, R.N.; Henderson, J.R.; Fyhn, H.J. Physiological energetics of developing embryos and yolk-sac larvae of Atlantic cod (Gadus morhua). II. Lipid metabolism and enthalpy balance. Mar. Biol. 1995, 124, 371–379. [Google Scholar] [CrossRef]
- Wiegand, M.D. Composition, accumulation and utilization of yolk lipids in teleost fish. Rev. Fish. Biol. Fish. 1996, 6, 259–286. [Google Scholar] [CrossRef]
- Rainuzzo, J.R.; Reitan, K.I.; Olsen, Y. The significance of lipids at early stages of marine fish: A review. Aquaculture 1997, 155, 103–115. [Google Scholar] [CrossRef]
- Rønnestad, I.; Koven, W.; Tandler, A.; Harel, M.; Fyhn, H.J. Utilisation of yolk fuels in developing eggs and larvae of European sea bass (Dicentrarchus labrax). Aquaculture 1998, 162, 157–170. [Google Scholar] [CrossRef]
- Carcupino, M.; Baldacci, A.; Mazzini, M.; Franzoi, P. Morphological organization of the male brood pouch epithelium of Syngnathus abaster Risso (Teleostea: Syngnathidae): Before, during and after egg incubation. Tissue and Cell 1997, 29, 21–30. [Google Scholar] [CrossRef]
- Haresign, T.W.; Shumway, S.E. Permeability of the marsupium of the pipefish Syngnathus fuscus to (14C)-alpha-amino-isobutyric acid. Comp. Biochem. Physiol. A 1981, 69, 603–604. [Google Scholar] [CrossRef]
- Ripley, J.L.; Foran, C.M. Direct evidence for embryonic uptake of paternally derived nutrients in two pipefishes (Syngnathidae: Syngnathus spp.). J. Comp. Physiol. B 2009, 179, 325–333. [Google Scholar] [CrossRef]
- Kvarnemo, C.; Mobley, K.B.; Partridge, C.; Jones, A.G.; Ahnesjö, I. Evidence of paternal nutrient provisioning to embryos in broad-nosed pipefish Syngnathus typhle. J. Fish. Biol. 2011, 78, 1725–1737. [Google Scholar] [CrossRef]
- Caut, S.; Fossette, S.; Guirlet, E.; Angulo, E.; Das, K.; Girondot, M.; Georges, J.Y. Isotope analysis reveals foraging area dichotomy for Atlantic leatherback turtles. PLoS ONE 2008, 3, e1845. [Google Scholar] [CrossRef]
- McMeans, B.C.; Olin, J.A.; Benz, G.W. Stable-isotope comparisons between embryos and mothers of a placentatrophic shark species. J. Fish. Biol. 2009, 75, 2464–2474. [Google Scholar] [CrossRef]
- Carpentier, A.S.; Booth, D.T.; Arthur, K.E.; Limpus, C.J. Stable isotope relationships between mothers, eggs and hatchlings in loggerhead sea turtles Caretta caretta. Mar. Biol. 2015, 162, 783–797. [Google Scholar] [CrossRef]
- de Lussanet, M.H.C.; Muller, M. The smaller your mouth, the longer your snout: Predicting the snout length of Syngnathus acus, Centriscus scutatus and other pipette feeders. J. R. Soc. Interface 2007, 4, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Roelke, D.L.; Sogard, S.M. Gender-based differences in habitat selection and activity level in the northern pipefish (Syngnathus fuscus). Copeia 1993, 2, 528–532. [Google Scholar] [CrossRef]
- Ahnesjö, I. Behavioural temperature preference in a brooding male pipefish Syngnathus typhle. J. Fish. Biol. 2008, 73, 1039–1045. [Google Scholar] [CrossRef]
- Stephens, P.A.; Boyd, I.L.; McNamara, J.M.; Houston, A.I. Capital breeding and income breeding: Their meaning, measurement, and worth. Ecology 2009, 90, 2057–2067. [Google Scholar] [CrossRef]
- Planas, M.; Olivotto, I.; González, M.J.; Laurà, R.; Zarantoniello, M. A multidisciplinary experimental study of the effects of breeders diet on newborn seahorses (Hippocampus guttulatus). Front Mar Sci 2020, 7, 638. [Google Scholar] [CrossRef]
- Planas, M. Carry-over effects of pre-breeding diets on seahorse (Hippocampus reidi) reproductive success. Aquaculture 2021, 533, 736148. [Google Scholar] [CrossRef]
- Sogabe, A.; Mohri, K.; Shoji, J. Reproductive seasonality of the seaweed pipefish Syngnathus schlegeli (Syngnathidae) in the Seto Inland Sea, Japan. Ichthyol. Res. 2012, 59, 223–229. [Google Scholar] [CrossRef]

| 2017 | 2018 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Captures | Total | A | B | C | Total | A | B | C | Total |
| Spring | 75 | 9 | 13 | 13 | 35 | 14 | 9 | 17 | 40 |
| Females | 43 | 8 | 7 | 9 | 24 | 6 | 2 | 11 | 19 |
| % Ovigerous | 84 | 88 | 86 | 56 | 75 | 100 | 100 | 91 | 95 |
| Males | 32 | 1 | 6 | 4 | 11 | 8 | 7 | 6 | 21 |
| % Pregnant | 91 | 100 | 83 | 73 | 82 | 100 | 100 | 100 | 100 |
| Summer | 62 | 4 | 7 | 12 | 23 | 7 | 8 | 24 | 39 |
| Females | 41 | 2 | 6 | 9 | 17 | 7 | 5 | 12 | 24 |
| % Ovigerous | 29 | 0 | 0 | 22 | 12 | 71 | 40 | 25 | 42 |
| Males | 21 | 2 | 1 | 3 | 6 | 0 | 3 | 12 | 15 |
| % Pregnant | 76 | 50 | 100 | 100 | 83 | - | 67 | 75 | 73 |
| Autumn | 16 | 0 | 8 | 4 | 12 | 0 | 3 | 1 | 4 |
| Females | 12 | 0 | 5 | 3 | 8 | 0 | 3 | 1 | 4 |
| % Ovigerous | 0 | - | 0 | 0 | 0 | - | 0 | 0 | 0 |
| Males | 4 | 0 | 3 | 1 | 4 | 0 | 0 | 0 | 0 |
| % Pregnant | 0 | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 153 | 13 | 28 | 29 | 70 | 21 | 20 | 42 | 83 |
| Females | 96 | 10 | 18 | 21 | 49 | 13 | 10 | 24 | 47 |
| % Ovigerous | 50 | 70 | 33 | 33 | 41 | 85 | 40 | 54 | 60 |
| Males | 57 | 3 | 10 | 8 | 21 | 8 | 10 | 18 | 36 |
| % Pregnant | 79 | 67 | 60 | 63 | 62 | 100 | 90 | 83 | 89 |
| OSR | |||||||||
| Spring | 0.45 | 0.12 | 0.45 | 0.37 | 0.33 | 0.57 | 0.78 | 0.37 | 0.54 |
| Summer | 0.57 | 1.00 | 1.00 | 0.60 | 0.71 | - | 0.50 | 0.75 | 0.52 |
| Spring + Summer | 0.48 | 0.22 | 0.50 | 0.42 | 0.39 | 0.42 | 0.69 | 0.54 | 0.53 |
| SL (cm) | WW (g) | δ13C (‰) | δ15N (‰) | |
|---|---|---|---|---|
| Females | 33.7 ± 6.7 | 29.5 ± 16.6 | −15.3 ± 0.5 | 11.0 ± 0.6 |
| Ovigerous | 36.2 ± 5.6 a | 34.8 ± 15.1 a | −15.3 ± 0.4 a | 11.3 ± 0.4 a |
| Nonovigerous | 31.8 ± 6.8 b | 24.2 ± 16.5 b | −15.3 ± 0.6 a | 10.7 ± 0.6 b |
| Males | 35.7 ± 6.8 | 39.6 ± 21.8 | −15.5 ± 0.5 | 11.3 ± 0.5 |
| Pregnant | 36.3 ± 3.2 a | 41.4 ± 20.6 a | −15.5 ± 0.4 a | 11.4 ± 0.3 a |
| Non-pregnant | 33.2 ± 8.8 ab | 32.6 ± 25.6 ab | −15.5 ± 0.5 a | 10.9 ± 0.4 b |
| Stage | n | SL (cm) | δ13C (‰) | δ15N (‰) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 1 | 2.03 | * | −16.6 | (−15.1) | 10.9 | (12.0) | |||||
| H | 6 | 2.18 | ± | 0.49 | −16.3 | ± | 0.5 | (−15.6 ± 0.6) | 11.7 | ± | 0.4 | (11.6 ± 0.3) |
| I | 5 | 2.79 | ± | 0.18 | −15.2 | ± | 0.7 | (−15.6 ± 0.3) | 11.8 | ± | 0.6 | (11.8 ± 0.4) |
| J | 3 | 3.02 | ± | 0.07 | −16.1 | ± | 1.2 | (−16.1 ± 0.2) | 11.5 | ± | 0.4 | (11.1 ± 0.4) |
| All Fish | n | Immature | n | Mature | n | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled | 3.36 | ± | 0.05 | 148 | 3.26 | ± | 0.05 | 58 | 3.41 | ± | 0.05 | 90 |
| Spring | 3.47 | ± | 0.08 | 71 | 3.37 | ± | 0.08 | 8 | 3.48 | ± | 0.07 | 63 |
| Summer | 3.20 | ± | 0.07 | 61 | 3.14 | ± | 0.07 | 34 | 3.26 | ± | 0.07 | 27 |
| Autumn | 3.36 | ± | 0.10 | 16 | 3.36 | ± | 0.10 | 16 | - | - | 0 | |
| Males | 3.39 | ± | 0.05 | 55 | 3.30 | ± | 0.05 | 12 | 3.42 | ± | 0.05 | 43 |
| Spring | 3.49 | ± | 0.08 | 31 | 3.34 | ± | 0.28 | 3 | 3.50 | ± | 0.08 | 28 |
| Summer | 3.26 | ± | 0.06 | 20 | 3.24 | ± | 0.08 | 5 | 3.26 | ± | 0.06 | 15 |
| Autumn | 3.34 | ± | 0.17 | 4 | 3.34 | ± | 0.17 | 4 | - | - | 0 | |
| Females | 3.33 | ± | 0.05 | 93 | 3.25 | ± | 0.05 | 46 | 3.39 | ± | 0.05 | 47 |
| Spring | 3.44 | ± | 0.07 | 40 | 3.39 | ± | 0.07 | 5 | 3.45 | ± | 0.08 | 35 |
| Summer | 3.17 | ± | 0.07 | 41 | 3.13 | ± | 0.07 | 29 | 3.26 | ± | 0.07 | 12 |
| Autumn | 3.36 | ± | 0.11 | 12 | 3.36 | ± | 0.11 | 12 | - | - | 0 | |
| TA | SEA | SEAc | TA | SEA | SEAc | ||
|---|---|---|---|---|---|---|---|
| Pooled | 5.60 | 0.88 | 0.89 | Sex | |||
| Year | Female | 5.23 | 0.95 | 0.96 | |||
| 2017 | 3.42 | 0.67 | 0.68 | Male | 2.39 | 0.64 | 0.66 |
| 2018 | 5.17 | 0.94 | 0.95 | Reproductive State | |||
| Season | Nonovigerous female | 4.32 | 1.16 | 1.19 | |||
| Spring | 2.80 | 0.61 | 0.62 | Non-pregnant male | 0.81 | 0.43 | 0.48 |
| Summer | 3.30 | 0.86 | 0.88 | Ovigerous female | 2.66 | 0.56 | 0.57 |
| Autumn | 2.03 | 0.67 | 0.71 | Pregnant male | 2.16 | 0.61 | 0.62 |
| Site | |||||||
| A | 2.58 | 0.83 | 0.85 | ||||
| B | 2.83 | 0.58 | 0.59 | ||||
| C | 4.55 | 1.03 | 1.05 |
| Year | 2017 | 2018 | Season | Spr | Sum | Aut | Site | A | B | C | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | - | 92.2 | Spr | - | 89.9 | 57.6 | A | - | 82.3 | 93.5 | |
| 2018 | 72.8 | - | Sum | 75.1 | - | 67.5 | B | 95.4 | - | 97.6 | |
| Aut | 68.2 | 87.5 | - | C | 83.7 | 73.9 | - | ||||
| Gender | Female | Male | Maturity | Mat | Imm | Rep Stage | nOvF | nPrM | OvF | PregM | |
| Female | - | 78.7 | Mature | - | 93.8 | nOvF | - | 64.8 | 76.6 | 73.7 | |
| Male | 95.8 | - | Immature | 66.7 | - | nPrM | 99.1 | - | 91.6 | 94.3 | |
| OvF | 99.4 | 77.9 | - | 97.6 | |||||||
| PregM | 98.2 | 67.8 | 95.6 | - |
| Females | Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Sources | Nonovigerous | Ovigerous | Non-pregnant | Pregnant | ||||
| 2017 | ||||||||
| Amphipoda (1) | 68 | (53–80) | 44 | (27–61) | 63 | (40–80) | 48 | (22–68) |
| Caridea (2) | 21 | (4–33) | 41 | (11–56) | 22 | (3–37) | 30 | (3–50) |
| Copepoda (3) | 4 | (0–16) | 6 | (0–21) | 7 | (0–25) | 9 | (0–27) |
| Isopoda (4) | 3 | (0–12) | 3 | (0–10) | 3 | (0–12) | 3 | (0–13) |
| Mysidacea (5) | 4 | (0–15) | 6 | (0–25) | 5 | (0–17) | 10 | (0–28) |
| 2018 | ||||||||
| Amphipoda | 46 | (0–73) | 28 | (0–48) | 36 | (0–62) | 21 | (0–38) |
| Caridea | 5 | (0–15) | 10 | (0–30) | 5 | (0–19) | 8 | (0–34) |
| Copepoda | 19 | (10–33) | 29 | (1–43) | 29 | (10–48) | 34 | (1–48) |
| Isopoda | 24 | (0.81) | 20 | (0–61) | 21 | (0–70) | 17 | (0–58) |
| Mysidacea | 6 | (2–12) | 13 | (1–25) | 9 | (1–21) | 20 | (1–32) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Planas, M. Ecological Traits and Trophic Plasticity in The Greater Pipefish Syngnathus acus in the NW Iberian Peninsula. Biology 2022, 11, 712. https://doi.org/10.3390/biology11050712
Planas M. Ecological Traits and Trophic Plasticity in The Greater Pipefish Syngnathus acus in the NW Iberian Peninsula. Biology. 2022; 11(5):712. https://doi.org/10.3390/biology11050712
Chicago/Turabian StylePlanas, Miquel. 2022. "Ecological Traits and Trophic Plasticity in The Greater Pipefish Syngnathus acus in the NW Iberian Peninsula" Biology 11, no. 5: 712. https://doi.org/10.3390/biology11050712
APA StylePlanas, M. (2022). Ecological Traits and Trophic Plasticity in The Greater Pipefish Syngnathus acus in the NW Iberian Peninsula. Biology, 11(5), 712. https://doi.org/10.3390/biology11050712







