Ontogenetic Patterning of Human Subchondral Bone Microarchitecture in the Proximal Tibia
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Morphology and Anatomy of Subchondral Bone
1.2. Subchondral Bone Loading and the Impact of Knee Joint Alignment
1.3. Aims and Hypotheses
2. Materials and Methods
2.1. Sample Composition
2.2. CT Imaging
2.3. Volume of Interest (VOI) Placement and Size
2.4. Age, Sex, and Body Mass Estimation
2.5. Trabecular Bone Morphometric Parameters
2.6. Cortical Masking
2.7. Statistical Analysis
- (1)
- Child (8–13.99 years, n = 6)
- (2)
- Adolescent (14–19.99 years, n = 10)
- (3)
- Young Adult (20–30.99 years, n = 4)
- (4)
- Middle Age (31–37.99 years, n = 11)
3. Results
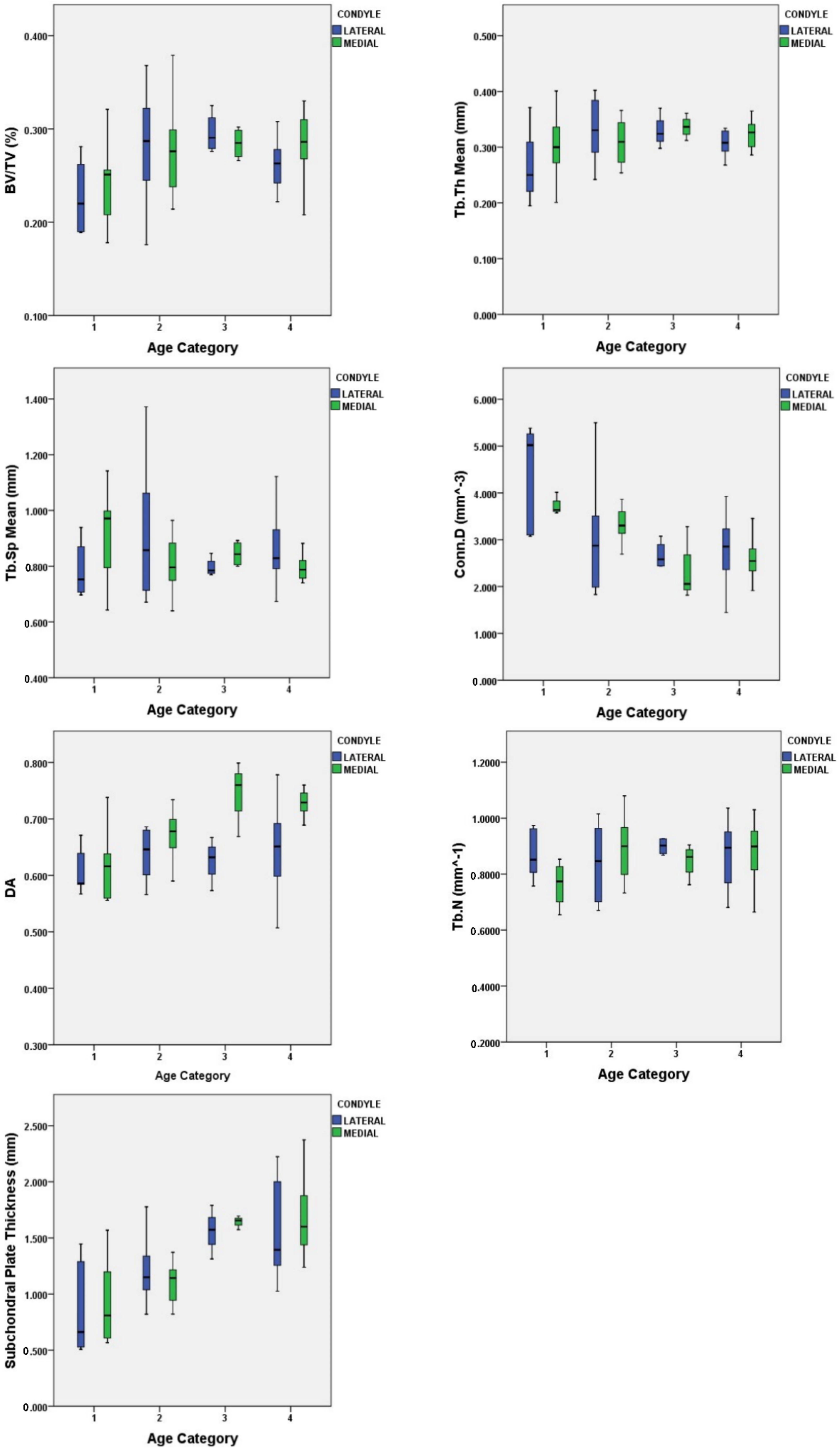
3.1. Quantification of Subchondral Bone Structure by Age Category
- Child
- Adolescent
- Young Adult
- Middle Age
3.2. Condyle Differences
4. Discussion
4.1. Characteristics of Subchondral Bone and Plate Ontogeny
4.2. Microarchitecture in Relation to Locomotion
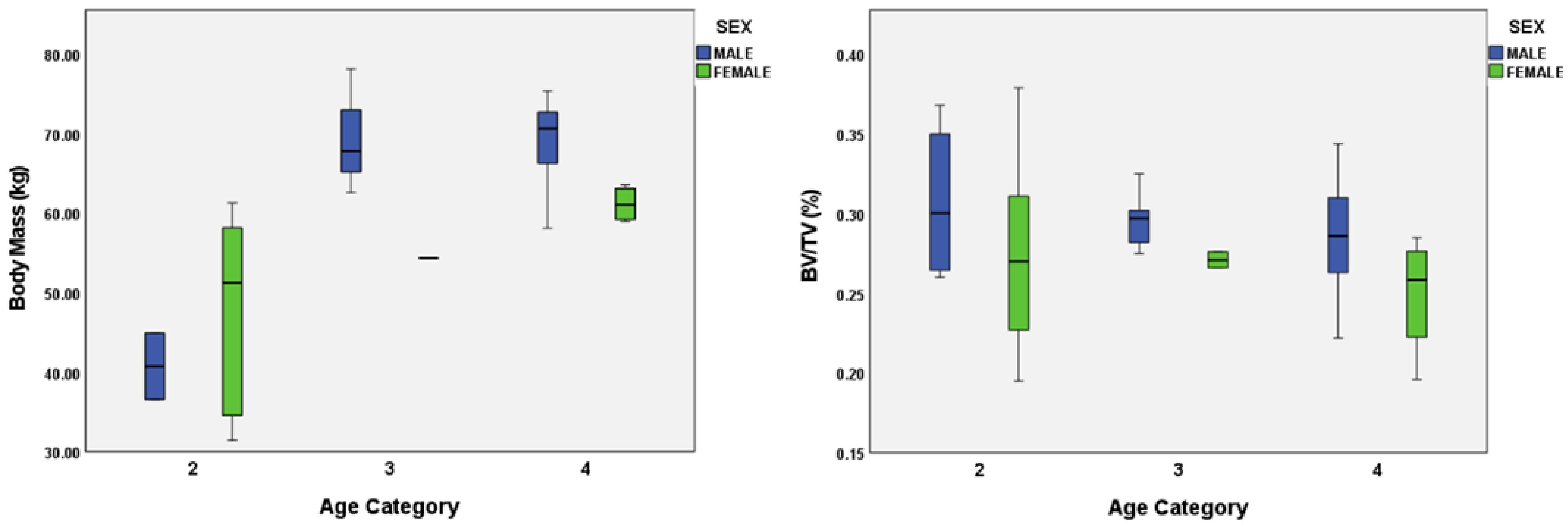
4.3. Body Mass and Sexual Dimorphism
4.4. Implications of this Study
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beresheim, A.C.; Pfeiffer, S.; Grynpas, M. Ontogenetic changes to bone microstructure in an archaeologically derived sample of human ribs. J. Anat. 2020, 236, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Chirchir, H.; Ruff, C.B.; Junno, J.-A.; Potts, R. Low trabecular bone density in recent sedentary modern humans. Am. J. Phys. Anthropol. 2017, 162, e23138. [Google Scholar] [CrossRef]
- Gosman, J.H.; Ketcham, R.A. Patterns in Ontogeny of Human Trabecular Bone from SunWatch Village in the Prehistoric Ohio Valley: General Features of Microarchitectural Change. Am. J. Phys. Anthropol. 2009, 138, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Griffin, N.L. Bone architecture of the hominin second proximal pedal phalanx: A preliminary investigation. J. Hum. Evol. 2008, 54, 162–168. [Google Scholar] [CrossRef] [PubMed]
- MacLatchy, L.; Müller, R. A comparison of the femoral head and neck trabecular architecture of Galago and Perodicticus using micro-computed tomography (μCT). J. Hum. Evol. 2002, 43, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Maga, M.; Kappelman, J.; Ryan, T.M.; Ketcham, R.A. Preliminary observations on the calcaneal trabecular microarchitecture of extant large-bodied hominoids. Am. J. Phys. Anthropol. 2006, 129, 410–417. [Google Scholar] [CrossRef]
- Mazurier, A.; Nakatsukasa, M.; Macchiarelli, R. The inner structural variation of the primate tibial plateau characterized by high-resolution microtomography. Implications for the reconstruction of fossil locomotor behaviours. Comptes Rendus Palevol 2010, 9, 349–359. [Google Scholar] [CrossRef]
- Mulder, B.; Stock, J.T.; Saers, J.P.P.; Inskip, S.A.; Cessford, C.; Robb, J.E. Intrapopulation variation in lower limb trabecular architecture. Am. J. Phys. Anthropol. 2020, 173, 112–129. [Google Scholar] [CrossRef] [Green Version]
- Perchalski, B.; Placke, A.; Sukhdeo, S.M.; Shaw, C.N.; Gosman, J.H.; Raichlen, D.A.; Ryan, T.M. Asymmetry in the Cortical and Trabecular Bone of the Human Humerus During Development. Anat. Rec. 2018, 301, 1012–1025. [Google Scholar] [CrossRef] [Green Version]
- Ragni, A.J. Trabecular architecture of the capitate and third metacarpal through ontogeny in chimpanzees (Pan troglodytes) and gorillas (Gorilla gorilla). J. Hum. Evol. 2020, 138, 102702. [Google Scholar] [CrossRef]
- Raichlen, D.A.; Gordon, A.D.; Foster, A.D.; Webber, J.T.; Sukhdeo, S.M.; Scott, R.S.; Gosman, J.H.; Ryan, T.M. An ontogenetic framework linking locomotion and trabecular bone architecture with applications for reconstructing hominin lifehistory. J. Hum. Evol. 2015, 81, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.M.; Shaw, C.N. Gracility of the modern Homo sapiens skeleton is result of decreased biomechanical loading. Proc. Natl. Acad. Sci. USA 2015, 112, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Ryan, T.M.; Walker, A. Trabecular bone structure in the humeral and femoral heads of anthropoid primates. Anat. Rec. 2010, 293, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Saers, J.P.P.; Cazorla-Bak, Y.; Shaw, C.N.; Stock, J.T.; Ryan, T.M. Trabecular bone structural variation throughout the human lower limb. J. Hum. Evol. 2016, 97, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Saers, J.P.P.; Ryan, T.M.; Stock, J.T. Trabecular Bone Functional Adaptation and Sexual Dimorphism in the Human Foot. Am. J. Phys. Anthropol. 2019, 168, 154–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saers, J.P.P.; Ryan, T.M.; Stock, J.T. Trabecular bone structure scales allometrically in the foot of four human groups. J. Hum. Evol. 2019, 135, 102654. [Google Scholar] [CrossRef]
- Saers, J.P.P.; Ryan, T.M.; Stock, J.T. Baby steps towards linking calcaneal trabecular bone ontogeny and the development of bipedal human gait. J. Anat. 2020, 236, 474–492. [Google Scholar] [CrossRef]
- Ahluwalia, K. Knee Joint Load as Determined by Tibial Subchondral Bone Density: It’s Relationship to Gross Morphology and Locomotor Behavior in Catarrhines. Ph.D. Thesis, Stony Brook University, Stony Brook, NY, USA, 2000. [Google Scholar]
- Su, A. The Functional Morphology of Subchondral and Trabecular Bone in the Hominoid Tibiotalar Joint. Ph.D. Thesis, Stony Brook University, Stony Brook, NY, USA, 2011. [Google Scholar]
- Rauch, F. The dynamics of bone structure development during pubertal growth. J. Musculoskelet. Neuronal Interact. 2012, 12, 1–6. [Google Scholar]
- Wang, Q.; Ghasem-Zadeh, A.; Wang, X.-F.; Iuliano-Burns, S.; Seeman, E. Trabecular Bone of Growth Plate Origin Influences Both Trabecular and Cortical Morphology in Adulthood. J. Bone Miner. Res. 2011, 26, 1577–1583. [Google Scholar] [CrossRef]
- Duren, D.L.; Seselj, M.; Froehle, A.W.; Nahhas, R.W.; Sherwood, R.J. Skeletal Growth and the Changing Genetic Landscape during Childhood and Adulthood. Am. J. Phys. Anthropol. 2013, 150, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Turner, C.H.; Robling, A.G. Designing exercise regimens to increase bone strength. Exerc. Sport Sci. Rev. 2003, 31, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Goliath, J.R.; Gosman, J.H.; Hubbell, Z.R.; Ryan, T.M. Epiphyseal versus Metaphyseal Trabecular Microarchitecture: Regional Ontogenetic Patterns in the Human Proximal Tibia. J. Bone Miner. Res. 2016, 31 (Suppl. S1), S400. [Google Scholar]
- Huiskes, R.; Ruimerman, R.; van Lenthe, G.H.; Janssen, J.D. Effects of mechanical forces on the maintenance and adaptation of form in trabecular bone. Nature 2000, 405, 704–706. [Google Scholar] [CrossRef]
- Mittra, E.; Rubin, C.; Qin, Y.-X. Interrelationships of trabecular mechanical and microstructural properties in sheep trabecular bone. J. Biomech. 2005, 38, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.M.; van Rietbergen, B.; Krovitz, G. Mechanical adaptation of trabecular bone in the growing human femur and humerus. Am. J. Phys. Anthropol. 2007, 132, 205. [Google Scholar]
- Saparin, P.; Scherf, H.; Hublin, J.-J.; Fratzl, P.; Weinkamer, R. Structural Adaptation of Trabecular Bone Revealed by Position Resolved Analysis of Proximal Femora of Different Primates. Anat. Rec. 2011, 294, 55–67. [Google Scholar] [CrossRef]
- Tsegai, Z.J.; Skinner, M.M.; Pahr, D.H.; Hublin, J.-J.; Kivell, T.L. Ontogeny and variability of trabecular bone in the chimpanzee humerus, femur and tibia. Am. J. Phys. Anthropol. 2018, 138, 318–332. [Google Scholar] [CrossRef]
- Acquaah, F.; Robson Brown, K.A.; Ahmed, F.; Jeffery, N.; Abel, R.L. Early trabecular development in human vertebrae: Overproduction, constructive regression, and refinement. Front. Endocrinol. 2015, 6, 67. [Google Scholar] [CrossRef] [Green Version]
- Beresheim, A.C.; Pfeiffer, S.; Grynpas, M.; Alblas, A. Sex-specific patterns in cortical and trabecular bone microstructure in the Kirsten Skeletal Collection, South Africa. Am. J. Hum. Biol. 2018, 30, e23108. [Google Scholar] [CrossRef]
- Cunningham, C.A.; Black, S.M. Anticipating bipedalism; trabecular organization in the newborn ilium. J. Anat. 2009, 214, 817–829. [Google Scholar] [CrossRef]
- Glorieux, F.; Travers, R.; Taylor, A.; Bowen, J.R.; Rauch, F.; Norman, M.; Parfitt, A.M. Normative data for iliac bone histomorphometry in growing children. Bone 2000, 26, 103–109. [Google Scholar] [CrossRef]
- Gosman, J.H.; Hubbell, Z.H.; Shaw, C.N.; Ryan, T.M. Development of Cortical Bone Geometry in the Human Femoral and Tibial Diaphysis. Anat. Rec. 2013, 296, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Kneissel, M.; Roschger, P.; Steiner, W.; Schamall, D.; Kalchhauser, G.; Boyde, A.; Teschler-Nicola, M. Cancellous bone structure in the growing and aging spine in a historic Nubian population. Calcif. Tissue Int. 1997, 61, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.M.; Krovitz, G.E. Trabecular bone ontogeny in the human proximal femur. J. Hum. Evol. 2006, 51, 591–602. [Google Scholar] [CrossRef]
- Terhune, C.E.; Kimbel, W.H.; Lockwood, C.A. Postnatal temporal bone ontogeny in Pan, Gorilla, and Homo, and the Implications for temporal bone ontogefny in Australopithecus afarensis. Am. J. Phys. Anthropol. 2013, 151, 630–642. [Google Scholar] [CrossRef]
- Frost, H.M. Bone’s Mechanostat: A 2003 Update. Anat. Rec. 2003, 275A, 1081–1101. [Google Scholar] [CrossRef]
- Forwood, M.R. Growing a Healthy Skeleton: The Importance of Mechanical Loading. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 8th ed.; Rosen, C.J., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; Chapter 19; pp. 149–155. [Google Scholar] [CrossRef]
- Gosman, J.H.; Stout, S.D.; Larsen, C.S. Skeletal biology over the life span: A view from the surfaces. Yearb. Phys. Anthropol. 2011, 54, 86–98. [Google Scholar] [CrossRef]
- Bousson, V.; Lowitz, T.; Laouisset, L.; Engelke, K.; Laredo, J.D. CT imaging for the investigation of subchondral bone in knee osteoarthritis. Osteoporos. Int. 2012, 23 (Suppl. S8), S861–S865. [Google Scholar] [CrossRef]
- Burr, D.B.; Gallant, M.A. Bone remodeling in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef]
- Carlson, K.J.; Patel, B.A. Habitual use of the primate forelimb is reflected in the material properties of subchondral bone in the distal radius. J. Anat. 2006, 208, 659–670. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Yu, Y.; Zhang, X.; Watts, T.; Zhou, B.; Wang, J.; Wang, T.; Zhao, W.; Chiu, K.Y.; et al. Subchondral Trabecular Rod Loss and Plate Thickening in the Development of Osteoarthritis. J. Bone Miner. Res. 2018, 32, 316–327. [Google Scholar] [CrossRef] [Green Version]
- Hamann, N.; Zaucke, F.; Dayakli, M.; Brüggemann, G.-P.; Niehoff, A. Growth-related structural, biochemical, and mechanical properties of the functional bone-cartilage unit. J. Anat. 2013, 222, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Pesesse, L.; Sanchez, C. Subchondral bone and osteoarthritis: Biological and cellular aspects. Osteoporos. Int. 2012, 23 (Suppl. S8), S847–S851. [Google Scholar] [CrossRef] [PubMed]
- Müller-Gerbl, M. The Subchondral Bone Plate. In Advances in Anatomy Embryology and Cell Biology 141: III-XI; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar] [CrossRef]
- Duncan, H.; Jundt, J.; Riddle, J.M.; Pitchford, W.; Christopherson, T. The tibial subchondral plate. A scanning electron microscope study. J. Bone Jt. Surg. 1987, 69, 1212–1220. [Google Scholar] [CrossRef]
- Madry, H.; van Dijk, C.N.; Müller-Gerbl, M. The basic science of the subchondral bone. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 419–433. [Google Scholar] [CrossRef]
- Milz, S.; Putz, R. Quantitative morphology of the subchondral plate of the tibial plateau. J. Anat. 1994, 185, 103–110. [Google Scholar]
- Odgaard, A.; Pedersen, C.M.; Bentzen, S.M.; Jorgensen, J.; Hvid, I. Density changes at the proximal tibia after menisectomy. J. Orthop. Res. 1989, 7, 744–753. [Google Scholar] [CrossRef]
- Clark, J.M.; Huber, J.D. The structure of the human subchondral plate. J. Bone Jt. Surg. 1990, 72, 866–873. [Google Scholar] [CrossRef] [Green Version]
- Hoemann, C.D.; Lafantaisie-Favreau, C.-H.; Lascau-Coman, V.; Chen, G.; Guzman-Morales, J. The Cartilage-Bone Interface. J. Knee Surg. 2012, 25, 85–97. [Google Scholar] [CrossRef]
- Zhang, L.-Z.; Zheng, H.-A.; Jiang, Y.; Tu, Y.-H.; Jiang, P.-H.; Yang, A.-L. Mechanical and Biologic Link Between Cartilage and Subchondral Bone in Osteoarthritis. Arthritis Care Res. 2012, 64, 960–967. [Google Scholar] [CrossRef]
- Burr, D.B. The importance of subchondral bone in osteoarthrosis. Curr. Opin. Rheumatol. 1998, 10, 256–262. [Google Scholar] [CrossRef]
- Martin, R.B.; Burr, D.B.; Sharkey, N.A.; Fyhrie, D.P. Skeletal Tissue Mechanics, 2nd. ed.; Springer: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Hamann, N.; Brüggemann, G.-P.; Niehoff, A. Topographical variations in articular cartilage and subchondral bone of the normal rat knee are age-related. Ann. Anat. 2014, 196, 278–285. [Google Scholar] [CrossRef]
- Layton, M.W.; Goldstein, S.A.; Goulet, R.W.; Feldkamp, L.A.; Kubinski, D.J.; Bole, G.G. Examination of subchondral bone architecture in experimental osteoarthritis by microscopic computed axial tomography. Arthritis Rheumatol. 1988, 31, 1400–1405. [Google Scholar] [CrossRef]
- Lories, R.J.; Luyten, F.P. The bone-cartilage unit in osteoarthritis. Rheumatology 2011, 7, 43–49. [Google Scholar] [CrossRef]
- Radin, E.L.; Rose, R.M. Role of subchondral bone in the initiation and progression of cartilage damage. Clin. Orthop. Relat. Res. 1986, 213, 34–40. [Google Scholar] [CrossRef]
- Barr, A.J.; Campbell, T.M.; Hopkinson, D.; Kingsbury, S.R.; Bowes, M.A.; Conaghan, P.G. A systematic review of the relationship between subchondral bone features, pain and structural pathology in peripheral joint osteoarthritis. Arthritis Res. Ther. 2015, 17, 228. [Google Scholar] [CrossRef] [Green Version]
- Gordon, K.D.; Duck, T.R.; King, G.J.; Johnson, J.A. Mechanical properties of subchondral cancellous bone of the radial head. J. Orthop. Trauma 2003, 17, 285–289. [Google Scholar] [CrossRef]
- Choi, K.; Kuhn, J.L.; Ciarelli, M.J.; Goldstein, S.A. The elastic moduli of human subchondral, trabecular, and cortical bone tissue and the size-dependency of cortical bone modulus. J. Biomech. 1990, 23, 1103–1113. [Google Scholar] [CrossRef] [Green Version]
- Gomoll, A.H.; Madry, H.; Knutsen, G.; van Dijk, N.; Seil, R.; Brittberg, M.; Kon, E. The subchondral bone in articular cartilage repair: Current problems in the surgical management. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 434–447. [Google Scholar] [CrossRef] [Green Version]
- McKinley, T.O.; Bay, B.K. Trabecular bone strain changes associated with cartilage defects in the proximal and distal tibia. J. Orthop. Res. 2001, 19, 906–913. [Google Scholar] [CrossRef]
- Suri, S.; Walsh, D.A. Osteochondral alterations in OA. Bone 2012, 51, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Lereim, P.; Goldie, I.; Dahlberg, E. Hardness of the subchondral bone of the tibial condyles in the normal state and in osteoarthritis and rheumatoid arthritis. Acta Orthop. 1974, 45, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Pontzer, H.; Lieberman, D.E.; Momin, E.; Devlin, M.J.; Polk, J.D.; Hallgrímsson, B.; Cooper, D.M.L. Trabecular Bone in the Bird Knee Responds with High Sensitivity to Changes in Load Orientation. J. Exp. Biol. 2006, 209, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.C.; Chan, P.S.; Chiang, S.C.; Hui, P.W. Angular and rotational profile of the lower limb in 2630 Chinese children. J. Pediatric Orthop. 1991, 11, 154–161. [Google Scholar] [CrossRef]
- Engel, G.M.; Staheli, L.T. The natural history of torsion and other factors influencing gait in childhood. A study of the angle of gait, tibial torsion, knee angle, hip rotation, and development of the arch in normal children. Clin. Orthop. Relat. Res. 1974, 99, 12–17. [Google Scholar] [CrossRef]
- Heath, C.H.; Staheli, L.T. Normal limits of knee angle in white children—Genu varum and genu valgum. J. Pediatric Orthop. 1993, 13, 259–262. [Google Scholar]
- Saini, U.C.; Bali, K.; Sheth, B.; Gahlot, N.; Gahlot, A. Normal development of the knee angle in healthy Indian children: A clinical study of 215 children. J. Child. Orthop. 2010, 4, 579–586. [Google Scholar] [CrossRef] [Green Version]
- Salenius, P.; Vankka, E. The development of the tibiofemoral angle in children. J. Bone Jt. Surg. 1975, 57, 259–261. [Google Scholar] [CrossRef]
- Van Rossom, S.; Wesseling, M.; Smith, C.R.; Thelen, D.G.; Vanwanseele, B.; Dieter, V.A.; Jonkers, I. The influence of knee joint geometry and alignment on the tibiofemoral load distribution: A computational study. Knee 2019, 26, 813–823. [Google Scholar] [CrossRef]
- Arazi, M.; Ogun, T.C.; Memik, R. Normal development of the tibiofemoral angle in children: A clinical study of 590 normal subjects from 3 to 17 years of age. J. Pediatric Orthop. 2001, 21, 264–267. [Google Scholar] [CrossRef]
- Cahuzac, J.P.; Vardon, D.; Sales de Gauzy, J. Development of the clinical tibiofemoral angle in normal adolescents. A study of 427 normal subjects from 10 to 16 years of age. J. Bone Jt. Surg. 1995, 77, 729–732. [Google Scholar] [CrossRef] [Green Version]
- Oginni, L.M.; Badru, O.S.; Sharp, C.A.; Davie, M.W.; Worsfold, M. Knee angles and rickets in Nigerian children. J. Pediatric Orthop. 2004, 24, 403–407. [Google Scholar] [CrossRef]
- Yoo, J.H.; Choi, I.H.; Cho, T.J.; Chung, C.Y.; Yoo, W.J. Development of tibiofemoral angle in Korean children. J. Korean Med. Sci. 2008, 23, 714–717. [Google Scholar] [CrossRef] [Green Version]
- Hurwitz, D.E.; Sumner, D.R.; Andriacchi, T.P.; Sugar, D.A. Dynamic knee loads during gait predict proximal tibial bone distribution. J. Biomech. 1998, 31, 423–430. [Google Scholar] [CrossRef]
- Wolff, J. The Law of Bone Remodeling; Translation of Wolff’s Das Gesetz der Transformation der Knochen Maquet P and Furlong R; Springer: Berlin, Germany, 1892. [Google Scholar]
- Pearson, O.M.; Lieberman, D.E. The aging of Wolff’s “Law”: Ontogeny and responses to mechanical loading in cortical bone. Yearb. Phys. Anthropol. 2004, 47, 63–99. [Google Scholar] [CrossRef]
- Sugiyama, T.; Price, J.S.; Lanyon, L.E. Functional adaptation to mechanical loading in both cortical and cancellous bone is controlled locally and is confined to the loaded bones. Bone 2010, 46, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Gosman, J.H. Growth and Development: Morphology, Mechanics, and Abnormalities. In Bone Histology: An Anthropological Perspective; Crowder, C., Stout, S.D., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 23–45. [Google Scholar] [CrossRef]
- Cowgill, L.W.; Warrener, A.; Pontzer, H.; Ocobock, C. Waddling and Toddling: The Biomechanical Effects of an Immature Gait. Am. J. Phys. Anthropol. 2010, 143, 52–61. [Google Scholar] [CrossRef]
- Bobinac, D.; Spanjol, J.; Zoricic, S.; Maric, I. Changes in articular cartilage and subchondral bone in osteoarthritic knee joints in humans. Bone 2003, 32, 284–290. [Google Scholar] [CrossRef]
- Buckland-Wright, C. Subchondral bone changes in hand and knee osteoarthritis detected by radiography. Osteoarthr. Cartil. 2004, 12, S10–S19. [Google Scholar] [CrossRef] [Green Version]
- Funck-Bretano, T.; Cohen-Solal, M. Subchondral Bone and Osteoarthritis. Curr. Opin. Rheumatol. 2015, 27, 420–426. [Google Scholar] [CrossRef]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Washimi, Y.; Kubo, K.; Onozuka, M. Gender-related changes in three-dimensional microstructure of trabecular bone at the human proximal tibia with aging. Histol. Histopathol. 2011, 26, 563–570. [Google Scholar] [CrossRef]
- Ding, M.; Dalstra, M.; Danielsen, C.C.; Kabel, J.; Hvid, I.; Linde, F. Age Variations in the Properties of Human Tibial Trabecular Bone. J. Bone Jt. Surg. 1997, 79, 995–1002. [Google Scholar] [CrossRef]
- Ding, M.; Odgaard, A.; Linde, F.; Hvid, I. Age-related variations in the microstructure of human tibial cancellous bone. J. Orthop. Res. 2002, 20, 615–621. [Google Scholar] [CrossRef]
- Gosman, J.H. Patterns in Ontogeny of Human Trabecular Bone from SunWatch Village in Prehistoric Ohio Valley. Ph.D. Thesis, Ohio State University, Columbus, OH, USA, 2007. [Google Scholar]
- Ding, M. Microarchitectural adaptations in aging and osteoarthritic subchondral bone tissues. Acta Orthop. Suppl. 2010, 81, 1–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hvid, I.; Hansen, S.L. Trabecular bone strength patterns at the proximal tibial epiphysis. J. Orthop. Res. 1985, 3, 464–472. [Google Scholar] [CrossRef]
- Freeman, M.; Pinskerova, V. The movement of the normal tibio-femoral joint. J. Biomech. 2005, 38, 197–208. [Google Scholar] [CrossRef]
- Santure, S.K.; Harn, A.D.; Esarey, D.; King, F.B. Archaeological Investigations at the Morton Village and Norris Farms 36 Cemetery; Illinois State Museum: Springfield, IL, USA, 1990. [Google Scholar]
- Milner, G.R.; Smith, V.G. Oneota Human Skeletal Remains. In Archaeological Investigations at the Morton Village and Norris Farms 36 Cemetery; Santure, S., Harn, A.D., Esarey, D., Eds.; Illinois State Museum: Springfield, IL, USA, 1990; pp. 111–148. [Google Scholar]
- Ryan, T.M.; Milner, G.R. Osteological applications of high-resolution computed tomography: A prehistoric arrow injury. J. Archaeol. Sci. 2006, 33, 871–879. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for Assessment of Bone Microstructure in Rodents Using Micro–Computed Tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Hubbell, Z.R. Developmental Mechanobiology of the Metaphyseal Cortical-Trabecular Interface in the Human Proximal Tibia and Proximal Humerus. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2016. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Domander, R.; Felder, A.A.; Doube, M. BoneJ2—Refactoring established research software. Wellcome Open Res. 2021, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Kontulainen, S.A.; Hughes, J.M.; Macdonald, H.M.; Johnston, J.D. The biomechanical basis of bone strength development during growth. In Optimizing Bone Mass and Strength; Daly, R.M., Petit, M.A., Eds.; Karger: Basel, Switzerland, 2007; pp. 13–32. [Google Scholar] [CrossRef]
- Kivell, T.L. A review of trabecular bone functional adaptation: What have we learned from trabecular analyses in extant hominoids and what can we apply to fossils. J. Anat. 2016, 228, 569–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazenby, R.A.; Skinner, M.M.; Kivell, T.L.; Hublin, J.-J. Scaling VOI Size in 3D μCT Studies of Trabecular Bone: A Test of the Over-Sampling Hypothesis. Am. J. Phys. Anthropol. 2011, 144, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Milner, G.R.; Humpf, D.A.; Harpending, H.C. Pattern matching of age-at-death distributions in paleodemographic analysis. Am. J. Phys. Anthropol. 1989, 80, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Moorrees, C.F.A.; Fanning, E.A.; Hunt, E.E. Formation and resorption of three deciduous teeth in children. Am. J. Phys. Anthropol. 1963, 21, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Moorrees, C.F.A.; Fanning, E.A.; Hunt, E.E. Age variation of formation stages for ten permanent teeth. J. Dent. Res. 1963, 42, 1490–1502. [Google Scholar] [CrossRef]
- Thoma, K.H.; Goldman, H.M. Oral Pathology; Mosby: St. Louis, MO, USA, 1960. [Google Scholar]
- Ubelaker, D.H. Human Skeletal Remains: Excavation, Analysis, Interpretation; Aldine Publishing: Chicago, IL, USA, 1978. [Google Scholar]
- Ruff, C.B. Body size prediction from juvenile skeletal remains. Am. Phys. Phys. Anthropol. 2007, 133, 698–716. [Google Scholar] [CrossRef]
- Burghardt, A.J.; Link, T.M.; Majumdar, S. High-resolution Computed Tomography for Clinical Imaging of Bone Microarchitecture. Clin. Orthop. Relat. Res. 2011, 469, 2179–2193. [Google Scholar] [CrossRef] [Green Version]
- Hildebrand, T.; Laib, A.; Müller, R.; Dequeker, J.; Rüegsegger, P. Direct three-dimensional morphometric analysis of human cancellous bone: Microstructural data from spine, femur, iliac crest, and calcaneus. J. Bone Miner. Res. 1999, 14, 1167–1174. [Google Scholar] [CrossRef]
- Hildebrand, T.; Rüegsegger, P. A new method for the model-independent assessment of thickness in three-dimensional images. J. Microsc. 1997, 185, 67–75. [Google Scholar] [CrossRef]
- Dougherty, R.; Kunzelmann, K. Computing local thickness of 3D structures with ImageJ. Microsc. Microanal. 2007, 13, 1678–1679. [Google Scholar] [CrossRef]
- Odgaard, A.; Gundersen, H.J. Quantification of connectivity in cancellous bone, with special emphasis on 3-D reconstructions. Bone 1993, 14, 173–182. [Google Scholar] [CrossRef]
- Toriwaki, J.; Yonekura, T. Euler number and connectivity indexes of a three dimensional digital picture. Forma 2002, 17, 183–209. [Google Scholar]
- Odgaard, A. Three-dimensional methods for quantification of cancellous bone architecture. Bone 1997, 20, 315–328. [Google Scholar] [CrossRef]
- Buie, H.R.; Campbell, G.M.; Klinck, R.J.; MacNeil, J.A.; Boyd, S.K. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone 2007, 41, 505–515. [Google Scholar] [CrossRef]
- Sutherland, D. The development of mature gait. Gait Posture 1997, 6, 163–170. [Google Scholar] [CrossRef]
- Tanck, E.; Homminga, J.; van Lenthe, G.H.; Huiskes, R. Increase in bone volume fraction precedes architectural adaptation in growing bone. Bone 2001, 28, 650–654. [Google Scholar] [CrossRef] [Green Version]
- Scheuer, L.; Black, S. The Juvenile Skeleton; Academic Press: London, UK, 2004. [Google Scholar]
- Carlson, K.J.; Lublinksy, S.; Judex, S. Do different locomotor modes during growth modulate trabecular architecture in the murine hind limb? Integr. Comp. Biol. 2008, 48, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Wallace, I.J.; Kwaczala, A.T.; Demes, J.B.; Carlson, K.J. Physical activity engendering loads from diverse directions augments the growing skeleton. J. Musculoskelet. Neuronal Interact. 2013, 13, 283–288. [Google Scholar]
- Griffin, N.L.; D’Aout, K.; Ryan, T.M.; Richmond, B.G.; Ketcham, R.A.; Postnov, A. Comparative forefoot trabecular bone architecture in extant hominids. J. Hum. Evol. 2010, 59, 202–213. [Google Scholar] [CrossRef]
- Ruff, C.B. Mechanical determinants of bone form: Insights from skeletal remains. J. Musculoskelet. Neuronal Interact. 2005, 5, 202–212. [Google Scholar] [PubMed]
- Ryan, T.M.; Ketcham, R.A. The three-dimensional structure of trabecular bone in the femoral head of strepsirrhine primates. J. Hum. Evol. 2002, 43, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Danielsen, C.C.; Hvid, I. Age-related three-dimensional microarchitectural adaptations of subchondral bone tissues in guinea pig primary osteoarthrosis. Calcif. Tissue Int. 2006, 78, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, S.; Jing, Y.; Su, J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021, 9, 20. [Google Scholar] [CrossRef]
- Mahedra, N.Y.; Putra, D.P.; Akbar, I.; Putra, R.U.; Prakaoso, A.T.; Yanis, M.; Chandra, H.; Syahrom, A.; Amrullah, M.I.; Jamari, J. Narrative Review of Subchondral Bone Morphology on Cartilage Damage (Osteoarthritis). In Atlantis Highlights in Engineering; Atlantis Press: Amsterdam, The Netherlands, 2021; Volume 9. [Google Scholar] [CrossRef]
- Rapagna, S.; Roberts, B.C.; Solomon, L.B.; Reynolds, K.J.; Thewlis, D.; Perilli, E. Tibial cartilage, subchondral bone plate and trabecular bone microarchitecture in varus- and valgus-osteoarthritis versus controls. J. Orthop. Res. 2021, 39, 1988–1999. [Google Scholar] [CrossRef] [PubMed]
- Zamil, Z.; Brown, K.R.; Tarlton, J.F.; Adams, M.A.; Torlot, G.E.; Cartwright, C.; Cook, W.A.; Vassilevskaja, K.; Sharif, M. Subchondral Bone Plate Thickening Precedes Chondrocyte Apoptosis and Cartilage Degradation in Spontaneous Animal Models of Osteoarthritis. BioMed Res. Int. 2014, 2014, 606870. [Google Scholar] [CrossRef]
- Zamil, Z.; Brown, K.R.; Sharif, M. Subchondral Bone Plate Changes More Rapidly than Trabecular Bone in Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 1496. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.D.; Vrahas, M.S. The apparent elastic modulus of the juxtarticular subchondral bone of the femoral head. J. Orthop. Res. 1984, 2, 32–38. [Google Scholar] [CrossRef]
- Lotz, J.C.; Gerhart, T.N.; Hayes, W.C. Mechanical properties of metaphyseal bone in the proximal femur. J. Biomech. 1991, 24, 317–329. [Google Scholar] [CrossRef]
- Abel, R.; Macho, G.A. Ontogenetic changes in the internal and external morphology of the ilium in modern humans. J. Anat. 2011, 218, 324–335. [Google Scholar] [CrossRef]
- Bogin, B. Patterns of Human Growth, 3rd ed.; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar] [CrossRef]
- Eckstein, F.; Matsuura, M.; Kuhn, V.; Priemel, M.; Muller, R.; Link, T.M.; Lochmuller, E.-M. Sex Differences of Human Trabecular Bone Microstructure in Aging Are Site-Dependent. J. Bone Miner. Res. 2007, 22, 817–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doershuk, L.J.; Saers, J.P.P.; Shaw, C.N.; Jashashvili, T.; Carlson, K.J.; Stock, J.T.; Ryan, T.M. Complex variation of trabecular bone structure in the proximal humerus and femur of five modern human populations. Am. J. Phys. Anthropol. 2019, 168, 104–118. [Google Scholar] [CrossRef] [Green Version]
- Ruff, C.B.; Holt, B.; Trinkaus, E. Who’s afraid of the big bad Wolff?: “Wolff’s law” and bone functional adaptation. Am. J. Phys. Anthropol. 2006, 129, 484–498. [Google Scholar] [CrossRef]
- Fajardo, R.J.; Muller, R.; Ketcham, R.A.; Colbert, M. Nonhuman anthropoid primate femoral neck trabecular architecture and its relationship to locomotor mode. Anat. Rec. 2007, 290, 422–436. [Google Scholar] [CrossRef]
- Roksandic, M.; Armstrong, S.D. Using the life history model to set the stage(s) of growth and senescence in bioarchaeology and paleodemography. Am. J. Phys. Anthropol. 2011, 145, 337–347. [Google Scholar] [CrossRef]
- Wood, J.W.; Milner, G.R.; Harpending, H.C.; Weiss, K.M. The Osteological Paradox: Problems of Inferring Prehistoric Health from Skeletal Samples. Curr. Anthropol. 1992, 33, 343–358. [Google Scholar] [CrossRef]
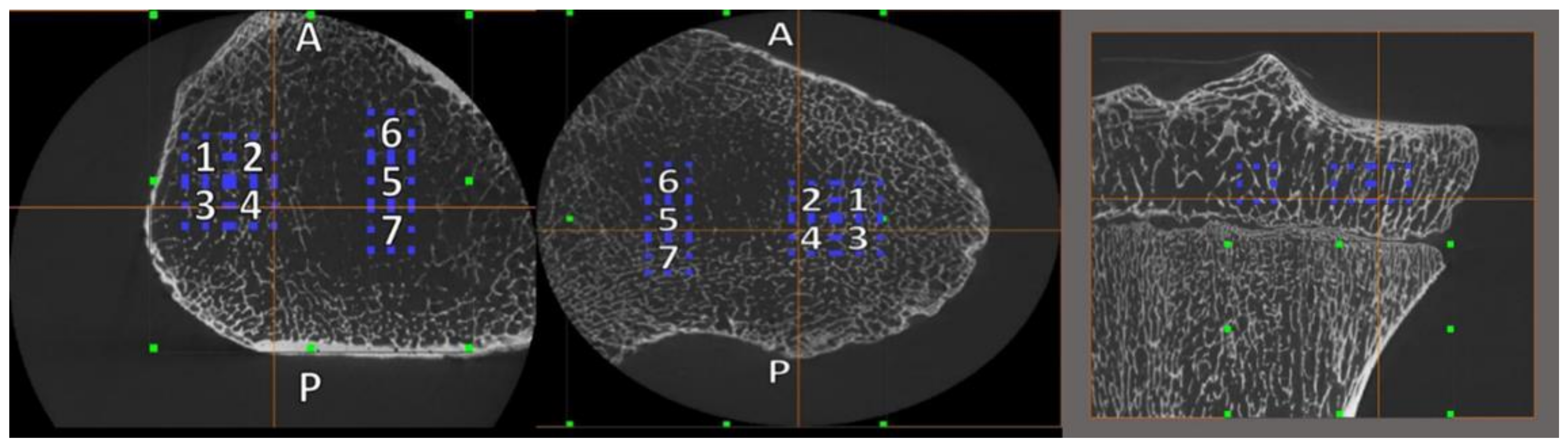
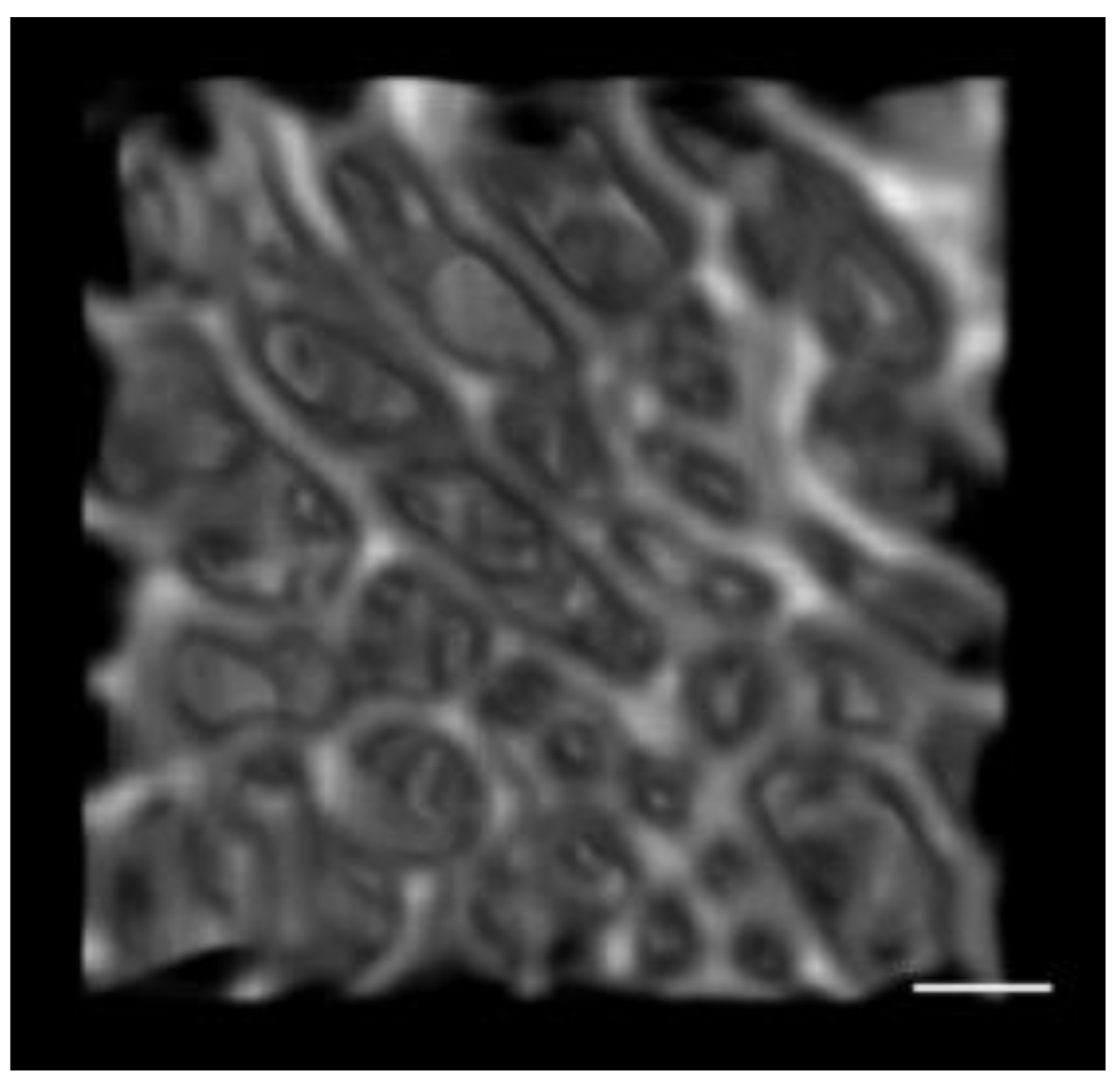
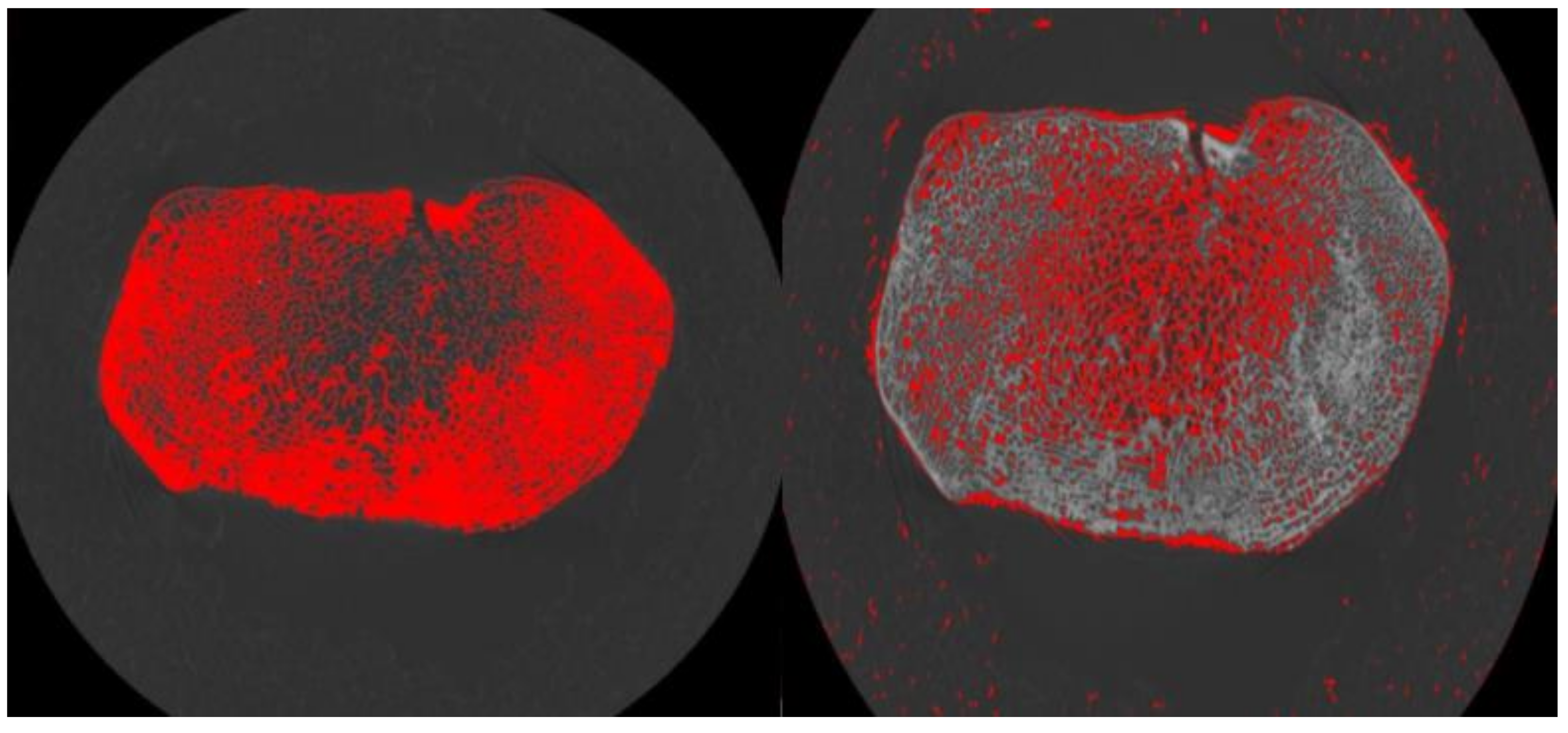
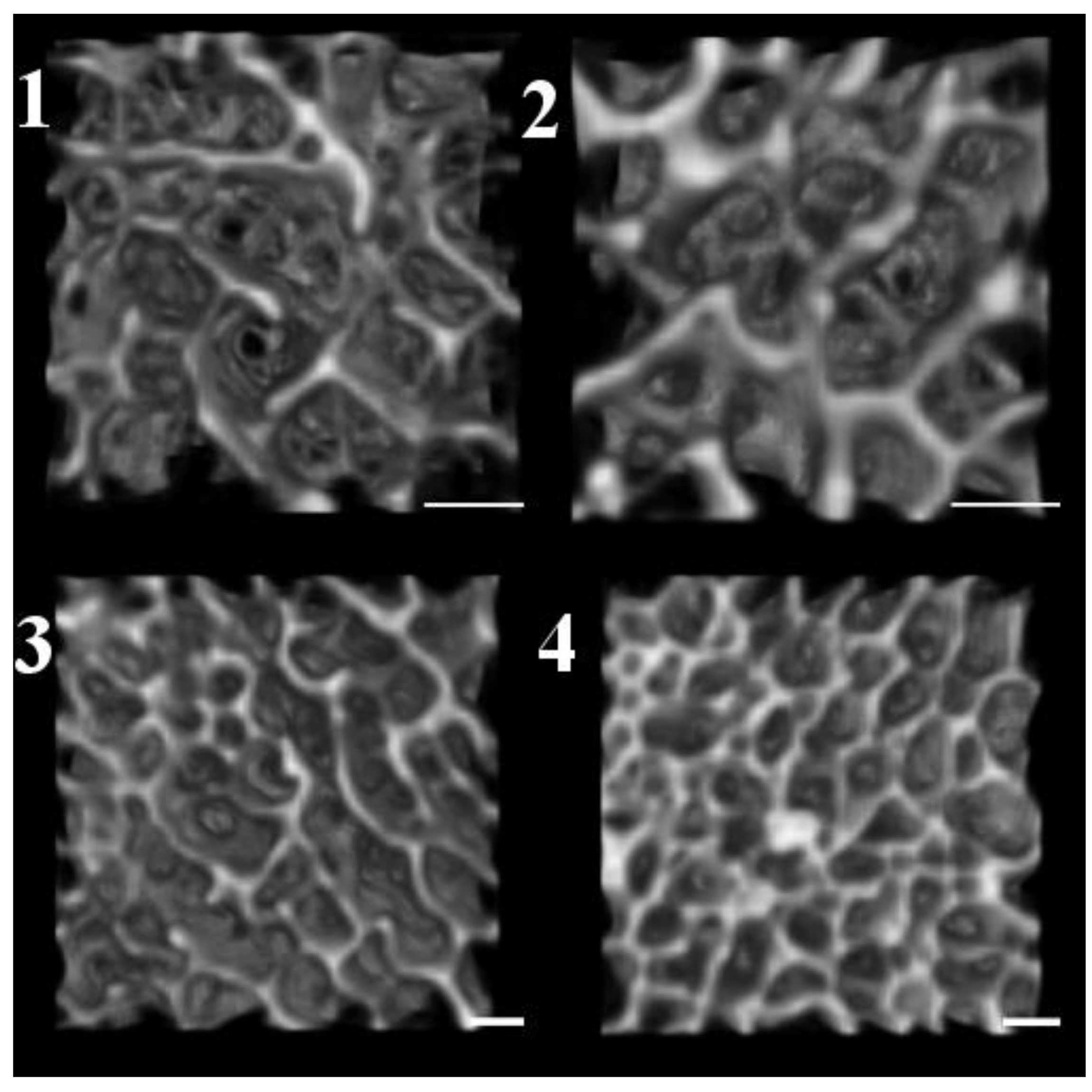
| ID | Estimated Age (Years) | Age Category | Sex | Body Mass Estimation (kg) | Voxel Size (mm) | VOI Cube Size (mm) | VOI Length (# of Slices) |
|---|---|---|---|---|---|---|---|
| 1 | 8 | 1 | U | 17.182 | 0.04 | 4.72 | 119 |
| 2 | 9 | 1 | 26.786 | 0.05 | 4.4 | 89 | |
| 3 | 9 | 1 | 20.152 | 0.04 | 5.16 | 130 | |
| 4 | 9.5 | 1 | 12.802 | 4 | 101 | ||
| 5 | 10.5 | 1 | 22.475 | 0.05 | 5.5 | 111 | |
| 6 | 11 | 1 | 20.481 | 5.7 | 115 | ||
| 7 | 15 | 2 | 33.687 | 5.6 | 113 | ||
| 8 | 15.5 | 2 | M? | 36.562 | 6.55 | 132 | |
| 9 | 16 | 2 | F | 34.549 | 6.2 | 124 | |
| 10 | 16 | 2 | M | 44.901 | 4.35 | 88 | |
| 11 | 16 | 2 | U | 39.148 | 6.15 | 124 | |
| 12 | 16.5 | 2 | F? | 31.407 | 6.3 | 127 | |
| 13 | 18 | 2 | F | 49.611 | 0.056 | 5.684 | 103 |
| 14 | 19.5 | 2 | 58.139 | 0.057 | 5.015 | 89 | |
| 15 | 19.5 | 2 | F? | 61.282 | 0.056 | 6.322 | 114 |
| 16 | 19.5 | 2 | F | 52.893 | 6.438 | 116 | |
| 17 | 21.5 | 3 | M | 62.587 | 0.057 | 7.772 | 137 |
| 18 | 22.5 | 3 | 78.169 | 0.056 | 8.178 | 147 | |
| 19 | 26.5 | 3 | 67.804 | 8.12 | 146 | ||
| 20 | 27.5 | 3 | F | 54.349 | 6.148 | 111 | |
| 21 | 32.5 | 4 | 58.970 | 6.902 | 124 | ||
| 22 | 32.5 | 4 | 59.480 | 0.057 | 6.844 | 121 | |
| 23 | 32.5 | 4 | M | 65.678 | 5.684 | 101 | |
| 24 | 32.5 | 4 | 58.092 | 0.056 | 6.554 | 118 | |
| 25 | 32.5 | 4 | F | 62.670 | 6.264 | 113 | |
| 26 | 32.5 | 4 | M | 73.941 | 8.294 | 149 | |
| 27 | 32.5 | 4 | 70.679 | 6.438 | 116 | ||
| 28 | 35 | 4 | F | 63.570 | 6.728 | 121 | |
| 29 | 37.5 | 4 | M | 71.477 | 0.057 | 7.328 | 130 |
| 30 | 37.5 | 4 | 66.862 | 0.056 | 7.192 | 129 | |
| 31 | 37.5 | 4 | 75.391 | 7.598 | 137 |
| Bone Morphometric Variable (Unit) | Description | References |
|---|---|---|
| Bone Volume Fraction (%) | Ratio showing what proportion of a volume is comprised of trabecular bone/bone tissue | [105,113] |
| Trabecular Thickness (mm) | Measure of the average thickness of trabecular struts | [105,114] |
| Subchondral Cortical Plate Thickness (mm) | Mean cortex thickness | |
| Trabecular Separation (mm) | Mean distance between trabeculae | [105,114,115] |
| Trabecular Number (mm−1) | Ratio of bone volume fraction to trabecular thickness, a measure of the number of traversals across a trabecular or solid structure | [105] |
| Connectivity Density (mm−3) | Measure of the ‘connectedness’ of trabeculae to one another within the VOI | [116,117] |
| Degree of Anisotropy (unitless) | Measure of the directional orientation of trabeculae, ranging from 0 (fully isotropic) to 1 (fully anisotropic) | [105,118] |
| Variables (Unit) | Age Category | n | Mean (Standard Deviation) |
|---|---|---|---|
| Bone Volume Fraction (%) | 1 | 11 | 0.234 (0.04) |
| 2 | 20 | 0.278 (0.06) | |
| 3 | 8 | 0.290 (0.02) | |
| 4 | 22 | 0.274 (0.03) | |
| Trabecular Thickness (mm) | 1 | 11 | 0.282 (0.07) |
| 2 | 20 | 0.320 (0.05) | |
| 3 | 8 | 0.333 (0.02) | |
| 4 | 22 | 0.316 (0.02) | |
| Trabecular Separation (mm) | 1 | 11 | 0.843 (0.15) |
| 2 | 20 | 0.859 (0.19) | |
| 3 | 8 | 0.820 (0.05) | |
| 4 | 22 | 0.832 (0.11) | |
| Connectivity Density (mm−3) | 1 | 8 | 4.133 (0.95) |
| 2 | 20 | 3.144 (0.84) | |
| 3 | 8 | 2.485 (0.51) | |
| 4 | 21 | 2.680 (0.57) | |
| Degree of Anisotropy (-) | 1 | 11 | 0.616 (0.06) |
| 2 | 20 | 0.654 (0.05) | |
| 3 | 8 | 0.687 (0.08) | |
| 4 | 22 | 0.684 (0.07) | |
| Trabecular Number (mm−1) | 1 | 11 | 0.826 (0.10) |
| 2 | 20 | 0.870 (0.12) | |
| 3 | 8 | 0.873 (0.05) | |
| 4 | 22 | 0.872 (0.11) | |
| Subchondral Cortical Plate Thickness (mm) | 1 | 11 | 0.888 (0.39) |
| 2 | 20 | 1.154 (0.24) | |
| 3 | 8 | 1.600 (0.16) | |
| 4 | 22 | 1.645 (0.39) | |
| Body Mass (kg) | 1 | 6 | 19.979 (1.93) |
| 2 | 10 | 44.219 (3.40) | |
| 3 | 4 | 65.727 (4.99) | |
| 4 | 11 | 66.074 (1.85) |
| Variable (Unit) | Condyle | Mean | Standard Deviation |
|---|---|---|---|
| Bone Volume Fraction (%) | 1 | 0.2651 | 0.0468 |
| 2 | 0.2753 | 0.0449 | |
| Trabecular Thickness (mm) | 1 | 0.3091 | 0.0505 |
| 2 | 0.317 | 0.0404 | |
| Trabecular Separation (mm) | 1 | 0.8538 | 0.1623 |
| 2 | 0.826 | 0.1108 | |
| Connectivity Density (mm−3) | 1 | 3.0826 | 1.0485 |
| 2 | 2.9423 | 0.6384 | |
| Degree of Anisotropy (-) | 1 | 0.634 | 0.0577 |
| 2 | 0.6936 | 0.0623 | |
| Trabecular Number (mm−1) | 1 | 0.8635 | 0.1083 |
| 2 | 0.8634 | 0.1066 | |
| Subchondral Cortical Plate Thickness (mm) | 1 | 1.300 | 0.4668 |
| 2 | 1.3387 | 0.4425 |
| Statistical Test | BV/TV (%) | Tb.Th (mm) | Tb.Sp (mm) | Conn.D (mm−3) | DA (-) | Tb.N (mm−1) | Plate Ct.Th (mm) | Body Mass (kg) |
|---|---|---|---|---|---|---|---|---|
| Kruskal–Wallis | 8.752 | 4.711 | 0.547 | 17.345 | 10.934 | 0.515 | 26.861 | 23.669 |
| df | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| p-value | 0.033 | 0.194 | 0.908 | <0.001 | 0.012 | 0.916 | <0.001 | <0.001 |
| Variable (Unit) | Age Category Comparison | p-Value |
|---|---|---|
| BV/TV (%) | 1 vs. 3 | 0.040 |
| Conn.D (mm−3) | 1 vs. 3 | 0.003 |
| 1 vs. 4 | 0.003 | |
| DA (-) | 1 vs. 4 | 0.011 |
| Plate Ct.Th (mm) | 1 vs. 3 | 0.004 |
| 1 vs. 4 | <0.001 | |
| 2 vs. 3 | 0.040 | |
| 2 vs. 4 | 0.030 |
| Variable (Unit) | Mean Rank (Negative) | Mean Rank (Positive) | Z | p-Value |
|---|---|---|---|---|
| BV/TV (%) | 18.75 | 13.03 | −0.649 | 0.516 |
| Tb.Th (mm) | 15.13 | 14.03 | −0.490 | 0.624 |
| Tb.Sp (mm) | 13.35 | 13.65 | −0.051 | 0.959 |
| Conn.D (mm−3) | 15.31 | 11.69 | −0.597 | 0.551 |
| Tb.N (mm−1) | 13.24 | 16.45 | −0.501 | 0.616 |
| DA (-) | 10.93 | 15.69 | −2.881 | 0.004 |
| Plate Ct.Th (mm) | 15.70 | 12.13 | −0.470 | 0.638 |
| Statistical Test | BV/TV (%) | Tb.Th Mean (mm) | Tb.Sp Mean (mm) | Conn.D (mm−3) | DA | Tb.N (mm−1) | Plate Ct.Th (mm) | Body Mass (kg) |
|---|---|---|---|---|---|---|---|---|
| Mann–Whitney U | 159.0 | 217.0 | 268.5 | 304.0 | 261.5 | 178.0 | 188.0 | 26.0 |
| Z | −2.309 | −1.034 | 0.099 | 1.183 | −0.055 | −1.891 | −1.671 | −2.462 |
| p-value | 0.021 | 0.301 | 0.921 | 0.237 | 0.956 | 0.059 | 0.095 | 0.013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goliath, J.R.; Gosman, J.H.; Stout, S.D.; Ryan, T.M. Ontogenetic Patterning of Human Subchondral Bone Microarchitecture in the Proximal Tibia. Biology 2022, 11, 1002. https://doi.org/10.3390/biology11071002
Goliath JR, Gosman JH, Stout SD, Ryan TM. Ontogenetic Patterning of Human Subchondral Bone Microarchitecture in the Proximal Tibia. Biology. 2022; 11(7):1002. https://doi.org/10.3390/biology11071002
Chicago/Turabian StyleGoliath, Jesse R., James H. Gosman, Sam D. Stout, and Timothy M. Ryan. 2022. "Ontogenetic Patterning of Human Subchondral Bone Microarchitecture in the Proximal Tibia" Biology 11, no. 7: 1002. https://doi.org/10.3390/biology11071002
APA StyleGoliath, J. R., Gosman, J. H., Stout, S. D., & Ryan, T. M. (2022). Ontogenetic Patterning of Human Subchondral Bone Microarchitecture in the Proximal Tibia. Biology, 11(7), 1002. https://doi.org/10.3390/biology11071002







