The Effects of Experimental Whole-Body Burning on Histological Age-at-Death Estimation from Human Cortical Bone and Dental Cementum
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. The Use of Skeletal Histology in Applied Contexts
1.1.1. Human vs. Nonhuman Differentiation
1.1.2. Age-at-Death Estimation
1.2. The Effect of Thermal Alteration on Skeletal Histology
2. Materials and Methods
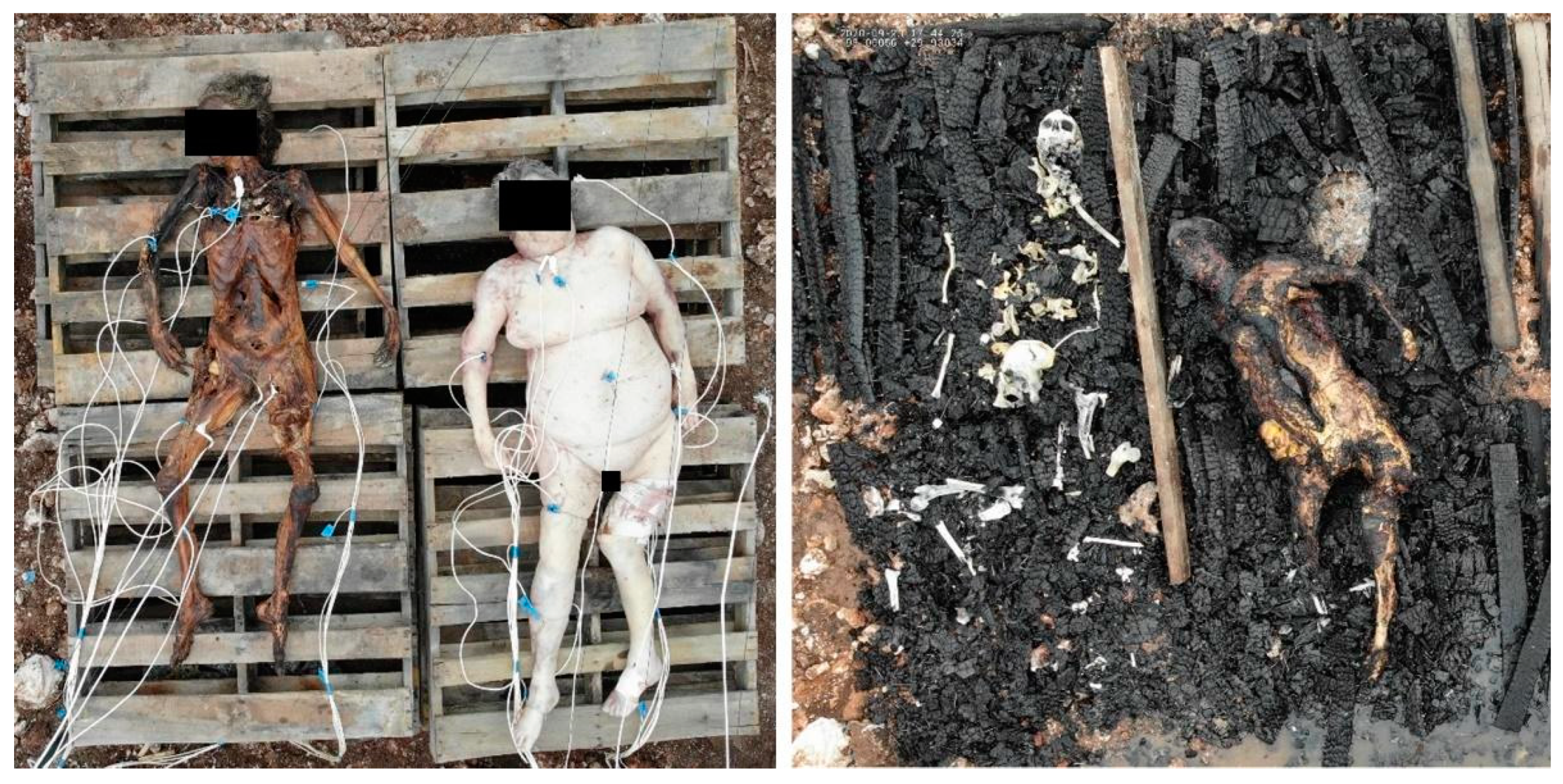
2.1. Experimental Design
2.1.1. Bone Samples
2.1.2. Dental Samples
2.2. Cortical Histomorphometric Analysis
2.3. Cortical Age-at-Death Analysis
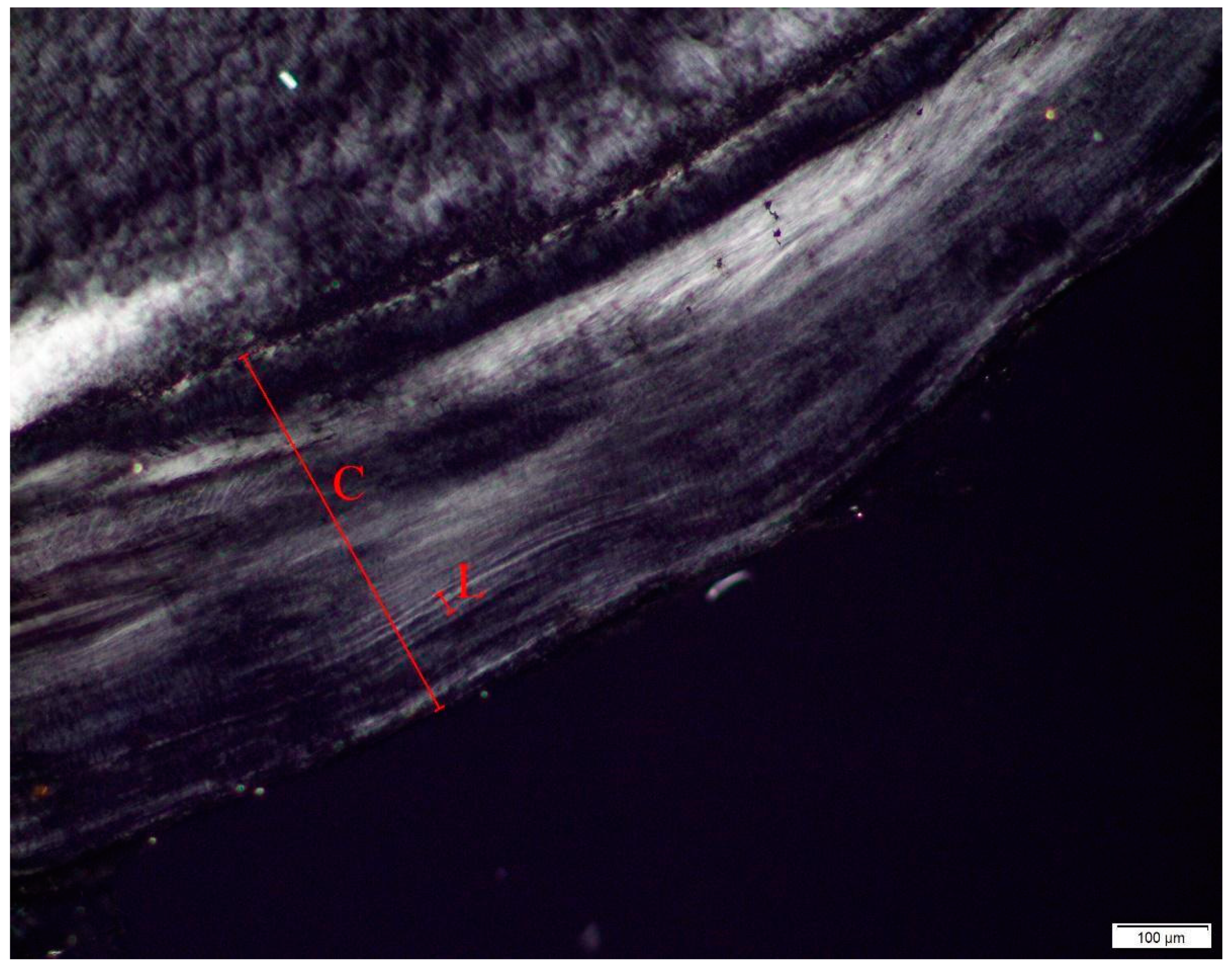
2.4. Dental Age-at-Death Analysis
3. Results
3.1. Thermocouple Readings
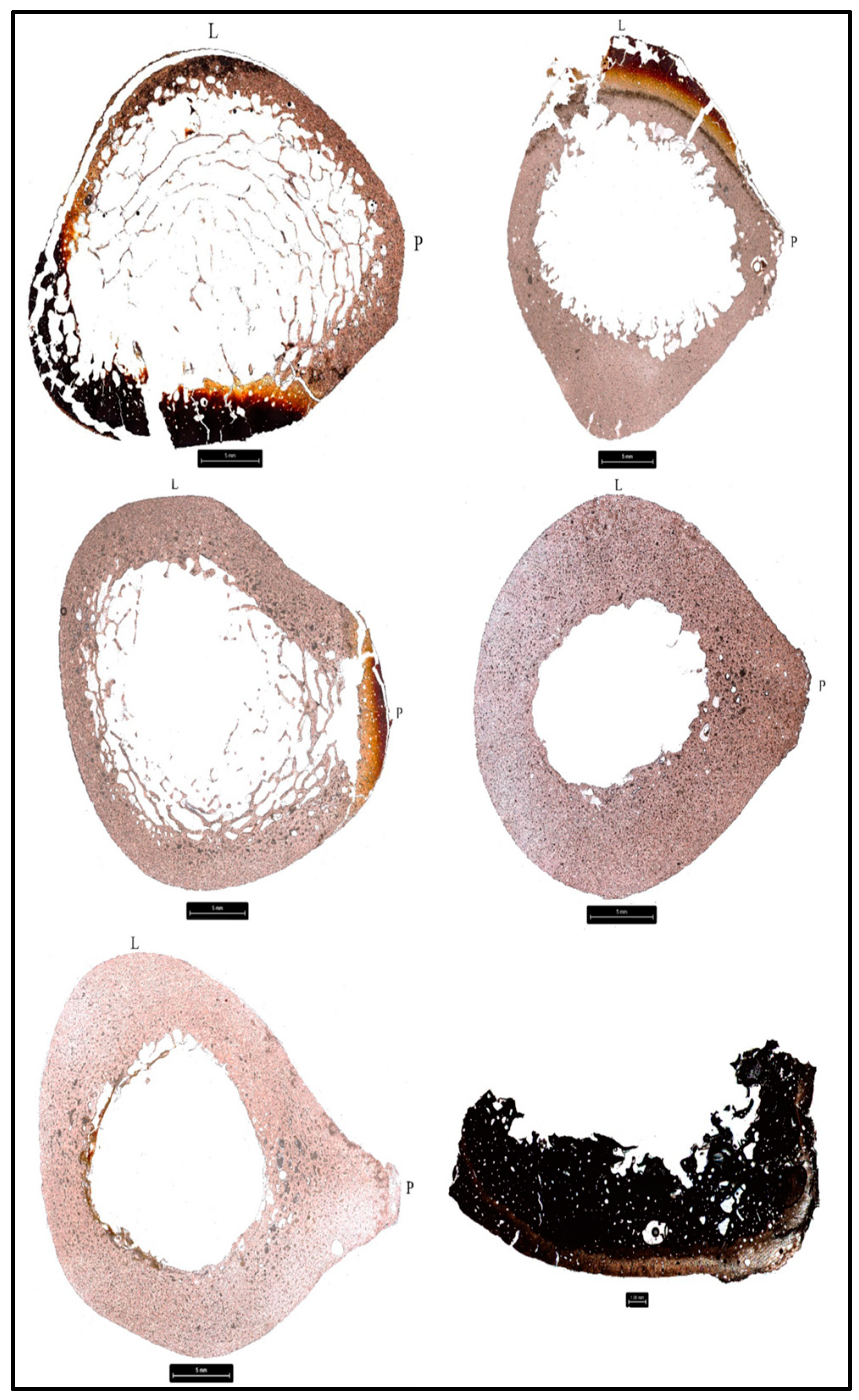
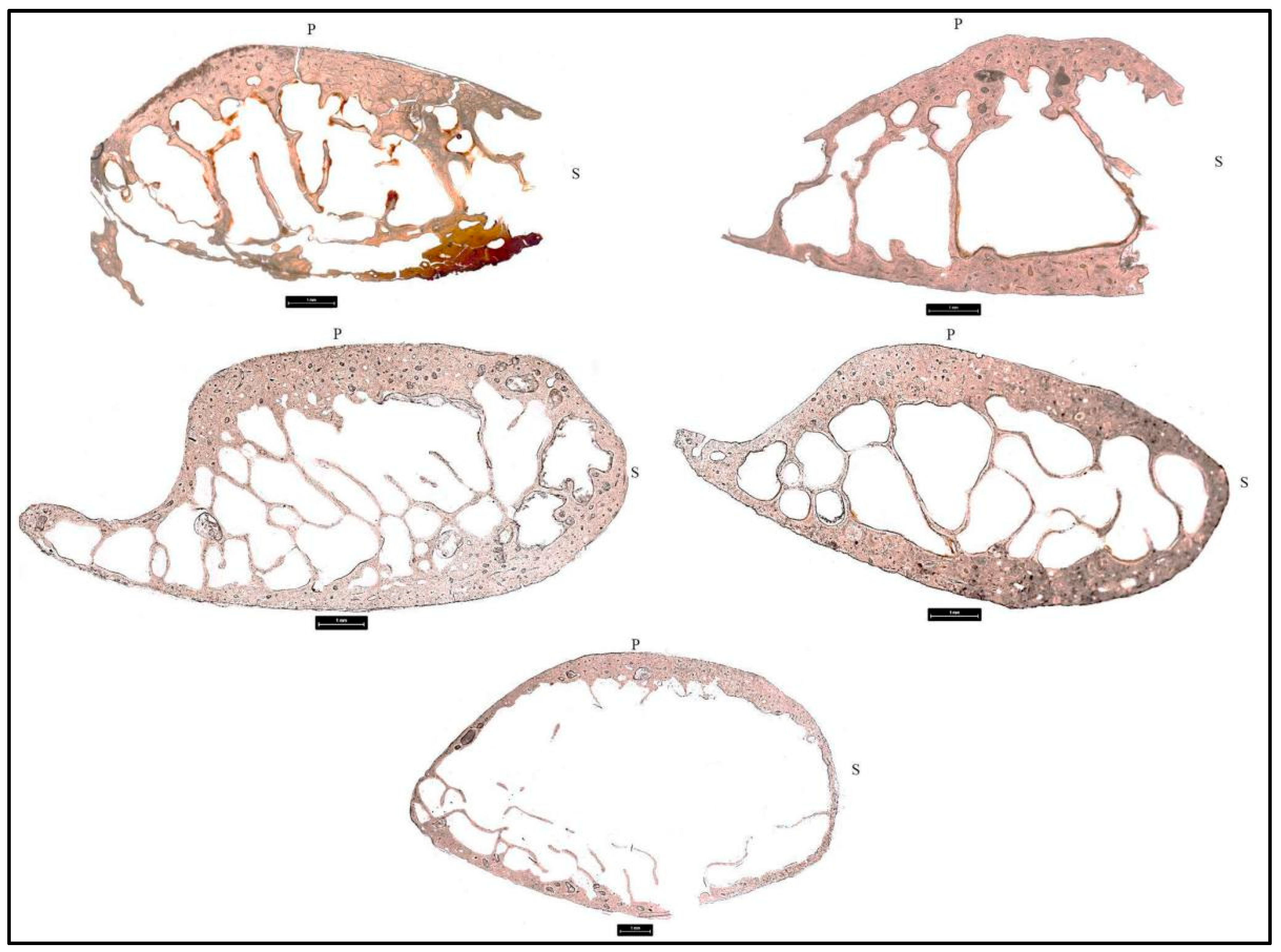
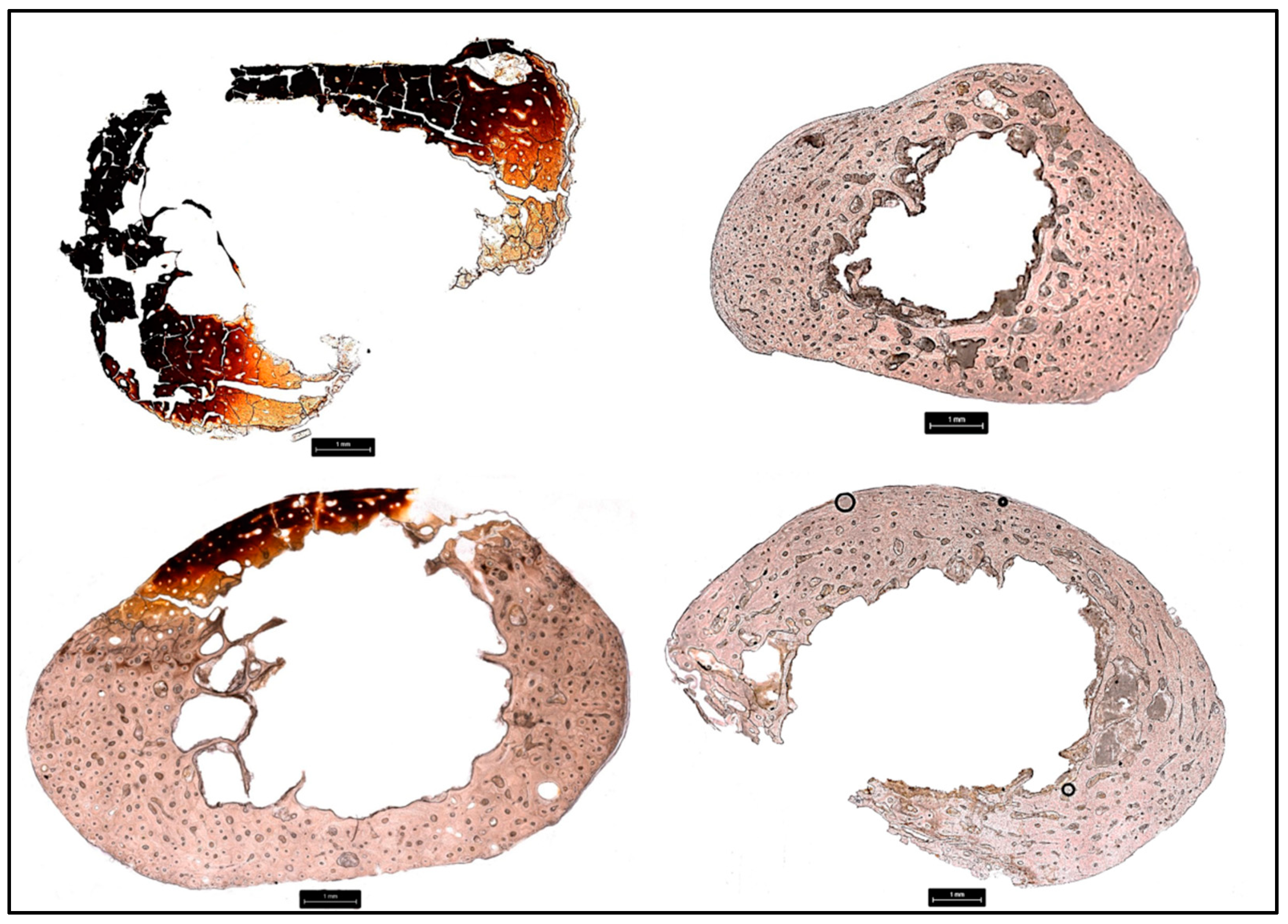
3.2. Cortical Bone Histomorphometric Analysis
3.2.1. Donor 1 (D1)
3.2.2. Donor 2 (D2)
3.2.3. Donor 3 (D3)
3.2.4. Donor 4 (D4)
3.2.5. Donor 5 (D5)
3.2.6. Donor 6 (D6)
3.3. Cortical Age-at-Death Estimation
3.3.1. Crowder and Dominguez [25] Femur Aging Method
3.3.2. Cho et al. [29] Rib Aging Method
3.3.3. Dental Age Estimation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fairgrieve, S.I. Forensic Cremation Recovery and Analysis; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Ubelaker, D. The Forensic Evaluation of Burned Skeletal Remains: A Synthesis. Forens. Sci. Int. 2009, 183, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.W.; Symes, S.A. The Analysis of Burned Human Remains, 2nd ed.; Schmidt, C.W., Symes, S.A., Eds.; Academic Press: London, UK, 2015. [Google Scholar]
- Nikita, E. Introduction to the Study of Burned Human Skeletal Remains; The Cyprus Institute Science and Technology in Archaeology and Culture Research Center (STARC): Nicosia, Cyprus, 2021. [Google Scholar]
- Mulhern, D.M.; Ubelaker, D.H. Differences in Osteon Banding between Human and Nonhuman Bone. J. Forens. Sci. 2001, 46, 220–222. [Google Scholar] [CrossRef]
- Burr, D. Chapter 1-Bone Morphology and Organization|Elsevier Enhanced Reader. In Basic and Applied Bone Biology; Burr, D., Allen, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–26. [Google Scholar] [CrossRef]
- Dominguez, V.M.; Crowder, C.M. The Utility of Osteon Shape and Circularity for Differentiating Human and Non-Human Haversian Bone. Am. J. Phys. Anthropol. 2012, 149, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Crescimanno, A.; Stout, S.D. Differentiating Fragmented Human and Nonhuman Long Bone Using Osteon Circularity. J. Forens. Sci. 2012, 57, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Stout, S.D.; Dietze, W.H.; Iscan, M.Y.; Loth, S.R. Estimation of Age at Death Using Cortical Histomorphometry of the Sternal End of the Fourth Rib. J. Forens. Sci. 1994, 39, 778–784. [Google Scholar] [CrossRef]
- Dominguez, V.M.; Mavroudas, S. Chapter 10-Bone Histology for Skeletal Age-at-Death Estimation. In Age Estimation: A Multidisciplinary Approach. An Introduction to Anthropological, Odontological, Histological, and Biochemical Methods for Assessment of Age; Adserias-Garriga, J., Ed.; Academic Press: London, UK, 2019; pp. 145–159. [Google Scholar] [CrossRef]
- Pfeiffer, S.; Heinrich, J.; Beresheim, A.; Alblas, M. Cortical Bone Histomorphology of Known-Age Skeletons from the Kirsten Collection, Stellenbosch University, South Africa. Am. J. Phys. Anthropol. 2016, 160, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Kerley, E.R. The Microscopic Determination of Age in Human Bone. Am. J. Phys. Anthropol. 1965, 23, 149–164. [Google Scholar] [CrossRef]
- Ahlqvist, J.F.; Damsten, O. A Modification of Kerley’s Method for the Microscopic Determination of Age in Human Bone. J. Forens. Sci. 1969, 14, 205–212. [Google Scholar]
- Singh, I.J.; Gunberg, D.L. Estimation of Age at Death in Human Males from Quantitative Histology of Bone Fragments. Am. J. Phys. Anthropol. 1970, 33, 373–381. [Google Scholar] [CrossRef]
- Kerley, E.R.; Ubelaker, D.H. Revisions in the Microscopic Method of Estimating Age at Death in Human Cortical Bone. Am. J. Phys. Anthropol. 1978, 49, 545–546. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.D. The Core Technique in the Determination of Age at Death of Skeletons. J. Forens. Sci. 1979, 24, 902–915. [Google Scholar] [CrossRef]
- Ericksen, M.F. Histologic Estimation of Age at Death Using the Anterior Cortex of the Femur. Am. J. Phys. Anthropol. 1991, 84, 171–179. [Google Scholar] [CrossRef]
- Fangwu, Z. Preliminary Study on Determination of Bone Age by Microscopic Method. Acta Anthropol. Sin. 1983, II, 142–151. [Google Scholar]
- Hauser, R.; Barres, D.; Durigon, M.; Derobert, L. Identification Par l’histomorphometrie Du Femur et Du Tibia. Acta Med. Leg. Soc. 1980, 30, 91097. [Google Scholar]
- Cera, F.; Drusini, A. Analisi Critica e Sperimentale Dei Metodi di Determinazione Dell’eta Attraverso Le Microstrutture Ossee. Quad. Anat. Prat. 1985, 41, 105–121. [Google Scholar]
- Sampson, C.; Branigan, K. A New Method of Estimating Age at Death from Fragmentary and Weathered Bone. In Death Decay and Reconstruction: Approaches to Archaeology and Forensic Science; Boddington, A., Garland, A., Janaway, R., Eds.; Manchester University Press: Manchester, UK, 1987; pp. 101–108. [Google Scholar]
- Narasaki, S. Estimation of Age at Death by Femoral Osteon Remodeling: Application of Thompson’s Core Technique to Modern Japanese. J. Anthrop. Soc. Nippon 1990, 98, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y.; Konishi, M.; Shimada, M.; Ohara, H.; Iwamoto, S. Estimation of Age from the Femur of Japanese Cadavers. Forens. Sci. Int. 1998, 98, 55–65. [Google Scholar] [CrossRef]
- Maat, G.J.; Maes, A.; Aarents, M.J.; Nagelkerke, N.J. Histological Age Prediction from the Femur in a Contemporary Dutch Sample. The Decrease of Nonremodeled Bone in the Anterior Cortex. J. Forens. Sci. 2006, 51, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Crowder, C.; Dominguez, V.M. A New Method for Histological Age Estimation of the Femur. In Proceedings of the American Academy of Forensic Sciences 64th Annual Meeting, Atlanta, GA, USA, 20–25 February 2012. [Google Scholar]
- Gocha, T.; Stout, S.; Agnew, A. Examining Accuracy of Age Estimates from New Histological Strategies at the Femoral Midshaft. In Proc. Am. Acad. Forens. Sci. 2016, 51, 230–237. [Google Scholar]
- Kenyhercz, M.; Crowder, C.M.; Dominguez, V.M. Histological Age Estimation of the Femur Using Random Forest Regression. In Proceedings of the American Academy of Forensic Sciences 72nd Annual Meeting, Anaheim, CA, USA, 17–22 February 2020. [Google Scholar]
- Stout, S.D.; Paine, R.R. Brief Communication: Histological Age Estimation Using Rib and Clavicle. Am. J. Phys. Anthropol. 1992, 87, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Stout, S.D.; Madsen, R.W.; Streeter, M.A. Population-Specific Histological Age-Estimating Method: A Model for Known African-American and European-American Skeletal Remains. J. Forens. Sci. 2002, 47, 12–18. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, D.-I.; Park, D.-K.; Lee, J.-H.; Chung, N.-E.; Lee, W.-T.; Han, S.-H. Assessment of Histomorphological Features of the Sternal End of the Fourth Rib for Age Estimation in Koreans. J. Forens. Sci. 2007, 52, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Pavon, M.V.; Cucina, A.; Tiesler, V. New Formulas to Estimate Age at Death in Maya Populations Using Histomorphological Changes in the Fourth Human Rib. J. Forens. Sci. 2010, 55, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Goliath, J.R.; Stewart, M.C.; Stout, S.D. Variation in Osteon Histomorphometrics and Their Impact on Age-at-Death Estimation in Older Individuals. Forens. Sci. Int. 2016, 262, 282.e1-6. [Google Scholar] [CrossRef]
- García-Donas, J.G.; Paine, R.R.; Bonicelli, A.; Kranioti, E.F. Age Estimation for Two Mediterranean Populations: Rib Histomorphometry Applied to Forensic Identification and Bone Remodelling Research. Int. J. Leg. Med. 2022, 136, 1469–1481. [Google Scholar] [CrossRef]
- Wittwer-Backofen, U.; Gampe, J.; Vaupel, J.W. Tooth Cementum Annulation for Age Estimation: Results from a Large Known-Age Validation Study. Am. J. Phys. Anthropol. 2004, 123, 119–129. [Google Scholar] [CrossRef]
- Gocha, T.P.; Schutkowski, H. Tooth Cementum Annulation for Estimation of Age-at-Death in Thermally Altered Remains. J. Forens. Sci. 2013, 58, S151–S155. [Google Scholar] [CrossRef]
- Kagerer, P.; Grupe, G. Age-at-death diagnosis and determination of life history parameters by incremental lines in human dental cementum as an identification aid. Forens. Sci. Int. 2001, 118, 75–82. [Google Scholar] [CrossRef]
- Aggarwal, P.; Saxena, S.; Bansal, P. Incremental Lines in Root Cementum of Human Teeth: An Approach to Their Role in Age Estimation Using Polarizing Microscopy. Indian J. Dent. Res. 2008, 19, 326. [Google Scholar] [CrossRef]
- Wedel, V.L.; Wescott, D.J. Using Dental Cementum Increment Analysis to Estimate Age and Season of Death in African Americans from an Historical Cemetery in Missouri. Int. J. Paleopathol. 2016, 15, 134–139. [Google Scholar] [CrossRef]
- Ristova, M.; Talevska, M.; Stojanovska, Z. Accurate Age Estimations from Dental Cementum and a Childbirth Indicator- a Pilot Study. J. Forens. Sci. Criminol. 2018, 6, 102. [Google Scholar]
- Wedel, V.L. Determination of season at death using dental cementum increment analysis. J. Forens. Sci. 2007, 52, 1334–1337. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, D.E. Life History Variables Preserved in Dental Cementum Microstructure. Science 1993, 261, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, D.E. The Biological Basis for Seasonal Increments in Dental Cementum and Their Application to Archaeological Research. J. Archaeol. Sci. 1994, 21, 525–539. [Google Scholar] [CrossRef]
- Garvin, H.M.; Passalacqua, N.V. Current Practices by Forensic Anthropologists in Adult Skeletal Age Estimation. J. Forens. Sci. 2012, 57, 427–433. [Google Scholar] [CrossRef]
- Forbes, G. The effects of heat on the histological structure of bone. Police J. 1941, 14, 50–60. [Google Scholar] [CrossRef]
- Bradtmiller, B.; Buikstra, J.E. Effects of Burning on Human Bone Microstructure: A Preliminary Study. J. Forens. Sci. 1984, 29, 535–540. [Google Scholar] [CrossRef]
- Shipman, P.; Foster, G.; Schoeninger, M. Burnt bones and teeth: An experimental study of color, morphology, crystal structure and shrinkage. J. Archaeol. Sci. 1984, 11, 307–325. [Google Scholar] [CrossRef]
- Nelson, R. A Microscopic Comparison of Fresh and Burned Bone. J. Forens. Sci. 1992, 37, 1055–1060. [Google Scholar]
- Hummel, S.; Schutkowski, H. Approaches to the Histological Age Determination of Cremated Human Remains. In Histology of Ancient Human Bone: Methods and Diagnosis; Grupe, G., Garland, A.N., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 111–123. [Google Scholar]
- Cattaneo, C.; DiMartino, S.; Scali, S.; Craig, O.E.; Grandi, M.; Sokol, R.J. Determining the Human Origin of Fragments of Burnt Bone: A Comparative Study of Histological, Immunological and DNA Techniques. Forens. Sci. Int. 1999, 102, 181–191. [Google Scholar] [CrossRef]
- Hanson, M.; Cain, C.R. Examining Histology to Identify Burned Bone. J. Archaeol. Sci. 2007, 34, 1902–1913. [Google Scholar] [CrossRef]
- Absolonova, K.; Veleminsky, P.; Dobisikova, M.; Beran, M.; Zocova, J. Histological Estimation of Age at Death from the Compact Bone of Burned and Unburned Human Ribs. J. Forens. Sci. 2013, 58, S135–S145. [Google Scholar] [CrossRef] [PubMed]
- Fernández Castillo, R.; Ubelaker, D.H.; Acosta, J.A.L.; de la Rosa, R.J.E.; Garcia, I.G. Effect of Temperature on Bone Tissue: Histological Changes. J. Forens. Sci. 2013, 58, 578–582. [Google Scholar] [CrossRef]
- Carroll, E.L.; Squires, K.E. Burning by Numbers: A Pilot Study Using Quantitative Petrography in the Analysis of Heat-Induced Alteration in Burned Bone. Int. J. Osteoarchaeol. 2020, 30, 691–699. [Google Scholar] [CrossRef]
- Labuschagne, L. The Use of Histological Examination Methods to Distinguish between the Burnt Remains of Human and Non-Human Bones. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2020. [Google Scholar]
- Oliveira-Santos, I.; Gouveia, M.; Cunha, E.; Gonçalves, D. The Circles of Life: Age at Death Estimation in Burnt Teeth through Tooth Cementum Annulations. Int. J. Leg. Med. 2017, 131, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Glassman, D.M.; Crow, R.M. Standardization Model for Describing the Extent of Burn Injury to Human Remains. J. Forens. Sci. 1996, 41, 152–154. [Google Scholar] [CrossRef]
- Crowder, C.; Heinrich, J.; Stout, S.D. Rib Histomorphometry for Adult Age Estimation. In Forensic Microscopy for Skeletal Tissues: Methods and Protocols; Bell, L.S., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; pp. 109–127. [Google Scholar] [CrossRef]
- Naji, S.; Colard, T.; Blondiaux, J.; Bertrand, B.; d’Incau, E.; Bocquet-Appel, J.-P. Cementochronology, to Cut or Not to Cut? Int. J. Paleopathol. 2016, 15, 113–119. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Crowder, C. Estimation of Age at Death Using Cortical Bone Histomorphometry; Report to the U.S. Department of Justice. 2013. Available online: https://www.ojp.gov/pdffiles1/nij/grants/240692.pdf (accessed on 5 October 2022).
- keRley: Histological Age Estimation. Available online: https://anthropologyapps.shinyapps.io/keRley/ (accessed on 26 August 2022).
- AlQahtani, S.J.; Hector, H.M.; Liversidge, H.M. Brief Communication: The London Atlas of Human Tooth Development and Eruption. Am. J. Phys. Anthropol. 2010, 142, 142–148. [Google Scholar] [CrossRef]
- Großkopf, B. Incremental lines in prehistoric cremated teeth. A technical note. Z. Morphol. Anthropol. 1989, 77, 309–311. [Google Scholar] [CrossRef]
- Meckel, L.A. The Utility of Dental Cementum Increment Analysis for Estimating Season-of-Death in Natura. Master’s Thesis, Texas State University, San Marcos, TX, USA, 2016. [Google Scholar]
- DeHaan, J.D.; Nurbakhsh, S. Sustained Combustion of an Animal Carcass and Its Implications for the Consumption of Human Bodies in Fires. J. Forens. Sci. 2001, 46, 1076–1081. [Google Scholar] [CrossRef]
- Lennartz, A.; Hamilton, M.D.; Weaver, R. Moisture Content in Decomposing, Desiccated, and Mummified Human Tissue. Forens. Anthropol. 2020, 3, 1. [Google Scholar] [CrossRef]
- Agnew, A.M. Histomorphometry of the Elderly Rib: A Methodological Approach with Implications for Biomechanics, Function and Fracture Risk. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2011. [Google Scholar]
- Havill, L.M.; Allen, M.R.; Harris, J.A.K.; Levine, S.M.; Coan, H.B.; Mahaney, M.C.; Nicolella, D.P. Intracortical Bone Remodeling Variation Shows Strong Genetic Effects. Calcif. Tissue Int. 2013, 93, 472–480. [Google Scholar] [CrossRef] [PubMed]
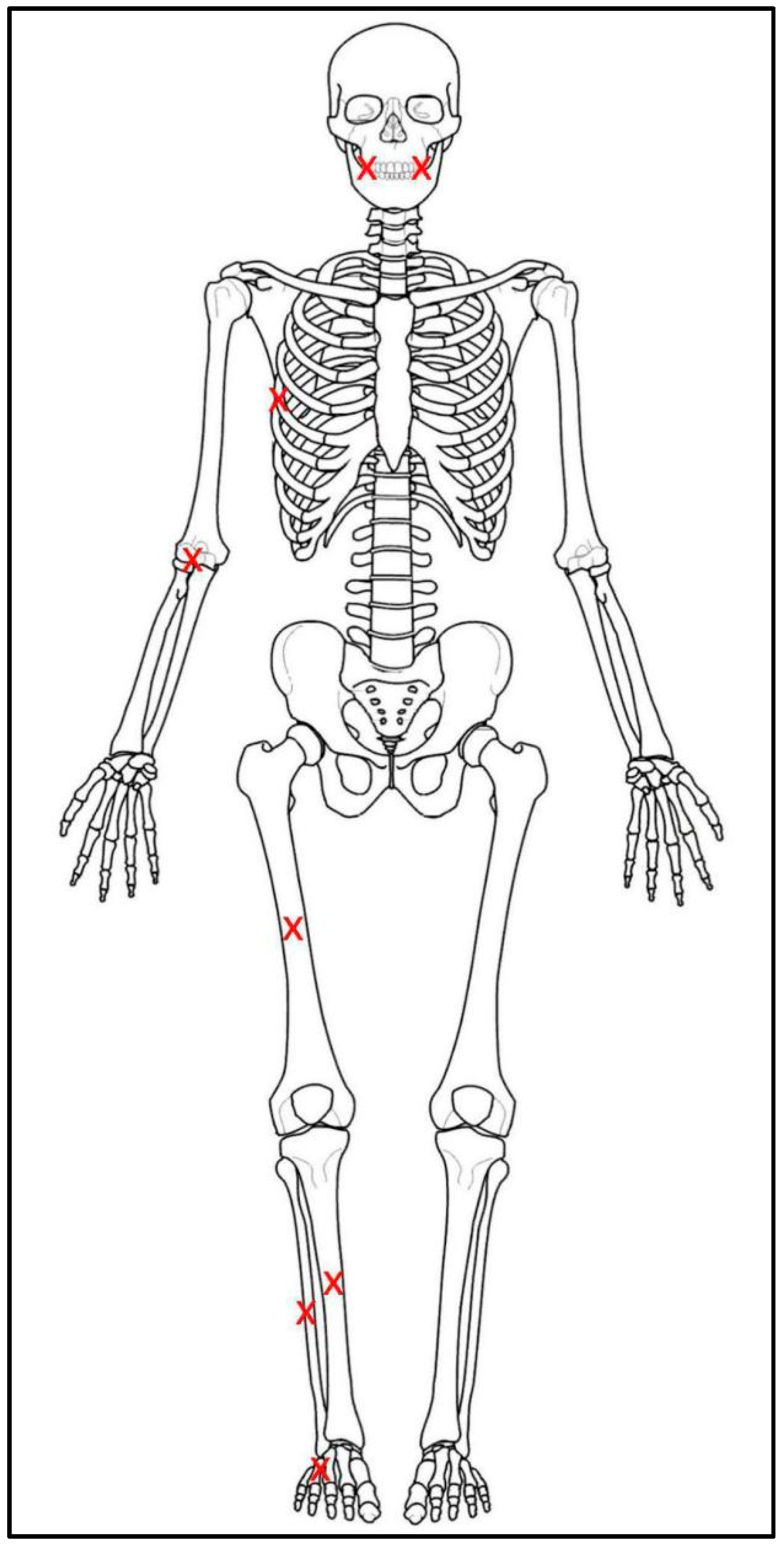
| Donor Number and Description | Sex | Age | Fire-Death Scenario |
|---|---|---|---|
| D1 Car Donor | F | 84 | Car |
| D2 Fresh Pit Donor | F | 81 | Pit, Fresh |
| D3 Pod Donor 1 | F | 74 | Pod 1, Couch |
| D4 Pod Donor 2 | F | 61 | Pod 2, Bed |
| D5 Pod Donor 3 | M | 65 | Pod 3, Bed |
| D6 Mummified Pit Donor | F | 67 | Pit, Mummified |
| Level | Stage | Description |
|---|---|---|
| 1 | Recognizable | Typically smoke death |
| 2 | Possibly recognizable | Charring on elements such as hand/feet, genitalia |
| 3 | Non-recognizable | Major destruction of head and extremities |
| 4 | Extensive burn destruction | Skull and extremities are severely fragmented or missing |
| 5 | Cremation | Little or no tissue remains |
| Donor | Pre-Burn Bone Samples | Pre-burn tooth samples | Post-Burn Bone Samples | Post-Burn Tooth Samples |
|---|---|---|---|---|
| D1 | Femur, Rib, Metatarsal | N/A | Femur, Rib | N/A |
| D2 | Femur, Rib, Metatarsal | N/A | Femur, Rib, Metatarsal | 14, 11, 43 |
| D3 | Femur, Rib, Metatarsal | N/A | Femur, Rib, Metatarsal | 23, 32 |
| D4 | Femur, Rib, Metatarsal | N/A | Femur, Rib, Metatarsal | 21, 23, 25 |
| D5 | Femur, Rib, Metatarsal | N/A | Femur, Rib, Metatarsal | 43, 44, 45 |
| D6 | Femur, Rib, Metatarsal | N/A | Femur | N/A |
| Donor | Mouth (Middle) | Mouth (Side) | 6th Rib | Elbow | Thigh | Shin | Calf | Foot | Structure | Burn Duration (min) | Mean Burn Temp |
|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | 801.8 | 795.2 | 1273.0 | 880.5 | 1053.8 | 1390.4 | 959.3 | 1384.6 | 1115.7 | 42 | 487.7 |
| D2 | 549.3 | 667.3 | 955.7 | 923.8 | 718.3 | 823.0 | 314.5 | 987.6 | 1036.6 | 53 | 206.6 |
| D3 | 821.3 | 860.8 | 324.4 | 307.6 | 440.4 | 931.4 | 885.6 | 781.0 | 880.5 | 32 | 193.0 |
| D4 | 479.2 | 457.6 | 532.0 | 83.5 | 29.8 | 105.8 | 731.2 | 827.2 | 832.8 | 32 | 152.0 |
| D5 | 1034.8 | 1072.0 | 25.8 | 452.4 | 45.9 | 756.0 | 684.4 | 817.5 | 1070.4 | 21 | 251.2 |
| D6 | 935.6 | 924.5 | 1074.1 | 1014.0 | 1045.3 | 1020.8 | 1014.6 | 1063.2 | 1080.2 | 53 | 352.9 |
| Donor | Body Score |
|---|---|
| D1 | 4 |
| D2 | 3 |
| D3 | 2 |
| D4 | 2 |
| D5 | 2 |
| D6 | 5 |
| Donor | Element | Number of Osteons Measured | Pre-Burn | Post-Burn | On.Ar t Statistic (df) | ||
|---|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | ||||
| D1 | Femur Rib Metatarsal | 19 39 0 | 0.042 0.027 N/A | 0.017 0.010 N/A | 0.055 0.020 N/A | 0.019 0.009 N/A | −2.7 (18) * 2.31 (39) * N/A |
| D2 | Femur Rib Metatarsal | 29 21 0 | 0.035 0.033 N/A | 0.010 0.014 N/A | 0.040 0.034 N/A | 0.010 0.013 N/A | −1.8 (28) −0.22 (20) N/A |
| D3 | Femur Rib Metatarsal | 40 49 39 | 0.030 0.025 0.020 | 0.010 0.010 0.010 | 0.030 0.025 0.030 | 0.010 0.012 0.010 | −0.23 (39) 0.04 (48) −3.46 (38) ** |
| D4 | Femur Rib Metatarsal | 40 41 41 | 0.045 0.030 0.035 | 0.014 0.020 0.011 | 0.049 0.030 0.033 | 0.021 0.010 0.011 | −0.71 (39) −0.73 (40) 0.83 (40) |
| D5 | Femur Rib Metatarsal | 40 42 39 | 0.050 0.030 0.038 | 0.020 0.010 0.020 | 0.050 0.030 0.030 | 0.020 0.010 0.010 | −0.14 (39) −0.53 (41) 1.69 (38) |
| D6 | Femur Rib Metatarsal | 21 0 0 | 0.056 N/A N/A | 0.020 N/A N/A | 0.036 N/A N/A | 0.014 N/A N/A | 3.29 (20) ** N/A N/A |
| Donor | Element | Number of Haversian Canals Measured | Pre-Burn | Post-Burn | H.Ar t Statistic (df) | ||
|---|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | ||||
| D1 | Femur Rib Metatarsal | 20 40 0 | 0.0035 0.0020 N/A | 0.0172 0.0013 N/A | 0.0037 0.0012 N/A | 0.0147 0.0010 N/A | −0.2 (19) 2.57 (39) * N/A |
| D2 | Femur Rib Metatarsal | 40 21 40 | 0.0338 0.0022 0.0017 | 0.0121 0.0014 0.0010 | 0.0380 0.0016 0.0024 | 0.0125 0.0011 0.0012 | 3.4 (39) ** 1.43 (20) −2.64 (39) * |
| D3 | Femur Rib Metatarsal | 40 49 41 | 0.0026 0.0020 0.0026 | 0.0018 0.0014 0.0017 | 0.0030 0.0017 0.0030 | 0.0024 0.0012 0.0020 | −1.06 (39) 1.35 (48) −1.54 (40) |
| D4 | Femur Rib Metatarsal | 40 42 40 | 0.0038 0.0013 0.0048 | 0.0018 0.0007 0.0028 | 0.0036 0.0016 0.0037 | 0.0022 0.0009 0.0020 | 0.54 (39) −1.47 (41) 2.09 (39) * |
| D5 | Femur Rib Metatarsal | 38 42 38 | 0.0043 0.0020 0.0034 | 0.0031 0.0017 0.0022 | 0.0037 0.0018 0.0059 | 0.0021 0.0012 0.0030 | 0.92 (37) 0.75 (41) 4.94 (39) ** |
| D6 | Femur Rib Metatarsal | 39 0 0 | 0.0033 N/A N/A | 0.0020 N/A N/A | 0.0019 N/A N/A | 0.0010 N/A N/A | 4.82 (38) ** N/A N/A |
| Donor | Known Age (Years) | Pre-burn Sample | Post-burn Sample | Post-Burn Sample Retest with no On.Ar | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IR | Mean Estimate | Variables Used | IR | Mean Estimate | Variables Used | IR | Mean Estimate | Variables Used | ||
| D1 | 85 | 59–91 * | 76 | iOPD, fOPD,On.Ar, Ant.Wi | 22–60 | 30 | iOPD, On.Ar | 24–50 | 29 | iOPD |
| D2 | 81 | 61–93 * | 78 | iOPD, fOPD,On.Ar, Ant.Wi | 57–86 * | 68 | iOPD, Ant.Wi | 57–86 * | 68 | iOPD, Ant.Wi |
| D3 | 74 | 53–90 * | 71 | iOPD, fOPD,On.Ar, Ant.Wi | 69–92 * | 78 | iOPD, On.Ar, Ant.Wi | 69–85 * | 77 | iOPD, Ant.Wi |
| D4 | 61 | 50–92 * | 73 | iOPD, fOPD,On.Ar, Ant.Wi | 55–82 * | 67 | iOPD, On.Ar, Ant.Wi | 54–80 * | 68 | iOPD, Ant.Wi |
| D5 | 65 | 23–74 * | 57 | iOPD, fOPD,On.Ar, Ant.Wi | 65–88 * | 77 | iOPD, Ant.Wi | 65–88 * | 77 | iOPD, Ant.Wi |
| D6 | 67 | 56–85 * | 71 | iOPD, fOPD,On.Ar, Ant.Wi | No anterior femur recovered | No anterior femur recovered | ||||
| Donor | Known Age (years) | Pre-Burn | Post-Burn | ||
|---|---|---|---|---|---|
| OPD | Point Age (Years) | OPD | Point Age (Years) | ||
| D1 | 85 | 22.06 | 53.23 | 24.99 | 63.06 * |
| D2 | 81 | 21.69 | 48.92 | 22.4 | 51.83 |
| D3 | 74 | 26.05 | 57.39 | 26.05 | 59.89 |
| D4 | 61 | 21.47 | 46.16 | 22.5 | 49.36 |
| D5 | 65 | 21.06 | 52.47 | 20.46 | 51.28 |
| D6 | 67 | 23.63 | 61.58 | N/A | N/A |
| Donor | Tooth | Average Number of TCA Lines | Average Age of Tooth Eruption (Years) | Estimated Age Based on Tooth (Years) | Global Age Estimate (Years) | Known Age (Years) |
|---|---|---|---|---|---|---|
| D2 | 11 | 82.54 | 7.5 | 90.04 | 79.21 | 81 |
| 43 | 56.89 | 11.5 | 68.38 | |||
| D3 | 23 | 39.14 | 12.5 | 51.64 | 56.31 | 74 |
| 32 | 49.48 | 11.5 | 60.98 | |||
| D4 | 21 | 55.09 | 7.5 | 62.59 | 69.19 | 61 |
| 23 | 63.29 | 12.5 | 75.79 | |||
| D5 | 43 | 52.58 | 11.5 | 64.08 | 60.42 | 65 |
| 44 | 65.90 | 11.5 | 77.40 | |||
| 45 | 27.38 | 12.5 | 39.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavroudas, S.R.; Meckel, L.A.; Gocha, T.P.; Goldstein, J.Z.; Garza, S.L. The Effects of Experimental Whole-Body Burning on Histological Age-at-Death Estimation from Human Cortical Bone and Dental Cementum. Biology 2022, 11, 1569. https://doi.org/10.3390/biology11111569
Mavroudas SR, Meckel LA, Gocha TP, Goldstein JZ, Garza SL. The Effects of Experimental Whole-Body Burning on Histological Age-at-Death Estimation from Human Cortical Bone and Dental Cementum. Biology. 2022; 11(11):1569. https://doi.org/10.3390/biology11111569
Chicago/Turabian StyleMavroudas, Sophia R., Lauren A. Meckel, Timothy P. Gocha, Justin Z. Goldstein, and Shelby L. Garza. 2022. "The Effects of Experimental Whole-Body Burning on Histological Age-at-Death Estimation from Human Cortical Bone and Dental Cementum" Biology 11, no. 11: 1569. https://doi.org/10.3390/biology11111569
APA StyleMavroudas, S. R., Meckel, L. A., Gocha, T. P., Goldstein, J. Z., & Garza, S. L. (2022). The Effects of Experimental Whole-Body Burning on Histological Age-at-Death Estimation from Human Cortical Bone and Dental Cementum. Biology, 11(11), 1569. https://doi.org/10.3390/biology11111569





