Microbial Consortia: An Engineering Tool to Suppress Clubroot of Chinese Cabbage by Changing the Rhizosphere Bacterial Community Composition
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biocontrol Bacterial Strains, Growth Medium, and Culture Conditions
2.2. Assembly of Microbial Consortia
2.3. Experimental Site, Experimental Conditions, and Design Descriptions
2.4. Assessment of Biocontrol Effect, Plant Growth Promotion, and Soil pH
2.5. Soil-Sample Collection and Extraction of DNA
2.6. PCR Amplification and Analysis of Rhizospheric Bacterial Diversity
2.7. Bioinformatics Analysis
3. Results
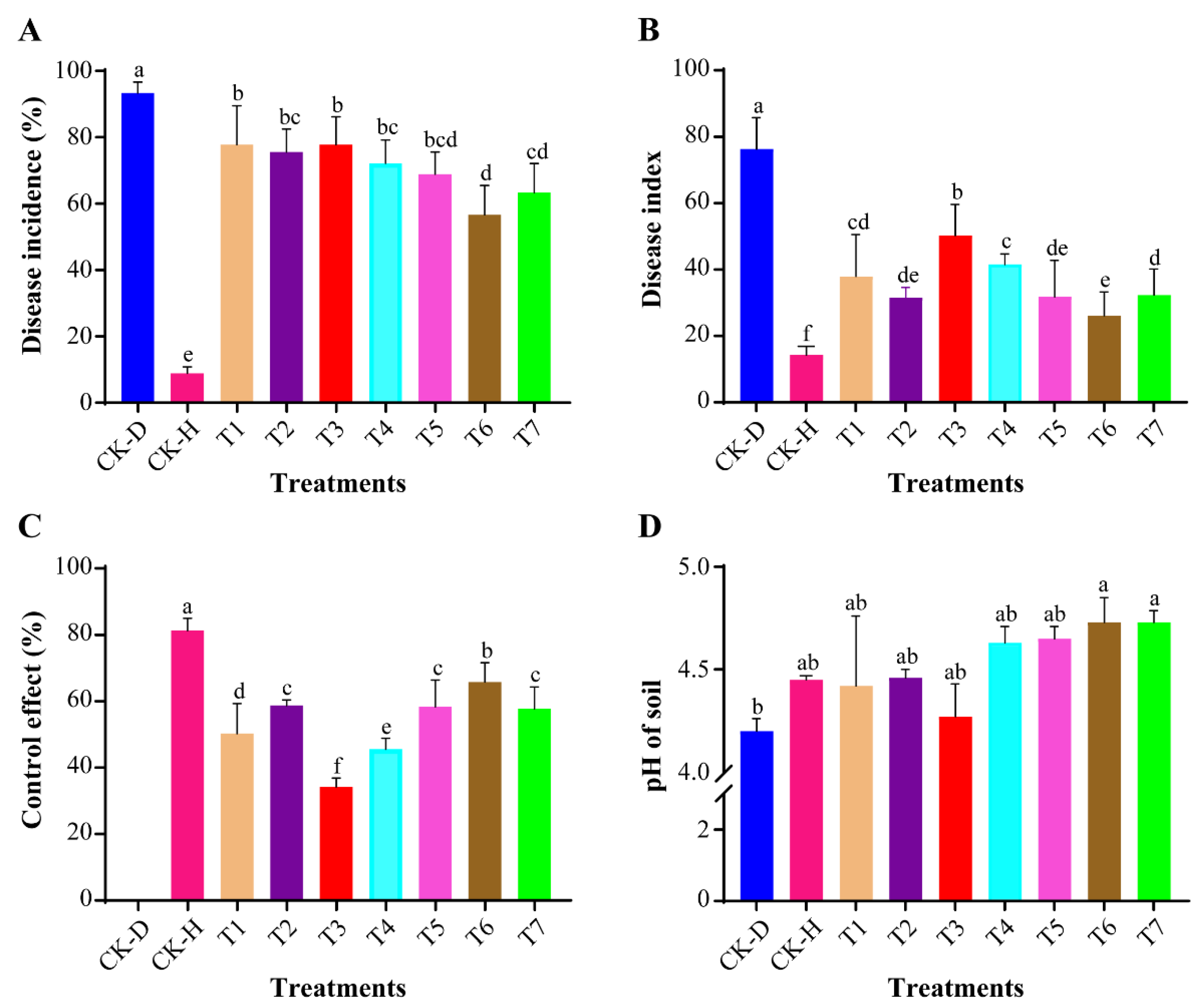
3.1. Assessment of Disease Incidence and Soil pH
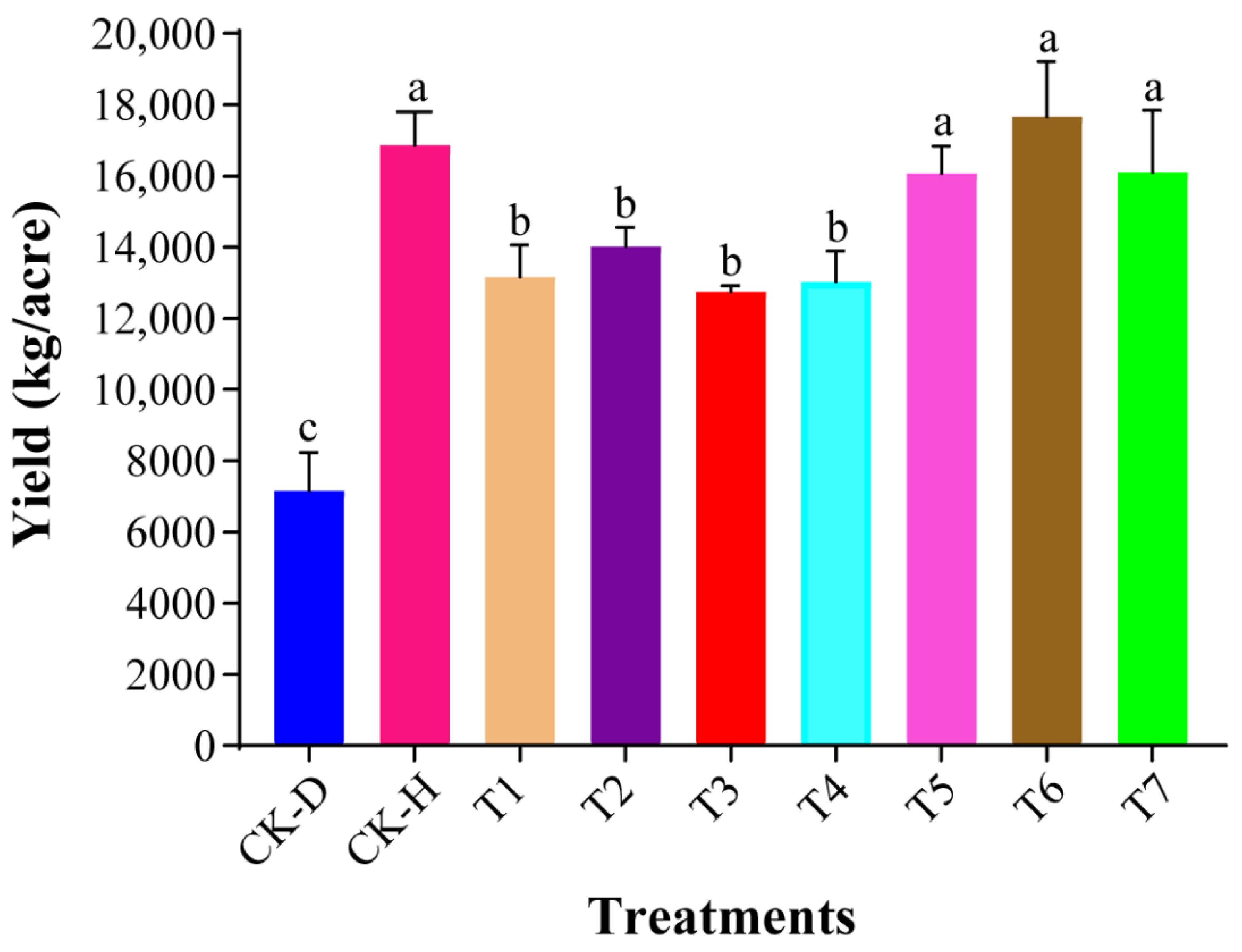
3.2. Evaluation of Plant Growth Promotion Potential of Biocontrol Strains
3.3. Assessment of Alpha Diversity of Bacterial Community Associated with Rhizosphere Soil of Chinese Cabbage
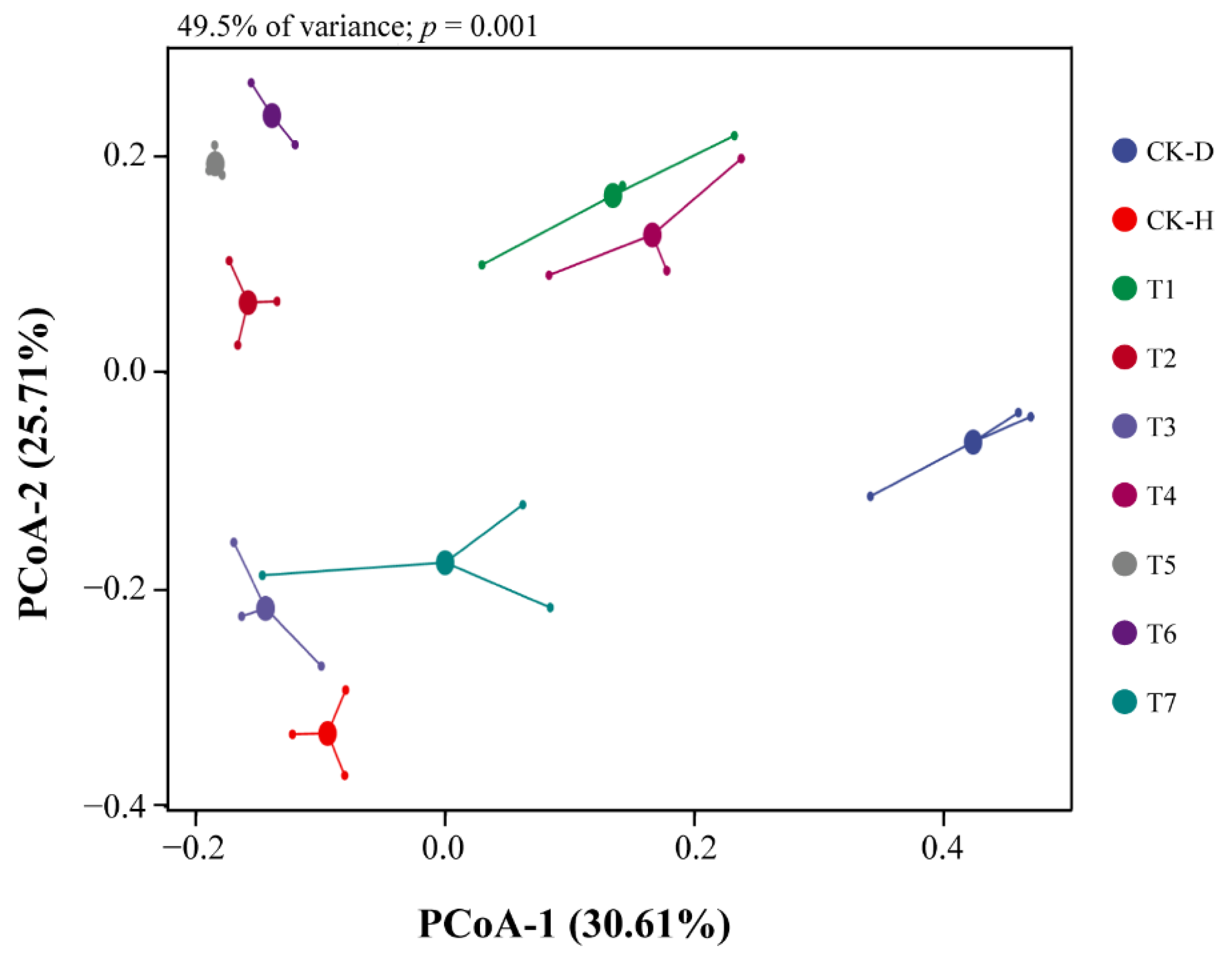
3.4. Investigation of Bacterial Community Structure in the Rhizosphere Soil of Chinese Cabbage
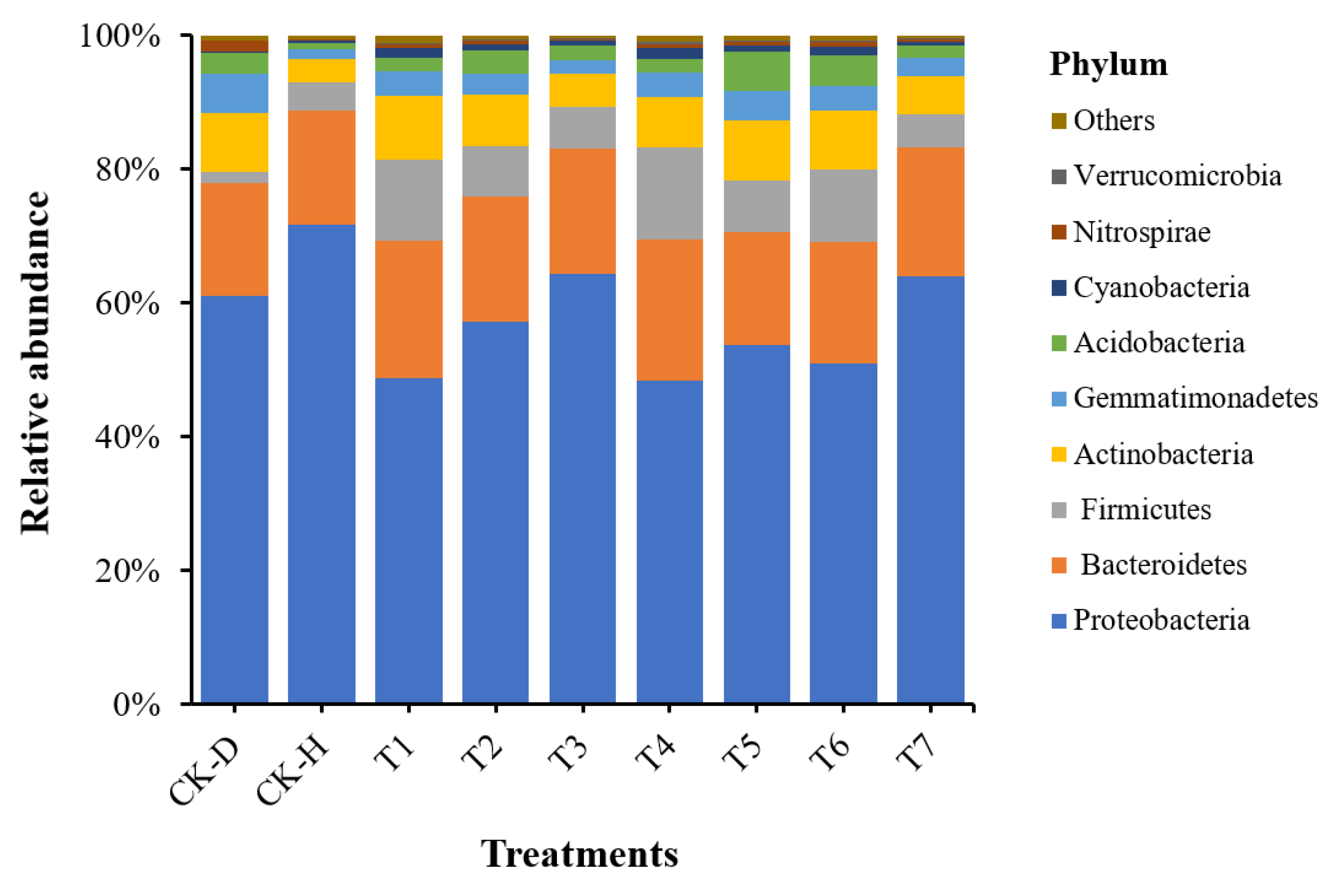
3.5. Analysis of Bacterial Community Composition at the Phylum Level
3.6. Changes in the Bacterial Community Composition at the Genus Level
3.7. Correlation Analysis of Disease Index, Yield, and pH between Bacterial Genera and Diversity Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, L.; Yang, J.; Ahmed, W.; Xiong, X.; Liu, Q.; Huang, Q.; Ji, G. Unraveling the Association between Metabolic Changes in Inter-Genus and Intra-Genus Bacteria to Mitigate Clubroot Disease of Chinese Cabbage. Agronomy 2021, 11, 2424. [Google Scholar] [CrossRef]
- Ren, L.; Xu, L.; Liu, F.; Chen, K.; Sun, C.; Li, J.; Fang, X. Host range of Plasmodiophora brassicae on cruciferous crops and weeds in China. Plant Dis. 2016, 100, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Chai, A.; Xie, X.; Shi, Y.; Li, B. Research status of clubroot (Plasmodiophora brassicae) on cruciferous crops in China. Can. J. Plant Pathol. 2014, 36, 142–153. [Google Scholar] [CrossRef]
- Kageyama, K.; Asano, T. Life cycle of Plasmodiophora brassicae. J. Plant Growth Regul. 2009, 28, 203–211. [Google Scholar] [CrossRef]
- Hwang, S.F.; Strelkov, S.E.; Feng, J.; Gossen, B.D.; Howard, R.J. Plasmodiophora brassicae: A review of an emerging pathogen of the Canadian canola (Brassica napus) crop. Mol. Plant Pathol. 2012, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Pageau, D.; Strelkov, S.E.; Gossen, B.D.; Hwang, S.-F.; Lahlali, R. A> 2-year crop rotation reduces resting spores of Plasmodiophora brassicae in soil and the impact of clubroot on canola. Eur. J. Agron. 2015, 70, 78–84. [Google Scholar] [CrossRef]
- Diederichsen, E.; Frauen, M.; Linders, E.G.; Hatakeyama, K.; Hirai, M. Status and perspectives of clubroot resistance breeding in crucifer crops. J. Plant Growth Regul. 2009, 28, 265–281. [Google Scholar] [CrossRef]
- Yang, X.X.; Huang, X.Q.; Wu, W.X.; Xiang, Y.J.; Lei, D.U.; Zhang, L.; Yong, L.I.U. Effects of different rotation patterns on the occurrence of clubroot disease and diversity of rhizosphere microbes. J. Integr. Agric. 2020, 19, 2265–2273. [Google Scholar] [CrossRef]
- Murakami, H.; Tsushima, S.; Kuroyanagi, Y.; Shishido, Y. Reduction of resting spore density of Plasmodiophora brassicae and clubroot disease severity by liming. Soil Sci. Plant Nutr. 2002, 48, 685–691. [Google Scholar] [CrossRef] [Green Version]
- Botero, A.; García, C.; Gossen, B.D.; Strelkov, S.E.; Todd, C.D.; Bonham-Smith, P.C.; Pérez-López, E. Clubroot disease in Latin America: Distribution and management strategies. Plant Pathol. 2019, 68, 827–833. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Qiu, L.; Zhang, Z.; Liu, K.; Xia, X.; Xiong, S.; Zhao, S.; Zhao, Z.; Hu, Y.; Liang, Y. Control of Streptomyces alfalfae XY25T Over Clubroot Disease and Its Effect on Rhizosphere Microbial Community in Chinese Cabbage Field Trials. Front. Microbiol. 2021, 12, 1504. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Hwang, S.-F.; Manolii, V.P.; Cao, T.; Fredua-Agyeman, R.; Harding, M.W.; Peng, G.; Gossen, B.D.; Mcdonald, M.R.; Feindel, D. Virulence and pathotype classification of Plasmodiophora brassicae populations collected from clubroot resistant canola (Brassica napus) in Canada. Can. J. Plant Pathol. 2018, 40, 284–298. [Google Scholar] [CrossRef]
- Ahmed, W.; Yang, J.; Tan, Y.; Munir, S.; Liu, Q.; Zhang, J.; Ji, G.; Zhao, Z. Ralstonia solanacearum, a deadly pathogen: Revisiting the bacterial wilt biocontrol practices in tobacco and other Solanaceae. Rhizosphere 2022, 21, 100479. [Google Scholar] [CrossRef]
- Xu, X.-M.; Jeffries, P.; Pautasso, M.; Jeger, M.J. A numerical study of combined use of two biocontrol agents with different biocontrol mechanisms in controlling foliar pathogens. Phytopathology 2011, 101, 1032–1044. [Google Scholar] [CrossRef] [Green Version]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Nguvo, K.J.; Gao, X. Weapons hidden underneath: Bio-control agents and their potentials to activate plant induced systemic resistance in controlling crop Fusarium diseases. J. Plant Dis. Prot. 2019, 126, 177–190. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Z.; He, P.; Munir, S.; Wu, Y.; Ho, H.; He, Y. Deciphering the bacterial and fungal communities in clubroot-affected cabbage rhizosphere treated with Bacillus subtilis XF-1. Agric. Ecosyst. Environ. 2018, 256, 12–22. [Google Scholar] [CrossRef]
- Lahlali, R.; Peng, G.; Gossen, B.; McGregor, L.; Yu, F.; Hynes, R.; Hwang, S.; McDonald, M.; Boyetchko, S. Evidence that the biofungicide Serenade (Bacillus subtilis) suppresses clubroot on canola via antibiosis and induced host resistance. Phytopathology 2013, 103, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wei, L.; Yang, J.; Ahmed, W.; Wang, Y.; Fu, L.; Ji, G. Probiotic consortia: Reshaping the rhizospheric microbiome and its role in suppressing root-rot disease of Panax notoginseng. Front. Microbiol. 2020, 11, 701. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Paulitz, T.C.; Steinberg, C.; Alabouvette, C.; Moënne-Loccoz, Y. The rhizosphere: A playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil 2009, 321, 341–361. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Gu, Y.; Friman, V.-P.; Kowalchuk, G.A.; Xu, Y.; Shen, Q.; Jousset, A. Initial soil microbiome composition and functioning predetermine future plant health. Sci. Adv. 2019, 5, eaaw0759. [Google Scholar] [CrossRef] [Green Version]
- Xue, C.; Ryan Penton, C.; Zhu, C.; Chen, H.; Duan, Y.; Peng, C.; Guo, S.; Ling, N.; Shen, Q. Alterations in soil fungal community composition and network assemblage structure by different long-term fertilization regimes are correlated to the soil ionome. Biol. Fertil. Soils 2018, 54, 95–106. [Google Scholar] [CrossRef]
- Cai, Q.; Zhou, G.; Ahmed, W.; Cao, Y.; Zhao, M.; Li, Z.; Zhao, Z. Study on the relationship between bacterial wilt and rhizospheric microbial diversity of flue-cured tobacco cultivars. Eur. J. Plant Pathol. 2021, 160, 265–276. [Google Scholar] [CrossRef]
- Ju, W.; Liu, L.; Fang, L.; Cui, Y.; Duan, C.; Wu, H. Impact of co-inoculation with plant-growth-promoting rhizobacteria and rhizobium on the biochemical responses of alfalfa-soil system in copper contaminated soil. Ecotoxicol. Environ. Saf. 2019, 167, 218–226. [Google Scholar] [CrossRef]
- Santoyo, G.; Guzmán-Guzmán, P.; Parra-Cota, F.I.; Santos-Villalobos, S.D.L.; Orozco-Mosqueda, M.; Glick, B.R. Plant growth stimulation by microbial consortia. Agronomy 2021, 11, 219. [Google Scholar] [CrossRef]
- Ahmed, W.; Dai, Z.; Liu, Q.; Munir, S.; Yang, J.; Karunarathna, S.C.; Li, S.; Zhang, J.; Ji, G.; Zhao, Z. Microbial Cross-Talk: Dissecting the Core Microbiota Associated With Flue-Cured Tobacco (Nicotiana tabacum) Plants Under Healthy and Diseased State. Front. Microbiol. 2022, 13, 397. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- He, P.; Cui, W.; Munir, S.; He, P.; Li, X.; Wu, Y.; Yang, X.; Tang, P.; He, Y. Plasmodiophora brassicae root hair interaction and control by Bacillus subtilis XF-1 in Chinese cabbage. Biol. Control. 2019, 128, 56–63. [Google Scholar] [CrossRef]
- Ahmed, A.; Munir, S.; He, P.; Li, Y.; He, P.; Yixin, W.; He, Y. Biocontrol arsenals of bacterial endophyte: An imminent triumph against clubroot disease. Microbiol. Res. 2020, 241, 126565. [Google Scholar] [CrossRef]
- Srivastava, R.; Khalid, A.; Singh, U.; Sharma, A. Evaluation of arbuscular mycorrhizal fungus, fluorescent Pseudomonas and Trichoderma harzianum formulation against Fusarium oxysporum f. sp. lycopersici for the management of tomato wilt. Biol. Control. 2010, 53, 24–31. [Google Scholar] [CrossRef]
- Sarma, B.K.; Yadav, S.K.; Singh, S.; Singh, H.B. Microbial consortium-mediated plant defense against phytopathogens: Readdressing for enhancing efficacy. Soil Biol. Biochem. 2015, 87, 25–33. [Google Scholar] [CrossRef]
- Palmieri, D.; Vitullo, D.; De Curtis, F.; Lima, G. A microbial consortium in the rhizosphere as a new biocontrol approach against Fusarium decline of chickpea. Plant Soil 2017, 412, 425–439. [Google Scholar] [CrossRef]
- Topalović, O.; Hussain, M.; Heuer, H. Plants and associated soil microbiota cooperatively suppress plant-parasitic nematodes. Front. Microbiol. 2020, 11, 313. [Google Scholar] [CrossRef] [Green Version]
- Chiaramonte, J.B.; Mendes, L.W.; Mendes, R. Rhizosphere microbiome and soilborne diseases. In Rhizosphere Biology: Interactions Between Microbes and Plants; Springer: Berlin/Heidelberg, Germany, 2021; pp. 155–168. [Google Scholar]
- Hu, J.; Wei, Z.; Friman, V.P.; Gu, S.H.; Wang, X.F.; Eisenhauer, N.; Yang, T.-J.; Ma, J.; Shen, Q.-R.; Xu, Y.-C.; et al. Probiotic diversity enhances rhizosphere microbiome function and plant disease suppression. MBio 2016, 7, e01790-16. [Google Scholar] [CrossRef] [Green Version]
- Kaushal, M.; Swennen, R.; Mahuku, G. Unlocking the microbiome communities of banana (Musa spp.) under disease stressed (Fusarium wilt) and non-stressed conditions. Microorganisms 2020, 8, 443. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Wang, N.-F.; Liu, H.-Y.; Zhang, Y.-Q.; Yu, L.-Y. Soil pH is a key determinant of soil fungal community composition in the Ny-Ålesund Region, Svalbard (High Arctic). Front. Microbiol. 2016, 7, 227. [Google Scholar] [CrossRef]
- Li, C.; Ahmed, W.; Li, D.; Yu, L.; Xu, L.; Xu, T.; Zhao, Z. Biochar suppresses bacterial wilt disease of flue-cured tobacco by improving soil health and functional diversity of rhizosphere microorganisms. Appl. Soil Ecol. 2022, 171, 104314. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Z.; Wang, X.; Sun, Q.; Dong, H.; Wang, G.; Chen, X.; Yin, C.; Han, Z.; Mao, Z. Effects of biochar on the growth of apple seedlings, soil enzyme activities and fungal communities in replant disease soil. Sci. Hortic. 2019, 256, 108641. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, H.; Sun, L.; Qi, G.; Chen, S.; Zhao, X. Microbial community composition is related to soil biological and chemical properties and bacterial wilt outbreak. Sci. Rep. 2017, 7, 343. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Yang, S.-Y.; Li, H.; Wang, H.; Li, Z.-L. Effects of calcium carbonate on the survival of Ralstonia solanacearum in soil and control of tobacco bacterial wilt. Eur. J. Plant Pathol. 2014, 140, 665–675. [Google Scholar] [CrossRef]
- Liu, L.; Huang, B.; Sun, J.; Guo, S.-R.; Li, L.-Q.; Guo, H.-W. Relationship between soil microbial quantity, enzyme activity and soil fertility in hot pepper greenhouse soils of different continuous cropping years. Soil Fertil. Sci. China 2013, 2, 5–10. [Google Scholar]
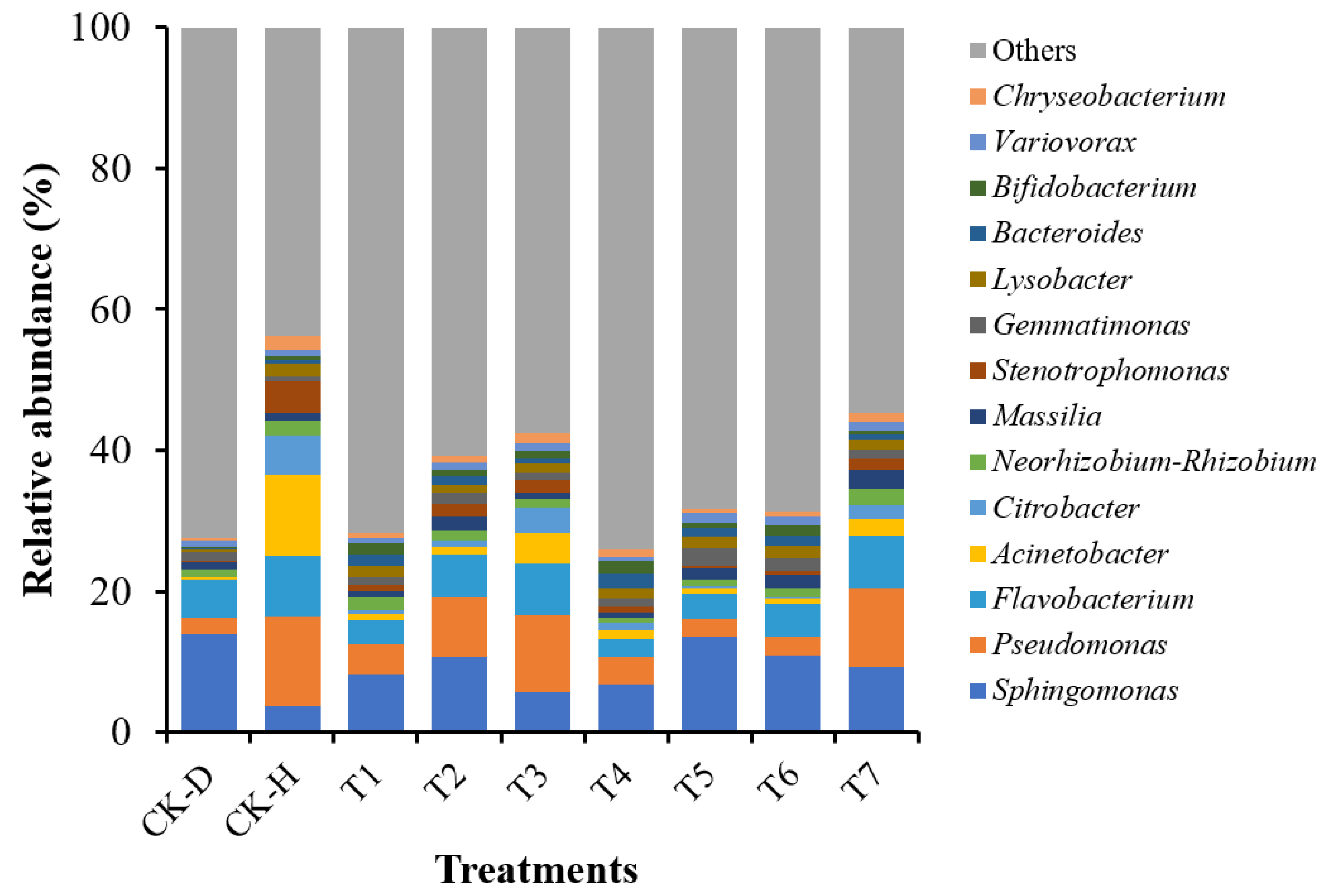
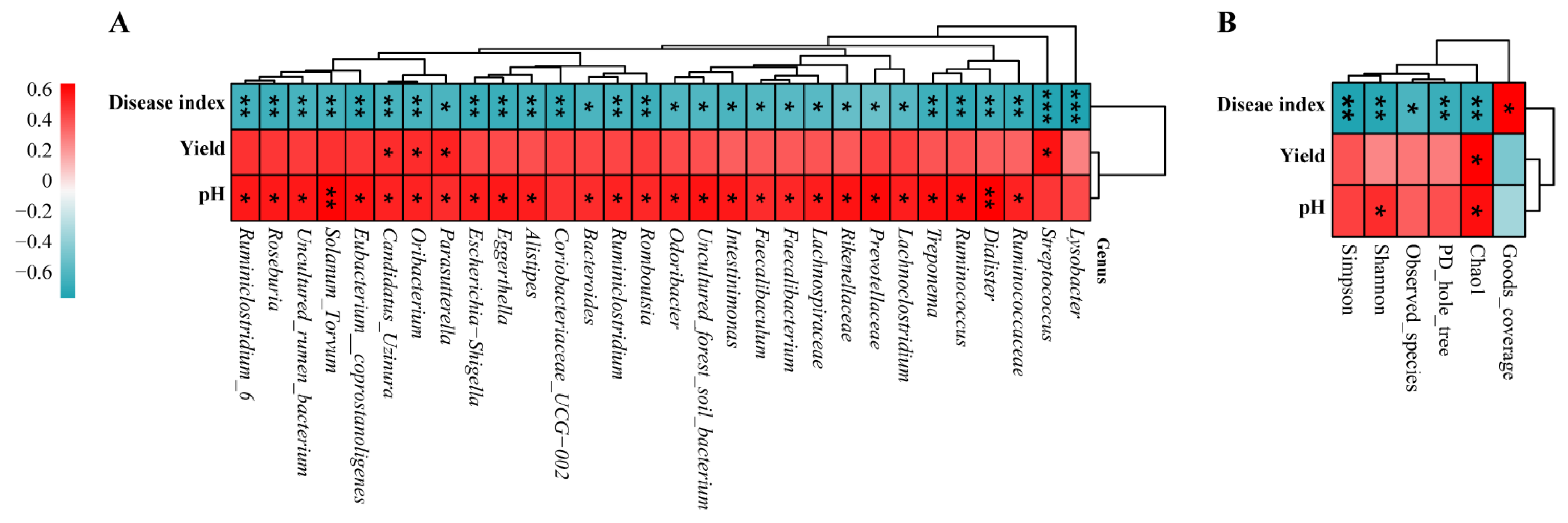
| Treatment | Chao1 | Shannon | Observed_Species | PD_Whole_Tree |
|---|---|---|---|---|
| CK-D | 2694.36 ± 159.32 c | 6.32 ± 0.24 d | 1862.87 ± 108.13 e | 72.13 ± 3.26 e |
| CK-H | 3995.2 ± 169.91 a | 9.53 ± 0.27 a | 3235.27 ± 211.87 a | 108.94 ± 7.04 a |
| T1 | 3433.88 ± 189.53 ab | 8.70 ± 1.08 a | 2632.2 ± 201.51 c | 92.95 ± 5 a |
| T2 | 3453.39 ± 143.99 ab | 8.84 ± 0.33 ab | 2602.9 ± 126.72 c | 91.84 ± 3.61 bc |
| T3 | 3089.13 ± 255.56 b | 8.06 ± 0.57 c | 2310.7 ± 233.38 d | 83.7 ± 5.85 d |
| T4 | 3619.18 ± 207.86 a | 9.46 ± 0.18 a | 2858.7 ± 139.39 b | 100.42 ± 3.76 a |
| T5 | 3411.68 ± 112.85 ab | 9.13 ± 0.27 ab | 2684.27 ± 117.31 c | 94.43 ± 2.49 b |
| T6 | 3487.64 ± 127.3 ab | 9.23 ± 0.06 a | 2655.1 ± 87.67 c | 94.33 ± 2.58 b |
| T7 | 3467.79 ± 257.75 ab | 8.5 ± 0.46 b | 2580.17 ± 214.62 cd | 90.37 ± 5.3 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Ahmed, W.; Dai, Z.; Zhou, X.; He, Z.; Wei, L.; Ji, G. Microbial Consortia: An Engineering Tool to Suppress Clubroot of Chinese Cabbage by Changing the Rhizosphere Bacterial Community Composition. Biology 2022, 11, 918. https://doi.org/10.3390/biology11060918
Zhang J, Ahmed W, Dai Z, Zhou X, He Z, Wei L, Ji G. Microbial Consortia: An Engineering Tool to Suppress Clubroot of Chinese Cabbage by Changing the Rhizosphere Bacterial Community Composition. Biology. 2022; 11(6):918. https://doi.org/10.3390/biology11060918
Chicago/Turabian StyleZhang, Jinhao, Waqar Ahmed, Zhenlin Dai, Xinghai Zhou, Zulei He, Lanfang Wei, and Guanghai Ji. 2022. "Microbial Consortia: An Engineering Tool to Suppress Clubroot of Chinese Cabbage by Changing the Rhizosphere Bacterial Community Composition" Biology 11, no. 6: 918. https://doi.org/10.3390/biology11060918
APA StyleZhang, J., Ahmed, W., Dai, Z., Zhou, X., He, Z., Wei, L., & Ji, G. (2022). Microbial Consortia: An Engineering Tool to Suppress Clubroot of Chinese Cabbage by Changing the Rhizosphere Bacterial Community Composition. Biology, 11(6), 918. https://doi.org/10.3390/biology11060918







