Impact of FLT3-ITD Insertion Length on Outcomes in Acute Myeloid Leukemia: A Propensity Score-Adjusted Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Analysis of FLT3 Mutation
2.3. Data Source
2.4. Variables and Comparison Groups
2.5. Propensity Score Estimation
2.6. Statistical Analysis
3. Results
3.1. Cohort Characteristics
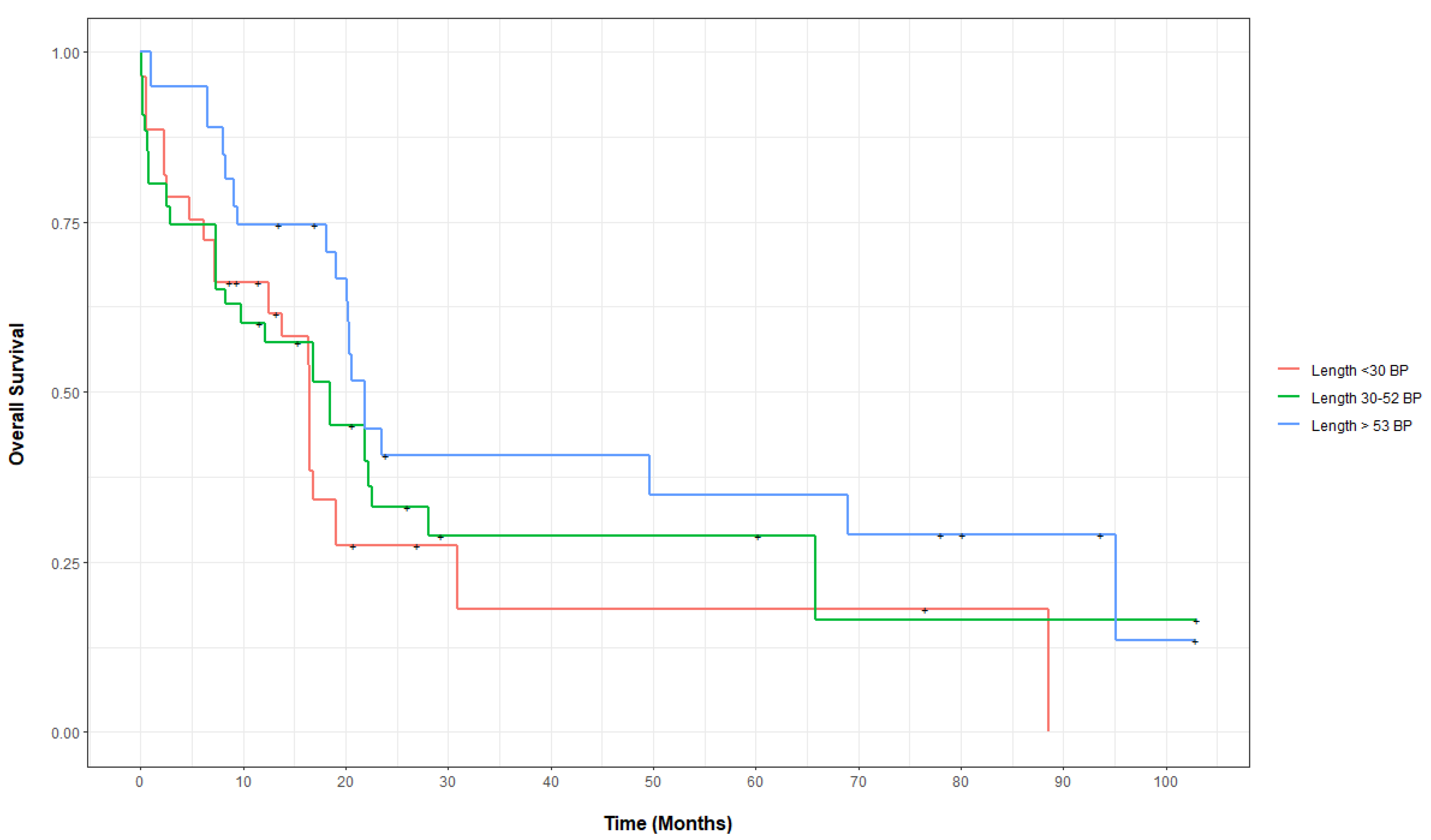
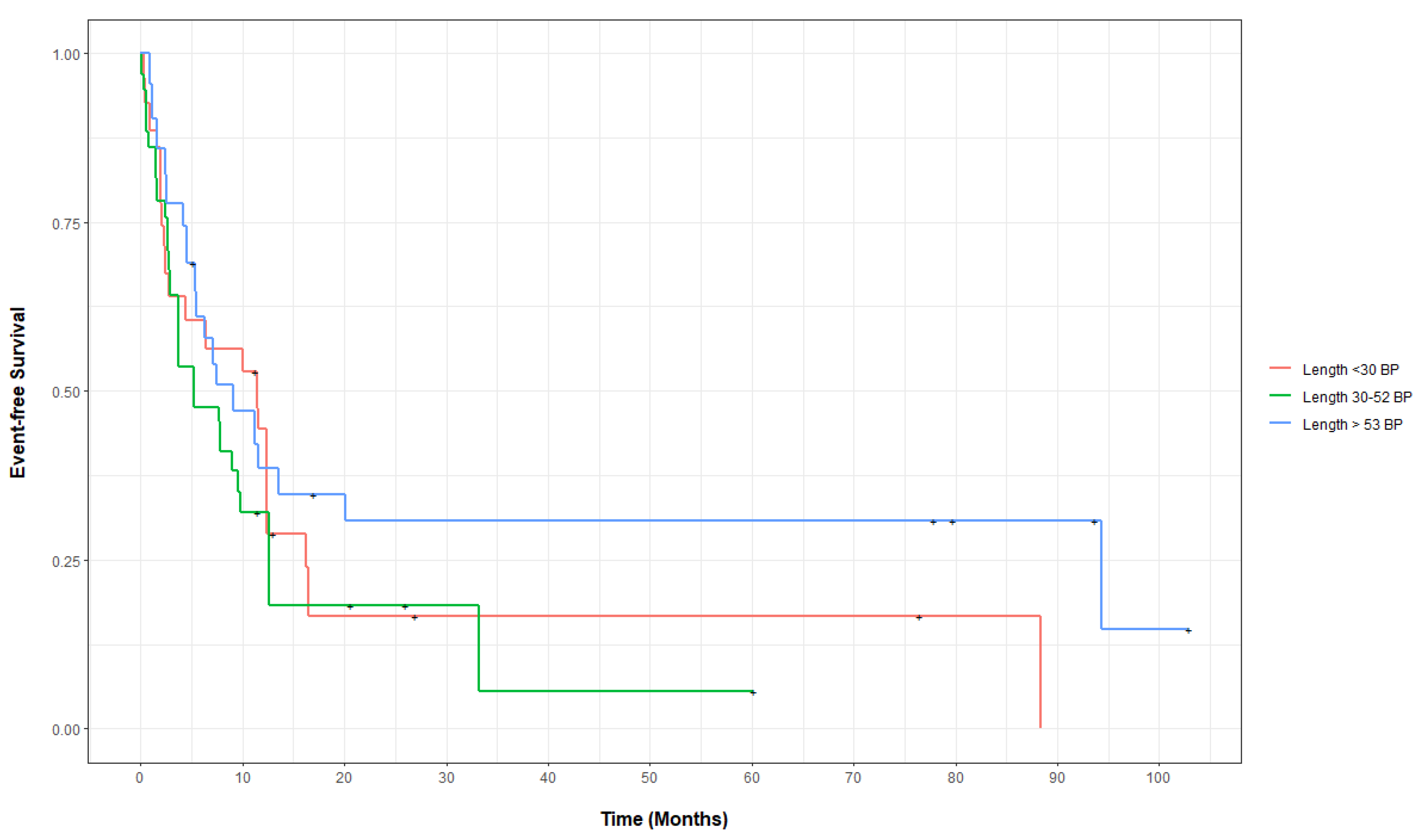
3.2. Outcomes of FLT3-ITD-Mutated AML, Categorized by Base-Pair Insertion Length
3.3. Outcomes of FLT3-ITD-Mutated and Wild-Type (WT) AML, Categorized by Insertion Length
3.4. Outcomes of FLT3-ITD-Mutated AML Categorized by Domain Insertion Expansion Categories
3.5. Outcomes after Using Midostaurin plus Induction Chemotherapy in FLT3-ITD AML, Categorized by Base-Pair Insertion Length
3.6. Outcomes after Using Gilteritinib Monotherapy in Relapsed or Refractory FLT3-ITD AML, Categorized by Base-Pair Insertion Length
3.7. Outcomes for Patients with FLT3-ITD and Mutated Nucleophosmin (NPM1) AML
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Donnell, M.R.; Tallman, M.S.; Abboud, C.N.; Altman, J.K.; Appelbaum, F.R.; Arber, D.A.; Bhatt, V.; Bixby, D.; Blum, W.; Coutre, S.E.; et al. Acute myeloid leukemia, version 3.2017: Clinical practice guidelines in oncology. JNCCN J. Natl. Compr. Cancer Netw. 2017, 15, 926–957. [Google Scholar] [CrossRef] [PubMed]
- Levis, M. FLT3 mutations in acute myeloid leukemia: What is the best approach in 2013? Hematology/Educ. Program Am. Soc. Hematol. Am. Soc. Hematol. Educ. Program 2013, 2013, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Schetelig, J.; Rollig, C.; Kayser, S.; Stoelzel, F.; Schaefer-Eckart, K.; Haenel, M.; Roesler, W.; Einsele, H.; Kaufmann, M.; Serve, H.; et al. Validation of the ELN 2017 Classification for AML with Intermediate Risk Cytogenetics with or without NPM1 -Mutations and High or Low Ratio FLT3-ITD s. Blood 2017, 130, 2694. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Ley, T.J.; Larson, D.E.; Miller, C.A.; Koboldt, D.C.; Welch, J.S.; Ritchey, J.K.; Young, M.A.; Lamprecht, T.; McLellan, M.D.; et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 2012, 481, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.; Black, J.; Faerman, C.; Swenson, L.; Wynn, M.; Lu, F.; Lippke, J.; Saxena, K. The Structural Basis for Autoinhibition of FLT3 by the Juxtamembrane Domain. Mol. Cell 2004, 13, 169–178. [Google Scholar] [CrossRef]
- Parcells, B.W.; Ikeda, A.K.; Simms-Waldrip, T.; Moore, T.B.; Sakamoto, K.M. FMS-Like Tyrosine Kinase 3 in Normal Hematopoiesis and Acute Myeloid Leukemia. Stem Cells 2006, 24, 1174–1184. [Google Scholar] [CrossRef]
- Pratz, K.W.; Sato, T.; Murphy, K.M.; Stine, A.; Rajkhowa, T.; Levis, M. FLT3-mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood 2010, 115, 1425–1432. [Google Scholar] [CrossRef] [Green Version]
- Stirewalt, D.L.; Kopecky, K.J.; Meshinchi, S.; Engel, J.H.; Pogosova-Agadjanyan, E.L.; Linsley, J.; Slovak, M.L.; Willman, C.L.; Radich, J.P. Size of FLT3 internal tandem duplication has prognostic significance in patients with acute myeloid leukemia. Blood 2006, 107, 3724–3726. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Lee, G.D.; Park, J.; Yoon, J.H.; Kim, H.J.; Min, W.S.; Kim, M. Quantitative fragment analysis of FLT3-ITD efficiently identifying poor prognostic group with high mutant allele burden or long ITD length. Blood Cancer J. 2015, 5, e336. [Google Scholar] [CrossRef] [Green Version]
- Meshinchi, S.; Stirewalt, D.L.; Alonzo, T.A.; Boggon, T.J.; Gerbing, R.B.; Rocnik, J.L.; Lange, B.J.; Gilliland, D.G.; Radich, J.P. Structural and numerical variation of FLT3/ITD in pediatric AML. Blood 2008, 111, 4930–4933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.B.; Dong, H.J.; Wang, J.; Qiu, Q.C.; Xue, S.L.; Li, L. Effect of FLT3-ITD Length on 32D Cell Proliferation, Apoptosis and Sensitivity to FLT3 Inhibitor. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2021, 29, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.B.; Dong, H.J.; Bao, X.B.; Qiu, Q.C.; Li, H.Z.; Shen, H.J.; Ding, Z.X.; Wang, C.; Chu, X.L.; Yu, J.Q.; et al. Impact of FLT3-ITD length on prognosis of acute myeloid leukemia. Haematologica 2019, 104, e9–e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayser, S.; Schlenk, R.F.; Londono, M.C.; Breitenbuecher, F.; Wittke, K.; Du, J.; Groner, S.; Späth, D.; Krauter, J.; Ganser, A.; et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood 2009, 114, 2386–2392. [Google Scholar] [CrossRef] [Green Version]
- Gale, R.E.; Green, C.; Allen, C.; Mead, A.J.; Burnett, A.K.; Hills, R.K.; Linch, D.C. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood 2008, 111, 2776–2784. [Google Scholar] [CrossRef] [Green Version]
- Schnittger, S.; Bacher, U.; Haferlach, C.; Alpermann, T.; Kern, W.; Haferlach, T. Diversity of the juxtamembrane and TKD1 mutations (Exons 13-15) in the FLT3 gene with regards to mutant load, sequence, length, localization, and correlation with biological data. Genes Chromosomes Cancer 2012, 51, 910–924. [Google Scholar] [CrossRef]
- Koszarska, M.; Meggyesi, N.; Bors, A.; Batai, A.; Csacsovszki, O.; Lehoczky, E.; Adam, E.; Kozma, A.; Lovas, N.; Sipos, A.; et al. Medium-sized FLT3 internal tandem duplications confer worse prognosis than short and long duplications in a non-elderly acute myeloid leukemia cohort. Leuk. Lymphoma 2014, 55, 1510–1517. [Google Scholar] [CrossRef]
- Kusec, R.; Jaksic, O.; Ostojic, S.; Kardum-Skelin, I.; Vrhovac, R.; Jaksic, B. More on prognostic significance of FLT3/ITD size in acute myeloid leukemia (AML). Blood 2006, 108, 405–406. [Google Scholar] [CrossRef]
- Rücker, F.G.; Du, L.; Luck, T.J.; Benner, A.; Krzykalla, J.; Gathmann, I.; Voso, M.T.; Amadori, S.; Prior, T.W.; Brandwein, J.M.; et al. Molecular landscape and prognostic impact of FLT3-ITD insertion site in acute myeloid leukemia: RATIFY study results. Leukemia 2021, 36, 90–99. [Google Scholar] [CrossRef]
- CRISP. 2021. Available online: https://www.crisphealth.org (accessed on 4 October 2021).
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corley, E.M.; Mustafa Ali, M.K.; Alharthy, H.; Kline, K.A.F.; Sewell, D.; Law, J.Y.; Lee, S.T.; Niyongere, S.; Duong, V.H.; Baer, M.R.; et al. Impact of IDH1 c.315C>T SNP on Outcomes in Acute Myeloid Leukemia: A Propensity Score-Adjusted Cohort Study. Front. Oncol. 2022, 12. Available online: https://www.crisphealth.org/applications/clinical-data/#health-records (accessed on 16 May 2022). [CrossRef] [PubMed]
- Greifer, N. WeightIt. 2021. Available online: https://github.com/ngreifer/WeightIt (accessed on 1 November 2021).
- Harder, V.S.; Stuart, E.A.; Anthony, J.C. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol. Methods 2010, 15, 234–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, P.C. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat. Med. 2016, 35, 5642–5655. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.H.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Burnett, A.K.; Dombret, H.; Fenaux, P.; Grimwade, D.; Larson, R.A.; et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010, 115, 453–474. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; Levis, M.J.; Perl, A.E.; Hill, J.E.; Rosales, M.; Bahceci, E. Molecular profile of FLT3 -mutated relapsed/refractory patients with AML in the phase 3 ADMIRAL study of gilteritinib. Blood Adv. 2022, 6, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Thiede, C.; Koch, S.; Creutzig, E.; Steudel, C.; Illmer, T.; Schaich, M.; Ehninger, G. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML). Blood 2006, 107, 4011–4020. [Google Scholar] [CrossRef] [Green Version]
- Verhaak, R.G.W.; Goudswaard, C.S.; van Putten, W.; Bijl, M.A.; Sanders, M.A.; Hugens, W.; Uitterlinden, A.G.; Erpelinck, C.A.J.; Delwel, R.; Löwenberg, B.; et al. Mutations in nucleophosmin (NPM1) in acute myeloid leukemia (AML): Association with other gene abnormalities and previously established gene expression signatures and their favorable prognostic significance. Blood 2005, 106, 3747–3754. [Google Scholar] [CrossRef]
- Döhner, K.; Schlenk, R.F.; Habdank, M.; Scholl, C.; Rücker, F.G.; Corbacioglu, A.; Bullinger, L.; Fröhling, S.; Döhner, H. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: Interaction with other gene mutations. Blood 2005, 106, 3740–3746. [Google Scholar] [CrossRef] [Green Version]
- Schnittger, S.; Schoch, C.; Kern, W.; Mecucci, C.; Tschulik, C.; Martelli, M.F.; Haferlach, T.; Hiddemann, W.; Falini, B. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood 2005, 106, 3733–3739. [Google Scholar] [CrossRef] [Green Version]
| <30 bp | Percentage/SD/IQR | 30–53 bp | Percentage/SD/IQR | >53 bp | Percentage/SD/IQR | p-Value | |
|---|---|---|---|---|---|---|---|
| Number of patients | 25 | - | 28 | - | 24 | - | - |
| Age (Average ± SD) | 63.9 | 18.40 | 63.8 | 15.2 | 58.6 | 15.2 | 0.43 |
| Age (Median, IQR) | 66.9 | 58–79.3 | 65.3 | 56.9–72.7 | 60.8 | 45.9–71.1 | 0.60 |
| Female | 5 | 20 | 12 | 43 | 17 | 71 | 0.002 |
| Blood Counts at Diagnosis | |||||||
| White Blood Cells (K/microL) (Median, IQR) | 18.5 | 8.8–79.9 | 75.9 | 21–105.6 | 70.7 | 13.2–130.3 | 0.03 |
| Hemoglobin (g/dL) (Median, IQR) | 8.4 | 7.3–9.6 | 8.6 | 7.1–9.3 | 8.2 | 7.3–9.5 | 0.75 |
| Platelets (K/microL) (Median, IQR) | 59.0 | 24–113 | 70.5 | 34.5–119 | 48.0 | 29.75–75 | 0.66 |
| Blast percentage (%) (Average ± SD) | 55.5 | 23.4 | 67.9 | 26.0 | 63 | 23.3 | 0.56 |
| Body Mass Index | 27.0 | 4.4 | 25.7 | 5.3 | 30 | 12.8 | 0.55 |
| Ethnicity | |||||||
| Causian | 18 | 72.0 | 20 | 71.4 | 17 | 70.8 | 0.99 |
| Other | 7 | 28.0 | 8 | 28.6 | 7 | 29.2 | |
| Comorbidities | |||||||
| Cardivascular disease | 6 | 24 | 4 | 14 | 6 | 25 | 0.57 |
| Diabetes mellitus | 7 | 28 | 4 | 14 | 5 | 20 | 0.47 |
| Hypertension | 11 | 44 | 11 | 39 | 9 | 38 | 0.89 |
| CKD stage III-V/ESRD | 1 | 4 | 2 | 7 | 1 | 4 | 0.84 |
| Active Cancer | 0 | 0 | 1 | 3.6 | 0 | 0 | 0.41 |
| AML type | 0.37 | ||||||
| AML, de novo | 17 | 68 | 22 | 78.6 | 19 | 79.2 | |
| AML with MDS/CMML changes | 8 | 32 | 4 | 14.3 | 4 | 16.7 | |
| Therapy-Related AML | 0 | 0 | 2 | 7.1 | 1 | 4.2 | |
| ELN 2017 Cytogenetic Category | 0.680 | ||||||
| Favorable Risk | 1 | 4 | 2 | 7.1 | 0 | 0 | |
| Intermediate Risk | 22 | 88 | 25 | 89.3 | 22 | 91.7 | |
| Unfavorable Risk | 0 | 0 | 0 | 0 | 0 | 0 | |
| Not performed/Poor banding, Inadequate | 2 | 8 | 1 | 3.6 | 2 | 8.3 | |
| FLT3-ITD status | 0.37 | ||||||
| FLT3-ITD-mutated allelic burden 1–49% | 18 | 72 | 15 | 53.6 | 15 | 62.5 | |
| FLT3-ITD-mutated allelic burden 50–100% | 7 | 28 | 13 | 46.4 | 8 | 33.3 | |
| FLT3 wild type | 0 | 0 | 0 | 0 | 1 | 4.2 | |
| FLT3-TKD mutated | 10 | 40 | 4 | 14.3 | 7 | 29.2 | 0.11 |
| TP53 mutational status | 0.01 | ||||||
| TP53 mutated | 1 | 4 | 0 | 0 | 0 | 0 | |
| TP53 wild type | 19 | 76 | 18 | 64.3 | 11 | 45.8 | |
| TP53 untested | 5 | 20 | 10 | 35.7 | 13 | 54.2 | |
| RUNX1 mutational status | 0.07 | ||||||
| RUNX1 mutated | 4 | 16 | 2 | 7.1 | 0 | 0 | |
| RUNX1 wild type | 16 | 64 | 16 | 57.1 | 11 | 45.8 | |
| RUNX1 untested | 5 | 20 | 10 | 35.7 | 13 | 54.2 | |
| ASXL1 mutational status | 0.02 | ||||||
| ASXL1 mutated | 3 | 12 | 0 | 0 | 0 | 0 | |
| ASXL1 wild type | 17 | 68 | 18 | 64.3 | 11 | 45.8 | |
| ASXL1 untested | 5 | 20 | 10 | 35.7 | 13 | 54.2 | |
| NPM1 mutational status | 0.08 | ||||||
| NPM1 mutated | 9 | 36 | 9 | 32.1 | 8 | 33.3 | |
| NPM1 wild type | 11 | 44 | 9 | 32.1 | 3 | 12.5 | |
| NPM1 untested | 5 | 20 | 10 | 35.7 | 13 | 54.2 | |
| CEBPA mutational status | 0.1 | ||||||
| CEBPA mutated | 1 | 4 | 4 | 11 | 1 | 4 | |
| CEBPA wild type | 19 | 76 | 15 | 53 | 10 | 42 | |
| CEBPA untested | 5 | 20 | 10 | 36 | 13 | 54 | |
| ECOG-PS status | 0.15 | ||||||
| I or II | 23 | 92 | 23 | 82.1 | 24 | 100 | |
| III or IV | 2 | 8 | 4 | 14.3 | 0 | 0 | |
| ECOG status unknown | 0 | 0 | 1 | 3.6 | 0 | 0 | |
| Types of first-line treatment | 0.28 | ||||||
| Anthracycline-based | 10 | 40 | 12 | 42.9 | 16 | 66.7 | |
| Non-anthracycline-based | 13 | 52 | 14 | 50 | 8 | 33.3 | |
| None | 2 | 8 | 2 | 7.1 | 0 | 0 | |
| Midostaurin with induction | 5 | 20 | 5 | 17.8 | 2 | 8.3 | 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corley, E.M.; Mustafa Ali, M.K.; Alharthy, H.; Kline, K.A.F.; Sewell, D.; Law, J.Y.; Lee, S.T.; Niyongere, S.; Duong, V.H.; Baer, M.R.; et al. Impact of FLT3-ITD Insertion Length on Outcomes in Acute Myeloid Leukemia: A Propensity Score-Adjusted Cohort Study. Biology 2022, 11, 916. https://doi.org/10.3390/biology11060916
Corley EM, Mustafa Ali MK, Alharthy H, Kline KAF, Sewell D, Law JY, Lee ST, Niyongere S, Duong VH, Baer MR, et al. Impact of FLT3-ITD Insertion Length on Outcomes in Acute Myeloid Leukemia: A Propensity Score-Adjusted Cohort Study. Biology. 2022; 11(6):916. https://doi.org/10.3390/biology11060916
Chicago/Turabian StyleCorley, Elizabeth M., Moaath K. Mustafa Ali, Hanan Alharthy, Kathryn A. F. Kline, Danielle Sewell, Jennie Y. Law, Seung Tae Lee, Sandrine Niyongere, Vu H. Duong, Maria R. Baer, and et al. 2022. "Impact of FLT3-ITD Insertion Length on Outcomes in Acute Myeloid Leukemia: A Propensity Score-Adjusted Cohort Study" Biology 11, no. 6: 916. https://doi.org/10.3390/biology11060916
APA StyleCorley, E. M., Mustafa Ali, M. K., Alharthy, H., Kline, K. A. F., Sewell, D., Law, J. Y., Lee, S. T., Niyongere, S., Duong, V. H., Baer, M. R., & Emadi, A. (2022). Impact of FLT3-ITD Insertion Length on Outcomes in Acute Myeloid Leukemia: A Propensity Score-Adjusted Cohort Study. Biology, 11(6), 916. https://doi.org/10.3390/biology11060916






