Breaking of Sitting Time Prevents Lower Leg Swelling—Comparison among Sit, Stand and Intermittent (Sit-to-Stand Transitions) Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Heart Rate and Blood Pressure Monitoring
2.4. Anthropometry
2.5. Dual-Energy X-ray Absorptiometry
2.6. Bioimpedance Analysis
2.7. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saunders, T.J.; McIsaac, T.; Douillette, K.; Gaulton, N.; Hunter, S.; Rhodes, R.E.; Prince, S.A.; Carson, V.; Chaput, J.-P.; Chastin, S. Sedentary behaviour and health in adults: An overview of systematic reviews. Appl. Physiol. Nutr. Metab. 2020, 45, S197–S217. [Google Scholar] [CrossRef]
- Kazi, A.; Duncan, M.; Clemes, S.; Haslam, C. A survey of sitting time among UK employees. Occup. Med. 2014, 64, 497–502. [Google Scholar] [CrossRef]
- Owen, N.; Healy, G.N.; Matthews, C.E.; Dunstan, D.W. Too much sitting: The population health science of sedentary behavior. Exerc. Sport Sci. Rev. 2010, 38, 105–113. [Google Scholar] [CrossRef]
- Wilkerson, A.H.; Usdan, S.L.; Knowlden, A.P.; Leeper, J.L.; Birch, D.A.; Hibberd, E.E. Workplace-related factors associated with employees’ standing time at work: A research brief. Am. J. Health Promot. 2019, 33, 606–610. [Google Scholar] [CrossRef]
- Anthony Ryan, G. The prevalence of musculo-skeletal symptoms in supermarket workers. Ergonomics 1989, 32, 359–371. [Google Scholar] [CrossRef]
- Cook, J.; Branch, T.; Baranowski, T.; Hutton, W. The effect of surgical floor mats in prolonged standing: An EMG study of the lumbar paraspinal and anterior tibialis muscles. J. Biomed. Eng. 1993, 15, 247–250. [Google Scholar] [CrossRef]
- Ngomo, S.; Messing, K.; Perrault, H.; Comtois, A. Orthostatic symptoms, blood pressure and working postures of factory and service workers over an observed workday. Appl. Ergon. 2008, 39, 729–736. [Google Scholar] [CrossRef]
- Stephens, B.R.; Granados, K.; Zderic, T.W.; Hamilton, M.T.; Braun, B. Effects of 1 day of inactivity on insulin action in healthy men and women: Interaction with energy intake. Metabolism 2011, 60, 941–949. [Google Scholar] [CrossRef]
- Halim, I.; Omar, A.R.; Saman, A.M.; Othman, I. A review on health effects associated with prolonged standing in the industrial workplaces. Ijrras 2011, 8, 14–21. [Google Scholar]
- Vena, D.; Rubianto, J.; Popovic, M.; Yadollahi, A. Leg fluid accumulation during prolonged sitting. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 4284–4287. [Google Scholar]
- Brodmann, M.; Gary, T.; Hafner, F.; Eller, P.; Deutschmann, H.; Pilger, E.; Seinost, G. Acute wiiitis representing as thrombosis of the inferior vena cava and left pelvic veins. Phlebology 2015, 30, 486–488. [Google Scholar] [CrossRef]
- Mercier, G.; Pastor, J.; Moffatt, C.; Franks, P.; Quéré, I. LIMPRINT: Health-related quality of life in adult patients with chronic edema. Lymphat. Res. Biol. 2019, 17, 163–167. [Google Scholar] [CrossRef]
- Saunders, T.J.; Atkinson, H.F.; Burr, J.; MacEwen, B.; Skeaff, C.M.; Peddie, M.C. The acute metabolic and vascular impact of interrupting prolonged sitting: A systematic review and meta-analysis. Sports Med. 2018, 48, 2347–2366. [Google Scholar] [CrossRef] [PubMed]
- Healy, G.N.; Dunstan, D.W.; Salmon, J.; Cerin, E.; Shaw, J.E.; Zimmet, P.Z.; Owen, N. Breaks in sedentary time: Beneficial associations with metabolic risk. Diabetes Care 2008, 31, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Loh, R.; Stamatakis, E.; Folkerts, D.; Allgrove, J.E.; Moir, H.J. Effects of interrupting prolonged sitting with physical activity breaks on blood glucose, insulin and triacylglycerol measures: A systematic review and meta-analysis. Sports Med. 2020, 50, 295–330. [Google Scholar] [CrossRef] [PubMed]
- Maw, G.; Mackenzie, I.; Taylor, N. Redistribution of body fluids during postural manipulations. Acta Physiol. Scand. 1995, 155, 157–163. [Google Scholar] [CrossRef]
- Gibson, A.; Beam, J.; Alencar, M.; Zuhl, M.; Mermier, C. Time course of supine and standing shifts in total body, intracellular and extracellular water for a sample of healthy adults. Eur. J. Clin. Nutr. 2015, 69, 14–19. [Google Scholar] [CrossRef]
- Dogra, S.; Wolf, M.; Jeffrey, M.P.; Foley, R.C.; Logan-Sprenger, H.; Jones-Taggart, H.; Green-Johnson, J.M. Disrupting prolonged sitting reduces IL-8 and lower leg swell in active young adults. BMC Sports Sci. Med. Rehabil. 2019, 11, 1–7. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chen, C.-Y.; Cho, M.-H. Effectiveness of leg movement in reducing leg swelling and discomfort in lower extremities. Appl. Ergon. 2012, 43, 1033–1037. [Google Scholar] [CrossRef]
- Pollack, A.A.; Wood, E.H. Venous pressure in the saphenous vein at the ankle in man during exercise and changes in posture. J. Appl. Physiol. 1949, 1, 649–662. [Google Scholar] [CrossRef]
- Campa, F.; Gobbo, L.A.; Stagi, S.; Cyrino, L.T.; Toselli, S.; Marini, E.; Coratella, G. Bioelectrical impedance analysis versus reference methods in the assessment of body composition in athletes. Eur. J. Appl. Physiol. 2022, 122, 561–589. [Google Scholar] [CrossRef]
- Francisco, R.; Matias, C.N.; Santos, D.A.; Campa, F.; Minderico, C.S.; Rocha, P.; Heymsfield, S.B.; Lukaski, H.; Sardinha, L.B.; Silva, A.M. The predictive role of raw bioelectrical impedance parameters in water compartments and fluid distribution assessed by dilution techniques in athletes. Int. J. Environ. Res. Public Health 2020, 17, 759. [Google Scholar] [CrossRef] [PubMed]
- Campa, F.; Toselli, S.; Mazzilli, M.; Gobbo, L.A.; Coratella, G. Assessment of body composition in athletes: A narrative review of available methods with special reference to quantitative and qualitative bioimpedance analysis. Nutrients 2021, 13, 1620. [Google Scholar] [CrossRef] [PubMed]
- Campa, F.; Silva, A.M.; Matias, C.N.; Monteiro, C.P.; Paoli, A.; Nunes, J.P.; Talluri, J.; Lukaski, H.; Toselli, S. Body water content and morphological characteristics modify bioimpedance vector patterns in volleyball, soccer, and rugby players. Int. J. Environ. Res. Public Health 2020, 17, 6604. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gomez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis--part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C.; Kyle, U.G.; Kondrup, J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: Phase angle and impedance ratio. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 330–339. [Google Scholar] [CrossRef]
- Garlini, L.M.; Alves, F.D.; Ceretta, L.B.; Perry, I.S.; Souza, G.C.; Clausell, N.O. Phase angle and mortality: A systematic review. Eur. J. Clin. Nutr. 2019, 73, 495–508. [Google Scholar] [CrossRef]
- Miura, T.; Matsumoto, Y.; Kawaguchi, T.; Masuda, Y.; Okizaki, A.; Koga, H.; Tagami, K.; Watanabe, Y.S.; Uehara, Y.; Yamaguchi, T.; et al. Low Phase Angle Is Correlated With Worse General Condition in Patients with Advanced Cancer. Nutr. Cancer 2019, 71, 83–88. [Google Scholar] [CrossRef]
- Campa, F.; Matias, C.N.; Marini, E.; Heymsfield, S.B.; Toselli, S.; Sardinha, L.B.; Silva, A.M. Identifying Athlete Body-Fluid Changes During a Competitive Season With Bioelectrical Impedance Vector Analysis. Int. J. Sports Physiol. Perform. 2019, 15, 1–21. [Google Scholar] [CrossRef]
- Ward, L.C. Segmental bioelectrical impedance analysis: An update. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 424–429. [Google Scholar] [CrossRef]
- Zhu, F.; Leonard, E.F.; Levin, N.W. Body composition modeling in the calf using an equivalent circuit model of multi-frequency bioimpedance analysis. Physiol. Meas. 2005, 26, S133. [Google Scholar] [CrossRef]
- Nescolarde, L.; Yanguas, J.; Lukaski, H.; Alomar, X.; Rosell-Ferrer, J.; Rodas, G. Localized bioimpedance to assess muscle injury. Physiol. Meas. 2013, 34, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Codognotto, M.; Piazza, M.; Frigatti, P.; Piccoli, A. Influence of localized edema on whole-body and segmental bioelectrical impedance. Nutrition 2008, 24, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Seo, A.; Kakehashi, M.; Tsuru, S.; Yoshinaga, F. Leg swelling during continuous standing and sitting work without restricting leg movement. J. Occup. Health 1996, 38, 186–189. [Google Scholar] [CrossRef]
- Chester, M.R.; Rys, M.J.; Konz, S.A. Leg swelling, comfort and fatigue when sitting, standing, and sit/standing. Int. J. Ind. Ergon. 2002, 29, 289–296. [Google Scholar] [CrossRef]
- Williams, J.R. The Declaration of Helsinki and public health. Bull. World Health Organ. 2008, 86, 650–652. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gomez, J.; Lilienthal Heitmann, B.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis-part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Wieling, W.; Schatz, I.J. The consensus statement on the definition of orthostatic hypotension: A revisit after 13 years. J. Hypertens. 2009, 27, 935–938. [Google Scholar] [CrossRef]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988. [Google Scholar]
- Han, T.; Lean, M. Lower leg length as an index of stature in adults. Int. J. Obes. Relat. Metab.Disord. J. Int. Assoc. Study Obes. 1996, 20, 21–27. [Google Scholar]
- Kushner, R.F.; Gudivaka, R.; Schoeller, D.A. Clinical characteristics influencing bioelectrical impedance analysis measurements. Am. J. Clin. Nutr. 1996, 64, 423S–427S. [Google Scholar] [CrossRef]
- Silva, A.M.; Matias, C.N.; Nunes, C.L.; Santos, D.A.; Marini, E.; Lukaski, H.C.; Sardinha, L.B. Lack of agreement of in vivo raw bioimpedance measurements obtained from two single and multi-frequency bioelectrical impedance devices. Eur. J. Clin. Nutr. 2019, 73, 1077–1083. [Google Scholar] [CrossRef]
- Rutkove, S.B. Electrical impedance myography: Background, current state, and future directions. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 2009, 40, 936–946. [Google Scholar] [CrossRef]
- Bogónez-Franco, P.; Nescolarde, L.; McAdams, E.; Rosell-Ferrer, J. Multifrequency right-side, localized and segmental BIA obtained with different bioimpedance analysers. Physiol. Meas. 2014, 36, 85. [Google Scholar] [CrossRef] [PubMed]
- Ellis, K.J.; Bell, S.J.; Chertow, G.M.; Chumlea, W.C.; Knox, T.A.; Kotler, D.P.; Lukaski, H.C.; Schoeller, D.A. Bioelectrical impedance methods in clinical research: A follow-up to the NIH Technology Assessment Conference. Nutrition 1999, 15, 874–880. [Google Scholar] [CrossRef]
- Masani, K.; Sayenko, D.G.; Vette, A.H. What triggers the continuous muscle activity during upright standing? Gait Posture 2013, 37, 72–77. [Google Scholar] [CrossRef] [PubMed]
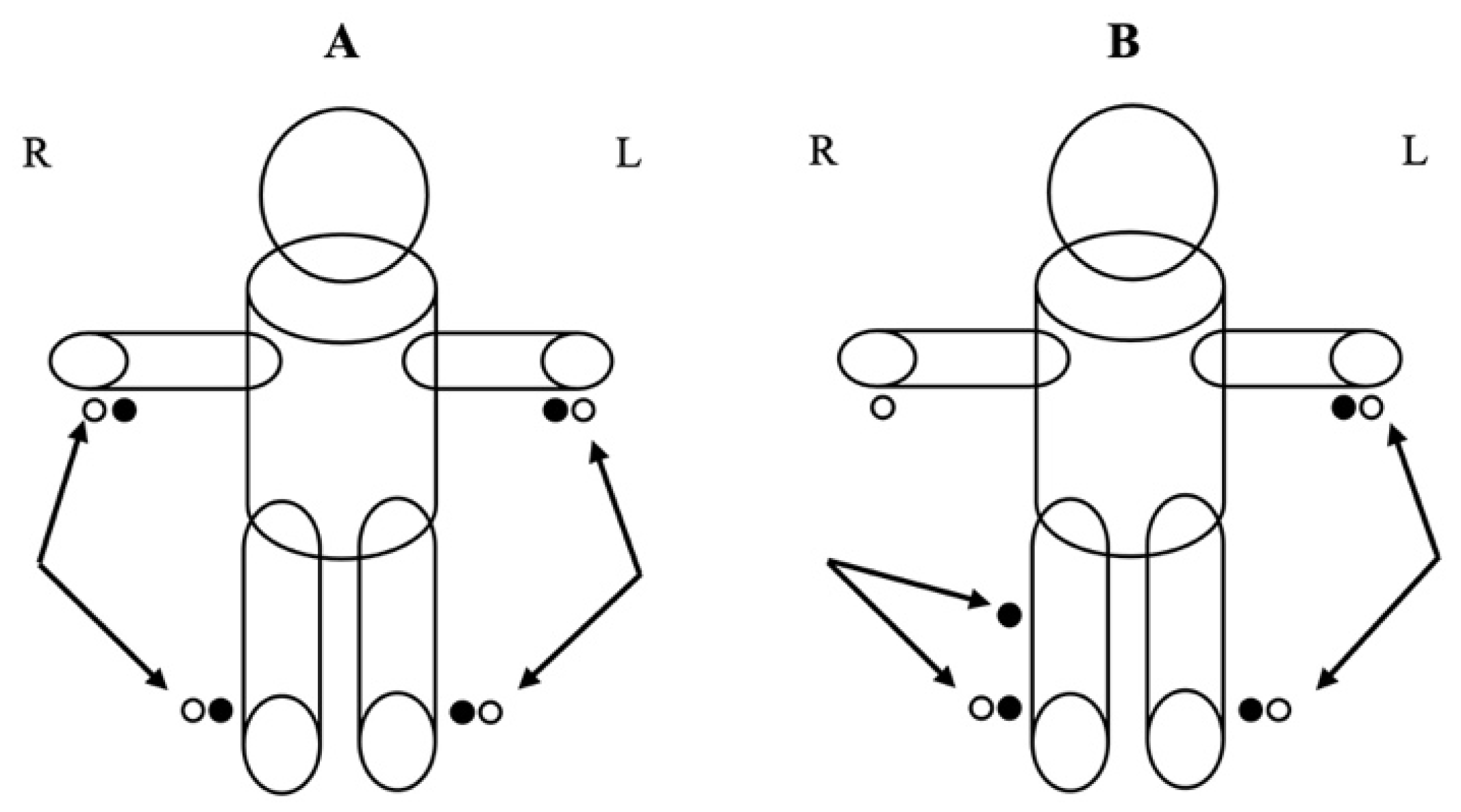
| Total Sample (n = 19) | Females (n = 9) | Males (n = 10) | |
|---|---|---|---|
| Demographic | |||
| Age | 27.5 ± 5.9 | 29.3 ± 7.4 | 25.8 ± 3.9 |
| Body Composition | |||
| Weight (kg) | 70.6 ± 12.0 | 66.2 ± 13.3 | 75.6 ± 10.0 |
| BMI (kg/m2) | 24.3 ± 3.6 | 24.1 ± 4.8 | 24.4 ± 2.3 |
| Fat mass (kg) | 17.4 ± 8.9 | 21.5 ± 11.1 | 13.7 ± 4.1 |
| Fat mass (%) | 24.5 ± 10.1 | 31.2 ± 10.6 | 18.6 ± 4.1 |
| Fat-free mass (kg) | 52.3 ± 10.2 | 43.8 ± 3.2 | 60.0 ± 7.8 |
| Blood Pressure | |||
| SBP (mmHg) | 118.8 ± 9.9 | 113.4 ± 10.0 | 124.1 ± 6.7 |
| DBP (mmHg) | 70.4 ± 10.0 | 68.3 ± 6.0 | 72.6 ± 13.0 |
| Whole-Body Bioimpedance | |||
| R (Ω) | 532.9 ± 116.7 | 614.8 ± 117.9 | 459.3 ± 46.6 |
| Xc (Ω) | 65.3 ± 14.9 | 67.5 ± 19.7 | 63.3 ± 9.4 |
| PhA (°) | 7.1 ± 1.3 | 6.3 ± 1.1 | 7.9 ± 1.0 |
| TBW (L) | 35.3 ± 7.6 | 28.8 ± 4.0 | 41.2 ± 4.5 |
| ECW (L) | 15.4 ± 3.0 | 13.0 ± 1.8 | 17.5 ± 2.2 |
| ICW (L) | 20.0 ± 4.6 | 15.8 ± 2.2 | 23.7 ± 2.3 |
| Sit | Stand | Intermittent | Difference-in-Differences | ||||
|---|---|---|---|---|---|---|---|
| Rlocalized | Baseline | 158.1 (4.0) | 157.4 (4.0) | 156.5 (4.0) | Sit − Stand | Sit − Int | Stand − Int |
| 10 min | 141.1 (4.0) † | 147.6 (4.0) † | 152.1 (4.0) | −7.2 (4.0) * | −12.7 (4.0) * | −5.5 (4.0) | |
| 20 min | 137.6 (4.0) ‡ | 146.4 (4.0) ‡ | 147.5 (4.0) | −9.5 (4.0) * | −11.6 (4.0) * | −2.0 (4.1) | |
| Xclocalized | Baseline | 16.8 (1.0) | 12.7 (1.0) | 13.2 (1.0) | Sit − Stand | Sit − Int | Stand − Int |
| 10 min | 15.5 (1.0) † | 12.8 (1.0) | 12.5 (1.0) | −1.3 (1.8) | −1.0 (1.7) | 0.4 (1.8) | |
| 20 min | 17.2 (1.0) | 14.1 (1.0) | 12.2 (1.0) ‡ | −0.6 (1.8) | 1.4 (1.8) | 2.0 (1.8) | |
| Zlocalized | Baseline | 159.0 (4.6) | 141.6 (4.6) | 131.7 (4.6) | Sit − Stand | Sit − Int | Stand − Int |
| 10 min | 158.1 (4.7) † | 148.1 (4.7) | 146.9 (4.7) | −7.3 (6.0) | −12.8 (6.0) * | −5.5 (6.1) | |
| 20 min | 157.4 (4.7) ‡ | 152.8 (4.6) | 148.1 (4.7) | −16.0 (6.0) * | −18.0 (6.1) * | −1.9 (6.2) | |
| PhAlocalized | Baseline | 6.1 (0.4) | 5.7 (0.4) | 6.0 (0.4) | Sit − Stand | Sit − Int | Stand − Int |
| 10 min | 5.2 (0.4) | 5.0 (0.4) | 5.2 (0.4) | −0.2 (0.5) | −0.2 (0.5) | <−0.1 (0.5) | |
| 20 min | 5.5 (0.4) | 4.9 (0.3) | 4.8 (0.4) | 0.3 (0.5) | 0.6 (0.5) | 0.3 (0.5) | |
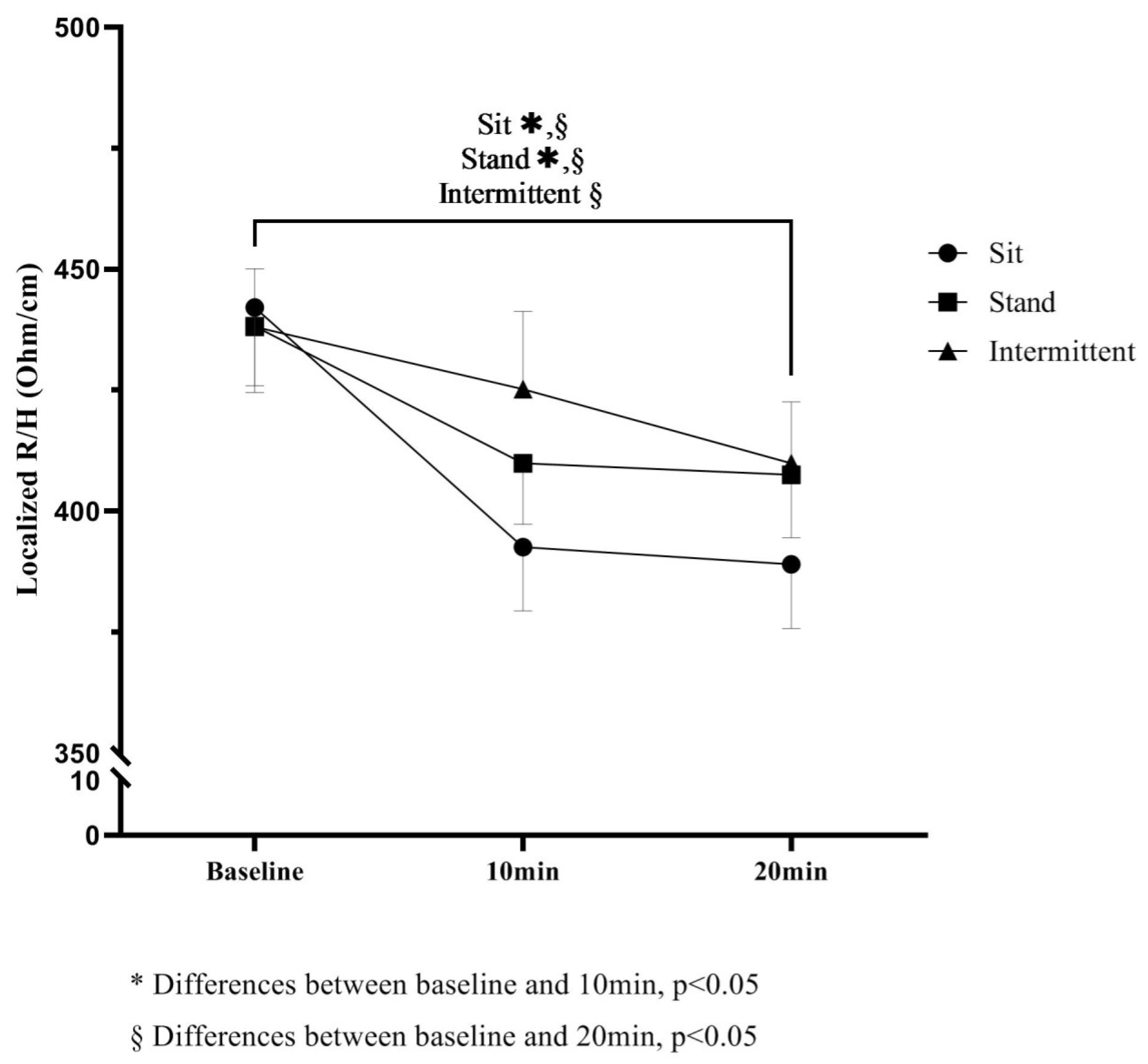
| R/Hlocalized | Baseline | 443.5 (13.8) | 439.8 (13.9) | 436.1 (14.0) | Sit − Stand | Sit − Int | Stand − Int |
| 10 min | 393.1 (13.8) † | 411.1 (13.8) | 426.1 (13.8) | −21.7 (13.3) | −40.5 (13.4) * | −18.7 (13.5) | |
| 20 min | 383.2 (13.9) ‡ | 410.1 (13.8) | 410.8 (13.8) | −30.5 (13.4) * | −35.0 (13.5) * | −4.5 (13.5) | |
| Xc/Hlocalized | Baseline | 47.8 (3.4) | 42.7 (3.4) | 48.9 (3.4) | Sit − Stand | Sit − Int | Stand − Int |
| 10 min | 35.4 (3.3) | 34.9 (3.3) | 38.9 (3.3) | −4.6 (5.7) | −2.4 (5.7) | −2.2 (5.8) | |
| 20 min | 35.8 (3.4) | 35.8 (3.4) | 33.1 (3.3) ‡ | −5.1 (5.8) | 3.7 (5.7) | 8.9 (5.8) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francisco, R.; Nunes, C.L.; Breda, J.; Jesus, F.; Lukaski, H.; Sardinha, L.B.; Silva, A.M. Breaking of Sitting Time Prevents Lower Leg Swelling—Comparison among Sit, Stand and Intermittent (Sit-to-Stand Transitions) Conditions. Biology 2022, 11, 899. https://doi.org/10.3390/biology11060899
Francisco R, Nunes CL, Breda J, Jesus F, Lukaski H, Sardinha LB, Silva AM. Breaking of Sitting Time Prevents Lower Leg Swelling—Comparison among Sit, Stand and Intermittent (Sit-to-Stand Transitions) Conditions. Biology. 2022; 11(6):899. https://doi.org/10.3390/biology11060899
Chicago/Turabian StyleFrancisco, Rúben, Catarina L. Nunes, João Breda, Filipe Jesus, Henry Lukaski, Luís B. Sardinha, and Analiza M. Silva. 2022. "Breaking of Sitting Time Prevents Lower Leg Swelling—Comparison among Sit, Stand and Intermittent (Sit-to-Stand Transitions) Conditions" Biology 11, no. 6: 899. https://doi.org/10.3390/biology11060899
APA StyleFrancisco, R., Nunes, C. L., Breda, J., Jesus, F., Lukaski, H., Sardinha, L. B., & Silva, A. M. (2022). Breaking of Sitting Time Prevents Lower Leg Swelling—Comparison among Sit, Stand and Intermittent (Sit-to-Stand Transitions) Conditions. Biology, 11(6), 899. https://doi.org/10.3390/biology11060899






