Comparative Study of Brain Size Ontogeny: Marsupials and Placental Mammals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
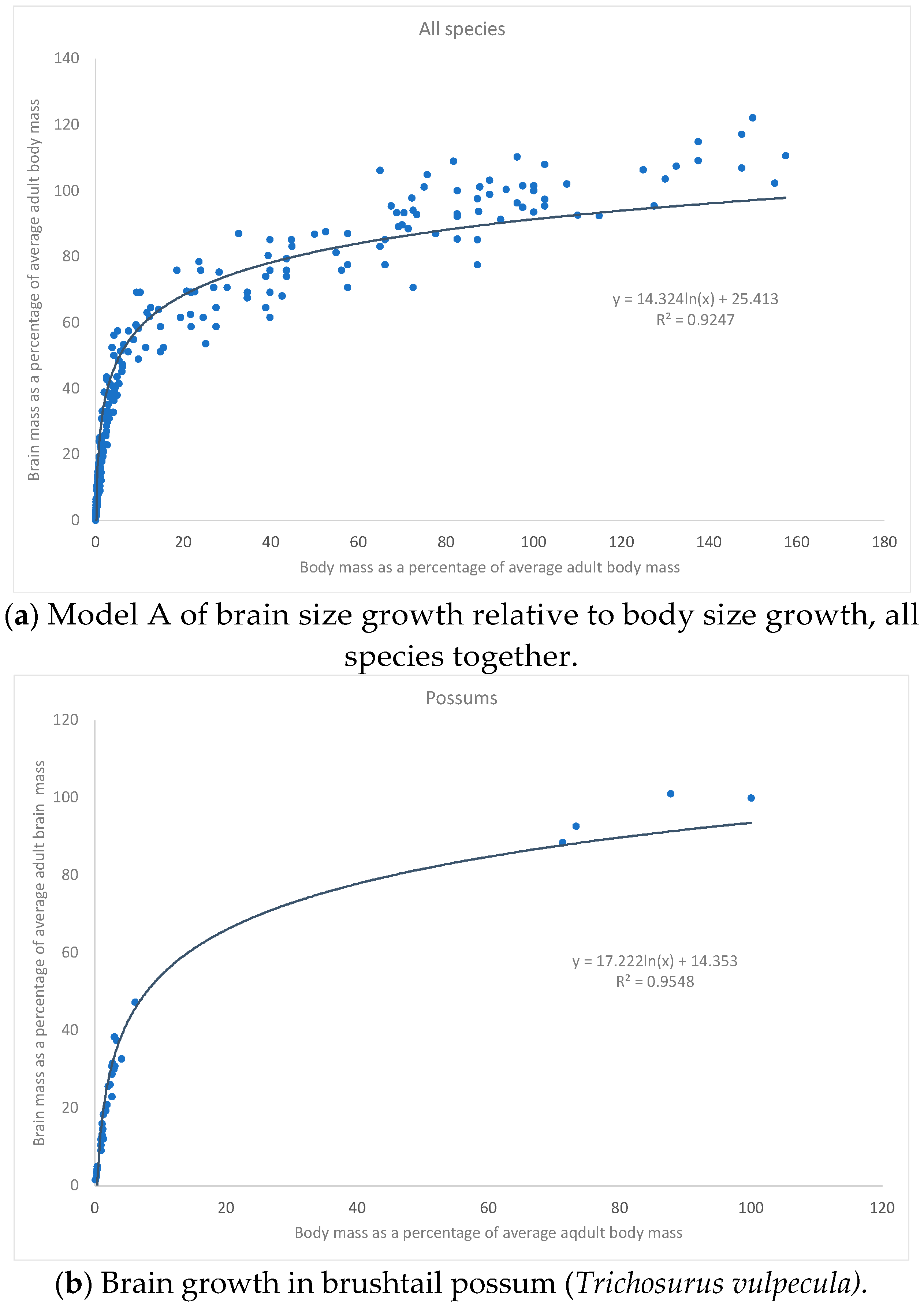
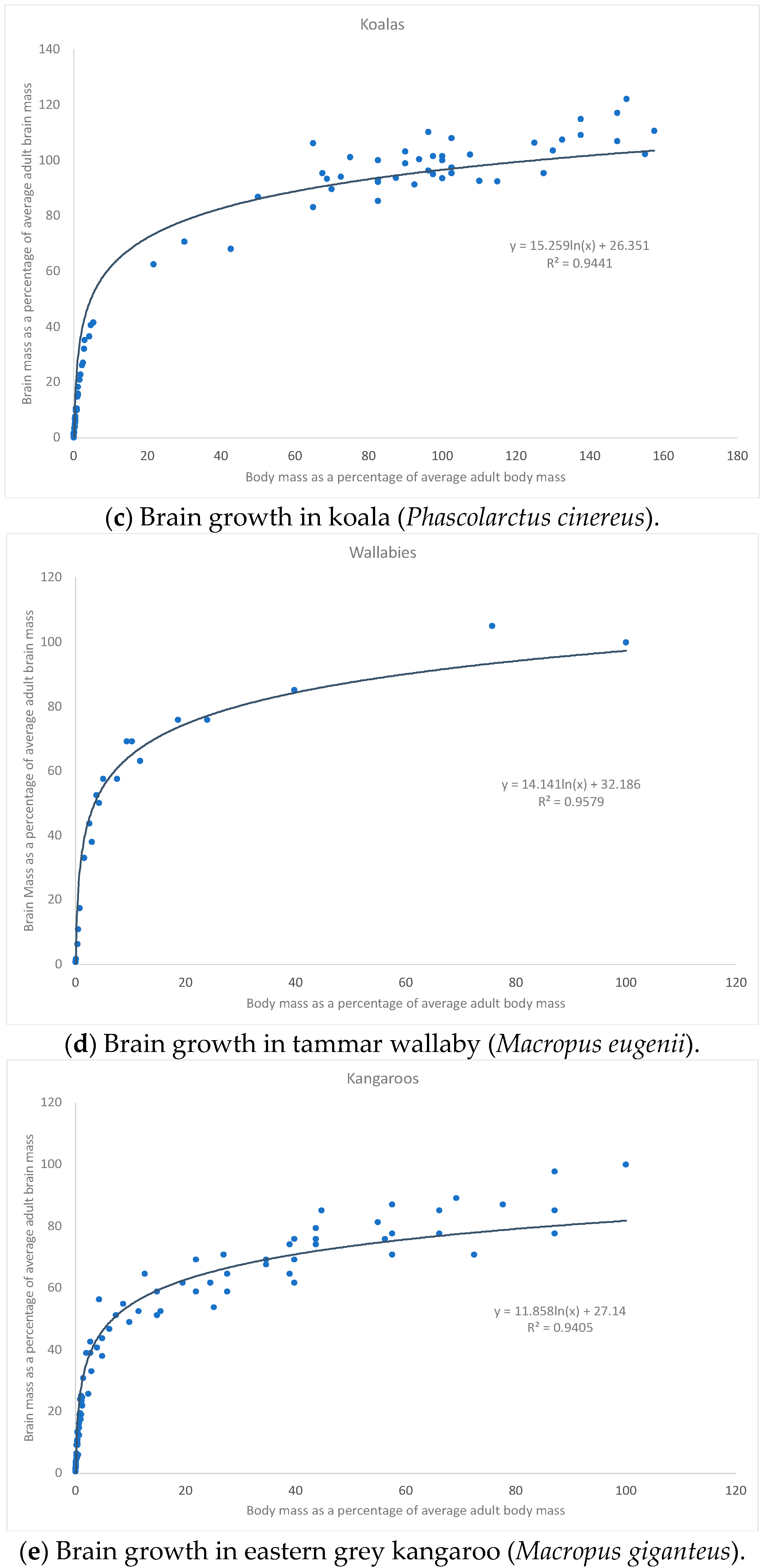
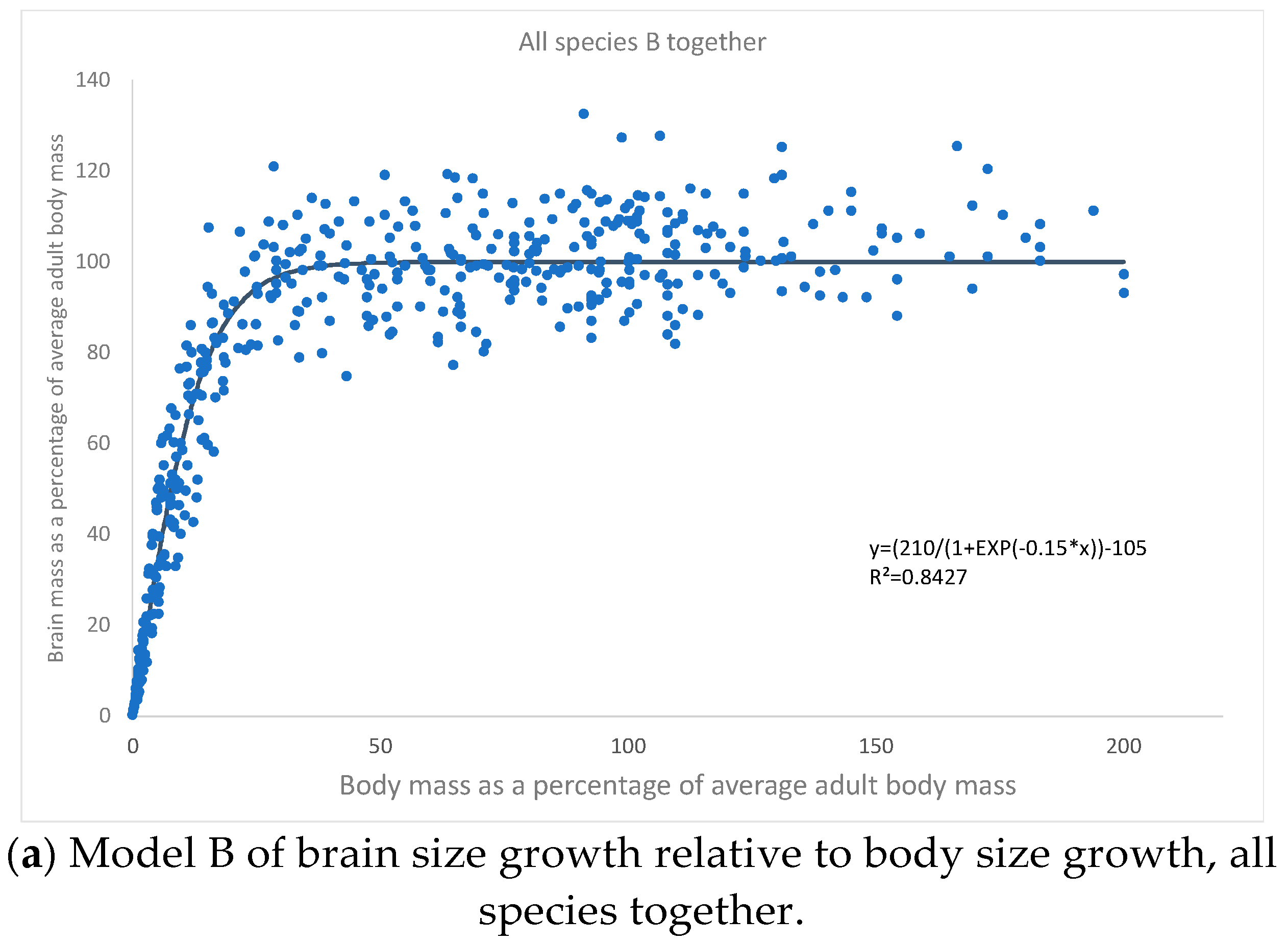
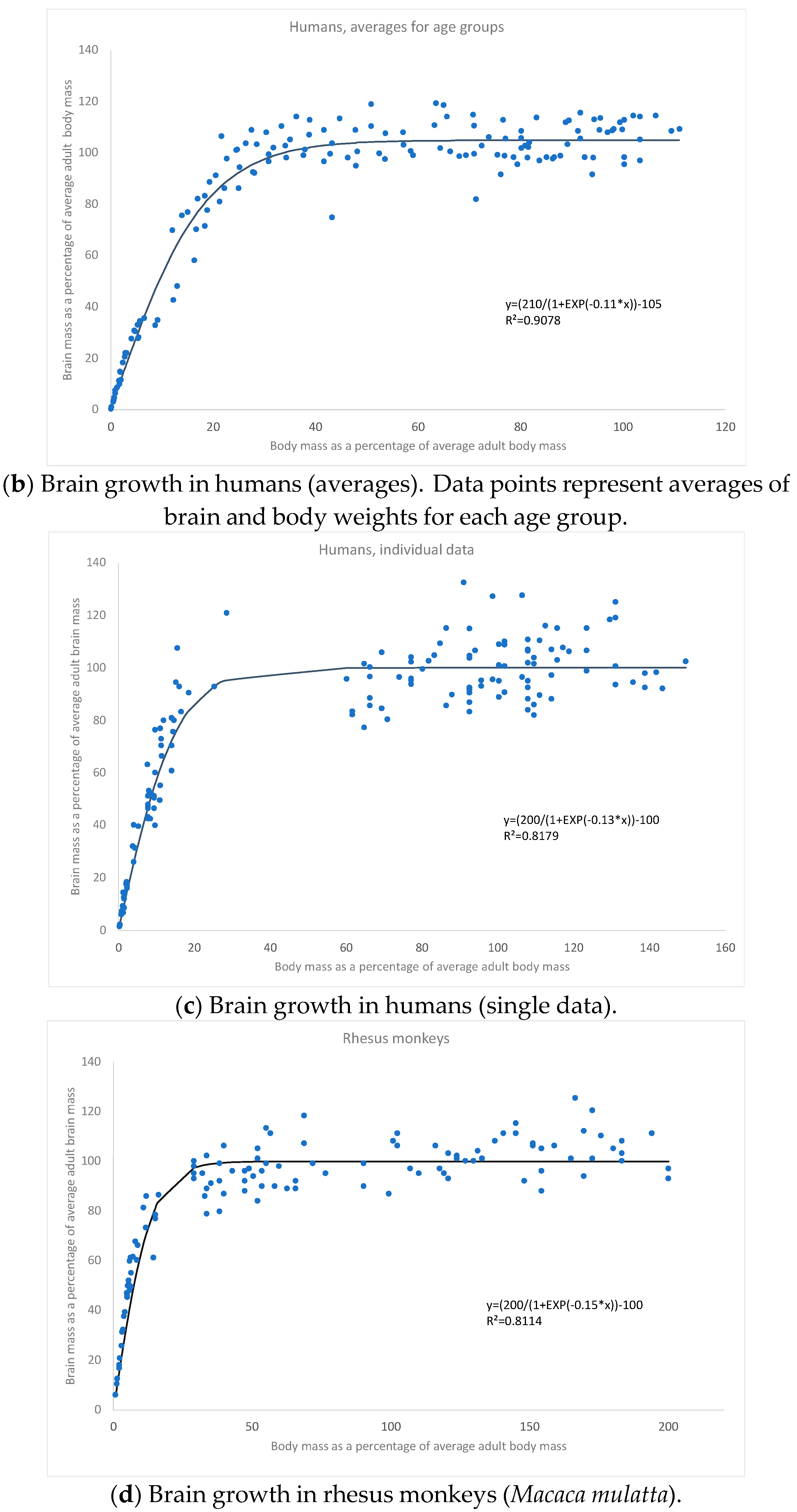
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Count, E.W. Brain and body weight in man: Their antecedents in growth and evolution. Ann. N. Y. Acad. Sci. 1947, 46, 993–1122. [Google Scholar] [CrossRef]
- Sacher, G.A.; Staffeldt, E.F. Elation of gestation time to brain weight for placental mammals: Implications for the theory of vertebrate growth. Am. Nat. 1974, 108, 593–615. [Google Scholar] [CrossRef]
- Holt, A.B.; Cheek, D.B.; Mellits, E.D.; Hill, D.E. Brain size and the relation of the primate to the non-primate. In Fetal and Postnatal Cellular Growth: Hormones and Nutrition; Cheek, D., Ed.; John Wiley & Sons: New York, NY, USA, 1975; pp. 23–44. [Google Scholar] [CrossRef]
- Passingham, R. Rates of Brain Development in Mammals Including Man. Brain Behav. Evol. 1975, 26, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Karlen, S.J.; Krubitzer, L. The functional and anatomical organization of marsupial neocortex: Evidence for parallel evolution across mammals. Prog. Neurobiol. 2007, 82, 122–141. [Google Scholar] [CrossRef]
- Weisbecker, V.; Goswami, A. Brain size, life history, and metabolism at the marsupial/placental dichotomy. Proc. Natl. Acad. Sci. USA 2010, 107, 16216–16221. [Google Scholar] [CrossRef]
- Hawkes, K.; Finlay, B.L. Mammalian brain development and our grandmothering life history. Physiol. Behav. 2018, 193, 55–68. [Google Scholar] [CrossRef]
- Thompson, D.W. On Growth and Form; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Jerison, H.J. Evolution of the Brain and Intelligence; Academic Press: New York, NY, USA, 1973. [Google Scholar]
- Hallgrímsson, B.; Katz, D.C.; Aponte, J.D.; Larson, J.R.; Devine, J.; Gonzales, P.N.; Young, N.M.; Roseman, C.C.; Marcucio, R.S. Integration and the developmental genetics of allometry. Integr. Comp. Biol. 2019, 59, 1369–1381. [Google Scholar] [CrossRef]
- Montgomery, S.H.; Mundy, N.I.; Barton, R.A. Brain evolution and development: Adaptation, allometry and constraint. Proc. R. Soc. B Boil. Sci. 2016, 283, 20160433. [Google Scholar] [CrossRef]
- Packard, G.C. The fallacy of biphasic growth allometry for the vertebrate brain. Biol. J. Linn. Soc. 2019, 128, 1057–1067. [Google Scholar] [CrossRef]
- Tsuboi, M. Biological interpretations of the biphasic model of ontogenetic brain–body allometry: A reply to Packard. Biol. J. Linn. Soc. 2019, 128, 1068–1075. [Google Scholar] [CrossRef]
- Dos Santos, S.E.; Porfirio, J.; Da Cunha, F.B.; Manger, P.R.; Tavares, W.; Pessôa, L.; Raghanti, M.A.; Sherwood, C.C.; Herculano-Houzel, S. Cellular Scaling Rules for the Brains of Marsupials: Not as “Primitive” as Expected. Brain Behav. Evol. 2017, 89, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Haug, H. Brain sizes, surfaces, and neuronal sizes of the cortex cerebri: A stereological investigation of man and his variability and a comparison with some mammals (primates, whales, marsupials, insectivores, and one elephant). Am. J. Anat. 1987, 180, 126–142. [Google Scholar] [CrossRef] [PubMed]
- Stolzenburg, J.-U.; Reichenbach, A.; Neumann, M. Size and density of glial and neuronal cells within the cerebral neocortex of various insectivorian species. Glia 1989, 2, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Darlington, R.B.; Dunlop, S.A.; Finlay, B.L. Neural development in metatherian and eutherian mammals: Variation and constraint. J. Comp. Neurol. 1999, 411, 359–368. [Google Scholar] [CrossRef]
- Renfree, M.B. Review: Marsupials: Placental Mammals with a Difference. Placenta 2010, 31, S21–S26. [Google Scholar] [CrossRef]
- Chavan, A.R.; Griffith, O.; Wagner, G.P. The inflammation paradox in the evolution of mammalian pregnancy: Turning a foe into a friend. Curr. Opin. Genet. Dev. 2017, 47, 24–32. [Google Scholar] [CrossRef]
- Smith, K.K. Going from s mall to large: Mechanical implications of body size diversity in terrestrial mammals. In Great Transformations in Vertebrate Evolution; Dial, K.P., Shubin, N., Brainerd, E.L., Eds.; University of Chicago Press: Chicago, IL, USA, 2015; pp. 205–225. [Google Scholar]
- Weisbecker, V.; Blomberg, S.; Goldizen, A.W.; Brown, M.; Fisher, D. The Evolution of Relative Brain Size in Marsupials Is Energetically Constrained but Not Driven by Behavioral Complexity. Brain Behav. Evol. 2015, 85, 125–135. [Google Scholar] [CrossRef]
- Renfree, M.; Holt, A.; Green, S.; Carr, J.; Cheek, D. Ontogeny of the Brain in a Marsupial (Macropus eugenii) Throughout Pouch Life. Brain Behav. Evol. 1982, 20, 57–71. [Google Scholar] [CrossRef]
- Nelson, J.E. Growth of the brain. In The Developing Marsupial: Models for Biomedical Research; Tyndale-Biscoe, C.H., Janssens, P.A., Eds.; Springer: Berlin/Heidelberg, Germnay, 1988; pp. 86–100. [Google Scholar]
- Janssens, P.A.; Hulbert, A.J.; Baudinette, R.V. Development of the pouch young from birth to pouch vacation. In Marsupial Biology: Recent Research, New Perspectives; Saunders, N., Hinds, L., Eds.; University of New South Wales Press: Sydney, Australia, 1997; pp. 71–89. [Google Scholar]
- Suárez, R.; Paolino, A.; Kozulin, P.; Fenlon, L.R.; Morcom, L.R.; Englebright, R.; O’Hara, P.J.; Murray, P.J.; Richards, L.J. Development of body, head and brain features in the Australian fat-tailed dunnart (Sminthopsis crassicaudata; Marsupialia: Dasyuridae); A postnatal model of forebrain formation. PLoS ONE 2017, 12, e0184450. [Google Scholar] [CrossRef]
- Tyndale-Biscoe, C.H.; Janssens, P.A. (Eds.) The Developing Marsupial: Models for Biomedical Research; Springer: Berlin, Germany, 1998; pp. 1–7. [Google Scholar]
- Todorov, O.S.; Blomberg, S.P.; Goswami, A.; Sears, K.; Drhlík, P.; Peters, J.; Weisbecker, V. Testing hypotheses of marsupial brain size variation using phylogenetic multiple imputations and a Bayesian comparative framework. Proc. R. Soc. B Boil. Sci. 2021, 288, 20210394. [Google Scholar] [CrossRef]
- De Miguel, C.; Henneberg, M. Encephalization of The Koala, Phascolarctos cinereus. Aust. Mammal. 1998, 20, 315–320. [Google Scholar] [CrossRef]
- Dobbing, J.; Sands, J. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 1970, 17, 115–123. [Google Scholar] [CrossRef]
- Harel, S.; Watanabe, K.; Linke, I.; Schain, R. Growth and Development of the Rabbit Brain. Neonatology 1972, 21, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, J.W.T.; Dobbing, J. Prenatal and postnatal growth and development of the central nervous system of the pig. Proc. R. Soc. London. Ser. B Boil. Sci. 1967, 166, 384–395. [Google Scholar] [CrossRef]
- Dobbing, J.; Sands, J. Quantitative growth and development of human brain. Arch. Dis. Child. 1973, 48, 757–767. [Google Scholar] [CrossRef]
- Connolly, C.J. External Morphology of the Primate Brain. Am. J. Biol. Anthropol. 1950, 8, 516–517. [Google Scholar]
- Spector, W.S. Handbook of Biological Data; W.B. Saunders Company: Philadelphia, PA, USA; London, UK, 1956. [Google Scholar]
- Dickerson, J.W.; Merat, A.; Yusuf, H.K.M. Effects of malnutrition on brain growth and development. In Brain and Behavioural Development; Dickerson, J.W.T., McGurk, H., Eds.; Surrey University Press: London, UK, 1982; pp. 73–108. [Google Scholar]
- Zilles, K. Biometrische analyse der frischvolumina verschiedener prosencephaler hirnregionen von 78 menschlichen, adulten gehirnen. Gegenbaurs Morphol. Jahrb. 1972, 118, 234–273. [Google Scholar]
- Kretschmann, H.J.; Kammradt, G.; Krauthausen, I.; Sauer, B.; Wingert, F. Brain growth in man. Bibl. Anat. 1986, 28, 1–26. [Google Scholar]
- Falk, D.; Froese, N.; Sade, D.S.; Dudek, B.C. Sex differences in brain/body relationships of Rhesus monkeys and humans. J. Hum. Evol. 1999, 36, 233–238. [Google Scholar] [CrossRef]
- Agrawal, H.C.; Davis, J.M.; Himwich, W.A. Water content of dog brain parts in relation to maturation of the brain. Am. J. Physiol. Content 1968, 215, 846–848. [Google Scholar] [CrossRef]
- Agrawal, H.C.; Davis, J.M.; Himwich, W.A. Water content of developing kitten brain. J. Neurochem. 1967, 14, 179–181. [Google Scholar] [CrossRef] [PubMed]
- De Souza, S.W.; Dobbing, J. Cerebral edema in developing brain: I. Normal water and cation content in developing rat brain and postmortem changes. Exp. Neurol. 1971, 32, 431–438. [Google Scholar] [CrossRef]
- Agrawal, H.C.; Davis, J.M.; Himwich, W.A. Developmental changes in mouse brain: Weight, water content and free amino acids. J. Neurochem. 1968, 15, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Karkach, A. Trajectories and models of individual growth. Demogr. Res. 2006, 15, 347–400. [Google Scholar] [CrossRef]
- Dzięciolowski, Z.M.; Clarke, C.M.H.; Fredric, B.J. Growth of feral pigs in New Zealand. Acta Theriol. 1990, 35, 77–88. [Google Scholar] [CrossRef]
- Blasco, A.; Gòmez, E. A note on growth curves of rabbit lines selected on growth rate or litter size. Anim. Prod. 1993, 57, 332–334. [Google Scholar] [CrossRef]
- Blasco, A.; Piles, M.; Varona, L. A Bayesian analysis of the effect of selection for growth rate growth curves in rabbits. Genet. Sel. Evol. 2003, 35, 21–41. [Google Scholar] [CrossRef]
- Salvador, C.H.; Fernandez, F.A.S. Reproduction and growth of a rare, Island-endemic cavy (cavia intermedia) from Southern Brazil. J. Mammal. 2008, 89, 909–915. [Google Scholar] [CrossRef]
- Radinsky, L. The Fossil Record of Primate Brain Evolution; American Museum of Natural History: New York, NY, USA, 1979. [Google Scholar]
- Assaf, Y.; Bouznach, A.; Zomet, O.; Marom, A.; Yovel, Y. Conservation of brain connectivity and wiring across the mammalian class. Nat. Neurosci. 2020, 23, 805–808. [Google Scholar] [CrossRef]
- Halley, A.C. Minimal variation in eutherian brain growth rates during fetal neurogenesis. Proc. R. Soc. B Boil. Sci. 2017, 284, 20170219. [Google Scholar] [CrossRef]
- Jarman, P.J. Sexual dimorphism in Macropodoidea. In Kangaroos, Wallabies and Rat-Kangaroos; Grigg, G., Jarman, P., Hume, I., Eds.; Surrey Beatty & Sons Pty Ltd.: Chipping Norton, Australia, 1989; pp. 433–447. [Google Scholar]
- Jarman, P.J. Social behavior and organization in the Macropodoidea. In Advances in the Study of Behavior; Slater, P.J.B., Rosenblatt, J.S., Beer, C., Milinski, M., Eds.; Academic Press: San Diego, CA, USA, 1991; pp. 1–47. [Google Scholar]
- Poole, W.; Carpenter, S.; Wood, J. Growth of Grey Kangaroos and the Reliability of Age Determination from Body Meausrements I. The Eastern Grey Kangaroo, Macropus giganteus. Wildl. Res. 1982, 9, 9–20. [Google Scholar] [CrossRef]
- Poole, W.; Carpenter, S.; Wood, J. Growth of Grey Kangaroos and the Reliability of Age Determination from Body Measurements II.* The Western Grey Kangaroos, Macropus fuliginosus fuliginosus, M. f. melanops and M. f. ocydromus. Wildl. Res. 1982, 9, 203–212. [Google Scholar] [CrossRef]
- Snell, R.S. Clinical Neuroanatomy, 7th ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010. [Google Scholar]
- De Miguel, C.; Henneberg, M. Variation in hominid brain size: How much is due to method? Homo 2001, 52, 3–58. [Google Scholar] [CrossRef] [PubMed]
- Henneberg, M. Evolution of the human brain: Is bigger better? Clin. Exp. Pharmacol. Physiol. 1998, 25, 745–749. [Google Scholar] [CrossRef]
- Henneberg, M.; Saniotis, A. Evolutionary origins of human brain and spirituality. Anthr. Anz. 2009, 67, 427–438. [Google Scholar] [CrossRef]
- Saniotis, A.; Henneberg, M. An Evolutionary Approach Toward Exploring Altered States of Consciousness, Mind–Body Techniques, and Non-Local Mind. World Futur. 2011, 67, 182–200. [Google Scholar] [CrossRef]
- Saniotis, A.; Grantham, J.P.; Kumaratilake, J.; Henneberg, M. Neuro-hormonal Regulation Is a Better Indicator of Human Cognitive Abilities Than Brain Anatomy: The Need for a New Paradigm. Front. Neuroanat. 2020, 13, 101. [Google Scholar] [CrossRef]
- Henneberg, M. Decrease of human skull size in the Holocene. Hum. Biol. 1988, 60, 395–405. [Google Scholar]
- Brown, P. Recent human evolution in East Asia and Australasia. Philos. Trans. R. Soc. B Biol. Sci. 1992, 337, 235–242. [Google Scholar] [CrossRef]
- Previc, F.H. The Dopaminergic Mind in Human Evolution and History; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Elston, G.N.; Garey, L.J. Prefrontal cortex: Brodmann and Cajal revisited. In Prefrontal Cortex: Roles, Interventions and Traumas Hauppauge; LoGrasso, L., Morretti, G., Eds.; Nova Science Pub Inc.: New York, NY, USA, 2019; pp. 245–259. [Google Scholar]
- Bonney, K.; Wynne, C. Studies of learning and problem solving in two species of Australian marsupials. Neurosci. Biobehav. Rev. 2004, 28, 583–594. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Miguel, C.; Saniotis, A.; Cieślik, A.; Henneberg, M. Comparative Study of Brain Size Ontogeny: Marsupials and Placental Mammals. Biology 2022, 11, 900. https://doi.org/10.3390/biology11060900
De Miguel C, Saniotis A, Cieślik A, Henneberg M. Comparative Study of Brain Size Ontogeny: Marsupials and Placental Mammals. Biology. 2022; 11(6):900. https://doi.org/10.3390/biology11060900
Chicago/Turabian StyleDe Miguel, Carmen, Arthur Saniotis, Agata Cieślik, and Maciej Henneberg. 2022. "Comparative Study of Brain Size Ontogeny: Marsupials and Placental Mammals" Biology 11, no. 6: 900. https://doi.org/10.3390/biology11060900
APA StyleDe Miguel, C., Saniotis, A., Cieślik, A., & Henneberg, M. (2022). Comparative Study of Brain Size Ontogeny: Marsupials and Placental Mammals. Biology, 11(6), 900. https://doi.org/10.3390/biology11060900





