Identification and Characterization of a New Microalga Dysmorphococcus globosus-HI from the Himalayan Region as a Potential Source of Natural Astaxanthin
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Isolation, and Growth Conditions
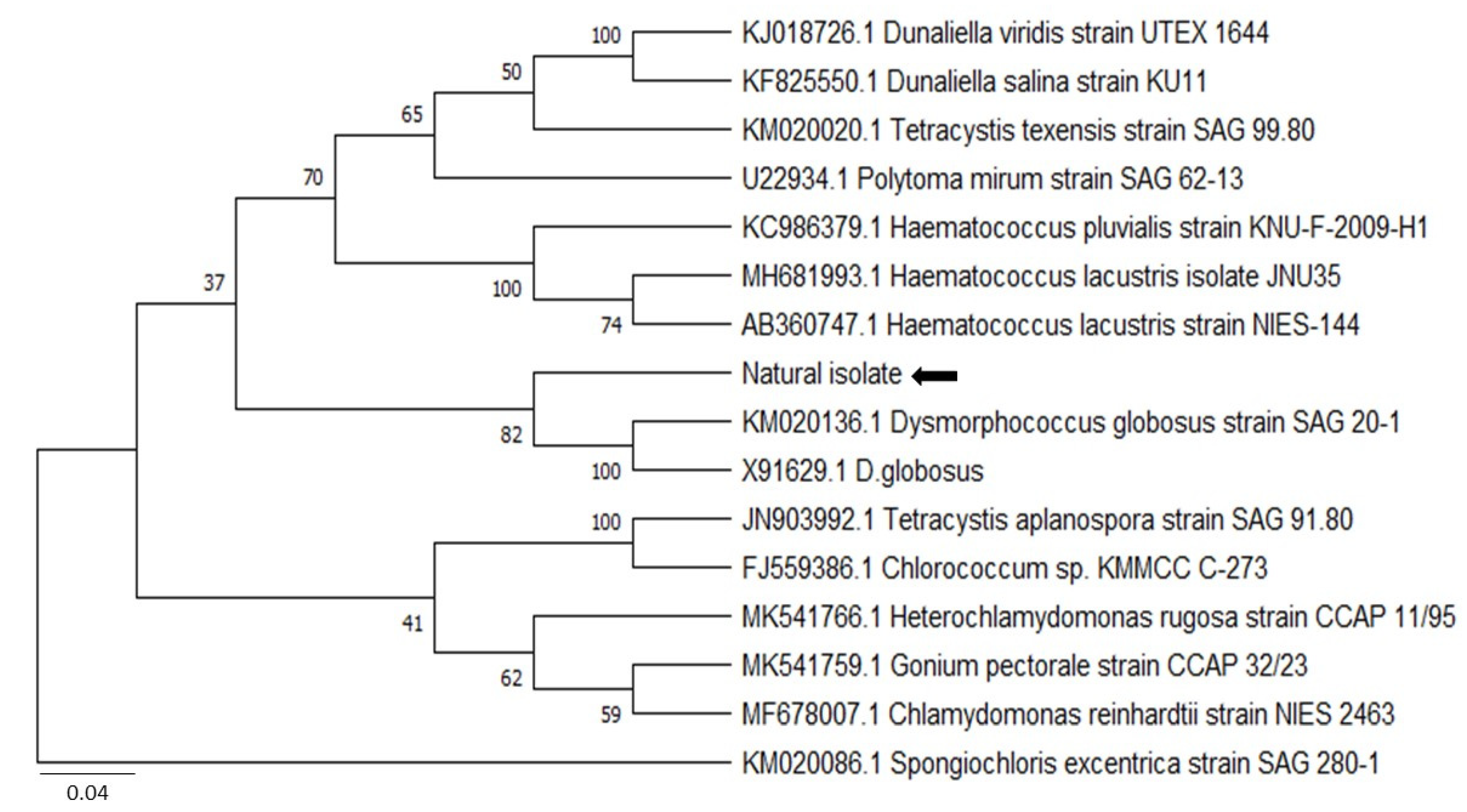
2.2. Molecular Identification and Phylogenetic Analysis
2.3. Morphological Characterization of Isolate by Light Microscopy
2.4. Assessing Different Growth Media for Optimum Growth
2.5. Growth Characteristics and Biochemical Analysis
2.5.1. Optical Density, Cell Dry Weight (CDW), and Growth Rate
2.5.2. Determination of Chlorophyll a and b and Total Carotenoid Content
2.5.3. Measurement of Protein and Carbohydrates
2.5.4. Total Lipid Content and Fatty Acids (FAs) Profile Analysis
2.5.5. Astaxanthin Analysis and Quantification
Thin Layer Chromatography (TLC)
High-Performance Liquid Chromatography (HPLC)
2.6. Statistical Analysis
3. Results
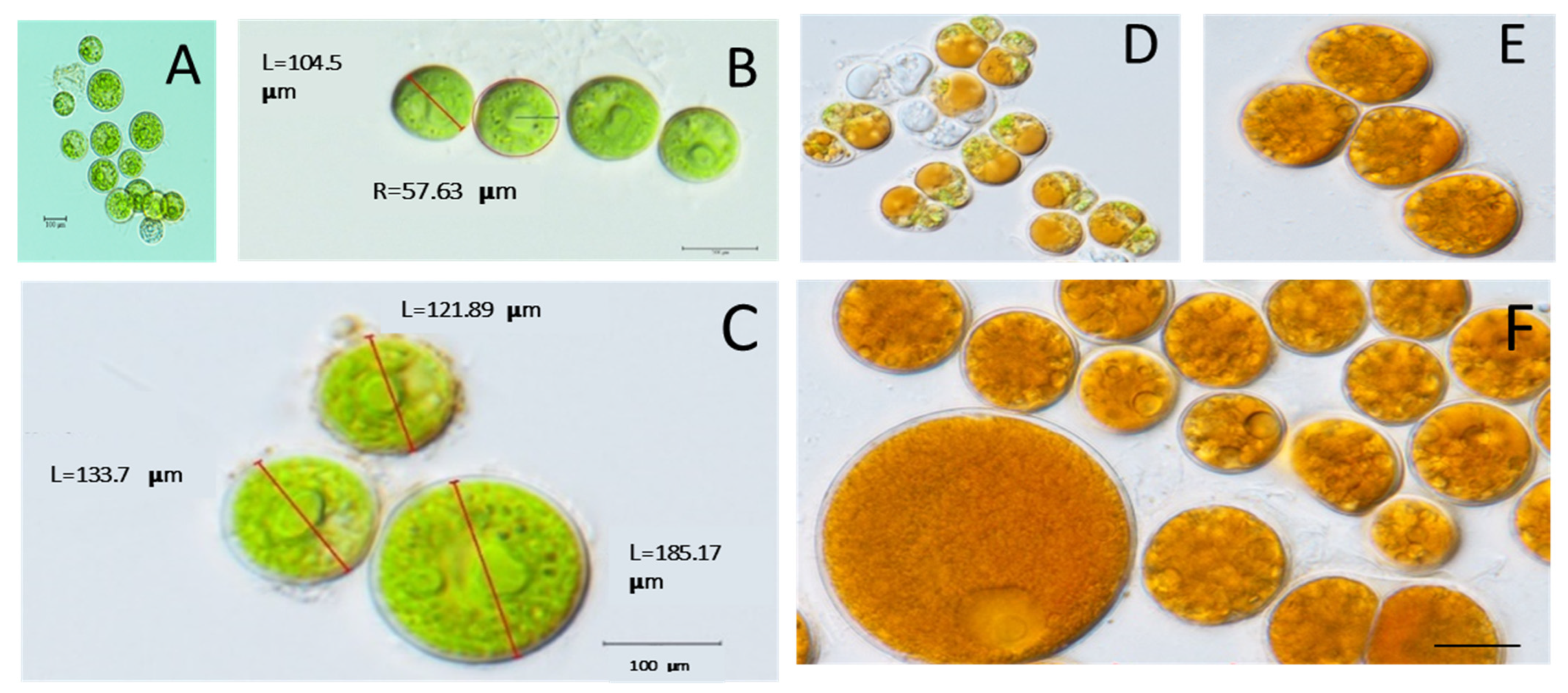
3.1. Cell Morphology
3.2. Molecular Identification and Phylogenetic Analysis
3.3. Growth Study in Different Media
3.4. Pigments and Biochemical Composition
3.4.1. Chlorophyll a, Chlorophyll b, and Total Carotenoids Content
3.4.2. Thin Layer Chromatography of Total Pigment Extracts
3.4.3. Proteins and Carbohydrates’ Content
3.5. Lipid and Fatty Acid Profile Analysis
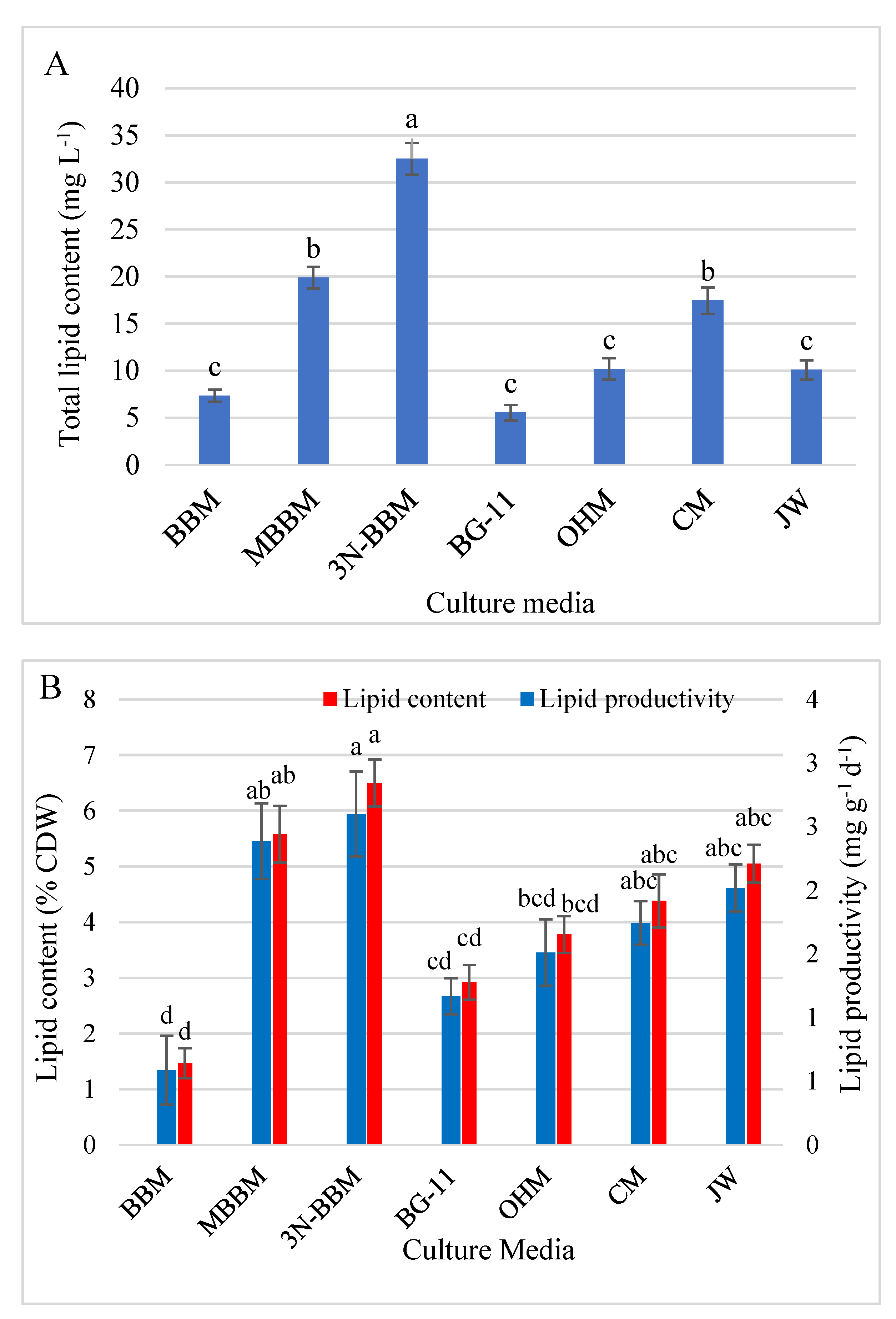
3.5.1. Lipid Content and Productivity
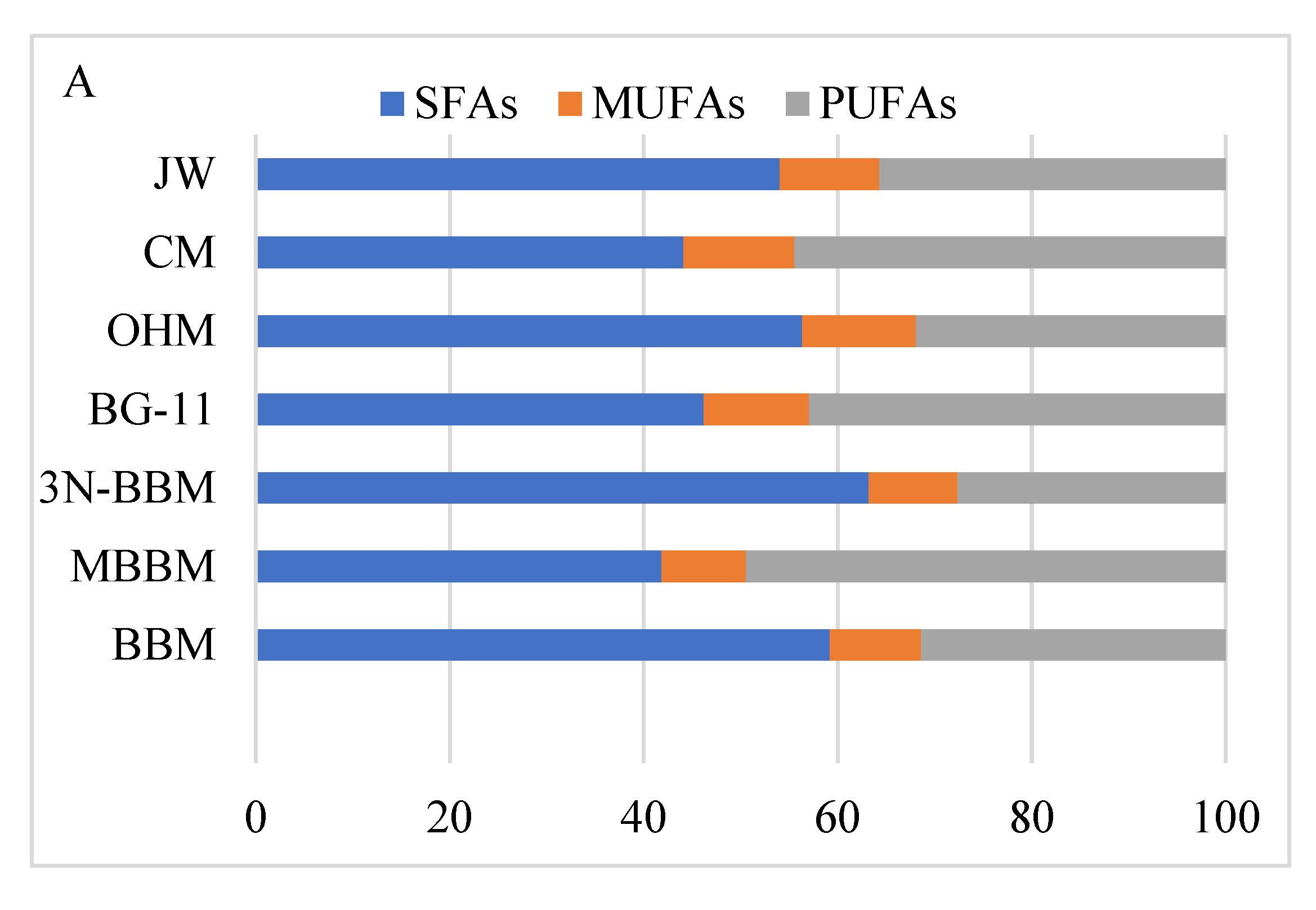
3.5.2. Profile of Fatty Acids
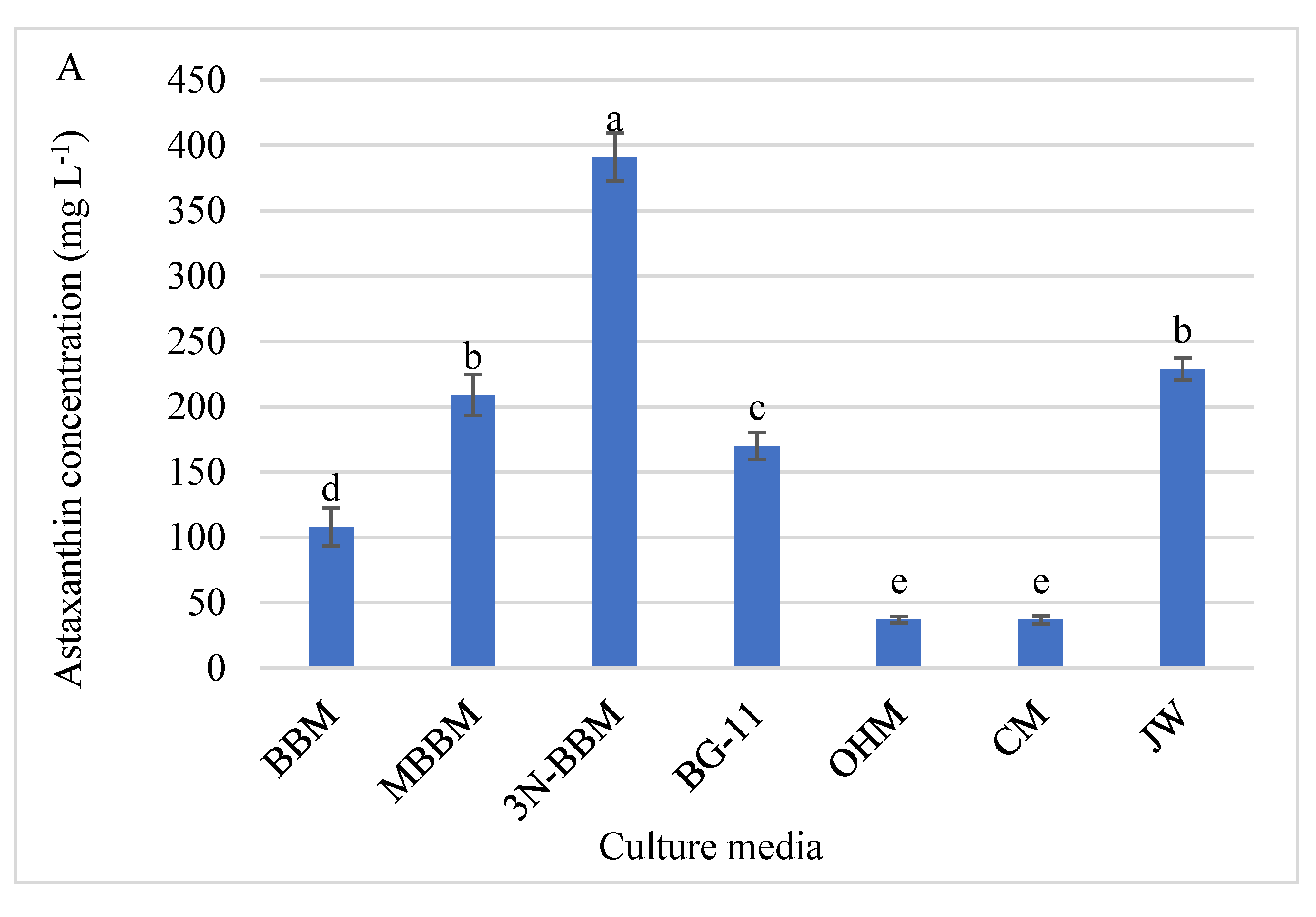
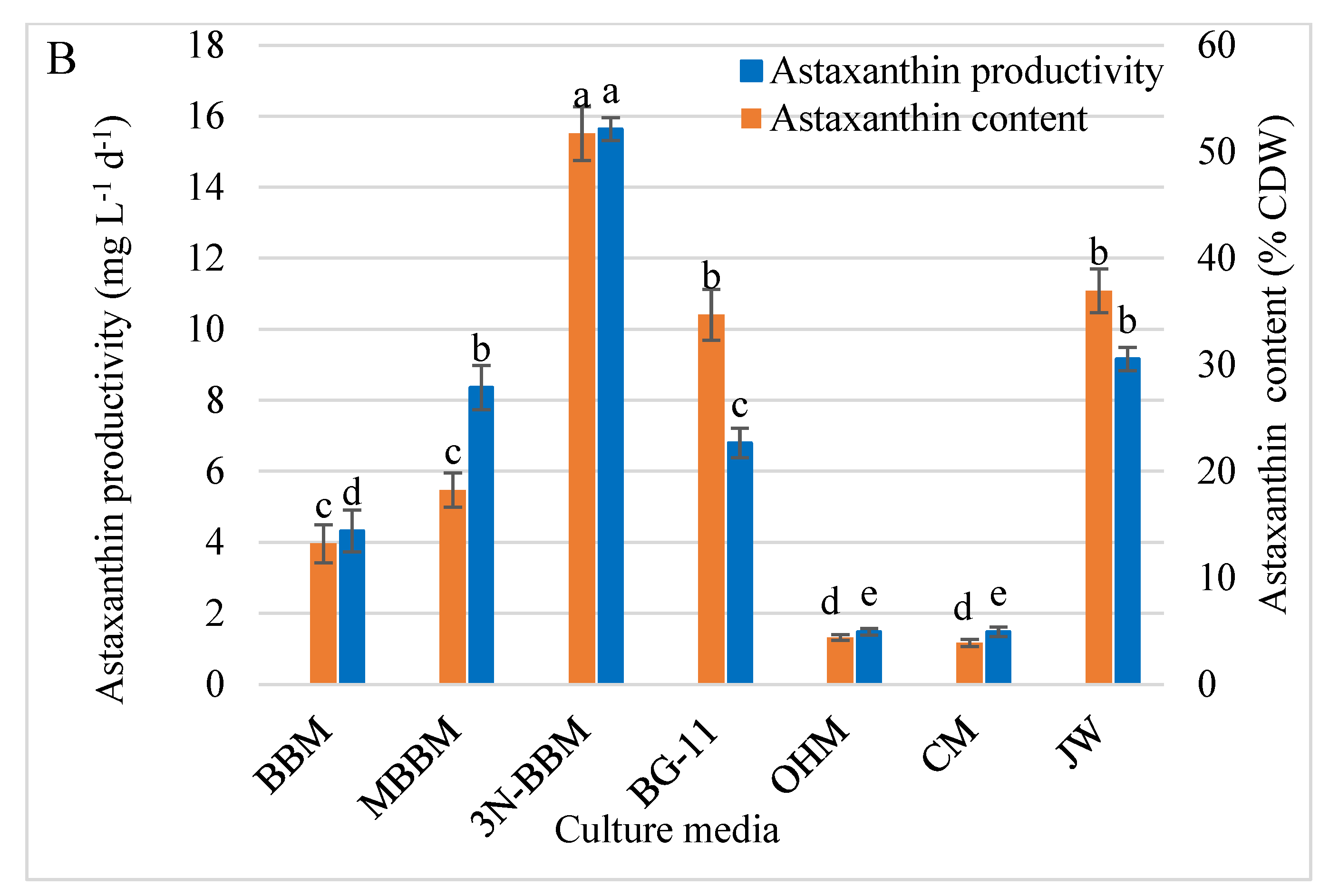
3.6. Astaxanthin Analysis by HPLC
4. Discussion
4.1. Morphological Study
4.2. Assessment of an Appropriate Culture Medium
4.3. Proteins and Carbohydrates Analysis
4.4. Lipids and FAs Profile
4.5. Astaxanthin Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, P.J.B.; Laurens, L.M.L. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry. energetics & economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar] [CrossRef]
- Singh, A.; Olsen, S.I. A critical review of biochemical conversion, sustainability and life cycle assessment of algal biofuels. Appl. Energy 2011, 88, 3548–3555. [Google Scholar] [CrossRef]
- Ravishankar, G.A.; Rao, A.R. Global Perspectives on Astaxanthin: From Industrial Production to Food, Health, and Pharmaceutical Applications; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Shah, M.R.; Liang, Y.; Jay, C.; Daroch, M. Astaxanthin-producing green from single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EL-Mohsnawy, E.; El-sheekh, M.; Mabrouk, M.; Zohir, W. Enhancing accumulation of omega 3 and 9 fatty acids in Chlorella vulgaris under mixotrophic nutrition. J. Anim. Plant Sci. 2020, 30, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Perez-Legaspi, I.A.; Valadez-Rocha, V.; Ortega-Clemente, L.A.; Jimenez-Garcıa, M.I. Microalgal pigment induction and transfer in aquaculture. Rev. Aquac. 2019, 12, 1–21. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Ammar, E.E.; Aioub, A.A.A.; Elesawy, A.E.; Karkour, A.M.; Mouhamed, M.S.; Amer, A.A.; EL-Shershaby, N.A. Algae as Bio-fertilizers: Between current situation and future prospective. Saudi J. Biol. Sci. 2022, 29, 3083–3096. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K.C. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, J.; Wu, W.; Cai, D.; Lv, J.; Jiang, G.; Huang, J.; Gao, J.; Hartmann, R.; Gabriels, D. Effects of conservation tillage practices on winter wheat water-use efficiency and crop yield on the Loess Plateau, China. Agric. Water Manag. 2007, 87, 307–314. [Google Scholar] [CrossRef]
- Lu, Q.; Li, H.; Zou, Y.; Liu, H.; Yang, L. Astaxanthin as a microalgal metabolite for aquaculture: A review on the synthetic mechanisms, production techniques, and practical application. Algal Res. 2021, 54, 102178. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Tan, J.S.; Oslan, S.N.; Matanjun, P.; Mokhtar, R.A.M.; Shapawi, R.; Huda, N. Haematococcus pluvialis as a potential source of astaxanthin with diverse applications in industrial sectors: Current research and future directions. Molecules 2021, 26, 6470. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Li, L.; Xu, W.; Wang, M.; Jiao, D.; Yao, B.; Xu, K.; Chen, Y.; Yang, S.; Long, M.; et al. Astaxanthin protects ochratoxin A-Induced oxidative stress and apoptosis in the heart via the Nrf2 pathway. Oxid. Med. Cell. Longev. 2020, 2020, 7639109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- JDonahoe, S.M.; Stewart, G.C.; McCabe, C.H.; Mohanavelu, S.; Murphy, S.A.; Cannon, C.P.; Antman, E. Diabetes and mortality following acute coronary syndromes. Clevel. Clin. Found. Intensive Rev. Intern. Med. Sixth Ed. 2007, 298, 765–775. [Google Scholar]
- Mehariya, S.; Sharma, N.; Iovine, A.; Casella, P.; Marino, T.; Larocca, V.; Molino, A.; Musmarra, D. An integrated strategy for nutraceuticals from Haematoccus pluvialis: From cultivation to extraction. Antioxidants 2020, 9, 825. [Google Scholar] [CrossRef]
- Xie, S.; Yin, P.; Tian, L.; Yu, Y.; Liu, Y.; Niu, J. Dietary supplementation of astaxanthin improved the growth performance, antioxidant ability and immune response of juvenile largemouth bass (Micropterus salmoides) fed high-fat diet. Mar. Drugs 2020, 18, 642. [Google Scholar] [CrossRef]
- Bhalamurugan, G.L.; Valerie, O.; Mark, L. Valuable bioproducts obtained from microalgal biomass and their commercial applications: A review. Environ. Eng. Res. 2018, 23, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Gao, B.; Wu, M.; Huang, L.; Zhang, C. A novel strategy for the hyper-production of astaxanthin from the newly isolated microalga Haematococcus pluvialis JNU35. Algal Res. 2019, 39, 101466. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Nie, K.; Jiang, H.; Fan, W. Effects of lutein supplementation in agerelated macular degeneration. PLoS ONE 2019, 14, e0227048. [Google Scholar] [CrossRef] [Green Version]
- Sztretye, M.; Dienes, B.; Gönczi, M.; Czirják, T.; Csernoch, L.; Dux, L.; Szentesi, P.; Keller-Pintér, A. Astaxanthin: A potential mitochondrial-targeted antioxidant treatment in diseases and with aging. Oxid. Med. Cell. Longev. 2019, 2019, 3849692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Cai, M.; Lin, M.; Huang, X.; Wang, J.; Zheng, X.; Wu, S.; An, Y. Accumulation of astaxanthin was improved by the nonmotile cells of Haematococcus pluvialis. Biomed Res. Int. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasunuma, T.; Takaki, A.; Matsuda, M.; Kato, Y.; Vavricka, C.J.; Kondo, A. Single-stage astaxanthin production enhances the nonmevalonate pathway and photosynthetic central metabolism in Synechococcus sp. PCC 7002. ACS Synth. Biol. 2019, 8, 2701–2709. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Li, Y.; Hu, Q. Astaxanthin in microalgae: Pathways, functions and biotechnological implications. Algae 2013, 28, 131–147. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Ferdous, U.T.; Yusof, Z. Medicinal prospects of antioxidants from algal sources in cancer therapy. Front. Pharmacol. 2021, 12, 593116. [Google Scholar] [CrossRef]
- Brotosudarmo, P.T.H.; Limantara, L.; Setiyono, E.; Heriyanto, T. Structures of astaxanthin and their consequences for therapeutic application. Int. J. Food Sci. 2020, 2020, 1–16. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of high added-value compounds-a brief review of recent work. Biotechnol. Prog. 2011, 27, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, K.; Shiratori, K.; Kotake, S.; Nishida, T.; Mizuki, N.; Yazawa, K.; Ohno, S. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2694–2701. [Google Scholar] [CrossRef] [Green Version]
- Young Park, S.; Binkley, R.M.; Jun, W.; Hee, M.; Yup, S. Metabolic engineering of Escherichia coli for high-level astaxanthin production with high productivity. Metab. Eng. 2018, 49, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Mirjana, M. Direct extraction of astaxanthin from the microalgae Haematococcus pluvialis using liquid-liquid chromatography. RSC Adv. 2019, 9, 22779–22789. [Google Scholar] [CrossRef] [Green Version]
- Molino, A.A.; Rimauro, J.; Casella, P.; Cerbone, A.; Larocca, V.; Chianese, S.; Karatza, D.; Mehariya, S.; Ferraro, A. Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognized as safe solvents and accelerated extraction. J. Biotechnol. 2018, 283, 51–61. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Too, H.-P. Microbial astaxanthin biosynthesis: Recent achievements, challenges, and commercialization outlook. Appl. Microbiol. Biotechnol. 2020, 104, 5725–5737. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef]
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent advances in biorefinery ofastaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019, 288, 121606. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Régnier, P.; Bastias, J.; Rodriguez-Ruiz, V.; Caballero-Casero, N.; Caballo, C.; Sicilia, D.; Fuentes, A.; Maire, M.; Crepin, M.; Letourneur, D.; et al. Astaxanthin from Haematococcus pluvialis prevents oxidative stress on human endothelial cells without toxicity. Mar. Drugs 2015, 13, 2857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, D.; Zhang, J.; Sawdon, A.J.; Peng, C.A. Enhanced astaxanthin accumulation in Haematococcus pluvialis using high carbon dioxide concentration and light illumination. Bioresour. Technol. 2018, 256, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Peng, J. Growth-associated biosynthesis of astaxanthin in heterotrophic Chlorella zofingiensis (Chlorophyta). World J. Microbiol. Biotechnol. 2008, 24, 1915–1922. [Google Scholar] [CrossRef]
- Chen, T.A.O.; Wei, D.; Chen, G.U.; Wang, Y.A.N.; Chen, F. Employment of organic acids to enhance astaxanthin formation in heterotrophic chlorella zofingiensis. J Food Process Pres 2009, 33, 271–284. [Google Scholar] [CrossRef]
- Aburai, N.; Sumida, D.; Abe, K. Effect of light level and salinity on the composition and accumulation of free and ester-type carotenoids in the aerial microalga Scenedesmus. Algal Res. 2015, 8, 30–36. [Google Scholar] [CrossRef]
- Orosa, M.; Torres, E.; Fidalgo, P.; Abalde, J. Production and analysis of secondary carotenoids in green algae. J. Appl. Phycol. 2000, 12, 553–556. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Chen, F. Influence of medium components on astaxanthin content and production of Haematococcus pluvialis. Process Biochem. 1998, 33, 385–391. [Google Scholar] [CrossRef]
- Li, L.; Chen, Z.; Huang, Q. Exogenous γ-aminobutyric acid promotes biomass and astaxanthin production in Haematococcus pluvialis. Algal Res. 2020, 52, 102089. [Google Scholar] [CrossRef]
- Nahidian, B.; Ghanati, F.; Shahbazi, M.; Soltani, N. Effect of nutrients on the growth and physiological features of newly isolated Haematococcus pluvialis TMU. Bioresour. Technol. 2018, 255, 229–237. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue—World vegetable center. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees’. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Bischoff, H.W.; Bold, H.C. Some Soil Algae from Enchanted Rock and Related Algal Species; University of Texas: Austin, TX, USA, 1963. [Google Scholar]
- Stein-Taylor, J.R. Handbook of Phycological Methods: Culture Methods and Growth Measurements; University Press: Cambridge, UK, 1979; ISBN 9780521297479. [Google Scholar]
- Mudimu, O.; Koopmann, I.K.; Rybalka, N.; Friedl, T.; Schulz, R.; Bilger, W. Screening of microalgae and cyanobacteria strains for α-tocopherol content at different growth phases and the influence of nitrate reduction on α-tocopherol production. J. Appl. Phycol. 2017, 29, 2867–2875. [Google Scholar] [CrossRef]
- Scibilia, L.; Girolomoni, L.; Berteotti, S.; Alboresi, A.; Ballottari, M. Photosynthetic response to nitrogen starvation and high light in Haematococcus pluvialis. Algal Res. 2015, 12, 170–181. [Google Scholar] [CrossRef]
- FaÂBregas, J.; DomõÂnguez, A.; Regueiro, M.; Maseda, A.; Otero, A. Optimization of culture medium for the continuous cultivation of the microalga Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2000, 53, 530–535. [Google Scholar] [CrossRef]
- Watanabe, S.; Mitsui, K.; Nakayama, T.; Inouye, I. Phylogenetic relationships and taxonomy of sarcinoid green algae: Chlorosarcinopsis, Desmotetra, Sarcinochlamys gen. nov., Neochlorosarcina, and Chlorosphaeropsis (Chlorophyceae, Chlorophyta). J. Phycol. 2006, 42, 679–695. [Google Scholar] [CrossRef]
- Šoštarič, M.; Golob, J.; Bricelj, M.; Klinar, D.; Pivec, A. Studies on the growth of Chlorella vulgaris in culture media with different carbon sources. Chem. Biochem. Eng. Q. 2009, 23, 471–477. [Google Scholar]
- Griffiths, M.J.; van Hille, R.P.; Harrison, S.T.L. Lipid productivity, settling potential and fatty acid profile of 11 microalgal species grown under nitrogen replete and limited conditions. J. Appl. Phycol. 2012, 24, 989–1001. [Google Scholar] [CrossRef]
- Rizwan, M.; Lee, J.H.; Gani, R. Optimal design of microalgae-based biorefinery: Economics, opportunities and challenges. Appl. Energy 2015, 150, 69–79. [Google Scholar] [CrossRef]
- Guillard, R. Methods for microflagellates and nannoplankton. In Handbook Phycological Methods Culture Methods and Growth Measurements; Stein, J.R., Ed.; Cambridge University Press: Cambridge, UK, 1973; pp. 69–85. [Google Scholar]
- Michelle Wood, A.; Everroad, R.C.; Wingard, L.M. Measuring Growth Rates in Microalgal Cultures. Algal Cult. Tech. 2005, 269–285. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Xue, J.; Balamurugan, S.; Li, D.; Liu, H.; Zeng, H.; Wang, L.; Yang, W.; Liu, J.; Li, H. Glucose-6-phosphate dehydrogenase as a target for highly efficient fatty acid biosynthesis in microalgae by enhancing NADPH supply. Metab. Eng. 2017, 41, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Fatma, Z.; Yazdani, S.S.; Kumar, S. DNA barcode and lipid analysis of new marine algae potential for biofuel. Algal Res. 2013, 2, 10–15. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kakizono, T.; Nagai, S. Astaxanthin production by a green alga, Haematococcus pluvialis accompanied with morphological changes in acetate media. J. Ferment. Bioeng. 1991, 71, 335–339. [Google Scholar] [CrossRef]
- Chekanov, K.; Litvinov, D.; Fedorenko, T.; Chivkunova, O.; Lobakova, E. Combined production of astaxanthin and β-carotene in a new strain of the microalga Bracteacoccus aggregatus BM5/15 (IPPAS C-2045) cultivated in photobioreactor. Biology 2021, 10, 643. [Google Scholar] [CrossRef]
- Taucher, J.; Baer, S.; Schwerna, P.; Hofmann, D.; Hümmer, M.; Buchholz, R.; Becker, A. Cell disruption and pressurized liquid extraction of carotenoids from microalgae. J. Thermodyn. Catal. 2016, 7, 158. [Google Scholar] [CrossRef] [Green Version]
- Casella, P.; Iovine, A.; Mehariya, S.; Marino, T.; Musmarra, D.; Molino, A. Smart method for carotenoids characterization in haematococcus pluvialis red phase and evaluation of astaxanthin thermal stability. Antioxidants 2020, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- SAS. The SAS Users Guide, Version 9.4; SAS Institute: Cary, NC, USA, 2013; Available online: https://support.sas.com/documentation/cdl/en (accessed on 3 April 2022).
- Bold, C.H.; Starr, R.C. A new member of the Phacotaceae. Bulltin Torrey Bot. Club 1953, 80, 178–186. [Google Scholar] [CrossRef]
- Dawson, J.T.; Harris, D.O. Notes on the life history of the planktonic alga, Dysmorphococcus globosus (Volvocales). J. Plankt. Res. Vol.9 1987, 9, 291–295. [Google Scholar] [CrossRef]
- Takeda, H. Dysmorphococcus variabilis, gen. et sp. nov. Ann. Bot. 1916, 30, 151–156. [Google Scholar] [CrossRef]
- Shyam, R. Studies on North Indian Volvocales. VI. On the life cycle and cytology of a new member of Phacotaceae, Dysmorphococcus sarmaii sp. nov. Can. J. Bot. 1981, 59, 726–734. [Google Scholar] [CrossRef]
- Korschikofaf, A. Beitrage zur Morphologie und Systematik der Volvocales. Archiv. Arch. Russ. Protistol 1925, 4, 153–193. [Google Scholar]
- Foot, B. Taxonomie der mikroskopischen Flora einheimischer Gewasser. Preslia 1957, 29, 278–319. [Google Scholar]
- Neofotis, P.; Huang, A.; Sury, K.; Chang, W.; Joseph, F.; Gabr, A.; Twary, S.; Qiu, W.; Holguin, O.; Polle, J.E.W. Characterization and classification of highly productive microalgae strains discovered for biofuel and bioproduct generation. Algal Res. 2016, 15, 164–178. [Google Scholar] [CrossRef] [Green Version]
- Buchheim, M.A.; Sutherland, D.M.; Buchheim, J.A.; Wolf, M. The blood alga: Phylogeny of Haematococcus (Chlorophyceae) inferred from ribosomal RNA gene sequence data. Eur. J. Phycol. 2013, 48, 318–329. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Brown, R.C.; Homsy, S.; Martinez, L.; Sidhu, S.S. Fast pyrolysis of microalgae remnants in a fluidized bed reactor for bio-oil and biochar production. Bioresour. Technol. 2013, 127, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Jena, U.; Das, K.C.; Kastner, J.R. Effect of operating conditions of thermochemical liquefaction on biocrude production from Spirulina platensis. Bioresour. Technol. 2011, 102, 6221–6229. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Huang, M.Y.; Chang, J.S.; Chen, C.Y. Thermal decomposition dynamics and severity of microalgae residues in torrefaction. Bioresour. Technol. 2014, 169, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Pirwitz, K.; Rihko-Struckmann, L.; Sundmacher, K. Valorization of the aqueous phase obtained from hydrothermally treated Dunaliella salina remnant biomass. Bioresour. Technol. 2016, 219, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.H.; Wu, Z.Y.; Chang, J.S. Isothermal and non-isothermal torrefaction characteristics and kinetics of microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2014, 155, 245–251. [Google Scholar] [CrossRef]
- Shuping, Z.; Yulong, W.; Mingde, Y.; Chun, L.; Junmao, T. Pyrolysis characteristics and kinetics of the marine microalgae Dunaliella tertiolecta using thermogravimetric analyzer. Bioresour. Technol. 2010, 101, 359–365. [Google Scholar] [CrossRef]
- Mahdy, A.; Mendez, L.; Ballesteros, M.; González-Fernández, C. Enhanced methane production of Chlorella vulgaris and Chlamydomonas reinhardtii by hydrolytic enzymes addition. Energy Convers. Manag. 2014, 85, 551–557. [Google Scholar] [CrossRef]
- Rendón Castrillón, L.J.; Ramírez Carmona, M.E.; Vélez Salazar, Y. Microalgas Para la Industria Alimenticia; Universidad Pontificia Bolivariana: Medellin, Colombia, 2015; ISBN 978-958-764-228. [Google Scholar]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Chong, W.T.; Lam, M.K.; Loh, P.K.; Vellayan, V. Microalgae biofuels as an alternative to fossil fuel for power generation. Renew. Sustain. Energy Rev. 2016, 58, 180–197. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, R.; Yu, T.; Li, T.; Zhou, J.; Cen, K. Biodiesel production from lipids in wet microalgae with microwave irradiation and bio-crude production from algal residue through hydrothermal liquefaction. Bioresour. Technol. 2014, 151, 415–418. [Google Scholar] [CrossRef]
- Verspreet, J.; Soetemans, L.; Gargan, C.; Hayes, M.; Bastiaens, L. Nutritional profiling and preliminary bioactivity screening of five micro-algae strains cultivated in northwest Europe. Foods 2021, 10, 1516. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Sun, X.M.; Ren, L.J.; Zhao, Q.Y.; Ji, X.J.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Affan, M.A.; Jang, J.; Kang, M.H.; Ko, A.R.; Jeon, S.M.; Oh, C.; Heo, S.J.; Lee, Y.H.; Ju, S.J.; et al. Morphological, molecular, and biochemical characterization of astaxanthin-producing green microalga Haematococcus sp. KORDI03 (haematococcaceae, chlorophyta) isolated from Korea. J. Microbiol. Biotechnol. 2015, 25, 238–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damiani, M.C.; Popovich, C.A.; Constenla, D.; Leonardi, P.I. Lipid analysis in Haematococcus pluvialis to assess its potential use as a biodiesel feedstock. Bioresour. Technol. 2010, 101, 3801–3807. [Google Scholar] [CrossRef] [PubMed]
- Folayan, A.J.; Anawe, P.A.L.; Aladejare, A.E.; Ayeni, A.O. Experimental investigation of the effect of fatty acids configuration, chain length, branching and degree of unsaturation on biodiesel fuel properties obtained from lauric oils, high-oleic and high-linoleic vegetable oil biomass. Energy Rep. 2019, 5, 793–806. [Google Scholar] [CrossRef]
- Stansell, G.R.; Gray, V.M.; Sym, S.D. Microalgal fatty acid composition: Implications for biodiesel quality. J. Appl. Phycol. 2012, 24, 791–801. [Google Scholar] [CrossRef]
- Tallima, H.; El, R. Arachidonic acid: Physiological roles and potential health benefits—A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef]
- Horrocks, L.A.; Yeo, Y.K. Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res. 1999, 40, 211–225. [Google Scholar] [CrossRef] [Green Version]
- Pryde, E.H.; Cowan, J.C.; Northern, T. Industrial chemical uses of polyunsaturated fatty acids. J. Am. Oil Chem. Soc. 1971, 48, 349–354. [Google Scholar] [CrossRef]
- Knothe, G. “Designer” biodiesel: Optimizing fatty ester composition to improve fuel properties. Energy Fuels 2008, 22, 1358–1364. [Google Scholar] [CrossRef]
- Choi, Y.E.; Yun, Y.S.; Park, J.M.; Yang, J.W. Determination of the time transferring cells for astaxanthin production considering two-stage process of Haematococcus pluvialis cultivation. Bioresour. Technol. 2011, 102, 11249–11253. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yu, C.; Zhong, D.B.; Zhao, Y.; Yu, X. Melatonin and calcium act synergistically to enhance the coproduction of astaxanthin and lipids in Haematococcus pluvialis under nitrogen deficiency and high light conditions. Bioresour. Technol. 2020, 305, 123069. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, K.; Hagen, C. β-carotene is the intermediate exported from the chloroplast during accumulation of secondary carotenoids in Haematococcus pluvialis. J. Appl. Phycol. 2001, 13, 89–93. [Google Scholar] [CrossRef]
- Zhekisheva, M.; Boussiba, S.; Khozin-goldberg, I.; Zarka, A.; Cohen, Z. Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters. J. Phycol. 2002, 331, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Wang, B.; Han, D.; Sommerfeld, M.; Lu, Y.; Chen, F.; Hu, Q. Molecular mechanisms of the coordination between astaxanthin and fatty acid biosynthesis in Haematococcus pluvialis (Chlorophyceae). Plant J. 2015, 81, 95–107. [Google Scholar] [CrossRef]
- Recht, L.; Töpfer, N.; Batushansky, A.; Sikron, N.; Gibon, Y.; Fait, A.; Nikoloski, Z.; Boussiba, S.; Zarka, A. Metabolite profiling and integrative modeling reveal metabolic constraints for carbon partitioning under nitrogen starvation in the green algae Haematococcus pluvialis. J. Biol. Chem. 2014, 289, 30387–30403. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, R.T.; Cysewsk, G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Boussiba, S.; Bing, W.; Yuan, J.; Zarka, A.; Chen, F. Changes in pigments profile in the green alga Haeamtococcus pluvialis exposed to environmental stresses. Biotechnol. Lett. 1999, 21, 601–604. [Google Scholar] [CrossRef]
- Steward, P.R.; Dougill, A.J.; Thierfelder, C.; Pittelkow, C.M.; Stringer, L.C.; Kudzala, M.; Shackelford, G.E. The adaptive capacity of maize-based conservation agriculture systems to climate stress in tropical and subtropical environments: A meta-regression of yields. Agric. Ecosyst. Environ. 2018, 251, 194–202. [Google Scholar] [CrossRef]
- Pan-utai, W.; Parakulsuksatid, P.; Phomkaivon, N. Effect of inducing agents on growth and astaxanthin production in Haematococcus pluvialis: Organic and inorganic. Biocatal. Agric. Biotechnol. 2017, 12, 152–158. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Shoparwe, N.F.; Yusoff, A.H.; Rahim, A.A.; Chang, C.S.; Tan, J.S.; Oslan, S.N.; Arumugam, K.; Ariff, A.B.; Sulaiman, A.Z.; et al. A review on Haematococcus pluvialis bioprocess optimization of green and red stage culture conditions for the production of natural astaxanthin. Biomolecules 2021, 11, 256. [Google Scholar] [CrossRef]





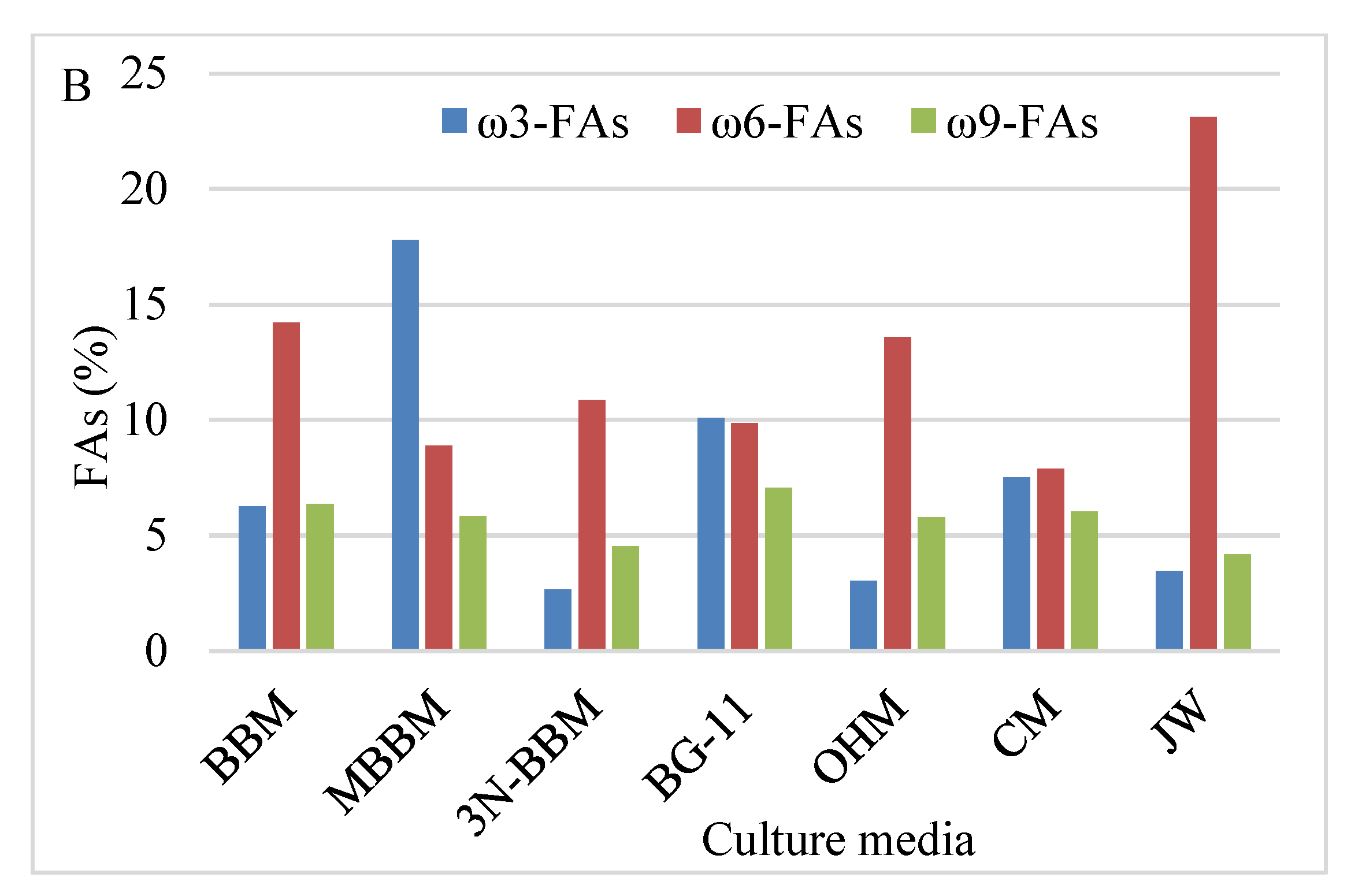
| Culture Media | Cell Dry Weight (g L−1) | Biomass Productivity (mg L−1 d−1) | Specific Growth Rate (day−1) | Division Per Day (K) | Maximum Cellular Yield (×106 cells mL−1) (R) | Doubling Time (h) |
|---|---|---|---|---|---|---|
| BBM | 0.817 ± 0.014c | 32.680 ± 0.545c | 0.065 ± 0.005d | 0.093 ± 0.011c | 3.600 ± 0.314d | 10.720 ± 0.020b |
| MBBM | 1.145 ± 0.009a | 45.820 ± 0.364a | 0.087 ± 0.002a | 0.125 ± 0.022a | 6.160 ± 0.294a | 7.994 ± 0.100e |
| 3N-BBM | 0.756 ± 0.051d | 30.240 ± 0.203d | 0.059 ± 0.003e | 0.085 ± 0.002d | 3.050 ± 0.483e | 11.773 ± 0.140a |
| BG-11 | 0.565 ± 0.011f | 22.600 ± 0.454f | 0.058 ± 0.009e | 0.083 ± 0.003 | 3.180 ± 0.108e | 12.032 ± 0.010a |
| OHM | 0.846 ± 0.006c | 33.440 ± 0.294c | 0.070 ± 0.006c | 0.101 ± 0.005b | 4.460 ± 0.312c | 9.901 ± 0.070c |
| CM | 0.950 ± 0.001b | 38.000 ± 0.605b | 0.081 ± 0.004b | 0.116 ± 0.040b | 5.300 ± 0.424b | 8.590 ± 0.090d |
| JW | 0.620 ± 0.007e | 24.800 ± 0.263e | 0.060 ± 0.009e | 0.086 ± 0.003d | 3.34 ± 0.122e | 11.601 ± 0.20a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zohir, W.F.; Kapase, V.U.; Kumar, S. Identification and Characterization of a New Microalga Dysmorphococcus globosus-HI from the Himalayan Region as a Potential Source of Natural Astaxanthin. Biology 2022, 11, 884. https://doi.org/10.3390/biology11060884
Zohir WF, Kapase VU, Kumar S. Identification and Characterization of a New Microalga Dysmorphococcus globosus-HI from the Himalayan Region as a Potential Source of Natural Astaxanthin. Biology. 2022; 11(6):884. https://doi.org/10.3390/biology11060884
Chicago/Turabian StyleZohir, Wafaa F., Vikas U. Kapase, and Shashi Kumar. 2022. "Identification and Characterization of a New Microalga Dysmorphococcus globosus-HI from the Himalayan Region as a Potential Source of Natural Astaxanthin" Biology 11, no. 6: 884. https://doi.org/10.3390/biology11060884
APA StyleZohir, W. F., Kapase, V. U., & Kumar, S. (2022). Identification and Characterization of a New Microalga Dysmorphococcus globosus-HI from the Himalayan Region as a Potential Source of Natural Astaxanthin. Biology, 11(6), 884. https://doi.org/10.3390/biology11060884






