Gut Microbiome Suffers from Hematopoietic Stem Cell Transplantation in Childhood and Its Characteristics Are Positively Associated with Intra-Hospital Physical Exercise
Abstract
Simple Summary
Abstract
1. Introduction
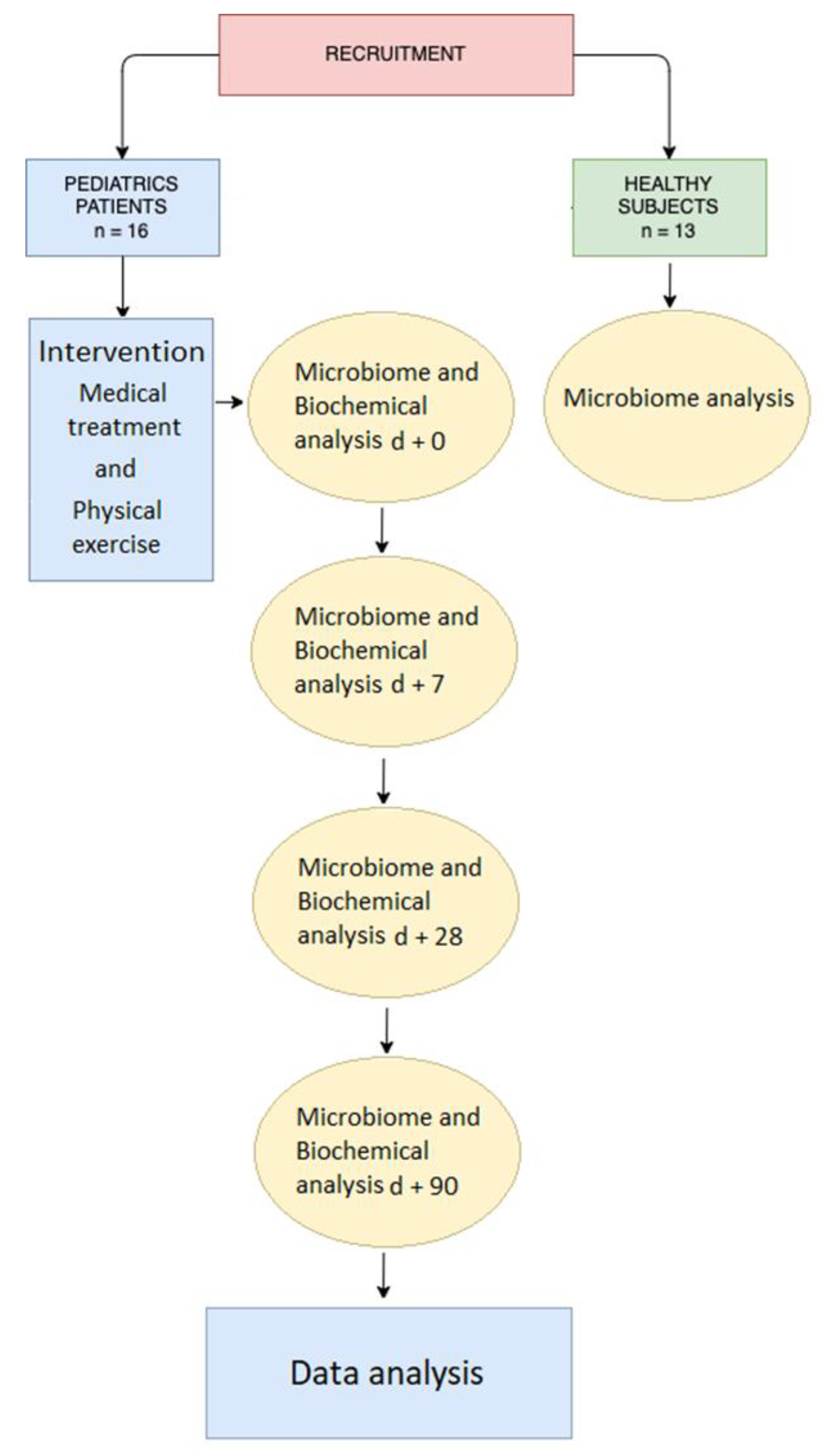
2. Materials and Methods
2.1. Subjects
2.2. Intervention
2.2.1. Medical Treatment
2.2.2. Physical Exercise
2.3. Sample Collection
2.4. Microbiome Analysis
2.5. Illumina Data Processing
2.6. Biochemical Analysis
2.7. Statistical Analysis
3. Results
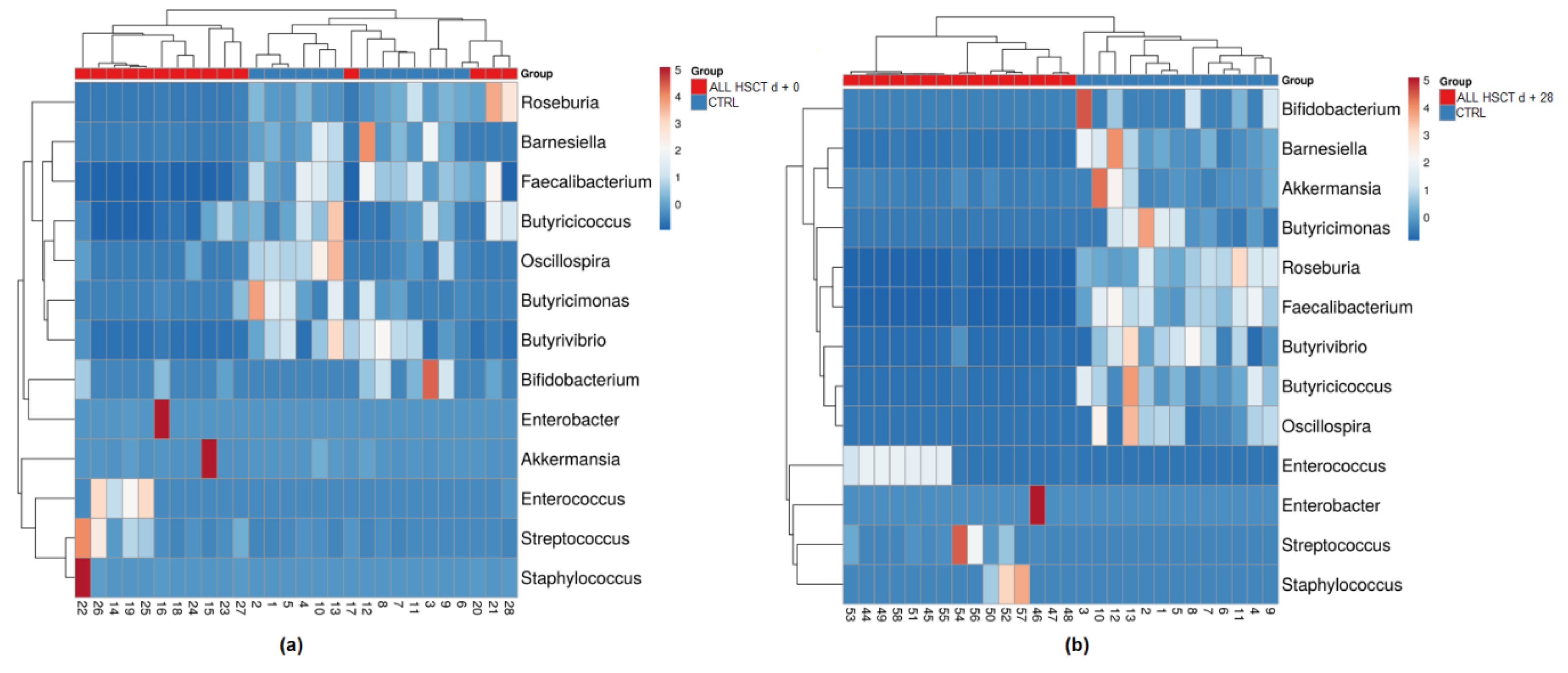
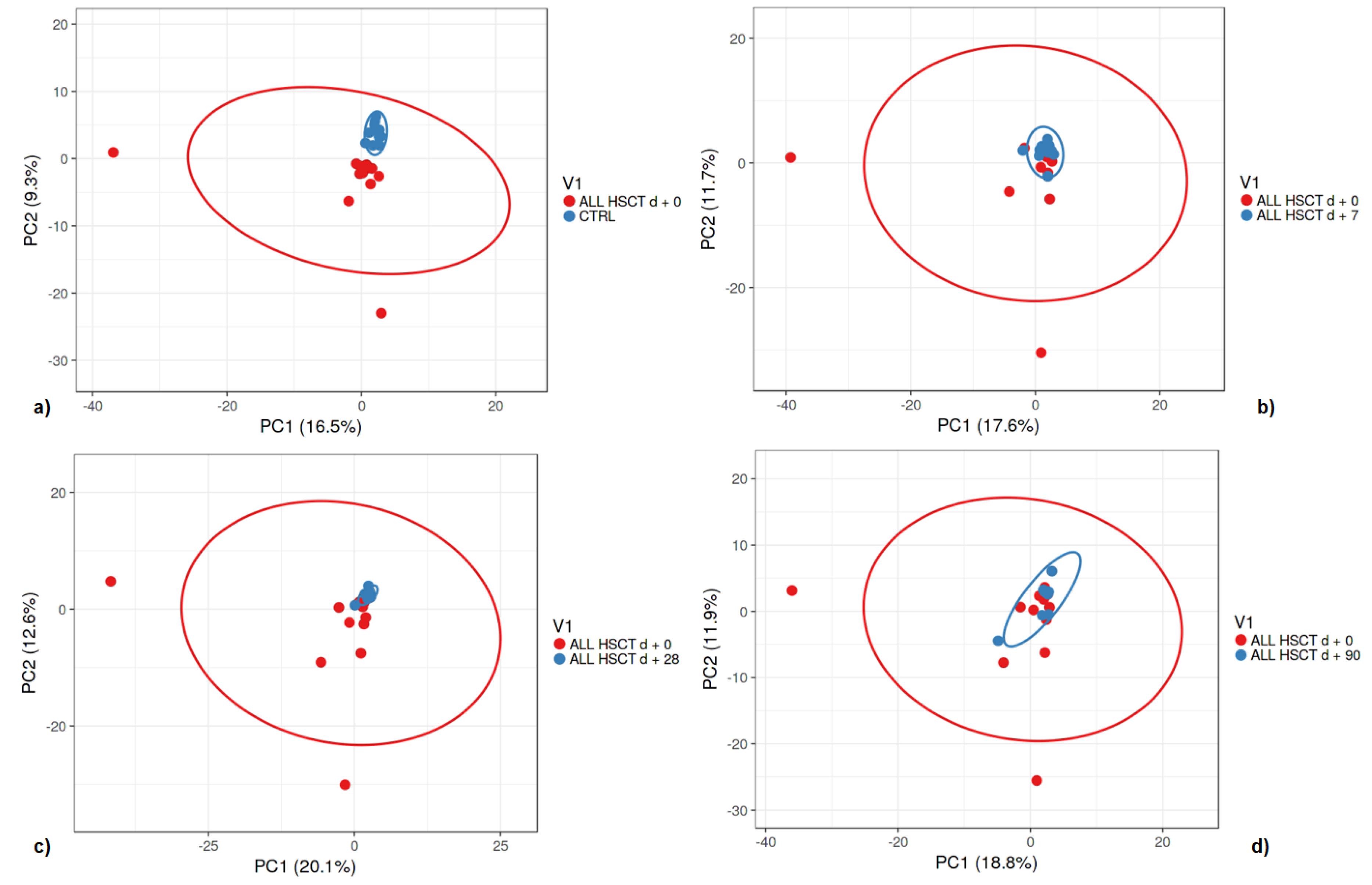
3.1. Fecal Microbiota
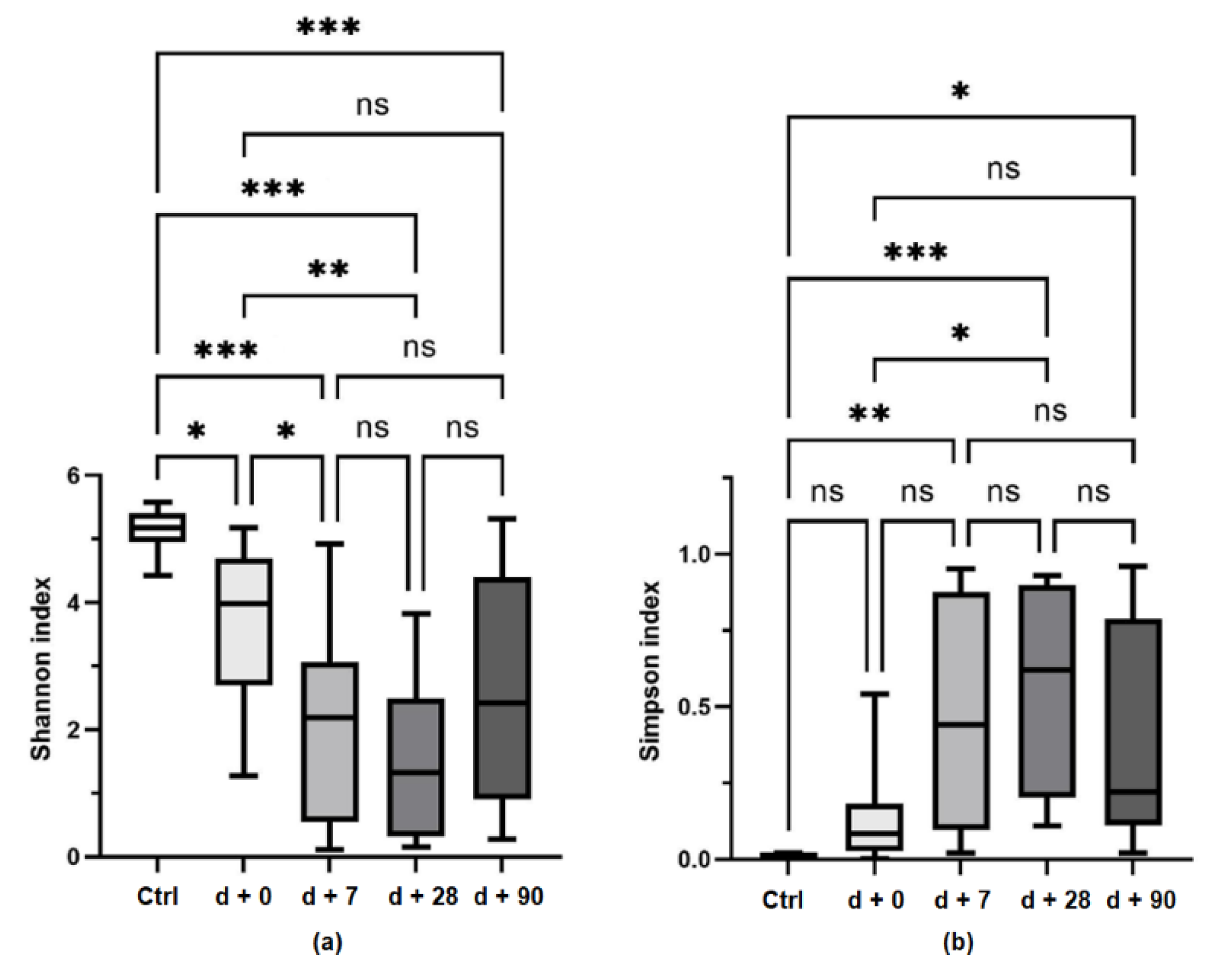
3.2. Bacterial Diversity
3.3. Clinical Signs and the Microbiome
3.4. Exercise Training
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khandelwal, P.; Millard, H.R.; Thiel, E.; Abdel-Azim, H.; Abraham, A.A.; Auletta, J.J.; Boulad, F.; Brown, V.I.; Camitta, B.M.; Chan, K.W.; et al. Hematopoietic Stem Cell Transplantation Activity in Pediatric Cancer between 2008 and 2014 in the United States: A Center for International Blood and Marrow Transplant Research Report. Biol. Blood Marrow Transplant. 2017, 23, 1342–1349. [Google Scholar] [CrossRef]
- Vitale, M.C.; Modaffari, C.; Decembrino, N.; Zhou, F.X.; Zecca, M.; Defabianis, P. Preliminary study in a new protocol for the treatment of oral mucositis in pediatric patients undergoing hematopoietic stem cell transplantation (HSCT) and chemotherapy (CT). Lasers Med. Sci. 2017, 32, 1423–1428. [Google Scholar] [CrossRef]
- Schots, R.; Kaufman, L.; Van Riet, I.; Ben Othman, T.; De Waele, M.; Van Camp, B.; Demanet, C. Proinflammatory cytokines and their role in the development of major transplant-related complications in the early phase after allogeneic bone marrow transplantation. Leukemia 2003, 17, 1150–1156. [Google Scholar] [CrossRef]
- Gilliam, L.A.A.; St Clair, D.K. Chemotherapy-induced weakness and fatigue in skeletal muscle: The role of oxidative stress. Antioxid. Redox Signal. 2011, 15, 2543–2563. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, A.; Banihashem, A.; Ghaemi, N.; Elmi, S.; Erfani Sayyar, R.; Elmi, S.; Esmaeili, H. Evaluation of Bone Mineral Density in Children with Acute Lymphoblastic Leukemia (ALL) and Non-Hodgkin’s Lymphoma (NHL): Chemotherapy with/without Radiotherapy. Int. J. Hematol. Stem Cell Res. 2016, 10, 153–160. [Google Scholar]
- Eissa, H.M.; Lu, L.; Baassiri, M.; Bhakta, N.; Ehrhardt, M.J.; Triplett, B.M.; Green, D.M.; Mulrooney, D.A.; Robison, L.L.; Hudson, M.M.; et al. Chronic disease burden and frailty in survivors of childhood HSCT: A report from the St. Jude Lifetime Cohort Study. Blood Adv. 2017, 1, 2243–2246. [Google Scholar] [CrossRef]
- Fridh, M.K.; Simonsen, C.; Schmidt-Andersen, P.; Nissen, A.A.; Christensen, J.F.; Larsen, A.; Mackey, A.L.; Larsen, H.B.; Müller, K. Cardiorespiratory fitness and physical performance after childhood hematopoietic stem cell transplantation: A systematic review and meta-analysis. Bone Marrow Transplant. 2021, 56, 2063–2078. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, R.; Skeens, M.; Garee, A.; Miao, Y.; Soni, S.; Pietryga, D.; Gross, T.; Termuhlen, A. Metabolic syndrome and endocrine dysfunctions after HSCT in children. Pediatr. Transplant. 2012, 16, 872–878. [Google Scholar] [CrossRef]
- Taur, Y.; Coyte, K.; Schluter, J.; Robilotti, E.; Figueroa, C.; Gjonbalaj, M.; Littmann, E.R.; Ling, L.; Miller, L.; Gyaltshen, Y.; et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci. Transl. Med. 2018, 10, eaap9489. [Google Scholar] [CrossRef]
- Lähteenmäki, K.; Wacklin, P.; Taskinen, M.; Tuovinen, E.; Lohi, O.; Partanen, J.; Mättö, J.; Vettenranta, K. Haematopoietic stem cell transplantation induces severe dysbiosis in intestinal microbiota of paediatric ALL patients. Bone Marrow Transplant. 2017, 52, 1479–1482. [Google Scholar] [CrossRef][Green Version]
- Simms-Waldrip, T.R.; Sunkersett, G.; Coughlin, L.A.; Savani, M.R.; Arana, C.; Kim, J.; Kim, M.; Zhan, X.; Greenberg, D.E.; Xie, Y.; et al. Antibiotic-Induced Depletion of Anti-inflammatory Clostridia Is Associated with the Development of Graft-versus-Host Disease in Pediatric Stem Cell Transplantation Patients. Biol. Blood Marrow Transplant. J. Am. Soc. 2017, 23, 820–829. [Google Scholar] [CrossRef]
- Ingham, A.C.; Kielsen, K.; Cilieborg, M.S.; Lund, O.; Holmes, S.; Aarestrup, F.M.; Müller, K.G.; Pamp, S.J. Specific gut microbiome members are associated with distinct immune markers in pediatric allogeneic hematopoietic stem cell transplantation. Microbiome 2019, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Masetti, R.; Muratore, E.; Leardini, D.; Zama, D.; Turroni, S.; Brigidi, P.; Esposito, S.; Pession, A. Gut microbiome in pediatric acute leukemia: From predisposition to cure. Blood Adv. 2021, 5, 4619–4629. [Google Scholar] [CrossRef] [PubMed]
- Masetti, R.; Zama, D.; Leardini, D.; Muratore, E.; Turroni, S.; Prete, A.; Brigidi, P.; Pession, A. The gut microbiome in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation. Pediatr. Blood Cancer 2020, 67, e28711. [Google Scholar] [CrossRef] [PubMed]
- Baghai Arassi, M.; Zeller, G.; Karcher, N.; Zimmermann, M.; Toenshoff, B. The gut microbiome in solid organ transplantation. Pediatr. Transplant. 2020, 24, e13866. [Google Scholar] [CrossRef] [PubMed]
- San Juan, A.F.; Chamorro-Viña, C.; Moral, S.; Fernández del Valle, M.; Madero, L.; Ramírez, M.; Pérez, M.; Lucia, A. Benefits of intrahospital exercise training after pediatric bone marrow transplantation. Int. J. Sports Med. 2008, 29, 439–446. [Google Scholar] [CrossRef]
- Buffart, L.M.; Sweegers, M.G.; May, A.M.; Chinapaw, M.J.; van Vulpen, J.K.; Newton, R.U.; Galvão, D.A.; Aaronson, N.K.; Stuiver, M.M.; Jacobsen, P.B.; et al. Targeting Exercise Interventions to Patients With Cancer in Need: An Individual Patient Data Meta-Analysis. J. Natl. Cancer Inst. 2018, 110, 1190–1200. [Google Scholar] [CrossRef]
- Moron, R.; Galvez, J.; Colmenero, M.; Anderson, P.; Cabeza, J.; Rodriguez-Cabezas, M.E. The Importance of the Microbiome in Critically Ill Patients: Role of Nutrition. Nutrients 2019, 11, 3002. [Google Scholar] [CrossRef]
- Hric, I.; Ugrayová, S.; Penesová, A.; Rádiková, Ž.; Kubáňová, L.; Šardzíková, S.; Baranovičová, E.; Klučár, Ľ.; Beke, G.; Grendar, M.; et al. The Efficacy of Short-Term Weight Loss Programs and Consumption of Natural Probiotic Bryndza Cheese on Gut Microbiota Composition in Women. Nutrients 2021, 13, 1753. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Rajagopala, S.V.; Yooseph, S.; Harkins, D.M.; Moncera, K.J.; Zabokrtsky, K.B.; Torralba, M.G.; Tovchigrechko, A.; Highlander, S.K.; Pieper, R.; Sender, L.; et al. Gastrointestinal microbial populations can distinguish pediatric and adolescent Acute Lymphoblastic Leukemia (ALL) at the time of disease diagnosis. BMC Genom. 2016, 17, 635. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, M.; Rüchel, N.; Janssen, S.; Borkhardt, A.; Gössling, K.L. The Microbiome in Childhood Acute Lymphoblastic Leukemia. Cancers 2021, 13, 4947. [Google Scholar] [CrossRef]
- Wei, L.; Wen, X.-S.; Xian, C.J. Chemotherapy-Induced Intestinal Microbiota Dysbiosis Impairs Mucosal Homeostasis by Modulating Toll-like Receptor Signaling Pathways. Int. J. Mol. Sci. 2021, 22, 9474. [Google Scholar] [CrossRef] [PubMed]
- De Pietri, S.; Ingham, A.C.; Frandsen, T.L.; Rathe, M.; Krych, L.; Castro-Mejía, J.L.; Nielsen, D.S.; Nersting, J.; Wehner, P.S.; Schmiegelow, K.; et al. Gastrointestinal toxicity during induction treatment for childhood acute lymphoblastic leukemia: The impact of the gut microbiota. Int. J. Cancer 2020, 147, 1953–1962. [Google Scholar] [CrossRef]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef]
- Holler, E.; Butzhammer, P.; Schmid, K.; Hundsrucker, C.; Koestler, J.; Peter, K.; Zhu, W.; Sporrer, D.; Hehlgans, T.; Kreutz, M.; et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: Loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol. Blood Marrow Transplant. 2014, 20, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Andermann, T.M.; Peled, J.U.; Ho, C.; Reddy, P.; Riches, M.; Storb, R.; Teshima, T.; van den Brink, M.R.M.; Alousi, A.; Balderman, S.; et al. The Microbiome and Hematopoietic Cell Transplantation: Past, Present, and Future. Biol. Blood Marrow Transplant. J. Am. Soc. 2018, 24, 1322–1340. [Google Scholar] [CrossRef]
- Thomas, R.; Wong, W.S.W.; Saadon, R.; Vilboux, T.; Deeken, J.; Niederhuber, J.; Hourigan, S.K.; Yang, E. Gut microbial composition difference between pediatric ALL survivors and siblings. Pediatr. Hematol. Oncol. 2020, 37, 475–488. [Google Scholar] [CrossRef]
- Rajagopala, S.V.; Singh, H.; Yu, Y.; Zabokrtsky, K.B.; Torralba, M.G.; Moncera, K.J.; Frank, B.; Pieper, R.; Sender, L.; Nelson, K.E. Persistent Gut Microbial Dysbiosis in Children with Acute Lymphoblastic Leukemia (ALL) during Chemotherapy. Microb. Ecol. 2020, 79, 1034–1043. [Google Scholar] [CrossRef]
- Taur, Y.; Xavier, J.B.; Lipuma, L.; Ubeda, C.; Goldberg, J.; Gobourne, A.; Lee, Y.J.; Dubin, K.A.; Socci, N.D.; Viale, A.; et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis. 2012, 55, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Papanicolaou, G.A.; Ustun, C.; Young, J.-A.H.; Chen, M.; Kim, S.; Woo Ahn, K.; Komanduri, K.; Lindemans, C.; Auletta, J.J.; Riches, M.L. Bloodstream Infection Due to Vancomycin-resistant Enterococcus Is Associated With Increased Mortality after Hematopoietic Cell Transplantation for Acute Leukemia and Myelodysplastic Syndrome: A Multicenter, Retrospective Cohort Study. Clin. Infect. Dis. 2019, 69, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.; Staudacher, H.; Weber, N.; Kennedy, G.; Varelias, A.; Banks, M.; Bauer, J. Pilot study investigating the effect of enteral and parenteral nutrition on the gastrointestinal microbiome post-allogeneic transplantation. Br. J. Haematol. 2020, 188, 570–581. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Biagi, E.; Rampelli, S.; Fiori, J.; Zama, D.; Soverini, M.; Barone, M.; Leardini, D.; Muratore, E.; Prete, A.; et al. Enteral Nutrition in Pediatric Patients Undergoing Hematopoietic SCT Promotes the Recovery of Gut Microbiome Homeostasis. Nutrients 2019, 11, 2958. [Google Scholar] [CrossRef]
- Zama, D.; Muratore, E.; Biagi, E.; Forchielli, M.L.; Rondelli, R.; Candela, M.; Prete, A.; Pession, A.; Masetti, R. Enteral nutrition protects children undergoing allogeneic hematopoietic stem cell transplantation from blood stream infections. Nutr. J. 2020, 19, 29. [Google Scholar] [CrossRef]
- Zama, D.; Gori, D.; Muratore, E.; Leardini, D.; Rallo, F.; Turroni, S.; Prete, A.; Brigidi, P.; Pession, A.; Masetti, R. Enteral versus Parenteral Nutrition as Nutritional Support after Allogeneic Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Transplant. Cell. Ther. 2021, 27, 180.e1–180.e8. [Google Scholar] [CrossRef]
- Azarnoush, S.; Bruno, B.; Beghin, L.; Guimber, D.; Nelken, B.; Yakoub-Agha, I.; Seguy, D. Enteral nutrition: A first option for nutritional support of children following allo-SCT? Bone Marrow Transplant. 2012, 47, 1191–1195. [Google Scholar] [CrossRef]
- Sakr, Y.; Sponholz, C.; Tuche, F.; Brunkhorst, F.; Reinhart, K. The role of procalcitonin in febrile neutropenic patients: Review of the literature. Infection 2008, 36, 396–407. [Google Scholar] [CrossRef]
- Andersen, S.; Banks, M.; Brown, T.; Weber, N.; Kennedy, G.; Bauer, J. Nutrition support during allogeneic stem cell transplantation: Evidence versus practice. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2020, 28, 5441–5447. [Google Scholar] [CrossRef]
- Zucchetti, G.; Rossi, F.; Chamorro Vina, C.; Bertorello, N.; Fagioli, F. Exercise program for children and adolescents with leukemia and lymphoma during treatment: A comprehensive review. Pediatr. Blood Cancer 2018, 65, e26924. [Google Scholar] [CrossRef]
- Coombs, A.; Schilperoort, H.; Sargent, B. The effect of exercise and motor interventions on physical activity and motor outcomes during and after medical intervention for children and adolescents with acute lymphoblastic leukemia: A systematic review. Crit. Rev. Oncol. Hematol. 2020, 152, 103004. [Google Scholar] [CrossRef] [PubMed]
- Rosenhagen, A.; Bernhörster, M.; Vogt, L.; Weiss, B.; Senn, A.; Arndt, S.; Siegler, K.; Jung, M.; Bader, P.; Banzer, W. Implementation of structured physical activity in the pediatric stem cell transplantation. Klin. Padiatr. 2011, 223, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Akyay, A.; Olcay, L.; Sezer, N.; Atay Sönmez, Ç. Muscle strength, motor performance, cardiac and muscle biomarkers in detection of muscle side effects during and after acute lymphoblastic leukemia treatment in children. J. Pediatr. Hematol. Oncol. 2014, 36, 594–598. [Google Scholar] [CrossRef]
- Götte, M.; Taraks, S.; Boos, J. Sports in pediatric oncology: The role(s) of physical activity for children with cancer. J. Pediatr. Hematol. Oncol. 2014, 36, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Braam, K.I.; van Dijk-Lokkart, E.M.; Kaspers, G.J.L.; Takken, T.; Huisman, J.; Buffart, L.M.; Bierings, M.B.; Merks, J.H.M.; van den Heuvel-Eibrink, M.M.; Veening, M.A.; et al. Effects of a combined physical and psychosocial training for children with cancer: A randomized controlled trial. BMC Cancer 2018, 18, 1289. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.S.; Valenzuela, P.L.; Herrera-Olivares, A.M.; Rincón-Castanedo, C.; Martín-Ruiz, A.; Castillo-García, A.; Fiuza-Luces, C.; Lucia, A. What are the effects of exercise training in childhood cancer survivors? A systematic review. Cancer Metastasis Rev. 2020, 39, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Esbenshade, A.J.; Ness, K.K. Dietary and Exercise Interventions for Pediatric Oncology Patients: The Way Forward. J. Natl. Cancer Inst. Monogr. 2019, 2019, 157–162. [Google Scholar] [CrossRef]
- Chamorro-Viña, C.; Ruiz, J.R.; Santana-Sosa, E.; González Vicent, M.; Madero, L.; Pérez, M.; Fleck, S.J.; Pérez, A.; Ramírez, M.; Lucía, A. Exercise during hematopoietic stem cell transplant hospitalization in children. Med. Sci. Sports Exerc. 2010, 42, 1045–1053. [Google Scholar] [CrossRef]
- Yildiz Kabak, V.; Cetinkaya, D.U.; Kuskonmaz, B.; Cetin, N.; Duger, T. Effects of multimodal exercise on clinical status and patient-reported outcomes in children undergoing hematopoietic stem cell transplantation. Pediatr. Hematol. Oncol. 2019, 36, 410–421. [Google Scholar] [CrossRef]
- Morales, J.S.; González Vicent, M.; Valenzuela, P.L.; Castillo-García, A.; Santana-Sosa, E.; Lassaletta, A.; Santos-Lozano, A.; Fiuza-Luces, C.; Lucia, A. Tailored Exercise during Hematopoietic Stem Cell Transplantation Hospitalization in Children with Cancer: A Prospective Cohort Study. Cancers 2020, 12, 3020. [Google Scholar] [CrossRef]
| Conditioning | |||
|---|---|---|---|
| TBI (n = 6) | FB (n = 10) | p | |
| Demographics and clinical characteristics at baseline | |||
| Female (n) | 3 | 3 | |
| Primary diagnosis | |||
| Acute leukemia (n) | 6 | 10 | |
| Donor type | |||
| Volunteer unrelated (n) | 4 | 8 | |
| Sibling (n) | 2 | 2 | |
| Clinical outcomes | |||
| Parenteral nutrition (days) | 32 (25–48) | 60 (45–90) | 0.05 |
| Per. os. (days) | 59 (30–66) | 31 (0–47) | 0.03 |
| Length of hospital stay from day + 0 | 47 (29–96) | 92 (62–122) | 0.01 |
| <30 days | 0 | 0 | |
| ≥30 days | 6 | 10 | |
| Developed GvHD | |||
| aGvHD (n) | 2 | 7 | |
| cGvHD (n) | 0 | 0 | |
| Occurrence of GvHD (day) | 32 (23–42) | 31 (13–55) | ns |
| Duration of GvHD (days) | 22 (18–26) | 42 (29–78) | ns |
| Survived to day + 90 | 5 | 10 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugrayová, S.; Švec, P.; Hric, I.; Šardzíková, S.; Kubáňová, L.; Penesová, A.; Adamčáková, J.; Pačesová, P.; Horáková, J.; Kolenová, A.; et al. Gut Microbiome Suffers from Hematopoietic Stem Cell Transplantation in Childhood and Its Characteristics Are Positively Associated with Intra-Hospital Physical Exercise. Biology 2022, 11, 785. https://doi.org/10.3390/biology11050785
Ugrayová S, Švec P, Hric I, Šardzíková S, Kubáňová L, Penesová A, Adamčáková J, Pačesová P, Horáková J, Kolenová A, et al. Gut Microbiome Suffers from Hematopoietic Stem Cell Transplantation in Childhood and Its Characteristics Are Positively Associated with Intra-Hospital Physical Exercise. Biology. 2022; 11(5):785. https://doi.org/10.3390/biology11050785
Chicago/Turabian StyleUgrayová, Simona, Peter Švec, Ivan Hric, Sára Šardzíková, Libuša Kubáňová, Adela Penesová, Jaroslava Adamčáková, Petra Pačesová, Júlia Horáková, Alexandra Kolenová, and et al. 2022. "Gut Microbiome Suffers from Hematopoietic Stem Cell Transplantation in Childhood and Its Characteristics Are Positively Associated with Intra-Hospital Physical Exercise" Biology 11, no. 5: 785. https://doi.org/10.3390/biology11050785
APA StyleUgrayová, S., Švec, P., Hric, I., Šardzíková, S., Kubáňová, L., Penesová, A., Adamčáková, J., Pačesová, P., Horáková, J., Kolenová, A., Šoltys, K., Kolisek, M., & Bielik, V. (2022). Gut Microbiome Suffers from Hematopoietic Stem Cell Transplantation in Childhood and Its Characteristics Are Positively Associated with Intra-Hospital Physical Exercise. Biology, 11(5), 785. https://doi.org/10.3390/biology11050785






