Simple Summary
Whitish lethal species of Amanita sect. Phalloideae (‘destroying angels’) are known to be among the most poisonous fungi worldwide due to their production of amatoxins. The taxonomy of species occurring in the Mediterranean region is here revised, clarifying the identity of several names. Amanita decipiens, A. porrinensis, and A. virosa var. levipes are here considered later heterotypic synonyms of A. verna, A. phalloides, and A. amerivirosa, respectively, while a new name, A. vidua, is proposed for a spring-occurring taxon. The amatoxins and phallotoxins present in Mediterranean destroying angels were characterized, and their epidemiology discussed on the basis of the case study of available data from Spain.
Abstract
In Europe, amatoxin-containing mushrooms are responsible for most of the deadly poisonings caused by macrofungi. The present work presents a multidisciplinary revision of the European species of Amanita sect. Phalloideae based on morphology, phylogeny, epidemiology, and biochemistry of amatoxins and phallotoxins. Five distinct species of this section have been identified in Europe to date: A. phalloides, A. virosa, A. verna, the recently introduced North American species A. amerivirosa, and A. vidua sp. nov., which is a new name proposed for the KOH-negative Mediterranean species previously described as A. verna or A. decipiens by various authors. Epitypes or neotypes are selected for species lacking suitable reference collections, namely A. verna and A. virosa. Three additional taxa, Amanita decipiens, A. porrinensis, and A. virosa var. levipes are here considered later heterotypic synonyms of A. verna, A. phalloides, and A. amerivirosa, respectively.
1. Introduction
Lethal species of the genus Amanita Pers. (Amanitaceae, Agaricales, Agaricomycetes, Basidiomycota, Fungi), including the white-coloured taxa in sect. Phalloideae, which are commonly called ‘destroying angels’, are among the most well-documented causes of intoxication by fungi, with reports dating back to ancient history. Despite the information campaigns periodically launched by public health services and mycological societies, intoxications caused by these species are still reported every year, resulting in the most severe cases of mushroom poisoning in Europe and North America [1,2,3]. The first lethal species to be formally named were A. phalloides (Vaill. ex Fr.) Link (basionym Agaricus phalloides Vaill. ex Fr.) and A. verna Bull. ex. Lam. (protonym Ag. bulbosus vernus Bull.), both found near Paris, France [4,5], and A. virosa Bertill. (basionym Ag. virosus Fr.) from Sweden [6]. Amatoxin-producing species, in most cases identified as Amanita phalloides (listed here among the ‘destroying angels’ because of its whitish forms: see below), are the most frequent cause of lethal intoxication by fungi in the USA [1], China [7], Bulgaria [8], Catalonia [9], Czech Republic [10], France [2,11], Northern Italy [12,13], Germany [14], South Korea [15], Switzerland [16], and Turkey [17].
However, poisonings with amatoxins in Europe are also caused by other taxa, such as A. verna, A. virosa, as well as multiple species of Lepiota [18], and the lineages around Galerina marginata (Batsch) Kühner [19,20,21,22,23]. Many lethal intoxication cases have been reported from Eastern and Central Asia, attributed either to A. verna [24,25] or A. virosa [26], and intoxications in spring seem to be even more frequent than those occurring in autumn in Iran [27]. In Lebanon, several spring intoxications were reported among Syrian migrants after the consumption of white phalloid-like Amanita (Bou Dagher-Kharrat, unpublished data). This was also the case in Turkey and Poland among Syrian and Afghan refugees, respectively [28,29]. A critical approach to treat these cases is to disentangle recurrent taxonomic controversies regarding the identity of A. verna and A. virosa, as well as to characterize the toxicological profiles of related taxa. For example, as noticed by [25], the amount of amatoxins in A. verna is surprisingly variable in the literature, leading Preston et al. [30] to hypothesize the existence of several species or varieties.
Recent phylogenetic accounts on sect. Phalloideae [31,32,33,34,35,36,37] have focused primarily on American, Asian, and African species, with very few European data included. According to Neville & Poumarat [38], the only species of sect. Phalloideae occurring in Europe were A. phalloides, A. verna, A. decipiens (Trimbach) Jacquet. (basionym A. verna var. decipiens Trimbach), A. dunensis Bon & Andary, and A. porrinensis Freire & M.L. Castro. In addition, a number of infraspecific taxa of these species were described: Amanita phalloides var. alba Costantin & L.M. Dufour, A. phalloides f. citrina J.E. Lange, A. phalloides var. euphalloides Maire, A. phalloides var. larroquei F. Massart & Beauvais ex F. Massart, A. phalloides var. moravecii Pilát, A. phalloides var. pulla Killerm., A. phalloides var. striatula Peck, A. phalloides f. umbrina Ferry, A. verna f. ellipticospora E.-J. Gilbert, A. verna var. grisea Massee, A. verna var. tarda Trimbach, A. virosa var. aculeata Voglino, A. virosa var. levipes Neville & Poumarat. The taxonomic status of most of these taxa has not yet been confirmed with genetic tools.
The aim of the present work is to address existing doubts about the taxonomic status of the most relevant species and varieties of Amanita sect. Phalloideae occurring in Europe and western Asia, by providing an accurate genetic profile of the type material existing in official herbaria or by designating new types selected among modern collections. The content in amatoxins and phallotoxins of all species analyzed was also chemically characterized, both qualitatively and quantitatively. Finally, an epidemiological analysis of data obtained from the Spanish health system was carried out to assess the risk of phalloidian poisonings in spring and fall in the different regions of this Mediterranean country.
2. Materials and Methods
2.1. Herbarium Collections and Morphological Studies
Basidiomata of white species of Amanita sect. Phalloideae were collected in France, Italy, Lebanon, Spain, and Sweden by various collectors. Samples to be studied were selected amongst available collections based on their conservation state, collection date, and additional documentation available (collection data, images). Samples were deposited in the herbaria of the Universidad de Alcalá (AH), Conservatoire et Jardin botaniques de la Ville de Genève (G), Université de Lille (LIP), Centro de Investigaciones Forestales de Lourizan (LOU), Real Jardín Botánico de Madrid (MA), Muséum National d’Histoire Naturelle de Paris (PC), Muséum d’Histoire Naturelle de Nice (NICE). Herbarium codes follow Thiers (continuously updated).
Microscopical observations of rehydrated herbarium specimens were conducted at the University of Lille by P.-A. Moreau and the University of Alcalá by G. Moreno, on hand-made longitudinal sections of lamellae, as well as cross-sections and tangential sections of pileipellis, universal veil, and partial veil in a 5% potassium hydroxide aqueous solution (5% KOH). Basidiospores naturally deposited on the surface of the partial veil or the apex of the stipe were observed in 5% KOH and Melzer’s reagent. Microscopic pictures were taken with a Nachet Andromède microscope equipped with a Motic 3.0 digital camera; spore dimensions were estimated with the software Piximètre 5.10 (A. Henriot, http://ach.log.free.fr/Piximetre accessed on 1 March 2022, continuously updated). Spore measurements include extreme measured values (in brackets) and 1st–9th deciles; Q represents the spore ratio (length/width), cited by 1st–9th deciles, and its mean value Qav. The shape of spores is interpreted following Bas [39]: subglobose means Q = [1.05–1.15], broadly ellipsoid [1.15–1.3], ellipsoid [1.3–1.6], and cylindrical [2.0–3.0].
2.2. Phylogenetic Studies
Total DNA was extracted from herbarium specimens using a standard method based on cetyltrimethylammonium bromide (CTAB) [40], or with the REDExtract-N-Amptm Plant PCR Kit (Sigma-Aldrich, St. Louis, MO, USA), following the manufacturer’s instructions. Polymerase chain reactions (PCRs) [41] included 35 cycles with an annealing temperature of 54 °C. Primers ITS1F and ITS4 [42,43] were employed to amplify the internal transcribed spacer nuc-rDNA region (ITS), while LR0R, LR1, and LR7 [44,45,46] were used for the 28S nuc-rDNA large ribosomal subunit (LSU). PCR products were checked in 1% agarose gels, and positive reactions were sequenced with one or both PCR primers (depending on the quality of the resulting sequences). Chromatograms were edited by hand to correct reads at heteromorphic sites and other putative errors using MEGA5 [47] or Codon Code Aligner 4.1.1 (CodonCode Corp., Centerville, MA, USA).
The ITS and LSU sequences obtained were aligned with the closest matches from BLAST searches in the public databases. The sequences retrieved (Supplementary Table S1) came mainly from [48,49,50,51,52], among others. The resulting dataset was aligned in MEGA5 software using its ClustalW application followed by manual correction. The concatenated loci (677 nt ITS, 554 nt LSU) were subjected to Gblocks [53], which removed 221 ambiguously aligned positions (165 nt ITS, 56 nt LSU), to obtain a final alignment with 512 nt from ITS (301 nt variable), and 498 nt from LSU (139 nt variable). Each region was loaded in PAUP* 4.0b10 [54] and subjected to MrModeltest 2.3 [55] to infer the best-fitting evolutionary model. MrBayes 3.2.6 [56] was employed to conduct a Bayesian inference (BI) analysis using model GTR + G+I (nst = 6, rates = invgamma, statefreqpr = dirichlet) in each partition (ITS and LSU), two simultaneous runs, four chains, temperature set to 0.2, and sampling every 100th generation. Finally, a full search for the best-scoring maximum likelihood (ML) tree was performed in RAxML 8.2.10 [57] using the standard search algorithm with the GTRCAT approximation as recommended by the manual for data sets >50 taxa, data partitioned as for Bayesian analysis, and 2000 bootstrap replications. All analyses were run locally. The significance threshold was set above 0.95 posterior probability (PP) for BI and above 70% bootstrap proportion (BP) for ML.
2.3. Toxicological Studies
High performance liquid chromatography (HPLC) was employed to quantify the amatoxins present in selected samples from the species inferred by phylogenetic analyses (Supplementary Table S2). All extractions were repeated three times. Since the pileus of poisonous species of Amanita is known to contain large amounts of toxins [58,59], a portion of the cap (50 mg of dry material) was taken from each sample and finely ground. One ml of a mixture of MeOH/H2O/HCl (5:4:1, v/v/v) was added as previously described [25,59,60,61,62], sonicated for 15 min, and then filtered and centrifuged. Three different fruitbodies of PAM19050401 (harvested in the same location and time, named a, b, and c) were analyzed, each one being extracted three times as the other samples.
Chromatographic separation and detection for quantitative analyses were performed on an Ultimate 3000 instrument that included a quaternary pump, a degasser, an automatic sampler, and a DAD detector (Thermo Fisher Scientific Inc., San Jose, CA, USA). The system was operated using the Chromeleon software, version 2.0. Chromatographic separation was achieved on an ODS Hypersyl C18 column (250 × 4.6 mm, 5 μm, Thermo Fisher Scientific Inc., San Jose, CA, USA), with a column temperature maintained at 35 °C. Analyses were performed at a flow rate of 1 mL/min, using solvent A (0.02 M ammonium acetate/acetonitrile 90:10 v/v) and solvent B (0.02 M ammonium acetate/acetonitrile 76:24 v/v) as previously described [60,61,63]. HPLC-grade acetonitrile (Carlo Erba Reagents, Val de Reuil, France) and water (PanReac Quimica SLU, Castellar del Valles, Spain) were employed. The gradient profile was set up as follows: 100% A for 4 min, then 57% B for 16 min, then 100% B for 10 min, and finally 100% A.
Standards of α-amanitin, β-amanitin, phallacidin, and phalloidin were purchased from Sigma-Aldrich (Saint-Louis, MO, USA) and stocked at −20 °C. The toxins were freshly prepared and mixed together to obtain calibration solutions at 1.95, 3.91, 7.81, 15.63, 31.25, 62.5, 125, 250, and 500 µg/mL in MeOH/H2O (50:50). Individual calibration curves were produced for each toxin and shown to be linear over the range of interest (R2 > 0.9995, n = 3, Supplementary Figure S1). The UV/Vis spectra were recorded in the 200–400 nm range and chromatograms were acquired at 254 nm (unspecific wavelength), 295 nm (λmax for phallacidin and phalloidin), and 305 nm (λmax for α-amanitin and β-amanitin). Extracts obtained from the different species of Amanita studied in the present work were analyzed directly without any prior dilution. For each peak, a UV spectrum was obtained and compared with those of commercial standards to confirm their identification. Contents of amatoxins and phallotoxins were expressed in mg of toxin/g of dried cap.
2.4. Epidemiological Studies
The epidemiological analysis focused on phalloidian intoxications in Spain, targeting two main indicators: (1) cumulative incidence of poisoning according to time of year and (2) geographical and seasonal distribution of poisoning incidence. Primary data were gathered from the following sources:
- Ministry of Science and Innovation–National Center of Epidemiology (CNE)–Sistema de Vigilancia de la Red Nacional de Vigilancia Epidemiológica (RENAVE), SiViEs platform: for the period 1980–2020, outbreaks in which the agent was a fungal toxin and those associated with another agent in which mushrooms were consumed. In this database, only clusters (n ≥ 2 patients) are registered. To target putative phalloidian cases, the criterion “Incubation time” was selected for values > 6 h (when recorded) coupled with “hospitalization”, assuming that phalloidian poisonings required hospitalizations. Cases involving non-phalloidian fungal species were discarded.
- Ministry of Health, Consumer Affairs and Social Welfare-Specialized Health Care Activity Register (CMBD and RAE-CMB data). These databases record individual hospital exits and summarize care pathways (diagnostics, hospitalization times, etc.). For the period 1997–2015 (MBDS-H; ICD 9), clinical criteria 988.1 (toxic effect of mushrooms and mushrooms) and 573.1 (hepatitis unspecified) were selected. For the period 2016–2020 (RAE-CMD; IC10), clinical criteria T62.0X1A (toxic effect of ingested mushrooms, unintentional/accidental) and K71.2 (toxic hepatopathy with acute hepatitis) were retained.
3. Results
3.1. Phylogeny
The Bayesian inference of phylogeny based on ITS and LSU data (Figure 1) is consistent with previously published organization of Amanita sect. Phalloideae [31,33,34,35,36,37,64], although the limited signal provided by the ribosomal regions alone does not resolve most supraspecific nodes supported by the analyses, including protein-coding genes. The Mediterranean ‘destroying angels’ include at least seven different species. Five of them cluster inside sect. Phalloideae: (1) Amanita phalloides (= A. phalloides var. alba, = A. porrinensis), (2) A. verna KOH+, (3) Amanita verna KOH-, (4) A. virosa, and (5) A. amerivirosa Tulloss, L.V. Kudzma & M. Tulloss (= A. virosa var. levipes). Another species (6) is nested within sect. Strobiliformes (Amanita strobiliformis), and the last one (7) belongs in sect. Validae (A. phalloides var. larroquei = A. citrina). Amanita phalloides is significantly related to A. subjunquillea S. Imai, which includes a white variety, A. subjunquillea var. alba Zhu L. Yang. While both species did not receive reciprocal significant support in the present analysis, they are considered independent taxa until a more complete analysis is done to test their actual status. Amanita virosa and A. amerivirosa/A. virosa var. levipes are significantly related to A. subpallidorosea Hai J. Li, but their phylogenetic relation with A. ocreata Peck, found in the multigenic analysis performed by Codjia et al. [34] could not be recovered with the present analysis. All Mediterranean species in sect. Phalloideae are nested within a ‘Northern Hemisphere lineage’, significantly distinct from the remaining taxa, which in previous works [34,35,36,37], constitute three different monophyletic lineages. ITS rDNA sequences of A. strobiliformis are 92% similar to those of A. sabulicola S. Morini et al., which is by now the closest relative [65]. In turn, the ITS sequence of A. phalloides var. larroquei (specimen provided by its author F. Massart) is almost 98% similar to sequences of A. citrina Pers. (i.e., MH508311), and therefore probably belongs to this species. The taxonomy of these species is updated below according to these results.
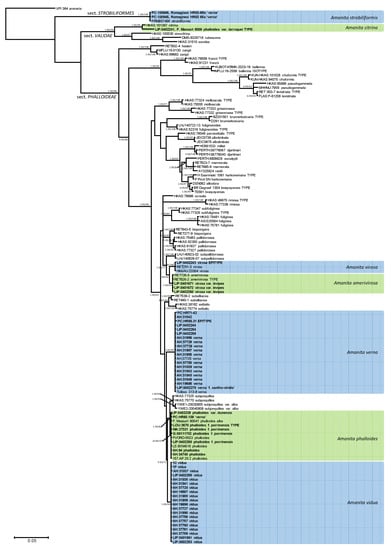
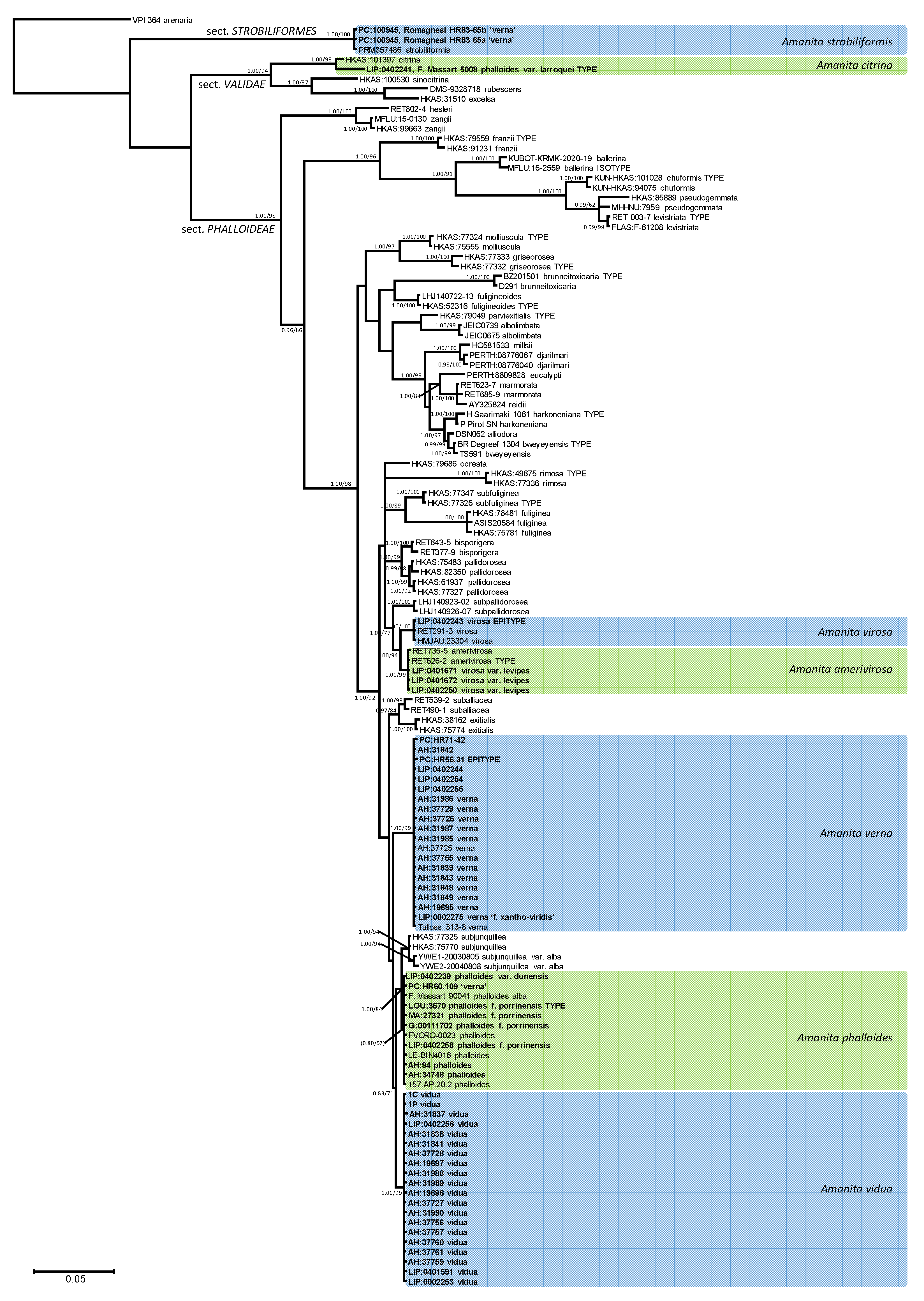
Figure 1.
A 50% majority rule ITS rDNA-28S rDNA consensus phylogram of Amanita sect. Phalloideae and selected lineages of the neighboring sections Strobiliformes and Validae (with Amanita arenaria from sect. Arenaria [66] as outgroup) obtained using MrBayes from 7050 sampled trees. Nodes were annotated if they were supported by ≥0.95 Bayesian posterior probability (left) or ≥70% maximum likelihood bootstrap proportions (right). Non-significant support values are exceptionally represented in parentheses. Sequences newly generated in this study are in bold face.
3.2. Taxonomy
- 1.
- Amanita phalloides (Vaill. ex Fr.) Link, Handb. Erk. Gew. 3: 272 (1833) Figure 2E, Figure 3E and Figure 4E
 Figure 2. Macroscopical features of ‘destroying angels’ (A,B). Amanita amerivirosa. (LIP:0402252), photo P.-A. Moreau. (C) Amanita amerivirosa, reaction to 10% KOH (LIP:0402250), photo N. Journe. (D) Amanita virosa, neotype (LIP:0402243), photo P.-A. Moreau. (E) Amanita phalloides (LIP:0401670). (F) Mixed basidiomes of Amanita verna (LIP:0402254) and A. vidua (LIP:0402256), photo M. Gelardi. (G) Amanita phalloides f. porrinensis (LIP:0402258), photo A. Mua. (H) Amanita verna (AH:31849), photo G. Moreno. (I) Amanita vidua (AH:19696), photo G. Moreno. (J) Amanita verna (LIP:0402254, top) and A. vidua (LIP:0402256, bottom) reaction to KOH, photo M. Gelardi. (K,L) A. verna ‘f. xanthoviridis’ ad. int. (LIP:0002275), photo M. Raumi. (M) Amanita vidua from Lebanon (1C and 1P).
Figure 2. Macroscopical features of ‘destroying angels’ (A,B). Amanita amerivirosa. (LIP:0402252), photo P.-A. Moreau. (C) Amanita amerivirosa, reaction to 10% KOH (LIP:0402250), photo N. Journe. (D) Amanita virosa, neotype (LIP:0402243), photo P.-A. Moreau. (E) Amanita phalloides (LIP:0401670). (F) Mixed basidiomes of Amanita verna (LIP:0402254) and A. vidua (LIP:0402256), photo M. Gelardi. (G) Amanita phalloides f. porrinensis (LIP:0402258), photo A. Mua. (H) Amanita verna (AH:31849), photo G. Moreno. (I) Amanita vidua (AH:19696), photo G. Moreno. (J) Amanita verna (LIP:0402254, top) and A. vidua (LIP:0402256, bottom) reaction to KOH, photo M. Gelardi. (K,L) A. verna ‘f. xanthoviridis’ ad. int. (LIP:0002275), photo M. Raumi. (M) Amanita vidua from Lebanon (1C and 1P). Figure 3. Drawings of basidiomes in Reumaux & Carteret (2013). (A) Amanita amerivirosa. (B) Amanita virosa. (C) Amanita verna. (D) Amanita vidua. (E) Amanita phalloides var. alba. Reproduced with permission of the authors and the editor from Patrick Reumaux and Xavier Carteret, Les Tueurs, published by Klincksieck editions, Paris, 2013 (except D, original drawing by X. Carteret). Bar = 1 cm.
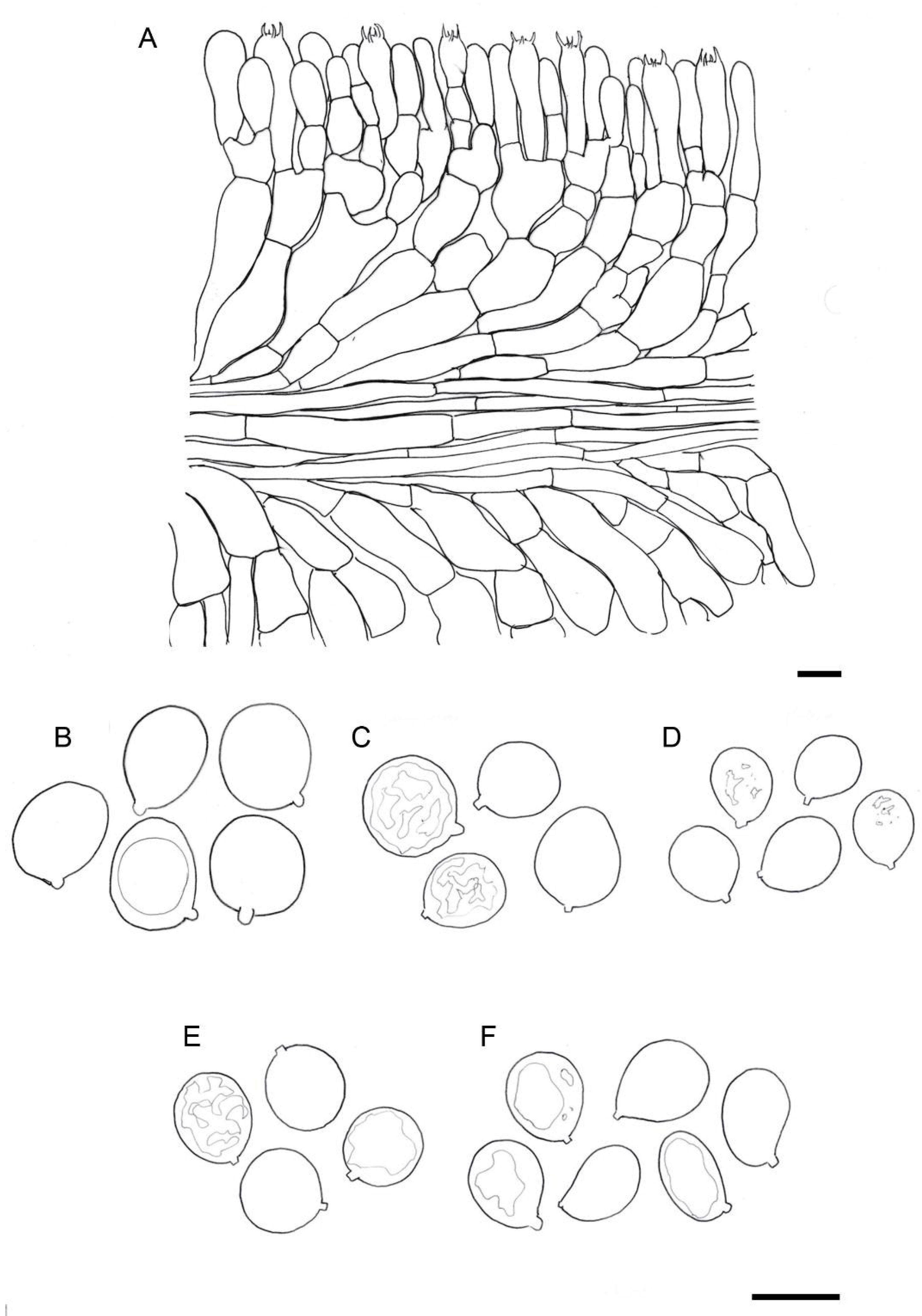
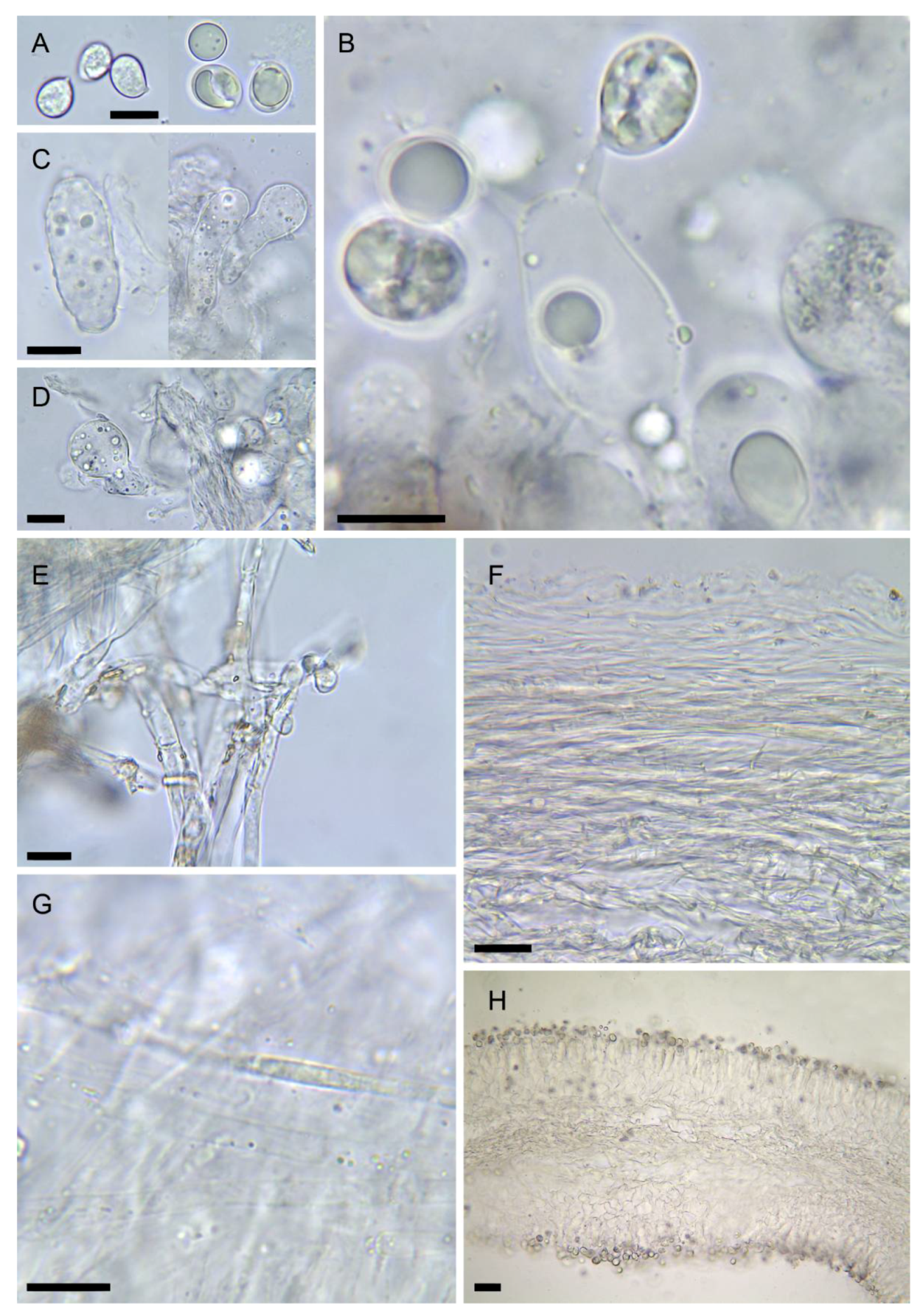
Figure 3. Drawings of basidiomes in Reumaux & Carteret (2013). (A) Amanita amerivirosa. (B) Amanita virosa. (C) Amanita verna. (D) Amanita vidua. (E) Amanita phalloides var. alba. Reproduced with permission of the authors and the editor from Patrick Reumaux and Xavier Carteret, Les Tueurs, published by Klincksieck editions, Paris, 2013 (except D, original drawing by X. Carteret). Bar = 1 cm.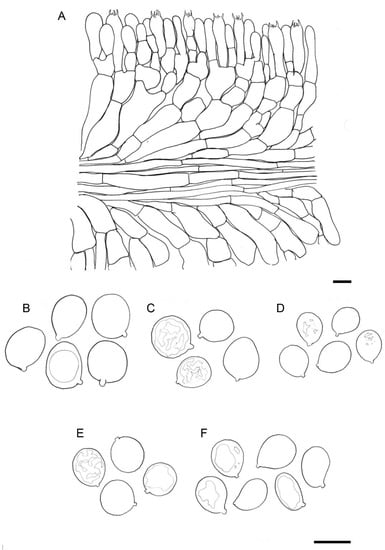 Figure 4. Drawings of microscopical features of destroying angels. (A) hymenophoral trama of Amanita vidua (LIP:0104591), longitudinal section. (B) Basidiospores of Amanita vidua (LIP:0401591). (C) Basidiospores of A. amerivirosa (LIP:0401671). (D) Basidiospores of A. virosa (LIP:0402243). (E) Basidiospores of A. phalloides (LIP:0401670). (F) Basidiospores of A. verna (LIP:0402249). Bar = 10 µm.
Figure 4. Drawings of microscopical features of destroying angels. (A) hymenophoral trama of Amanita vidua (LIP:0104591), longitudinal section. (B) Basidiospores of Amanita vidua (LIP:0401591). (C) Basidiospores of A. amerivirosa (LIP:0401671). (D) Basidiospores of A. virosa (LIP:0402243). (E) Basidiospores of A. phalloides (LIP:0401670). (F) Basidiospores of A. verna (LIP:0402249). Bar = 10 µm.- ≡
- Agaricus phalloides Vaill. ex Fr., Syst. mycol. (Lundae) 1: 13 (1821), nom. sanct. [protonym Fungus phalloides Vaill., Bot. paris. (Paris): 74, Table 14, Figure 5 (1723), inval.]; Venenarius phalloides (Vaill. ex Fr.) Murrill, Mycologia 4(5): 240 (1912); Amanitina phalloides (Vaill. ex Fr.) E.-J. Gilbert, in Bresadola, Iconogr. mycol., Suppl. I (Milan) 27: 78 (1940)
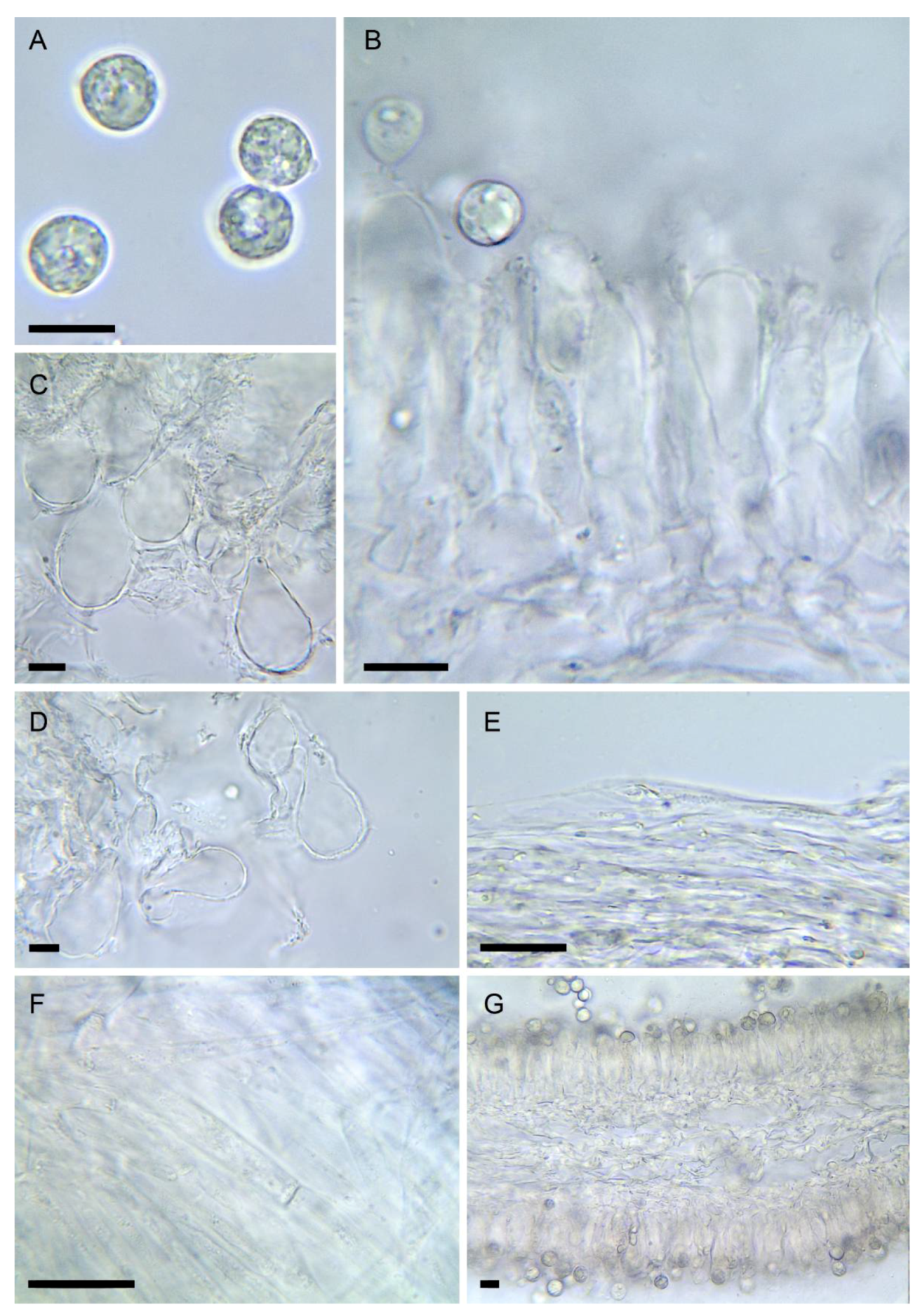
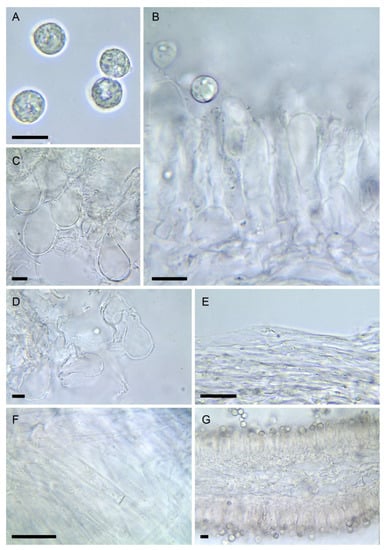 Figure 5. Amanita amerivirosa (LIP:0401671). (A) Basidiospores. (B) Hymenium and subhymenium. (C) Lamella edge with terminal cells. (D) Elements of partial veil (upper surface). (E) Elements of suprapellis. (F) Pileipellis, radial section. (G) Hymenophore, longitudinal section. All observations in 5% KOH solution. Bar = 10 µm.
Figure 5. Amanita amerivirosa (LIP:0401671). (A) Basidiospores. (B) Hymenium and subhymenium. (C) Lamella edge with terminal cells. (D) Elements of partial veil (upper surface). (E) Elements of suprapellis. (F) Pileipellis, radial section. (G) Hymenophore, longitudinal section. All observations in 5% KOH solution. Bar = 10 µm.- =
- Agaricus insidiosus Letell., Annls Sci. Nat., Bot., sér. 2 4: 35 (1835); Amanitopsis insidiosa (Letell.) Sacc., Syll. Fung. 9: 2 (1891); Amanita insidiosa (Letell.) Bigeard & Guillemin, Fl. champ. sup. France Edn 2: 14 (1913); Amanita phalloides f. insidiosa (Letell.) E.-J. Gilbert, Le genre Amanita: 41 (1918)
- =
- Amanita phalloides var. alba Costantin & L.M. Dufour, Nouv. Fl. Champ., Edn 2 (Paris): 256 (1895)
- =
- Amanita phalloides f. citrina J.E. Lange, Dansk bot. Ark. 2(3): 8 (1915)
- =
- Amanita phalloides var. pulla Killerm., Denkschr. Bayer. Botan. Ges. in Regensb. 18: 4 (1930)
- =
- Amanita phalloides var. euphalloides Maire, Mém. Soc. Sci. Nat. Maroc. 45: 103 (1937)
- =
- Amanita phalloides var. moravecii Pilát, Česká Mykol. 20(1): 25 (1966)
- =
- Amanita dunensis R. Heim ex Bon & Andary, Docums Mycol. 13(50): 13 (1983); Amanita phalloides f. dunensis R. Heim, Revue Mycol., Paris 28: 9 (1963) [nom. inval., Art. 36.1] (synonymy proposed here)
- =
- Amanita verna var. tarda Trimbach, Annales du Muséum d’Histoire Naturelle de Nice 91(3): 85 (1999); Amanita tarda (Trimbach) Contu, Boll. Gruppo Micol. ‘G. Bresadola’ (Trento) 43(2): 83 (2000)
Invalid names
- =
- Hypophyllum virosum Paulet Traité champ. Comest. (Paris): 2: 326 (1793) [inval., op. rejic.]
- =
- Amanita viridis Pers., Tent. disp. meth. fung. (Lipsiae): 67 (1797) [illegit., non A. viridis Huds.)]
- =
- Agaricus virosus Vittad., Descr. fung. mang. Italia: 135 (1835) [illegit., non A. virosus Sow.]
- =
- Amanita andaryi Mornand, Docums Mycol. 22(88): 12 (1993) [inval., introduced as nom. nov. based on “A. virosus var. albus Vittad. 1835: 143”, but this name does not exist in the cited book]
Pileus subglobose at first, then convex-flattened, lacking an umbo, 6–15 cm in diam.; surface easily detachable, smooth, shiny, thin, frequently greenish with a darker centre, but also yellowish-greenish, pale with greenish tinges, or rarely pure white (A. phalloides var. alba); with adnate radial fibrils, olivaceous to dark olivaceous in color; margin not striated, incurved when young, flat when mature. Lamellae very crowded, free, with pure-white or sometimes greenish lamellulae; edge white, but slightly yellowish or greenish near the pileal margin. Stipe 7–18 × 1–2 cm, cylindrical to subcylindrical, white with greenish tinges; with a white, sack-like, membranous volva, detachable, formed by 3–4 lobes, free in their upper half. Partial veil an annulus hanging high on the stipe, longitudinally striate, membranous, thick, white with citrine tinges. Universal veil without remnants on pileus surface. Flesh white, with greenish or yellowish tinges under the cuticle; odourless or with a slightly pleasant smell (resembling that of roses) when young, urine-like and disagreeable when mature; taste sweetish. Reactions to NaOH or KOH nil to faintly yellowish on white specimens.
Spores [3/72] mostly broadly ellipsoid (60%), also subglobose (30%) or ellipsoid (20%), (7.5–) 8.0–9.5 (–10.0) × 6.7–7.8 (–8.5) µm, Q = 1.1–1.3, Qav = 1.2, smooth, hyaline and amyloid. Basidia 4-spored, 38–52 × 9–12 µm, clampless. Pileipellis an ixocutis formed by gelified filamentous hyphae 1.5–5 µm in diam., clampless. Partial veil formed by cylindrical hyphae 1.5–5 µm in diam., clampless. Volva filamentous, formed by cylindrical hyphae 2–5.5 µm in diam., clampless, with rare sphaerocytes.
Habitat and distribution: very frequent, isolated, or scattered basidiomes can be found in autumn, forming mycorrhizal associations with Quercus as well as with other broadleaved trees, such as Castanea, Corylus, Betula or Fagus, less commonly with conifers (Cedrus, Pinus). Found in temperate, Mediterranean, and boreal habitats.
Examined material: FRANCE: Haute-Corse, Ghisonaccia, Forêt de Piniu, under Pinus pinaster in fixed sand dune, 5 November 2019, leg. P.-A. Moreau, PAM19110516, 0401670 (LIP). Oise, near Saint-Sauveur, forêt de Compiègne, broadleaved forest, 22 August 1960, leg. H. Romagnesi, HR60.109 (PC, as ‘Amanita verna’). SPAIN: Cádiz, Jimena de la Frontera, Parque Natural de los Alcornocales, under Quercus suber and Q. canariensis, 29 November 2003, leg. F. Prieto & A. González, 34,748 (AH). Madrid, between Bustarviejo and Pto. de Canencia, under Quercus pyrenaica, 1 October 1975, leg. G. Moreno, 94 (AH).
Notes: Amanita phalloides is a very common autumnal species described in detail in many publications and web sites, i.e., [38,67,68]. Freire & Castro [69] did not recognize A. phalloides in the odd-shaped specimen described by them (and validated by Castro [70]) as A. porrinensis; these two species cannot be separated from one another with the current sequencing results, and so they are considered conspecific. Therefore, we propose to downgrade the unusual Mediterranean taxon observed by Freire & Castro [69] and redescribed by Neville et al. [71] to a form of A. phalloides.
- 2.
- Amanita phalloides f. porrinensis (Freire & M.L. Castro) G. Moreno & Olariaga, comb. nov. Figure 2G and Figure 3EMycoBank number: MB 842794
- ≡
- Amanita porrinensis Freire & M.L. Castro, Mykes 1: 59 (1998) [also An. Jard. bot. Madr. 44(2): 533 (1987), inval., art. 39.1]
Examined material: ITALY: Ferrara, Comacchio, Bosco di Volano, forest with Pinus pinaster and P. pinea, 17 October 1998, leg. G. Monterumici, P. Neville 00111702 (G). Sardegna, Seneghe, forest of Quercus suber and Q. ilex, 1 November 2020, leg. S. Nioi, ALV30282, 0402258 (LIP). SPAIN: Pontevedra, Vigo, A Madroa, mixed forest with Quercus robur and Pinus pinaster, 28 October 1984, leg. J. Diz (G. M. ‘O Porriño’), Fungi-3670 (LOU, holotype). Idem, 7 November 1991, leg. L. Freire, M. Castro & J. Diz, Fungi-27321 (MA).
Pileus campanulate, with a wide and obtuse central umbo, 5–7.5 cm in diam.; surface pure white, not changing, smooth, not striate nor fibrillose, lacking any remnants of the universal veil, difficult to separate from the context underneath; margin not striated, inrolled at first, then flat when mature. Lamellae very crowded free, with pure white lamellulae; edge white; spore print white. Stipe 10–20 × 2–3 cm, cylindrical, sinuose, enlarged towards the base, pure white, sericeous to slightly floccose; annulus white, hanging high on the stipe, membranous, thin, soon disappearing; universal veil forming a fragile sack-like membranous volva, adhering, detachable, without remnants on the pileus surface. Flesh white, not changing, odourless; taste slightly sweet. Reactions: pileipellis turning citrine yellow with KOH 10% according to Freire & Castro [69], although the same authors report a weak pale yellowish reaction in the herbarium notes. Neville et al. [71] report an intensely yellow reaction on lamellae, weaker in the pileus and stipe; positive to Wieland test.
Spores broadly ellipsoid to ovoid or rarely subglobose, 7–10 × 6–7.5 µm, smooth, hyaline and amyloid. Basidia 4-spored, 35–40 × 10–11 µm, clavate, clampless. Pileipellis an ixocutis formed by filamentous hyphae 2–6 µm in diam., clampless, slightly gelified. Annulus formed by cylindrical hyphae 2.5–8 µm in diam., lacking sphaerocytes and clampless. Volva filamentous, formed by cylindrical hyphae 2–5.5 µm in diam., clampless, without sphaerocytes, but sometimes with clavate elements.
Notes: a very rare albino form of A. phalloides with a characteristic obtuse wide umbo and squamose or sericeous stipe. Only a few collections are known, found in autumn, in Spain [69], as well as Italy and Switzerland [71,72,73]. A toxic species according to the results of the Wieland test performed by the original authors [69]. The present results based on ribosomal data suggest that A. porrinensis is conspecific with A. phalloides. Sequences from several protein-coding genes (tef1, rpb2) obtained from a recent collection from Italy (ALV30282) do not show differences with those obtained from A. phalloides available in public databases (data not shown). Amanita porrinensis is here downgraded to a form of A. phalloides to warn about this unusual, but probably lethal, morphological variant.
- 3.
- Amanita amerivirosa Tulloss, L.V. Kudzma & M. Tulloss, Amanitaceae 1(4): 5 (2021) Figure 2A–C, Figure 3A, Figure 4C and Figure 5
- =
- Amanita virosa var. levipes Neville & Poumarat, Fungi europ. 9: 600 (2004)
Examined material: FRANCE: Loire-Atlantique, Nantes, under Quercus petraea, November 2019, leg. R. Chéreau, PAM19110001, 0401671 (LIP). Idem, under Quercus sp., November 2019, leg. G. Ouvrard, PAM19110002, 0401672 (LIP). Pas-de-Calais, Baincthun, forêt domaniale de Boulogne, under Quercus petraea and Carpinus betulus on a wet clay-calcareous soil, 24 July 2021, A. Gasch & P.-A. Moreau, PAM21072401, 0402251 (LIP). Val-d’Oise, Montmorency, forêt domaniale, under Quercus spp. and other broadleaved trees, 14 June 2019, leg. N. Journe FR2019667, 0402250 (LIP). Mayenne, Forêt communale d’Ombrée D’Anjou-Combrée, acidophilic atlantic Quercus petraea forest, 11 August 2021, A. Gasch & P.-A. Moreau, PAM21081110, 0402252 (LIP).
Pileus 4–15 cm in diam., originally conical-campanulate, expanding to convex-flat with a persistently convex center, never depressed; surface pure white and remaining so or sometimes becoming light ochraceous-pinkish at the center with age, greasy to nearly dry, shiny-silky when dry, smooth; margin incurved then expanded and flexuose when mature, smooth, never striated. Lamellae crowded, 80–100, reaching the stipe, typically with one lamellula per lamella, smooth to nearly smooth, cream white, with slight pinkish tones with age; edge white, floccose. Stipe 9–20 (–25) × 0.8–2.5 cm, cylindrical throughout or progressively thinner towards the apex, bulbous-sphaerical at the base (which can be up to 3.5 cm thick), pure white, slightly floccose towards the apex, below the annulus subtly silky-zebrate, becoming glabrous with age; partial veil a membranous, persistent annulus hanging 2–3 cm down the apex, rarely laciniate and then hanging at the margin, not adhering to the edges; universal veil forming an ample membranous volva, rarely dissociated on the pileus, white, with pale pinkish-ochraceous patches on outer surface. Context white, slightly foxy-spotted on damaged surfaces, light lemon yellow when dry; smell distinctly iodine-like in the bulb, elsewhere not distinct, fungoid by alteration; taste mild, fungoid. Reaction to 5% KOH chrome yellow on all surfaces and context. Exsiccates turn entirely light lemon yellow after a few days. Spore print pure white.
Spores [2/40] (7.1–) 8.1–10.2 (–11.8) × (6.7–) 7.3–9.3 (–11.1) µm, Q = 1.00–1.20, Qav = 1.1, subglobose (60–80%) to broadly ellipsoid (20–40%), smooth, amyloid, with dense yellow content in KOH at maturity, rather thick-walled and not easily collapsing. Hymenophoral trama a 50–60 µm thick mediostratum, reaching 80 µm towards the pileus, made of broad physaloid hyphae 8–25 µm wide, mixed with slender hyphae 4–8 µm wide, divergent towards the hymenium. Subhymenium 10–15 µm thick, ramose, faintly developed. Basidia 35–43 × 9–12 µm, mostly 4-spored with a significant number of 2-spored basidia. Lamellae edge sterile, terminal inflated cells measuring 18–45 × 12–18 µm, shortly clavate to sphaeropedunculate, wall yellowish up to 0.5 µm thick, often incrusted by yellowish mucoid deposits. Pileipellis scarcely differentiated, 30–50 µm thick, an ixotutis of slender hyphae 1.5–4 µm wide, with yellow wall irregularly thickened, forming internal and external yellow concretions; subpellis not distinct. Universal veil 2-layered; outer layer made of yellowish hyphae 3–12 µm wide, with yellow wall covered by mucoid deposits; inner layer made of colourless hyphae 2.5–14 µm wide, with smooth wall up to 0.5 µm thick, mostly long and fasciculate. Partial veil on the upper surface made of intricate filamentous hyphae with numerous globose, cylindrical elements with yellow, strongly granulose surface, 3–12 µm wide. Lower surface with numerous globose, cylindrical to elongate elements 20–60 × 7–25 µm.
Notes: Amanita amerivirosa is a very recently described taxon [37], so far only documented by DNA sequences from NE America where it has been recorded for a long time under the name A. virosa. We confirm here Neville & Poumarat’s [38] hypothesis that Amanita virosa var. levipes described from the French Atlantic coast is the same fungus, likely not indigenous in Europe.
- 4.
- Amanita verna Bull. ex Lam., Encycl. Méth. Bot. (Paris) 1(1): 113 (1783) Figure 2F,I–M, Figure 3C, Figure 4F,G and Figure 6
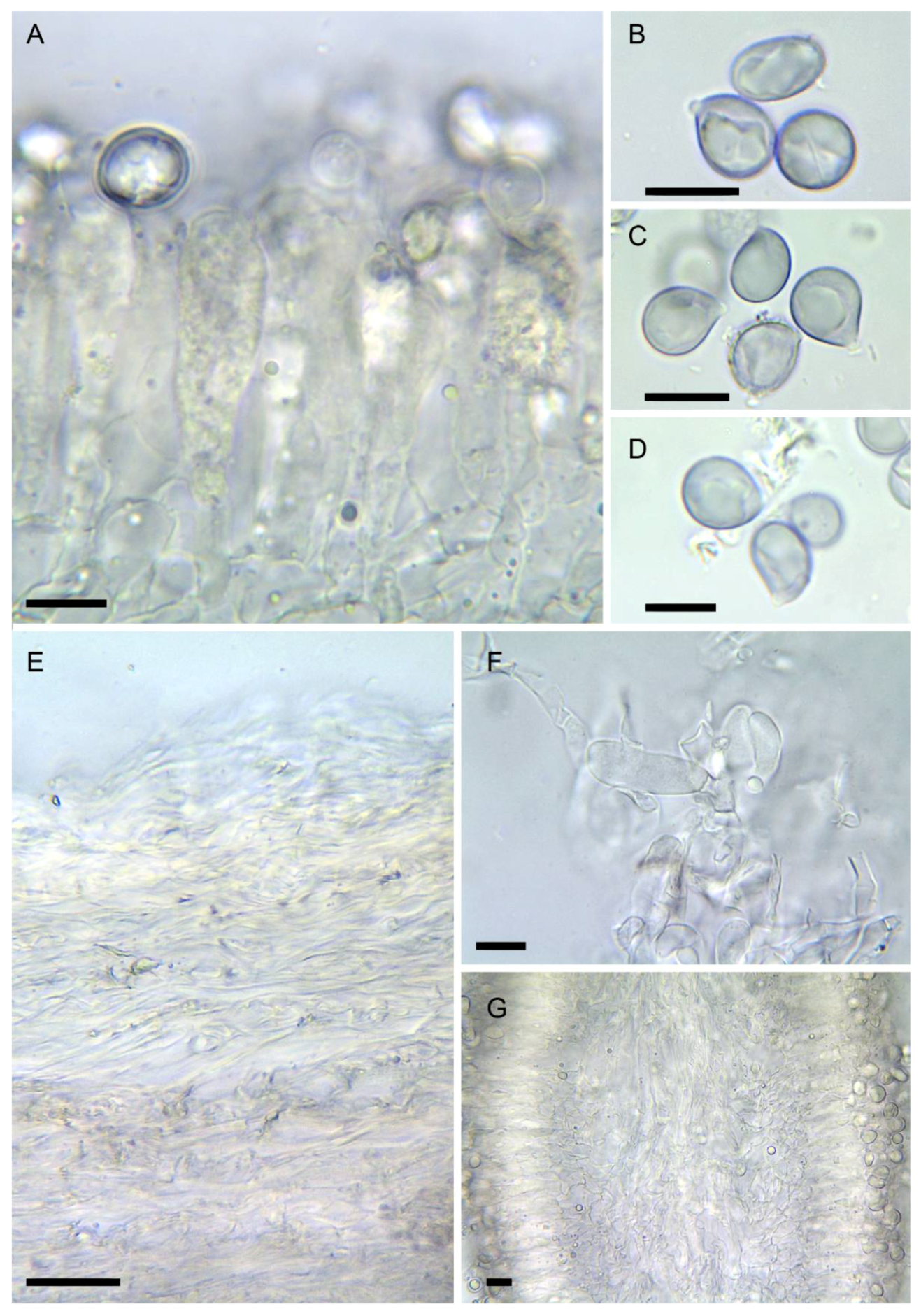
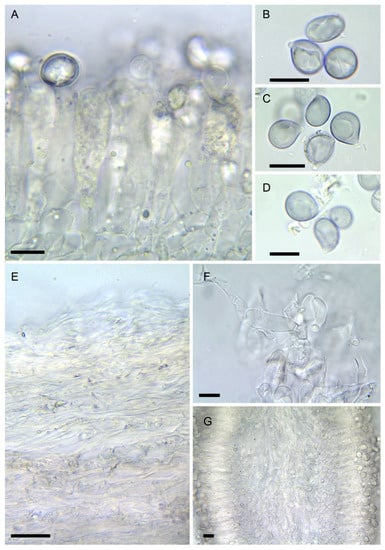 Figure 6. Amanita verna (LIP:0402244, except B: LIP:0402249. (A) Hymenium and subhymenium. (B–D) Basidiospores. (E) Pileipellis, radial section. (F) Elements of partial veil (upper surface). (G) Hymenophore, longitudinal section. Observations in 5% KOH solution except A, B: Melzer. Bar = 10 µm.
Figure 6. Amanita verna (LIP:0402244, except B: LIP:0402249. (A) Hymenium and subhymenium. (B–D) Basidiospores. (E) Pileipellis, radial section. (F) Elements of partial veil (upper surface). (G) Hymenophore, longitudinal section. Observations in 5% KOH solution except A, B: Melzer. Bar = 10 µm.
Protonym: Agaricus bulbosus vernus Bull., Herb. Fr. (Paris) 3: Table 108 (1782–1783) [inval., Art. 32.1(c)]; Agaricus virosus [unranked] vernus (Bull. ex Lam.) Fr., Epicr. syst. mycol. (Uppsala): 4 (1838); Amanita phalloides [unranked] verna (Bull. ex Lam.) Fr., Hymenom. Europ. (Uppsala): 18 (1874); Amanita phalloides var. verna (Bull. ex Lam.) Lanzi, Bull. Soc. Hist. nat. Afr. N. 7(6): 145 (1916); Amanitina verna (Bull. ex Lam.) E.-J. Gilbert, in Bresadola, Iconogr. mycol., Suppl. I (Milan) 27: 78 (1940); Venenarius vernus (Bull. ex Lam.) Murrill, Lloydia 11: 104 (1948)
Typification: lectotype (designated here): Bulliard, Herb. Fr. 2: Table 108 (1782–1783), MycoBank MBT 10005511. Epitype (designated here): FRANCE: Oise, Apremont, 19 Jul 1956, leg. H. Romagnesi, HR56.31 (PC), as a support to the lectotype designated above, MycoBank MBT 10005512.
- =
- Agaricus vernalis Bolton, Hist. fung. Halifax (Huddersfield) 2: 48, Table XLVIII (1788)
- =
- Amanita verna var. ochroleuca Forq. ex Quél., 1888, Fl. mycol. Fr.: 309; Amanita phalloides var. ochroleuca (Forq. ex Quél.) Quél. & Bataille, 1902, Fl. Amanites et lépiotes: 32; Amanita ochroleuca (Forq. ex Quél.) Bigeard & Guillemin, 1913, Fl. champ. sup. France 2nd ed.: 14
- =
- Amanita verna var. decipiens Trimbach, Riviéra Scientifique, 1970(1): 18 (1970); Amanita decipiens (Trimbach) Jacquet., Docums Mycol. 22(86): 30 (1992)
Invalid names
- =
- Amanita verna f. ellipticospora E.-J. Gilbert, in Bresadola, Iconogr. Mycol., Suppl. II (Milan) 27: 320 (1941) [inval., art. 38.1 and art. 39.1]
Pileus 3–9 cm diam., globose at first then convex to flat, soon flattened to somewhat depressed at center; surface smooth, slightly greasy when moist, pure white at first, somewhat ochraceous towards disk with age; margin convex, only later expanding, never striate. Lamellae crowded, 70–80 reaching the stipe, 1 (-2) lamellulae per lamella, free to somewhat adnexed, pure white when young, often with a subtle pinkish tone when ageing; edge white, thin, smooth to somewhat serrulate. Stipe 6–15 × 0.8–1.5 cm, equal to slightly narrowed at apex, abruptly bulbous-sphaerical at base, not or only faintly striate at apex, smooth, silky to subtly floccose below annulus; partial veil a membranous, persistent but thin annulus usually hanging high on the stipe, white, slightly striate on the upper side, smooth; universal veil a membranous, rather thin volva, white to somewhat ochraceous on surface. Flesh white, unchanging; smell none, somewhat fungoid on adults; taste mild. Reaction chrome-yellow to 5% KOH on all surfaces and context, except inner layer of volva.
Spores [3/60] usually mostly broadly ellipsoid (50%) and partly subglobose and ellipsoid (20–30% each), some lacrymoid or pyriform in side view, (7.4–) 8.0–11.1 (–11.3) × (6.1–) 6.4–9.1 (–9.7) µm, Q = 1.15–1.50, Qav= 1.2, smooth, amyloid. Basidia 4-spored, 42–58 × 7.5–10 µm, clavate with tapering base, with yellow granular content at maturity, then filled with yellow, amorphous to crystalloid necropigment. Hymenophoral trama made up of a mediostratum 30–50 µm wide (on exsiccate), with parallel slender hyphae, more divergent towards subhymenium, 2–12 µm wide. Subhymenium 30–45 µm thick, with polygonal, lobate to cordiform elements, 12–24 × 7–18 µm. Lamellae edge sterile to substerile, consisting of clusters of terminals inflated, sphaeropedunculate cells measuring 10–25 × 8–15 µm, with yellowish wall up to 1.2 µm thick, with abundant yellow granulations on surface. Pileipellis 100–120 µm thick, with a suprapellis 30–40 µm thick made of slender hyphae 3–4 µm wide, with abundant yellowish mucoid deposits in KOH. Subpellis not differentiated. Context of the pileus made up of thick, intricate hyphae up to 10–12 µm broad. Partial veil made up of intricate cylindrical hyphae 3–8 µm wide, with scattered strongly granular content; upper side with fascicles of globose to piriform elements measuring 15–35 × 10–14 (–20) µm, mostly with thickened walls; outer surface made up of more or less parallel hyphae. Universal veil 1-layered, made up of both slender and thick hyphae 3–18 µm wide, intricate, smooth, with interspersed yellow granulations.
Ecology and distribution: ectomycorrhizal with Fagaceae, especially Quercus ilex, Q. pubescens, Q. pyrenaica, Q. robur and Q. suber, and also Pinus pinea, exceptionally with Castanea sativa or Fagus sylvatica, on acidic to moderately basic soils, known from thermophilous regions in Western Europe and North Africa, probably widespread across the Mediterranean Basin; mostly fruiting in spring (March to June), occasionally observed in late autumn.
Examined material: FRANCE: Oise, Apremont, in the humus of a broadleaved forest, 19 July 1956, leg. H. Romagnesi, HR56.31 (PC). Oise, Coye-la-Forêt, near Poteau du Crochet, broadleaved forest, 20 June 1971, leg. H. Romagnesi, HR71.42 (PC). Alpes-Maritimes, Berre-les-Alpes, June 1969, J. Trimbach, 2010.2.148 (NICE, holotype of A. verna var. decipiens). Landes, Bias, under Quercus pubescens on sandy soil, J.-C. Déiana, J. Guinberteau & P.-A. Moreau, PAM01042903, 0402249 (LIP, not sequenced). ITALY: Lazio, Anguillara Sabazia, among litter in acidic soil, along a sidetrack on a steep slope facing north in a pure stand of Quercus ilex, 185 m a.s.l., ca 100 m from the shore of lake Bracciano, 16 May 2021, leg. M. Gelardi, F. Costanzo & O. Gelardi, MG846-BIS, 0402254 (LIP). Lazio, Bracciano, Bosco di S. Celso, among litter in acidic soil in a pure stand of Quercus cerris, 190 m a.s.l., ca 200 m from the shore of lake Bracciano, 1 May 2021, leg. A. Appolloni & M. Tosoni, MG847, 0402255 (LIP). Tuscany, Pisa, Vecchiano, San Piero a Grado, Tenuta di Salviati, in a forest of Pinus pinea and Quercus ilex, 17 May 2019, leg. M. Raumi, Raumi ‘Amanita Salviati’ ALV21204, 0002275 (LIP). SPAIN: Ávila, Casillas, under Castanea sativa, leg. L. Rubio-Casas, 25 May 2008, 31,839 (AH). Ávila, El Tiemblo, under Quercus pyrenaica, leg. L. Rubio-Casas, J.C. Campos, L. Rubio-Roldán & J. Hernanz, 14 June 2008, 31,848 (AH). Idem, 31,849 (AH). Cáceres, Miramontes, Cerro la Garrapata, under Quercus pyrenaica and Cistus ladanifer, leg. G. Moreno & E. Arrojo, 6 May 2000, 19,695 (AH). Madrid, leg. Soc. Micol. Madrid, 5 May 2002, 31,985 (AH). Idem. 31,986 (AH). Madrid, El Escorial, under Quercus pyrenaica, leg. B. Nilsson, 6 June 2008, 31,843 (AH). Madrid, El Pardo, under Quercus ilex subsp. ballota, leg. V. Sánchez, 2 June 2008, 31,842 (AH). Madrid, Villa del Prado, under Quercus ilex subsp. ballota, leg. J.C. Campos, 25 April 2003, 37,755 (AH). Idem, leg. J. Hernanz, J.C. Campos & J. Campos, 1 May 2004, 37,726 (AH). Salamanca, under Quercus spp., June 2019, leg. P. García Jiménez, PAM19060001, 0402244 (LIP). Toledo, Hinojosa de San Vicente, leg. L. Rubio-Casas, 22 May 2004, 31,987 (AH). Toledo, Hormigos, under Quercus ilex subsp. ballota, leg. J.C. Campos & J. Hernanz, 1 May 2004, 31,991 (AH). Idem, 37,725 (AH). Idem, leg. A. Zapata, 8 May 2004, 37,729 (AH).
Notes: this lethal species can be easily confused with immature specimens of Agaricus. Unexpanded young basidiomata can also be confused with the edible species Amanita ponderosa Malençon & R. Heim (‘gurumelo’), a highly regarded mushroom in south-eastern Spain (Huelva, Badajoz, Cáceres; [74]). The results obtained in the present work (Figure 1) show that KOH+ and KOH- specimens of A. verna actually belong to distinct species. All KOH- specimens examined were found in Mediterranean habitats of southern Europe. Romagnesi’s collection HR83.65 at PC herbarium (supposed A. verna KOH- from northern France; [75]) was actually made of two specimens, one with white pileus, the second with distinct greenish shades. Sequence data obtained from them revealed that both are actually specimens of A. strobiliformis with an atypical membranous volva.
If the original concept of A. verna is considered a synonym of A. phalloides var. alba [76,77], the name A. decipiens would be available for the KOH+ specimens (see Discussion below). However, we think that this option would not contribute to stabilizing the nomenclature of this group, and the interpretation of A. verna as a KOH+ species is here preferred. Two samples of A. verna collected in Oise department, 50–60 km north of Paris, were found in Romagnesi’s herbarium: HR56.31 from Apremont and HR71.42 from Coye-la-Forêt. To close the debate and fix the nomenclature of A. verna, we epitypify this name with collection HR56.31.
The variability in spores of A. verna caught the attention of various mycologists, from Gilbert [78] to Malençon & Bertault ([79]; Figure 7) or Neville & Poumarat [38]. In the present study, no spore print was available, and spores were observed on exsiccata as deposits at the apex of the stipe or near the edge. Most collections (including the epitype) studied here displayed only a moderate variability of spore shape, which ranged from subglobose to ellipsoid (Figure 6A,C). Specimens with narrowly ellipsoid to cylindrical spores such as those described as A. verna f. ellipticospora by Gilbert [78] or those described by Bertault [80] from specimens collected in Morocco, could not be revised here and a special attention should be given to such collections in the future. The description of A. gilbertii f. subverna Bertault & Parrot from Morocco, about which Bertault [81] wrote: “seules les spores cylindriques permettent de les séparer” (only the cylindrical spores make a difference [with A. verna]), suggests that this taxon, which Neville & Poumarat [38] validated by selecting a holotype found in the island of Porquerolles (south eastern France), could be related also to A. verna.
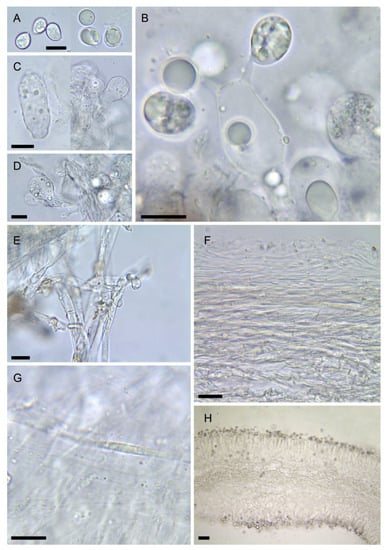
Figure 7.
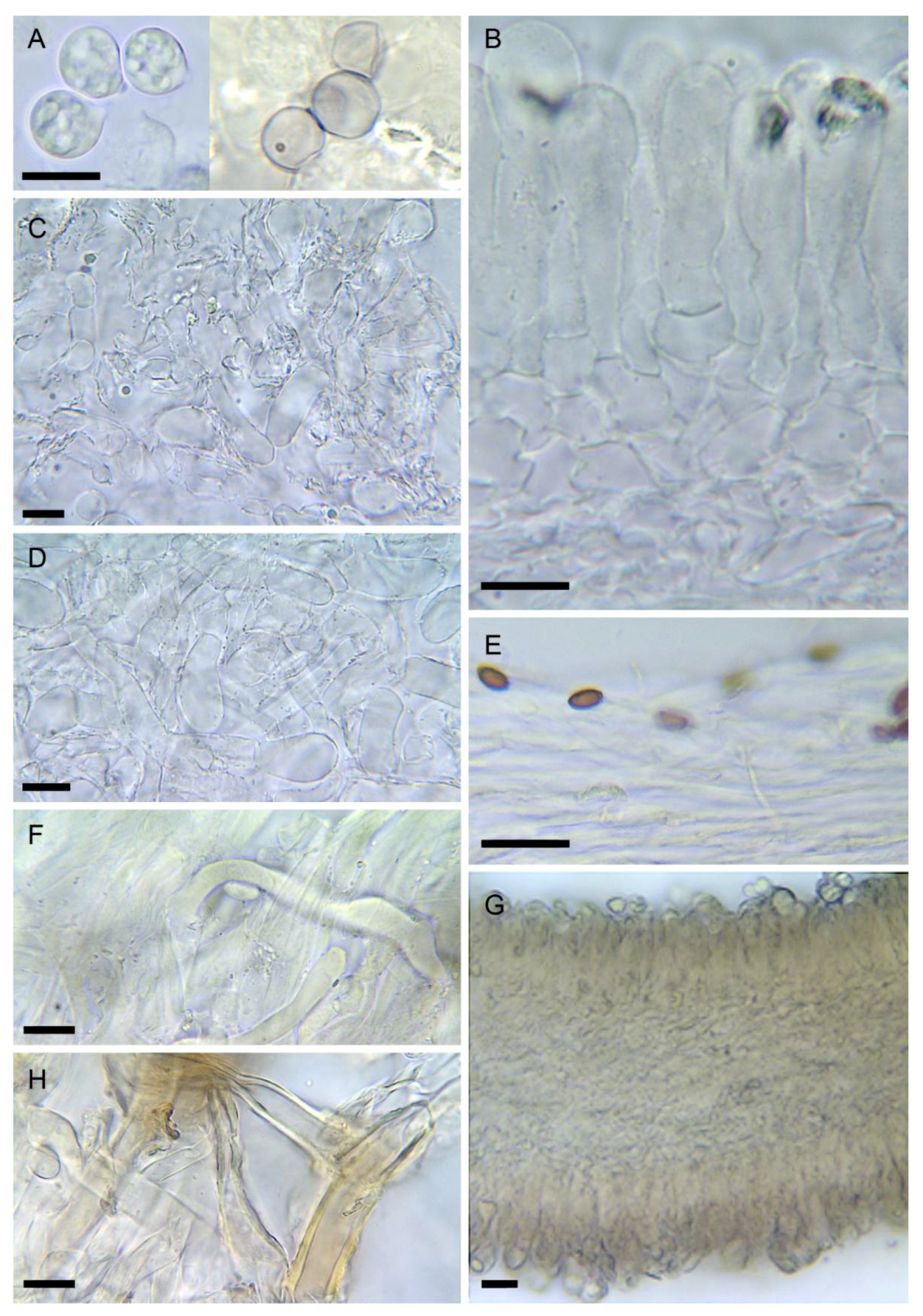
Amanita vidua (LIP:0401591). (A) Basidiospores. (B) Hymenium. (C) Terminal cells on lamella edge. (D) Elements of partial veil (upper surface). (E) Universal veil, outer surface. (F) Elements of suprapellis. (G) Pileipellis, radial section. (H) Hymenophore, longitudinal section. All observations in 5% KOH solution. Bar = 10 µm.
Although Amanita verna is univocally described in the literature as having a white to more or less ochre pileus, several collections from Italy with a greenish yellow pileus have been found by Raumi [82], described under the provisional name ‘Amanita verna f. xanthoviridis’ ad. int. The ITS rDNA, TEF1, and RPB2 sequences from one of these collections (ALV21204, kindly shared by M. Raumi, 1 pileus deposited at LIP n°0002275) do not differ from those of typical samples of A. verna (Figure 1). The yellow tinge seems to be due to the presence in the subpellis of radially oriented hyphae 5–6.5 µm wide with thick, yellowish walls (up to 1 µm thick), which were not observed in white specimens; the spores are in the range of the type, [1/22] 9.2–10.4 × 7.0–8.2 µm, Q = 1.2–1.3, Qav = 1.2, mostly broadly elliptical, with a few macrospores likely issued from 2-spored basidia, up to 13.5 × 10.5 µm. This remarkable form might be confused with A. phalloides, from which it differs by the fruiting season and the strong yellow reaction of all parts of the basidiome to KOH (Figure 2). No complete specimen could be obtained for the purpose of this article, and thus a full description of this form and the validation of its provisional name could not be provided here.
- 5.
MycoBank number: MB 842793
Misapplied names: Amanita verna sensu Bertault [80,83], Amanita verna var. verna sensu Trimbach [84], La Chiusa [85], Neville & Poumarat [38], etc.; Amanita tarda sensu Contu [86]
Etymology: from Latin noun vidua: widow, an ironic reference to its high toxicity and to the fact that this species became ‘widowed’ after the name A. verna could not be employed for it anymore.
Diagnosis: Differs from Amanita verna by the absence of yellow reaction with KOH on all tissues, as well as its less distinctly bulbous stipe, and often umbonate pileus early tinged with ochraceous tones. Typically occurring in spring in siliceous soils of the Mediterranean regions, under Quercus ilex or Q. suber.
Holotype: SPAIN: Andalucia, Galaroza, under Quercus ilex on siliceous soil, 4 May 2019, A. Gasch & P.-A. Moreau, PAM19050401 (coll. P.-A. Moreau, LIP 0401591).
Description: Basidiomata solitary to scattered. Pileus at first subglobose, then expanding to flat-convex or broadly convex-umbonate, pileipellis easily detachable, smooth, shiny, thin, off-white to cream-white, sometimes developing ochre-yellow or ochre-pinkish tones at the centre, or uniformly isabelle ochre-brown (reminding of A. eliae) when old or dry, lacking adnate fibrils; margin not striated, inrolled at first, soon expanded, persistently white. Lamellae pure white, crowded, free with broad collarium at maturity, with lamellulae, white; edge floccose to serrulate, white, becoming cream-yellow to salmon ochre towards margin when old. Spore print white. Stipe 7.5–16 × 1–1.8 cm, cylindrical to subcylindrical, white, smooth, pruinose-floccose at the apex and slightly striated above the ring, somewhat zebrate below, with a slightly bulbose base about 2.5 cm diam., with a sack-like membranous white volva at the base, usually buried, without internal limb; partial veil forming a descending ring, membranous, ample but early adpressed on stipe and collapsed in adults, thick, white, often doubled by an oblique bandlet some mm below. Universal veil without remnants on the pileus. Flesh white, becoming cream to pinkish with age. Odour fungoid, not remarkable, even with alteration. Taste fungoid. Reaction to 10% KOH pale ochre on pileipellis, nil elsewhere.
Spores [6/140] variable in shape, from subglobose (20–60%) to broadly ellipsoid (30–50%) and ellipsoid (10–40%), measuring (8.8–) 9.0–10.8 (–11.2) × (6.8–) 7.2–8.5 (–9.1) µm, Q = 1.15–1.30 (–1.60), Qav = 1.2 [from the holotype], smooth, hyaline content before maturity, some spores filled with yellowish droplets with age, amyloid. Basidia 4-spored, cylindrical with tapering base, measuring 32–58 × 9–12 µm, clampless. Subhymenium 30–50 µm thick, nearly pseudoparenchymatous with lobate, constricted or cordiform elements measuring 15–25 × 12–20 µm. Hymenophoral trama with a mediostratum 120–150 µm thick in fresh specimens, collapsing to 40–50 µm in herbarium samples, filamentous, made up of parallel slender hyphae 2.5–5 µm wide mixed with broad, inflated hyphae 7–11 µm wide; hymenopodium not differentiated. Lamellae edges fertile (mature specimens); terminal cells sparse, cylindrical to clavate-capitate, measuring 30–38 × 11–14 µm. Pileipellis thick, 2-layered; suprapellis a 30–40 µm thick ixocutis formed by gelatinized filamentous hyphae 2–5 µm diam., clampless, some of them with yellow granular content in KOH; subpellis not gelatinized, otherwise similar to suprapellis, 30–50 µm thick, mostly made up of parallel, slender hyphae 1–2.5 µm wide and also sparse broader hyphae up to 5 (–7) µm wide, all of them smooth with slightly thickened, yellowish walls. Partial veil (ring) made of intricate cylindrical hyphae 2–8 µm wide, smooth, clampless, with walls thickened up to 1.5 µm; upper surface filamentous with sparse sphaeropedunculate elements measuring 12–15 × 9–11 µm. Volva distinctly 2-layered; inner layer filamentous, about 150–300 µm thick, made up of intricate cylindrical hyphae 3–7.5 µm diam., often branched, clampless, mixed with very rare sphaerocytes or inflated elements up to 45 × 22 µm, all smooth and colourless; outer layer up to 40 µm thick, made up of cylindrical slender hyphae 3–7 µm wide, smooth with slightly thickened walls (up to 0.2 µm), yellowish in KOH, locally incrusted with yellowish deposits.
Habitat and distribution: infrequent vernal species that probably establishes ectotrophic mycorrhizae with Mediterranean species of Quercus (Q. ilex ssp. ballota, Q. pyrenaica, Q. suber, etc.), rarely also with other trees, i.e., Castanea, Fagus, Pinus. Known from acidic soils of the Mediterranean basin, from Spain to Lebanon, as well as Morocco [76], from April to early June.
Examined material: FRANCE: Var, la Garde-Freinet, under Quercus suber and Castanea sativa on siliceous soil, leg. S. Kizlik & Société Mycologique de Provence, 7 June 1987, La Garde Freinet nº83, 0002253 (LIP). Var, Pourrières, Quercus ilex in garrigue, M. Bon, November 1976, herb. M. Bon 761,022 (LIP, not sequenced). ITALY: Lazio, Anguillara Sabazia, among litter in acidic soil, along a sidetrack on a steep slope facing north in a pure stand of Quercus ilex, 185 m a.s.l., ca 100 m from the shore of lake Bracciano, 16 May 2021, leg. M. Gelardi, F. Costanzo & O. Gelardi, MG846, 0402256 (LIP). LEBANON: Bnebil, Mount-Lebanon, in a mixed stand of Pinus pinea and Quercus infectoria, 1000 m a.s.l., 18 April 2018. SPAIN: Madrid, Chapineria, under Quercus ilex subsp. ballota, leg. Soc. Micol. Madrid, 14 Apr 2002, 31,984 (AH). Madrid, leg. Soc. Micol. Madrid, 5 May 2002, 31,988 (AH). Madrid, El Pardo, under Quercus ilex subsp. ballota, leg. V. Sánchez, 24 May 2008, 31,838 (AH). Idem, 2 June 2008, 31,841 (AH). Segovia, Revenga, under Quercus ilex subsp. ballota, leg. G. Moreno, F. Esteve-Raventós, A. Sánchez, J. Díez, V. D’Angelo & M.M. Dios, 5 June 1998, 31,989 (AH). Madrid, Sierra de Guadarrama, under Quercus pyrenaica, leg. Soc. Micol. Madrid, 22 April 2001, 31,990 (AH). Madrid, Villa del Prado, under Quercus ilex subsp. ballota, leg. G. Moreno, 20 April 2002, 19,696 (AH). Idem, leg. J.C. Campos, 25 April 2003, 37,756 (AH). Idem, 37,757 (AH). Idem 37,758 (AH). Idem 37,759 (AH). Idem, 20 April 2004, 37,760 (AH). Idem, 37,761 (AH). Idem, leg. J. Hernanz, J.C. Campos & J. Campos, 1 May 2004, 37,727 (AH). Idem, 37,728 (AH). Idem, leg. J.C. Campos, 18 May 2008, 19,697 (AH). Idem, 31,837 (AH).
Notes: Amanita vidua is very similar to A. verna, both sometimes fruiting in the same areas. No evident diagnostic morphological features could be found to distinguish these species, which differ mainly in their reaction to KOH (chrome yellow in A. verna, negative to faintly ochre in A. vidua), in the narrower and less differentiated bulb at the base of the stipe, and in the exclusively Mediterranean distribution of A. vidua, which does not reach temperate areas where only A. verna is present. Genetically, ITS rDNA, rpb2, and tef1 sequences (introns excluded) of A. vidua are 94–96% similar to those of A. verna or A. phalloides, while 28S rDNA is 99–100% similar to those of these species.
- 6.
- Amanita virosa Bertill., Dict. Encyclop. Sci. Médic. (Paris) 1(3): 497 (1866) [nom. nov. based on Ag. virosus Fr. 1838, illegit.], Figure 2D, Figure 3B, Figure 4D and Figure 8
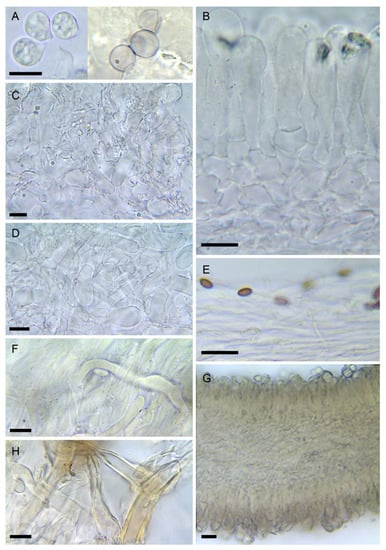 Figure 8. Amanita virosa (LIP:0402243). (A) Basidiospores. (B) Hymenium and subhymenium. (C) Elements of lamella edge. (D) Elements of partial veil (upper surface). (E) Pileipellis, radial section. (F) Hyphae in subpellis showing two thromboplerous hyphae. (G) Hymenophore, longitudinal section. (H) Universal veil, outer surface. All observations in 5% KOH solution except A (left): Melzer. Bar = 10 µm.
Figure 8. Amanita virosa (LIP:0402243). (A) Basidiospores. (B) Hymenium and subhymenium. (C) Elements of lamella edge. (D) Elements of partial veil (upper surface). (E) Pileipellis, radial section. (F) Hyphae in subpellis showing two thromboplerous hyphae. (G) Hymenophore, longitudinal section. (H) Universal veil, outer surface. All observations in 5% KOH solution except A (left): Melzer. Bar = 10 µm.
Basionym: Agaricus virosus Fr., Epicr. Syst. mycol. (Upsaliae): 3 (1838), illegit. [non-Ag. virosus Sow., 1809]; Amanitina virosa (Bertill.) E.-J. Gilbert, in Bresadola, Iconogr. mycol., Suppl. I (Milan) 27: 78 (1940)
Typification: no type designated by Fries [8]; no original material known to us. Neotype here designated: SWEDEN: Fiby Urskog, old-growth Picea abies forest, leg. A. Dahlberg & P.-A. Moreau, 12 August 2006, coll. P.-A. Moreau SU12-06-19, 0402243 (LIP), MycoBank MBT 10005510.
- =
- Amanita virosa var. aculeata Voglino, Boll. Soc. bot. ital., 1894: 120 (1894)
Description of the neotype: Pileus 4–11 cm, cylindrical to conical-obtuse at first, later flexuose with the center persistently convex or sometimes umbonate, never flattened or depressed, greasy to almost viscid, shiny-silky when dry, pure white, disc slightly ochraceous when old; margin incurved for a long time, then irregularly expanded, smooth, never striated, white, usually with traces of a floccose partial veil hanging. Lamellae crowded, rather thick, free, collariate at maturity, pure white, with subtle pinkish tones when mature; edge thick, heavily floccose, usually with adherent floccose patches of the partial veil. Stipe 7–18 × 0.6–1.8 cm, cylindrical or narrowed towards the apex, equal, slender, often curved at maturity, inflated at the base with a sphaerical bulb up to 3 (–4) cm wide; surface entirely floccose, with bandlets or ascendant scales on the lower part with age, somewhat striate at the apex, pure white and remaining so with age; partial veil membranose-floccose, fragile, easily dissociated, usually partly hanging high on stipe, often absent in mature specimens, partly adherent to lamellae and to the pileus margin, white; universal veil forming a resistant membranous volva, white to somewhat ochraceous on surface. Context white, unchanging with age. Herbarium samples remaining white, or faintly pale yellow at the center of the pileus; fresh specimens lack any smell, but they can develop a fungoid, unpleasant odor (especially in the bulb) with age; taste mild, fungoid. Reaction to 5% KOH chrome yellow on all surfaces and context except the inner layer of the volva.
Spores [2/78] mostly subglobose (50%), also broadly ellipsoid (30%) and rarely globose or ellipsoid (<10% each), (7.0–) 8.1–10.2 (–11.7) × (5.6–) 7.0–8.9 (–11) µm, Q = 1.1–1.2 (–1.4), Qav = 1.1, smooth, amyloid, with a dense yellow content in KOH at maturity, thin-walled and mostly collapsed on exsiccata. Basidia 4-spored, 32–52 × 9–12 µm, clavate, with a dense yellowish content. Subhymenium poorly developed, 10–15 µm wide, made up of small polygonal elements. Hymenophoral trama with a mediostratum made up of slender hyphae 2–7 µm wide mixed with numerous physaloid hyphae 18–25 µm wide arranged in diverging orientations; hymenopodium not differentiated. Lamellae edges thick, floccose, similar in structure to the partial veil. Pileipellis an ixocutis 100–120 µm thick, made up of slender hyphae measuring 2–4 µm wide, towards subpellis mixed with broader hyphae with yellow content, up to 9 µm wide. Subpellis not gelatinized, 60–80 µm thick, with parallel hyphae mixed with numerous oleipherous hyphae 3–4 µm wide with homogeneous yellow content in KOH. Context of the pileus distinct from subpellis, made up of intricate hyphae, often inflated up to 15 µm wide, without oleiferous hyphae. Partial veil homogeneous, pulverulent, made of mostly short cylindrical elements measuring 15–25 × 9–11 µm, mixed with cylindrical hyphae 4–7 µm wide, with slightly thickened yellowish walls. Universal veil 2-layered: outer layer thin, irregular, made up of hyphae embedded in a deep yellow extraparietal amorphous pigment, strongly thick-walled, wall up to 2 µm thick, deep yellow, with frequent cylindrical terminal elements, sometimes lobate, with rounded apex up to 6 µm wide; inner layer made up of rather broad hyphae, 7–14 µm wide, with very rare large sphaerocysts up to 85 × 55 µm, intricate, becoming more parallel towards the outer layer; no oleipherous hyphae observed.
Notes: In Europe, Amanita virosa cannot be easily confused with other species, being the only Amanita sect. Phalloideae growing on acidic organic soils, with a creamy ring and conical pileus. However, although being a strictly Eurasian species (as suggested by the sequences available in GenBank and UNITE databases), its name has been misapplied to various white extra-European species. Its neotypification with a collection from Sweden will fix the name and avoid further misidentifications.
Examined material: FRANCE: Nord, Condé-sur-Escaut, forêt domaniale de Bonsecours, 15 September 2013, R. Courtecuisse & P.-A. Moreau, PAM13091506 (LIP), not sequenced.
Other species examined: Amanita citrina Pers. Tent. disp. meth. fung. (Lipsiae): 66 (1797) (as Amanita phalloides var. larroquei F. Massart & Beauvais ex F. Massart, Bull. Soc. linn. Bordeaux 31(4): 223 (2004)). FRANCE: Landes, Bombannes, under Pinus pinaster in sand dune, Massart 05008, 0402241 (LIP, authentic material of A. phalloides var. larroquei). Amanita strobiliformis (Paulet) Bertill. Dict. Encyclop. Sci. Médic. (Paris) 1(3): 499 (1866) (as Amanita verna ss. Romagnesi [71]). FRANCE: Manche, Saint-Sauveur-le-Vicomte, église de Taillepied, in sand and gravels along a broadleaved wood, de Bernon & Guérin, 17 Aug 1983, coll. H. Romagnesi HR83.65a and HR83.65b (PC, as ‘A. verna’).
- Key to European species of sect. Phalloideae
- 1.
- KOH reaction bright yellow on surfaces…………………………………………………………2
- 1.
- KOH reaction none or only pale cream yellow…………………………………………………5
- 2.
- Stipe smooth. Ring complete, not lacerated at maturity, membranous, persistent on stipe, made up of mostly filamentous hyphae…………………………………………………………3
- 2.
- Stipe woolly-scaly. Ring thin, usually lacerate, hanging at margin or disappearing at maturity, mostly made up of short cylindrical elements. Smell none till late maturity, finally slightly nitrous. Spores mostly subglobose (Qav < 1.15). Moist, acidic, often peaty forests under conifers (Pinus, Picea) or broadleaved (Betula, Fagus)…………………A. virosa
- 3.
- Pileus soon flattened to depressed. Smell not perceptible in the bulb. Basidiomata white to ochraceous when dry [pileus yellowish green in ‘f. xanthoviridis’]. Subhymenium 30–45 µm thick, of puzzle-like structure. Spores mostly broadly elliptical (Qav > 1.15). Calcareous or mineral-rich soils, mostly under Quercus spp., usually in spring……………………A. verna
- 3.
- Pileus conical to campanulate……………………………………………………………………4
- 4.
- Strong smell of iodine in the bulb. Basidiomata turning uniformly lemon yellow when dry. Subhymenium poorly developed, ramose, less than 20 µm thick. Spores mostly subglobose (Qav < 1.15). Acidic, mesophilic forests, mostly under Quercus…………A. amerivirosa
- 4.
- Smell not of iodine. Basidiomata turning brownish when dry. Subhymenium well-developed, with puzzle-like elements. Spores mostly broadly ellipsoid (Qav > 1.15). Mediterranean forests…………………………………………………A. phalloides f. porrinensis
- 5.
- Fruiting in spring or early summer. KOH reaction dirty cream-yellow. Pileus white turning ochraceous at disc when old or dry, often gibbose at maturity. Under oaks on mineral-rich soils, in spring, Mediterranean…………………………………………A. vidua
- 5.
- Fruiting in autumn. KOH reaction nil or pale yellowish. Pileus usually greenish with dark fibrils (typical form), but also yellowish, ochraceous, greenish brown to pure white or with only faint greenish shade, convex to flattened but only exceptionally gibbose (A. phalloides f. porrinensis). Under various deciduous (especially Fagaceae) or coniferous (Abies, Cedrus, Pinus, Picea) trees on acidic to moderately calcareous soils, summer to early winter, Mediterranean to cold-temperate…………………………………………A. phalloides
3.3. Toxicology
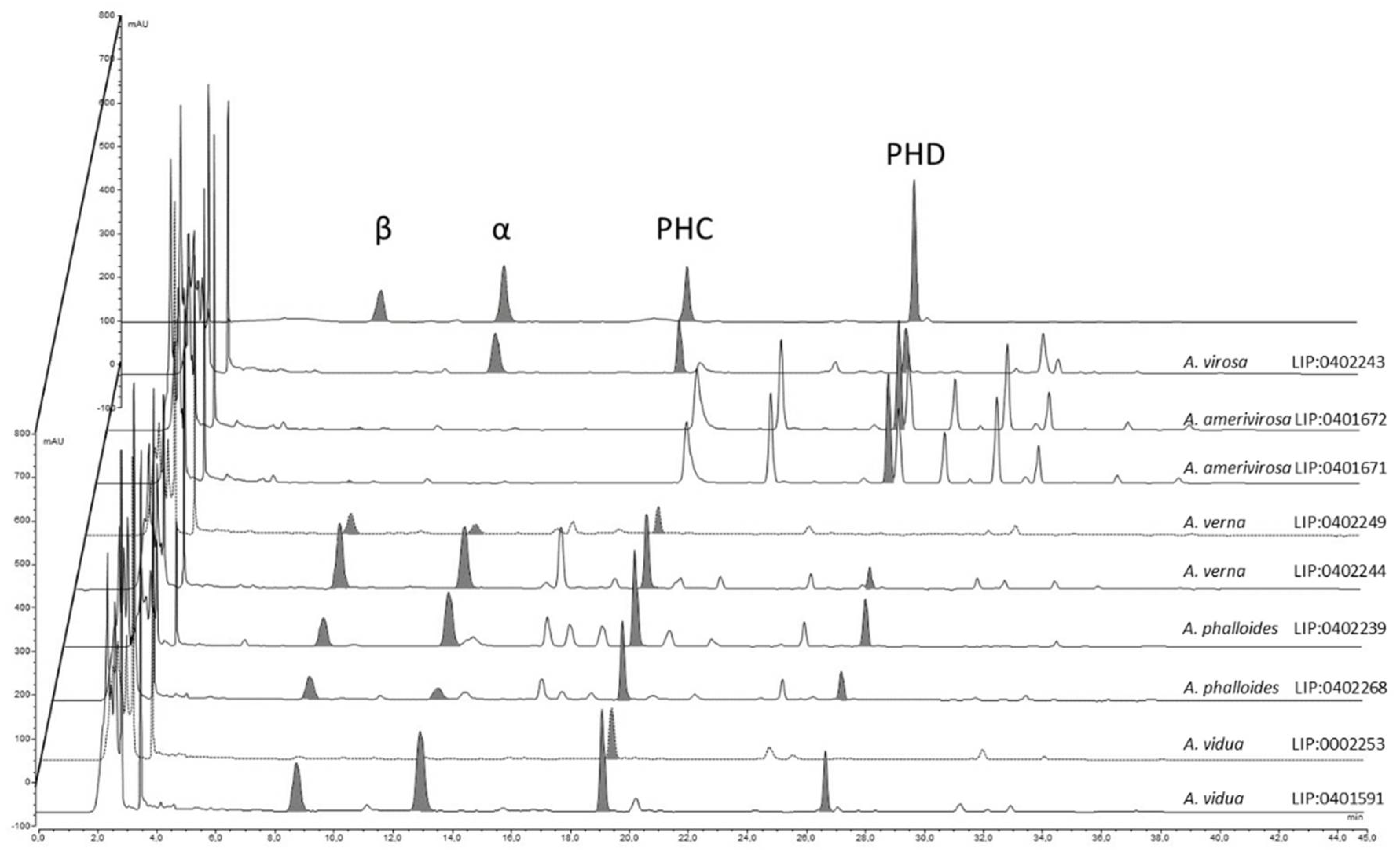
Results from the HPLC analysis (Figure 9) show that the contents of amatoxins and phallotoxins are strongly associated with the genetic identity of the samples. Amanita virosa contains α-amanitin, phallacidin, and phalloidin but lacks β-amanitin. This is partially in accordance with the literature [62,87,88,89]. However, according to other authors, some samples of A. virosa from North America [90,91] or Japan [92] contain β-amanitin, but no sequencing was performed to check the actual identity of these samples, which could represent other taxa. Samples of A. amerivirosa analyzed in the present work do not show amanitins or phallacidin, resembling the profile exhibited by other samples of A. virosa from North America [90]. Samples of A. verna analyzed in the present work contain large amounts of α- and β-amanitins (5.47 and 10.26 mg/g dry matter, respectively) and phallacidin (4.69 mg/g dry matter), and a lower amount of phalloidin (0.64 mg/g dry matter) in accordance with other works [25,89]. With regard to A. phalloides, different contents of α-amanitin were found in samples LIP:0402239 (from a Pinus pinaster forest with sandy soil) and SMM2018-10 (from a Quercus ilex stand with Arbutus unedo). Soil properties are thought to influence the toxicological profile of A. phalloides [58,93], and this could explain the differences found. However, Bon & Andary [94] did not find α- or β-amanitins by thin layer chromatography (TLC) in the type specimen of Amanita dunensis (=A. phalloides). The method of extraction and/or quantification (HPLC vs. TLC) might account for these differences. In addition, what remains of A. dunensis type collection at LIP (Bon 4119) is a half-specimen in bad condition, probably already very mature when collected. This could be another factor that influences the toxicological results. More samples from the Atlantic coast should be analyzed to check whether actual differences exist or not. Finally, the analyses conducted on specimens of A. vidua PAM1905041 at various developmental stages revealed low inter-specimen variations. Large amounts of all amatoxins (α-amanitin: 4.73–7.18 mg/g dry matter, β-amanitins: 5.02–8.50 mg/g dry matter) and phallotoxins were detected (phallacidin: 6.53–7.78 mg/g dry matter), suggesting that A. vidua is highly toxic and must be considered as another deadly ‘destroying angel’, maybe the most poisonous of the entire group.
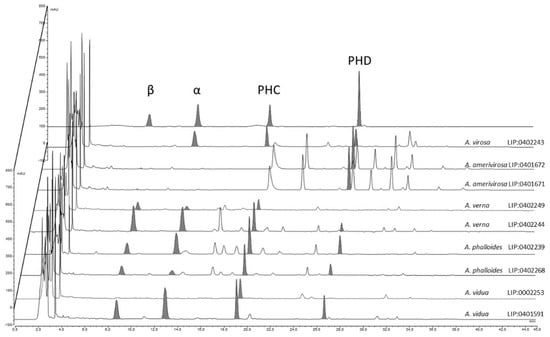
Figure 9.
Contents of amatoxins and phallotoxins in the different species of Mediterranean ‘destroying angels’ inferred from phylogenetic analysis: α = α-amanitin, β = β-amanitin, PHC = phallacidin, PHD = phalloidin.
3.4. Epidemiology
The analysis of records obtained from Spanish databases was divided into two periods: (1) spring period (from 1 February to 30 July), and (2) fall period (from 1 August to 31 January). These periods are clearly separated by the cold or dry periods when Amanita species do not fruit, i.e., mid-winter (January), in which no case of phalloidian poisoning was registered in any database, and mid-summer (July) with only one case known, attributable to A. verna. Ninety-four fungal poisoning clusters (≥2 patients), representing 406 symptomatic cases, were retrieved from the RENAVE database (1980–2020, source 1). Five clusters in the spring period (Feb–Jul) and ten clusters in the fall period contained cases compatible with phalloidian poisonings. Spring clusters led to 10 hospitalizations without registered deaths and originated in the provinces of Aragon (2 clusters, 5 cases), Andalusia (2 clusters, 3 cases), and Castile and León (1 cluster, 2 cases). During the fall (August-January), 10 clusters were retained as possibly phalloidian, representing 28 hospitalizations and 2 deaths. One fall case was attributed explicitly to Lepiota brunneoincarnata, while the others were either attributed to Amanita spp. or not identified in RENAVE; fall intoxications occurred in Andalusia (3 clusters, 9 cases), Castile-La Mancha (2 clusters, 4 cases), Catalonia (2 clusters, 4 cases), Aragon (1 cluster, 2 cases), Galicia (1 cluster, 2 cases), and Castile and León (1 cluster, 2 cases).
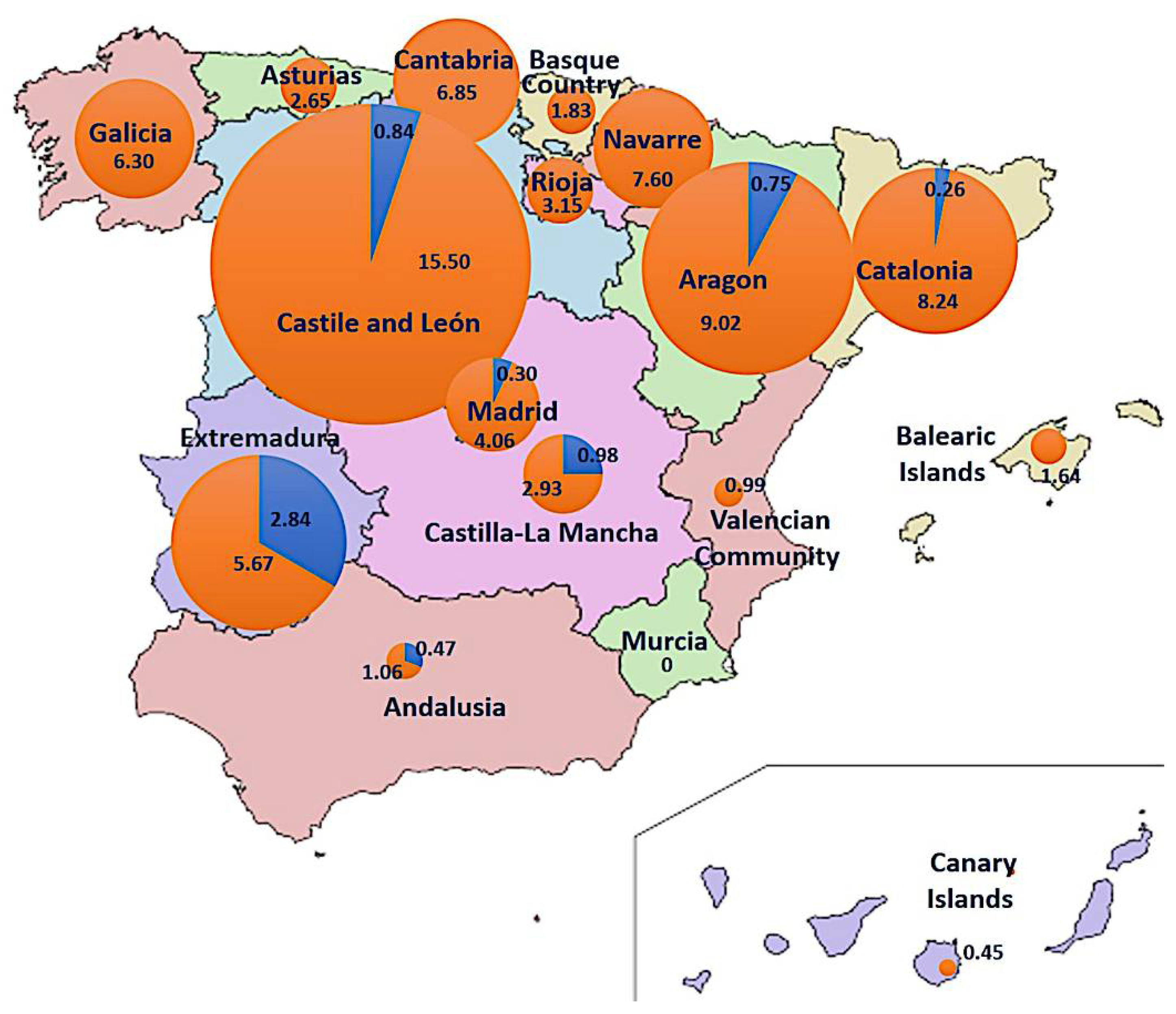
The CMBD (1997–2015) and RAE-CMBD (2016–2020) databases (source 2) contain 1817 cases of putative fungal intoxications, 219 of them presenting acute hepatitis, characteristic of the phalloidian syndrome. During the spring period (Feb–Jul), 16 cases were recorded, which originated in Andalusia (4), Extremadura (3), Madrid (2), Castile and León (2), Castile-La Mancha (2), Catalonia (2), and Aragon (1). No spring cases were recorded in 10/17 regions, including all those in the northwest, the Spanish Levant, and the Islands. During the fall (Aug–Jan), 203 cases were recorded all over Spain, mainly occurring in the Catalonia (64), Castile and León (37), Madrid (27), Galicia (17), Aragon (12), and Andalusia (9) regions; Murcia is the only community without any phalloidian cases in the fall. It is noticeable that all spring cases in Andalusia occurred in the western part of the region (Huelva, Seville) close to Extremadura, which has a spring incidence nearly 10 times higher than the national average. Global cumulative incidence of phalloidian poisonings in Spain for the 1997–2020 period was 4.8/1,000,000 hab. (0.34 in spring and 4.34 in fall), calculated from the population in the middle of the period (detailed for each community in Figure 10), and average cumulative incidence was 0.20/1,000,000 inhabit. per year.
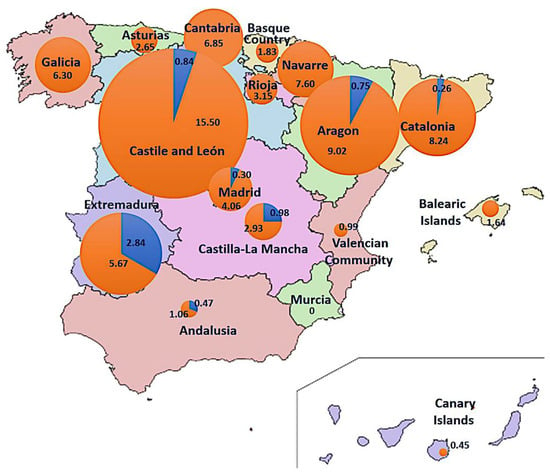
Figure 10.
Cumulative incidence of phalloidian poisonings in Spain per region and season (blue: spring, orange: autumn). Values indicate Incidence (per million of habitants) in 1997–2020 period (circumference radius are proportional to the incidence value).
4. Discussion
Amanita verna was originally described by Bulliard ([5], pl. 108) under the trinomial name Agaricus bulbosus vernus. The original plate illustrates four complete, entirely white basidiomes and a longitudinal section of the biggest one. In the extensive legend of the plate, the author describes a spring-fruiting Amanita with pure white, convex to depressed pileus, rounded bulb, and a well-formed membranous ring placed high on a smooth stipe. Bulliard indicates that the species is common in the woods around Paris, and cites a greenish form, which he later described in detail (pl. 506) under the name Agaricus bulbosus Bull. Shortly after this publication, the taxon was upgraded to species status by Lamarck [95] as Amanita verna (Bull.) Lam., with a similar description.
The history of the name Amanita verna, sanctioned by Fries [96] at species rank, has been reviewed by various authors, with opposing conclusions about the real identity of Bulliard’s species, according to personal interpretations of the aforementioned elements (Table 1). Konrad & Maublanc [76], for instance, interpreted A. verna as the pigmentless form of A. phalloides, also named A. phalloides var. alba or A. andaryi (invalid name), based on the fact that Bulliard included in his Ag. bulbosus white and yellow specimens without other distinction. However, most authors accepted A. verna as a vernal species, much rarer than mentioned by Bulliard (at least in Northern France), which differs from A. virosa by its smooth stipe.

Table 1.
Main interpretations of Amanita verna in literature.
In a posthumous publication (a catalogue of macrochemical reactions of many species without descriptions), Bataille [77] revealed a new feature observed in specimens identified by him as A. verna: the absence of reaction to 10% KOH on the pileus, in contrast to A. virosa, which turned bright chrome-yellow to this reagent. However, many authors after him reported a positive reaction on their own collections of A. verna [67,86,97], among others, and so did Trimbach [84], who accommodated the KOH+ samples in a separate variety, A. verna var. decipiens Trimb., expressing his own doubts about the taxonomic value of the KOH reaction. A couple of years later, Trimbach coined the name A. verna var. tarda for a variety found in Switzerland with less truncate lamellae, fruiting in autumn, and lacking any visible reaction to KOH.
Bertault [80,83] reported KOH- collections of A. verna with more or less uncinate lamellae in Mediterranean cork oak (Quercus suber) forests of Morocco. Contu [84] applied the name Amanita tarda to this taxon, retaining the name A. verna for the KOH+ species. The truncate lamellulae emphasized by Trimbach [98] for A. verna var. decipiens were discussed and re-evaluated by Russi & Josserand [97] in a detailed study of KOH+ A. verna from the Lyon area. Specimens of KOH- Amanita verna were known only from southern Europe and Morocco, until Romagnesi [75] published an initially convincing record from Normandy, northern France (coll. HR83.65). However, after a long discussion, Romagnesi concluded that KOH- A. verna was a thermophilic species unlikely to have been seen by Bulliard around Paris, suggesting keeping the name A. verna for the KOH+ species, and applying the name A. verna f. ellipsospora E.-J. Gilbert to his KOH- collection. Neville & Poumarat [38] again considered A. verna as KOH- after checking three collections sent to them from southern France and employed the name A. verna var. decipiens for KOH+ collections.
In the present work, KOH+ and KOH- specimens of A. verna are shown to belong to distinct species. KOH- specimens are typically found in Mediterranean habitats of southern Europe (apparently only in acidic soils), while KOH+ samples are found in Mediterranean and temperate climates. Unfortunately, we could not find any preserved specimens of A. verna from near Paris after 1971. Guy Redeuilh (pers. comm. to P.-A. Moreau) reported that A. decipiens/KOH+ A. verna was usually collected in some relict oak woodland in sandy places along the Seine River some 30 years ago; the extensive urbanization during the last century destroyed most of the favorable places where Bulliard might have observed it. One KOH+ collection from Oise (North of Paris), HR56.31, is proposed here as an epitype to fix this ancient taxonomic issue. Amanita verna var. tarda is interpreted as a synonym of A. phalloides var. alba, as suggested by Neville & Poumarat ([38], pag. 567); Romagnesi also misidentified one of his collections of A. phalloides var. alba as Amanita verna (PC:HR60.109).
The KOH- samples are here named A. vidua, since they lack any other existing name. The spectacular yellow reaction to KOH displayed by A. verna is present also in A. virosa and A. amerivirosa, and therefore this macrochemical feature could have been present in the common ancestor of the whole ‘North Hemisphere lineage’ but lost in A. phalloides (although somewhat present in f. porrinensis) and strongly weakened in A. vidua. This reaction appears under optical microscope to be mainly located at the abundant epiparietal mucoid deposits on most hyphae, and extends to spore content, which shows a yellowish lipidic content in 5% KOH, in all species with the exception of A. phalloides, and is only scarcely visible in A. vidua. The spores themselves have a distinctive shape, mainly subglobose in A. amerivirosa and A. virosa (Qav = 1–1.15), mainly broadly ellipsoid with a higher Qav (1.13–1.22) and usually narrower (<7.8 µm wide) in A. phalloides and A. vidua. Amanita verna has a remarkable variability in spore shape, observed among different collections as described above (see Figure 6A,C,D), but also among spores obtained from the same basidiome as illustrated by Malençon & Bertault [79].
The four toxins studied in the present work are apparently occurring across the whole sect. Phalloideae, suggesting that all species are probably toxic, but there are some remarkable differences among them. In the virosa clade, β-amanitin is not detectable or at a very low concentration, and A. amerivirosa has a very low content of α-amanitin, completely lacking phallacidin. In addition, chromatograms of A. amerivirosa display some peaks absent in A. virosa, with UV spectra corresponding to compounds lacking 6-hydroxytryptophan [62]. The identification of these compounds would be of great toxicological interest. The actual amount of toxins is highly dependent on the extraction conditions, with major impacts of solvent, temperature, and processing times during the maceration step. Freshness of the samples analyzed also influences the results of HLPC, but little information exists about the degradation of amatoxins and phallotoxins during storage. Stijve & Seeger [99] observed a decrease in amanitins (α- and β-) and phalloidin contents over time in specimens collected in the same location in different years and suggest that phallotoxins might be less stable during storage than amanitins, whose stability is known to depend on treatment and storage of the samples [100,101,102]. Results obtained in the present work support this hypothesis, since at least α- and β-amanitins seem less abundant in older specimens (i.e., A. vidua from 1987 vs. A. verna from 2001). However, these observations were not the purpose of the present work, and therefore a more detailed study should be carried out to further clarify this issue.
Amanita amerivirosa has been reported from western France for more than 30 years, first as A. decipiens [103], then as ‘American A. virosa’, and finally named A. virosa var. levipes [38]. Carefully surveyed by local mycologists who could follow its fast expansion from the Nantes area to Bordeaux and Normandy [104,105], it has become especially abundant in the last 15 years, recently reaching the Paris area [106]. Fortunately, in spite of the abundance of this species in western France, the number of poisoning reports caused by A. amerivirosa in France is still limited [2,107], with only two cases confirmed in 2018 (identified as A. virosa var. levipes; Moreau, unpublished data), and they lacked associated symptomatology. This could be related to the low amount of toxins detected in the collections of A. amerivirosa studied by Beutler & Der Marderosian [90], Bonnet & Basson [91], and the present work. However, this needs to be confirmed by additional analyses, and so this species should not be excluded from the list of lethal ‘destroying angels’ for the time being. On the other hand, A. vidua and A. verna are evidently highly poisonous on the basis of the amatoxins detected in these species. Both species may grow in the same sites and season as A. ponderosa Malençon & R. Heim (the so-called “gurumelo”), a highly prized edible fungus endemic in southern Portugal and south-western Spain (Huelva, Badajoz, Cáceres; [74,108]). This could explain the abnormally high incidence of spring intoxications compared to fall intoxications in the Spanish provinces of Extremadura and Andalusia (Figure 10). Amanita boudieri Barla (Amanita sect. Lepidella), which can cause another lethal syndrome (acute renal failure; [109]), has also been mistaken for A. ponderosa but differs morphologically by the absence of a membranous volva and ring [108].
The average incidence of phalloidian poisonings (mostly due to lethal Amanita species) in Spain during the period analyzed in the present work was 0.20/1,000,000 inhabit. per year. This value is comparable to botulism (0.20 per year in the last decade; [110]), another food-borne disease, and thus represents a comparable challenge for public health. More detailed and homogeneous data on similar poisoning cases in Europe, as well as more precise identification of the responsible species, would be necessary to evaluate and manage the risk, which seems to differ between regions. Especially, information campaigns targeting unexperienced mushroom collectors should warn them of the presence of the lethal ‘destroying angels’. Since A. vidua can be found in the whole Mediterranean basin (from Spain to Levant, and probably also in North Africa) and shows a high toxicity, a special focus should be placed on this species in the entire area. However, spring intoxications are apparently not relevant in some areas, such as Apulia (southern Italy; [111]), suggesting that either A. vidua and A. verna are not present there, or else that in the absence of similar edible early-fruiting species or of local picking traditions attached to them, they are not accidentally consumed.
5. Conclusions
- (a)
- Five distinct species of Amanita section Phalloideae can be found in Mediterranean Europe: A. phalloides, A. virosa, A. verna, A. amerivirosa, and A. vidua sp. nov., a new name proposed for the KOH-negative Mediterranean species previously described as A. verna or A. decipiens by various authors.
- (b)
- Three taxa, Amanita decipiens, A. porrinensis, and A. virosa var. levipes are here considered later heterotypic synonyms of A. verna, A. phalloides, and A. amerivirosa, respectively.
- (c)
- Samples identified with the provisional name ‘Amanita verna f. xanthoviridis’ are not genetically different from A. verna f. verna, suggesting that this species can produce distinctly colored basidiomata.
- (d)
- All Mediterranean species of Amanita sect. Phalloideae contain amatoxins and phallotoxins, although their identity and quantity differ between species. The newly described A. vidua is the most amanitin-rich European species of the section. No amatoxin was detected in the analyzed samples of Amanita amerivirosa from western France.
- (e)
- In Spain, the incidence of mushroom poisonings in spring attributed to species of Amanita sect. Phalloideae is about 10 times higher in regions where other similar edible species are collected in the same season, representing up to 1/3 of all mushroom poisoning during the year in these regions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology11050770/s1, Supplementary Table S1: Sequences employed in the phylogenetic analysis; Supplementary Table S2: Amatoxins present in the species inferred by phylogenetic analyses; Supplementary Figure S1: Calibration curves of toxins analyzed.
Author Contributions
Conceptualization, A.G.-I., G.M. and P.-A.M.; methodology, P.A., J.-M.B., X.C., M.B.D.-K., A.G.-I., M.G., J.L.M., P.-A.M., S.M. and S.R.; formal analysis, P.A. and J.-M.B.; writing—original draft preparation, P.A., A.G.-I., G.M. and P.-A.M.; writing—review and editing, P.A., A.G.-I., G.M., S.M. and S.R. All authors have read and agreed to the published version of the manuscript.
Funding
Part of the work was financed by the Spanish Ministry of Education and Culture through the FPU grant AP2006-00890 and the “Subprograma AGR del Ministerio de Ciencia y Innovación (Plan Nacional I + D+i)” research project AGL2009-12884-C03-03, and the Associazione Micologica Ecologica Romana (AMER) APS, which economically covered the expenses of the sequences obtained from the Italian material of Amanita verna and A. vidua.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors express their gratitude to Jean Alesandri, Didier Borgarino, René Chéreau, Anders Dahlberg, Prudencio Garcia, Jacques Guinberteau, Nathalie Journe, Alberto Mua, Marco Raumi and Gilbert Ouvrard for field collaboration or for sharing their samples for the present study, and Régis Courtecuisse and Raphaël Hervé for bibliographic and nomenclatural complements. The following curators (and their herbaria) are also acknowledged for their collaboration: Javier Rejos (AH), Francisco Javier Silva (LOU), Margarita Dueñas (MA), Olivier Gerriet (NICE), Marisa Castro (Oporto) and Bart Buyck (PC). Ibai Olariaga obtained sequences of Amanita porrinensis (including the holotype). The authors thank also Luis Monje and Ángel Pueblas of the Department of Drawing and Scientific Photography at the Alcalá University for their help in the digital preparation of the photographs. The editions Klincksieck (Paris) are acknowledged for the permission of reproduction of some figures published in the book “Les Tueurs” by P. Reumaux and X. Carteret (2013) in Figure 3. Laetitia Marey (Société Mycologique de France) is also acknowledged for kindly providing bibliographic assistance, and Geoffrey Kibby for correcting the language of the final draft. Authors finally thank the Spanish Ministerio de Sanidad, Consumo y Bienestar Social for the data obtained from the Registro de Actividad de Atención Sanitaria Especializada (CMBD and RAE-CMB), as well as the Ministerio de Ciencia e Innovación-Centro Nacional de Epidemiología for the data obtained from the Sistema de Vigilancia de la Red Nacional de Vigilancia Epidemiológica (RENAVE, SiViEs platform).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brandenburg, W.E.; Ward, K.J. Mushroom poisoning epidemiology in the United States. Mycologia 2018, 110, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Sinno-Tellier, S.; Bruneau, C.; Daoudi, J.; Greillet, C.; Verrier, A.; Bloch, J. Surveillance nationale des intoxications alimentaires par des champignons: Bilan des cas rapportés au réseau des centres antipoison de 2010 à 2017 en France métropolitaine. Bull Epidemiol. Hebd. 2019, 33, 666–678. [Google Scholar]
- Horowitz, B.Z.; Moss, M.J. Amatoxin Mushroom Toxicity; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Vaillant, S. Botanicon Parisiense, ou Dénombrement par Ordre Alphabétique des Plantes qui se Trouvent aux Environs de Paris; Verbeek, J., Verbeek, H., Lakeman, B., Eds.; A.-C. Briasson: Leiden/Amsterdam, The Netherlands, 1727; pp. 1–205. [Google Scholar]
- Bulliard, J.B.F. Herbier de la France; Bulliard, J.B.G., Ed.; “Chez l’auteur, Didot, Debure et Belin”: Paris, France, 1782–1783; Volume 3, pp. 97–128. [Google Scholar]
- Fries, E.M. Epicrisis Systematis Mycologici; Gleerup, C.W.K., Ed.; “Typographia Academica”: Uppsala, Sweden, 1838; pp. 1–610. [Google Scholar]
- Ye, Y.; Liu, Z.; Zhao, M. CLIF-OF > 9 predicts poor outcome in patients with Amanita phalloides poisoning. Am. J. Emerg. Med. 2021, 39, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Marinov, P.; Bonchev, G.; Ivanov, D.; Zlateva, S.; Dimitrova, T.; Georgiev, K. Mushrooms intoxications. J. IMAB 2018, 24, 1887–1890. [Google Scholar] [CrossRef]
- Piqueras, J. Actualidades en intoxicaciones por setas. Butl. Soc. Catalana Micol. 1983, 7, 19–23. [Google Scholar]
- Pelclová, D.; Rakovcová, H. Amanita phalloides poisoning queries recorded at the toxicology information center in Prague. Cas. Lek. Ces. 1993, 132, 470–472. [Google Scholar]
- Lecot, J.; Bruneau, C.; Courtois, A.; Vodovar, D.; Landreau, A.; Le Roux, G.; D’Escatha, A. Syndrome phalloïdien: Série rétrospective de 204 cas. Toxicol. Anal. Clin. 2021, 33, S13–S39. [Google Scholar] [CrossRef]
- Cervellin, G.; Comelli, I.; Rastelli, G.; Sanchis-Gomar, F.; Negri, F.; De Luca, C.; Lippi, G. Epidemiology and clinics of mushroom poisoning in Northern Italy: A 21-year retrospective analysis. Hum. Exp. Toxicol. 2018, 37, 697–703. [Google Scholar] [CrossRef]
- Giusti, A.; Ricci, E.; Gasperetti, L.; Galgani, M.; Polidori, L.; Verdigi, F.; Narducci, R.; Armani, A. Building of an Internal Transcribed Spacer (ITS) gene dataset to support the Italian Health Service in mushroom identification. Foods 2021, 10, 1193. [Google Scholar] [CrossRef]
- Wennig, R.; Eyer, F.; Schaper, A.; Zilker, T.; Andresen-Streichert, H. Mushroom poisoning. Dtsch. Ärztebl. 2020, 117, 701–708. [Google Scholar] [CrossRef]
- Ahn, C.; Kang, H.; Lim, T.H.; Oh, J. Poisoning due to ingestion of amatoxin-containing mushrooms in South Korea: A systematic review and meta-analysis. Signa Vitae 2021, 17, 25–33. [Google Scholar]
- Keller, S.A.; Klukowska-Rötzler, J.; Schenk-Jaeger, K.M.; Kupferschmidt, H.; Exadaktylos, A.K.; Lehmann, B.; Liakoni, E. Mushroom poisoning—A 17 year retrospective study at a level I University Emergency Department in Switzerland. Int. J. Environ. Res. Public Health 2018, 15, 2855. [Google Scholar] [CrossRef] [PubMed]
- Yardan, T.; Baydin, A.; Eden, A.O.; Akdemir, H.U.; Aygun, D.; Acar, E.; Arslan, B. Wild mushroom poisonings in the Middle Black Sea region in Turkey: Analyses of 6 years. Hum. Exp. Toxicol. 2010, 29, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Sarawi, S.; Shi, Y.-N.; Lotz-Winter, H.; Reschke, K.; Bode, H.B.; Piepenbring, M. Occurrence and chemotaxonomical analysis of amatoxins in Lepiota spp. (Agaricales). Phytochemistry 2022, 195, 113069. [Google Scholar] [CrossRef]
- Enjalbert, F.; Gallion, C.; Jehl, F.; Monteil, H.; Faulstich, H. Amatoxins and phallotoxins in Amanita species: High-performance liquid chromatographic determination. Mycologia 1993, 85, 579–584. [Google Scholar] [CrossRef]
- Enjalbert, F.; Rapior, S.; Nouguier-Soulé, J.; Guillon, S.; Amouroux, N.; Cabot, C. Treatment of amatoxin poisoning: 20-year retrospective analysis. J. Toxicol. Clin. Toxicol. 2002, 40, 715–757. [Google Scholar] [CrossRef]
- Enjalbert, F.; Cassanas, G.; Rapior, S.; Renault, C.; Chaumont, J.P. Amatoxins in wood-rotting Galerina marginata. Mycologia 2004, 96, 720–729. [Google Scholar] [CrossRef]
- Xiang, H.; Zhou, Y.; Zhou, C.; Lei, S.; Yu, H.; Wang, Y.; Zhu, S. Investigation and analysis of Galerina sulcipes poisoning in a canteen. Clin. Toxicol. 2018, 56, 365–369. [Google Scholar] [CrossRef]
- Landry, B.; Whitton, J.; Bazzicalupo, A.L.; Ceska, O.; Berbee, M.L. Phylogenetic analysis of the distribution of deadly amatoxins among the little brown mushrooms of the genus Galerina. PLoS ONE 2021, 16, e0246575. [Google Scholar] [CrossRef]
- Hazani, E.; Taitelman, U.; Shasha, S.M. Amanita verna poisoning in Israel-Report of a rare case out of time and place. Arch. Toxicol. 1983, 6, 186–189. [Google Scholar]
- Yilmaz, I.; Kaya, E.; Sinirlioglu, Z.A.; Bayram, R.; Surmen, M.G.; Colakoglu, S. Clinical importance of toxin concentration in Amanita verna mushroom. Toxicon 2014, 87, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, M.; Afshari, A.; Arsene, A.L.; Mégarbane, B.; Dumanov, J.; Bastos Paoliello, M.M.; Tsatsakis, A.; Carvalho, F.; Hashemzaei, M.; Karimi, G.; et al. Toxicological profile of Amanita virosa-A narrative review. Toxicol. Rep. 2019, 6, 143–150. [Google Scholar] [CrossRef]
- Khatir, I.G.; Hosseininejad, S.M.; Ghasempouri, S.K.; Asadollahpoor, A.; Moradi, S.; Jahanian, F. Demographic and epidemiologic evaluation of mushroom poisoning: A retrospective study in 4-year admissions of Razi Hospital (Qaemshahr, Mazandaran, Iran). Med. Glas. 2020, 17, 117–122. [Google Scholar]
- Petekkaya, S.; Börk, T.; Ayaz, N.; Göktürk, C.; Şamdancı, E.; Celbiş, O. Fatal mushroom poisoning in Syrian refugees. Azerbaijan Med. Assoc. J. 2016, 1, 30–35. [Google Scholar] [CrossRef]
- Euronews. Available online: https://www.euronews.com/2021/09/02/afghan-refugee-5-dies-after-eating-poisonous-mushrooms-in-poland (accessed on 2 September 2021).
- Preston, J.F.; Stark, H.J.; Kimbrough, J.W. Quantitation of amanitins in Amanita verna with calf thymus RNA polymerase B. Lloydia 1975, 38, 153–161. [Google Scholar]
- Cai, Q.; Tulloss, R.E.; Tang, L.P.; Tolgor, B.; Zhang, P.; Chen, Z.H.; Yang, Z.L. Multi-locus phylogeny of lethal amanitas: Implications for species diversity and historical biogeography. BMC Evol. Biol. 2014, 14, 143. [Google Scholar] [CrossRef]
- Cai, Q.; Cui, Y.-Y.; Yang, Z.L. Lethal Amanita species in China. Mycologia 2016, 108, 993–1009. [Google Scholar] [CrossRef]
- Cui, Y.-Y.; Cai, Q.; Tang, L.-P.; Liu, J.-W.; Yang, Z.L. The family Amanitaceae: Molecular phylogeny, higher-rank taxonomy and the species in China. Fungal Divers. 2018, 91, 5–230. [Google Scholar] [CrossRef]
- Codjia, J.E.I.; Cai, Q.; Zhou, S.W.; Luo, H.; Ryberg, M.; Yorou, N.S.; Yang, Z.L. Morphology, multilocus phylogeny, and toxin analysis reveal Amanita albolimbata, the first lethal Amanita species from Benin, West Africa. Front. Microbiol. 2020, 11, 599047. [Google Scholar] [CrossRef]
- Fraiture, A.; Amalfi, M.; Raspé, O.; Kaya, E.; Akata, I.; Degreef, J. Two new species of Amanita sect. Phalloideae from Africa, one of which is devoid of amatoxins and phallotoxins. MycoKeys 2019, 53, 93–125. [Google Scholar]
- Codjia, J.E.I.; Wang, P.M.; Ryberg, M.; Yorou, N.S.; Yang, Z.L. Amanita sect. Phalloideae: Two interesting non-lethal species from West Africa. Mycol. Progr. 2022, 21, 39. [Google Scholar]
- Tulloss, R.E.; Kudzma, L.V.; Tulloss, M.K.; Rockefeller, A. Amanita amerivirosa—A new toxic North American species of Amanita section Phalloideae. Amanitaceae 2021, 1, 1–15. [Google Scholar]
- Neville, P.; Poumarat, S. Amaniteae. Amanita, Limacella & Torrendia. Fungi Europaei; Candusso, M., Ed.; Edizioni Candusso: Alassio, Italy, 2004; pp. 1–1120. [Google Scholar]
- Bas, C. Morphology and subdivision of Amanita and a monograph on its section Lepidella. Persoonia 1969, 5, 385–579. [Google Scholar]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef]
- Mullis, K.; Faloona, F.A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987, 155, 335–350. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for Basidiomycetes-Application to the identification of mycorrhizae and rust. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically ampliWed ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Cubeta, M.A.; Echandi, E.; Abernethy, T.; Vilgalys, R. Characterization of anastomosis groups of binucleate Rhizoctonia species using restriction analysis of an amplified ribosomal RNA gene. Phytopathology 1991, 81, 1395–1400. [Google Scholar] [CrossRef]
- van Tuinen, D.; Zhao, B.; Gianinazzi-Pearson, V. PCR studies of AM fungi, from studies to application. In Mycorrhiza Manual; Varma, A., Ed.; Springer: Heidelberg, Germany, 1998; pp. 387–399. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Weiß, M.; Yang, Z.L.; Oberwinkler, F. Molecular phylogenetic studies in the genus Amanita. Can. J. Bot. 1998, 76, 1170–1179. [Google Scholar]
- Oda, T.; Tanaka, C.; Tsuda, M. Molecular phylogeny of Japanese Amanita species based on nucleotide sequences of the internal transcribed spacer region of nuclear ribosomal DNA. Mycoscience 1999, 40, 57–64. [Google Scholar] [CrossRef]
- Drehmel, D.; Moncalvo, J.M.; Vilgalys, R. Molecular phylogeny of Amanita based on large-subunit ribosomal DNA sequences: Implications for taxonomy and character evolution. Mycologia 1999, 91, 610–618. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Yang, Z. Molecular phylogeny of eastern Asia species of Amanita (Agaricales, Basidiomycota): Taxonomic and biogeographic implications. Fungal Divers. 2004, 17, 219–238. [Google Scholar]
- Zhang, P.; Chen, Z.H.; Xiao, B.; Tolgor, B.; Bao, H.Y.; Yang, Z.L. Lethal amanitas of East Asia characterized by morphological and molecular data. Fungal Divers. 2010, 42, 119–133. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP *. Phylogenetic Analysis Using Parsimony (*and Other Methods); Sinauer Associates: Sunderland, MA, USA, 2003; Version 4. [Google Scholar]
- Nylander, J.A.A. MrModeltest v2; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Enjalbert, F.; Cassanas, G.; Salhi, S.L.; Guinchard, C.; Chaumont, J.P. Distribution of the amatoxins and phallotoxins in Amanita phalloides. Influence of the tissues and the collection site. Comptes Rendus Séances Acad. Sci. Sér. 3 Sci. Vie 1999, 322, 855–862. [Google Scholar] [CrossRef]
- Kaya, E.; Yilmaz, I.; Sinirlioglu, Z.A.; Karahan, S.; Bayram, R.; Yaykasli, K.O.; Colakoglu, S.; Saritas, A.; Severoglu, Z. Amanitin and phallotoxin concentration in Amanita phalloides var. alba mushroom. Toxicon 2013, 76, 225–233. [Google Scholar] [CrossRef]
- Enjalbert, F.; Gallion, C.; Jehl, F.; Monteil, H. Simultaneous assay for amatoxins and phallotoxins in Amanita phalloides Fr. by high-performance liquid chromatography. J. Chromatogr. 1992, 598, 227–236. [Google Scholar] [CrossRef]
- McKnight, T.A.; McKnight, K.B.; Skeels, M.C. Amatoxin and phallotoxin concentration in Amanita bisporigera spores. Mycologia 2010, 102, 763–765. [Google Scholar] [CrossRef] [PubMed]
- Sgambelluri, R.M.; Epis, S.; Sassera, D.; Luo, H.; Angelos, E.R.; Walton, J.D. Profiling of amatoxins and phallotoxins in the genus Lepiota by liquid chromatography combined with UV absorbance and mass spectrometry. Toxins 2014, 6, 2336–2347. [Google Scholar] [CrossRef] [PubMed]
- Morel, S.; Fons, F.; Rapior, S.; Dubois, V.; Vitou, M.; Portet, K.; Doré, J.-C.; Poucheret, P. Decision-making for the detection of amatoxin poisoning: A comparative study of standard analytical methods. Cryptogam. Mycol. 2016, 37, 217–239. [Google Scholar] [CrossRef]
- Davison, E.M.; Giustiniano, D.; Busetti, F.; Gates, G.M.; Syme, K. Death cap mushrooms from southern Australia: Additions to Amanita (Amanitaceae, Agaricales) section Phalloideae Clade IX. Aust. Syst. Bot. 2017, 30, 371–389. [Google Scholar] [CrossRef]
- Morini, S.; Gennari, A.; Agnello, C. Amanita sabulicola, una nuova specie descritta dal Salento (Puglia-Italia). Riv. Micol. 2020, 62, 239–251. [Google Scholar]
- Davison, E.M.; Giustiniano, D.; Bougher, N.L.; McGurk, L.E.; Watkin, E.L.J. Additions to Amanita (Amanitaceae, Agaricales) section Arenariae from south-western Australia. Aust. Syst. Bot. 2021, 34, 541–569. [Google Scholar] [CrossRef]
- Marchand, A. Champignons du Nord et du Midi. Tome 1: Les Meilleurs Comestibles et les Principaux Vénéneux; Marchand, A., Ed.; Diffusion Hachette: Perpignan, France, 1971; pp. 1–282. [Google Scholar]
- Moreno, G.; Manjón, J.L. Guía de Hongos de la Península Ibérica; Omega: Barcelona, Spain, 2010; pp. 1–1417. [Google Scholar]
- Freire, L.; Castro, M.L. Nueva especie del género “Amanita” Anales Jard. Bot. Madrid 1987, 44, 533–534. [Google Scholar]
- Castro, M.L. Amanita porrinensis L. Freire et M.L. Castro, estudio comparativo con otros taxons da sección Phalloideae (Fr.) Quél. Mykes 1998, 1, 57–59. [Google Scholar]
- Neville, P.; Poumarat, S.; Monterumici, G. Una rara Amanita della sezione Phalloideae, nuova per l’Italia: Amanita porrinensis. Boll. Gr. Micol. G. Bresadola Trento 2000, 43, 143–150. [Google Scholar]
- Miceli, A. Amanita porrinensis, una specie rarissima ritrovata sui Monti Peloritani. MicoPonte 2019, 12, 27–33. [Google Scholar]
- Gasparini, F.; Serafin, F. La quarta Amanita mortale. Boll. Gr. Micol. G. Bresadola Trento Sez. Vincenza 2012, 32, 40–44. [Google Scholar]
- Moreno, G.; Platas, G.; Peláez, F.; Bernedo, M.; Vargas, A.; Daza, A.; Santamaría, C.; Camacho, M.; de la Osa, L.R.; Manjón, J.L. Molecular phylogenetic analysis shows that Amanita ponderosa and A. curtipes are distinct species. Mycol. Progr. 2008, 7, 41–47. [Google Scholar] [CrossRef]
- Romagnesi, H. Contribution à la solution du problème d’Amanita verna Bull. Bull. Soc. Mycol. Franc. 1984, 100, 237–241. [Google Scholar]
- Konrad, P.; Maublanc, A. Icones Selectae Fungorum, Vols. I-IV, Encyclopédie Mycologique 1; P. Lechevallier: Paris, France, 1924–1937. [Google Scholar]
- Bataille, F. Les réactions macrochimiques chez les champignons: Suivies d’indications sur la morphologie des spores. Bull. Soc. Mycol. France 1947, 63 (Suppl. Sl), 1–172. [Google Scholar]
- Gilbert, E.-J. Iconographia mycologica, Amanitaceae. Iconogr. Mycol. 1941, 27 (Suppl. Sl), 203–427. [Google Scholar]
- Malençon, G.; Bertault, R. Flore des champignons supérieurs du Maroc, tome 1. Trav. Inst. Sci. Chérifien Sér. Bot. Biol. 1970, 32, 1–601. [Google Scholar]
- Bertault, R. Amanites du Maroc (deuxième contribution). Bull. Soc. Mycol. Franc. 1965, 81, 345–371. [Google Scholar]
- Bertault, R. Amanites du Maroc. Bull. Soc. Mycol. Franc. 1964, 80, 364–384. [Google Scholar]
- Raumi, M. Amanita verna f. xantho-viridis, ad Interim. Available online: www.gruppomicologicocecinese.it/SchedaFunghi.php?id=933 (accessed on 1 March 2022).
- Bertault, R. Amanites du Maroc (troisième contribution et additions). Bull. Soc. Mycol. Franc. 1980, 96, 271–286. [Google Scholar]
- Trimbach, J. Amanita verna variété decipiens n. var. Riviera Sci. 1970, 1, 15–18. [Google Scholar]
- La Chiusa, L. Amanite bianche tossiche. Boll. Gr. Micol. G. Bresadola Trento 2000, 43, 109–120. [Google Scholar]
- Contu, M. Saggio di una ciave per la determinazione delle specie del genere Amanita osservate in Sardegna. Boll. Gr. Micol. G. Bresadola Trento 2000, 43, 67–86. [Google Scholar]
- Tang, S.; Zhou, Q.; He, Z.; Luo, T.; Zhang, P.; Cai, Q.; Yang, Z.; Chen, J.; Chen, Z. Cyclopeptide toxins of lethal amanitas: Compositions, distribution and phylogenetic implication. Toxicon 2016, 120, 78–88. [Google Scholar] [CrossRef]
- Wei, J.; Wu, J.; Chen, J.; Wu, B.; He, Z.; Zhang, P.; Li, H.; Sun, C.; Liu, C.; Chen, Z.; et al. Determination of cyclopeptide toxins in Amanita subpallidorosea and Amanita virosa by high-performance liquid chromatography coupled with high-resolution mass spectrometry. Toxicon 2017, 133, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Walton, J. The Cyclic Peptide Toxins of Amanita and other Poisonous Mushrooms. Chapter 3. In Distribution and Taxonomic Variation in the Amanita Cyclic Peptide Toxins; Springer International Publishing AG: New York, NY, USA, 2018; pp. 59–91. [Google Scholar]
- Beutler, J.A.; Der Marderosian, A.H. Chemical variation in Amanita. J. Nat. Prod. 1981, 44, 422–431. [Google Scholar] [CrossRef]
- Bonnet, M.S.; Basson, P.W. The toxicology of Amanita virosa: The destroying angel. Homeopathy 2004, 93, 216–220. [Google Scholar] [CrossRef]
- Ahmed, W.H.A.; Gonmori, K.; Suzuki, M.; Watanabe, K.; Suzuki, O. Simultaneous analysis of α-amanitin, β-amanitin, and phalloidin in toxic mushrooms by liquid chromatography coupled to time-of-flight mass spectrometry. Forensic Toxicol. 2010, 28, 69–76. [Google Scholar] [CrossRef]
- Garcia, J.; Oliveira, A.; de Pinho, P.G.; Freitas, V.; Carvalho, A.; Baptista, P.; Pereira, E.; Bastos, M.D.L.; Carvalho, F. Determination of amatoxins and phallotoxins in Amanita phalloides mushrooms from northeastern Portugal by HPLC-DAD-MS. Mycologia 2015, 107, 679–687. [Google Scholar] [CrossRef]
- Bon, M.; Andary, C. A new species of Amanita: Amanita dunensis Heim ex Bon et Andary. Doc. Mycol. 1983, 50, 13–14. [Google Scholar]
- Lamarck, J.-B.M. Encyclopédie Méthodique. Botanique. Tome Premier, Partie 1; Panckoucke: Paris, France, 1783; pp. 1–344. [Google Scholar]
- Fries, E.M. Systema Mycologicum I; “Ex Officina Berlingiana”: Lund, Sweden, 1821. [Google Scholar]
- Russi, M.; Josserand, M. Etude sur Amanita verna (Bull.) Persoon (Basidiomycète Agaricales) récoltée dans la région lyonnaise. Bull. Mens. Soc. Linn. Lyon 1983, 52, 6–10. [Google Scholar] [CrossRef]
- Trimbach, J. Amanita verna (Bull. ex Fr.) ss. str. et ses variétés. Ann. Mus. Hist. Nat. Nice 1972, 72, 83–86. [Google Scholar]
- Stijve, T.; Seeger, R. Determination of α-, ß- and γ-amanitin by high performance thin-layer chromatography in Amanita phalloides (Vaill. ex Fr.) Secr. from various origin. Z. Naturforsch. 1979, 34, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Hallen, H.E.; Adams, G.C.; Eicker, A. Amatoxins and phallotoxins in indigenous and introduced South African Amanita species. S. Afr. J. Bot. 2002, 68, 322–326. [Google Scholar] [CrossRef]
- Kaya, E.; Hancı, M.; Karahan, S.; Bayram, S.; Yaykaşlı, K.O.; Sürmen, M.G. Alfa amanitinin su ve metanoldeki termostabilitesi. Eur. J. Basic Med. Sci. 2012, 2, 106–111. [Google Scholar] [CrossRef]
- Sharma, S.; Aydin, M.; Bansal, G.; Kaya, E.; Singh, R. Determination of amatoxin concentration in heat-treated samples of Amanita phalloides by high-performance liquid chromatography: A forensic approach. J. Forensic Leg. Med. 2021, 78, 102111. [Google Scholar] [CrossRef] [PubMed]
- Hervé, H.; Mabon, G. Une amanite printanière à la Toussaint! Amanita decipiens (Trimbach) Jacquetant. Cahiers Mycol. Nantais 1996, 8, 3–9. [Google Scholar]
- Berger, C. Surprenantes poussées d’une amanite blanche mortelle Amanita virosa ss. auct. améric. Cahiers Mycol. Nantais 2002, 14, 5–6. [Google Scholar]
- Surault, J.-L. Récoltes intéressantes en 1998. Bull. Soc. Mycol. Poitou 1999, 22, 31–35. [Google Scholar]
- Dayrou, G.; Rioult, J.-P.; Shorten, D. Amanita virosa var. levipes Neville et Poumarat: Une espèce en progression dans l’Ouest de la France. Bull. Ann. Féd. Assoc. Mycol. Ouest 2018, 7, 25–34. [Google Scholar]
- Villa, A. Phytolistes, mycoliste: Les listes d’experts en appui des toxicologues. Toxicol. Anal. Clin. 2018, 30, 166–167. [Google Scholar] [CrossRef]
- Gravito Henriques, J.L. Estudo sobre casos de intoxicação por ingestão de cogumelos silvestres de Primavera: Equívocos entre Amanita ponderosa e Amanita boudieri. AÇAFA On-Line 2019, 12, 272–294. [Google Scholar]
- Kirchmair, M.; Carrilho, P.; Pfab, R.; Haberl, B.; Felgueiras, J.; Carvalho, F.; Cardoso, J.; Melo, I.; Vinhas, J.; Neuhauser, S. Amanita poisonings resulting in acute, reversible renal failure: New cases, new toxic Amanita mushrooms. Nephrol. Dial. Transplant. 2012, 27, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Centro Nacional de Epidemiología (CNE). Resultados de la Vigilancia Epidemiologica de las Enfermedades Tasmisibles. Informe Annual, años 2017–2018; Instituto de Salud Carlos III: Madrid, Spain, 2020. [Google Scholar]
- Pennisi, L.; Lepore, A.; Gagliano-Candela, R.; Santacroce, L.; Charitos, I.A. A report on mushrooms poisonings in 2018 at the Apulian Regional Poison Center. Maced. J. Med. Sci. 2020, 8E, 616–622. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).