Expression and Role of β3-Adrenergic Receptor during the Differentiation of 3T3-L1 Preadipocytes into Adipocytes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Cell Culture and Differentiation
2.3. β3-AR Short-Hairpin RNA (shRNA) Transfection
2.4. Oil Red O Staining
2.5. Cell Survival Assay
2.6. Measurement of Intracellular TG
2.7. Preparation of Whole-Cell Lysates
2.8. Immunoblot Analysis
2.9. Quantitative Real-Time RT-PCR
2.10. Statistical Analyses
3. Results
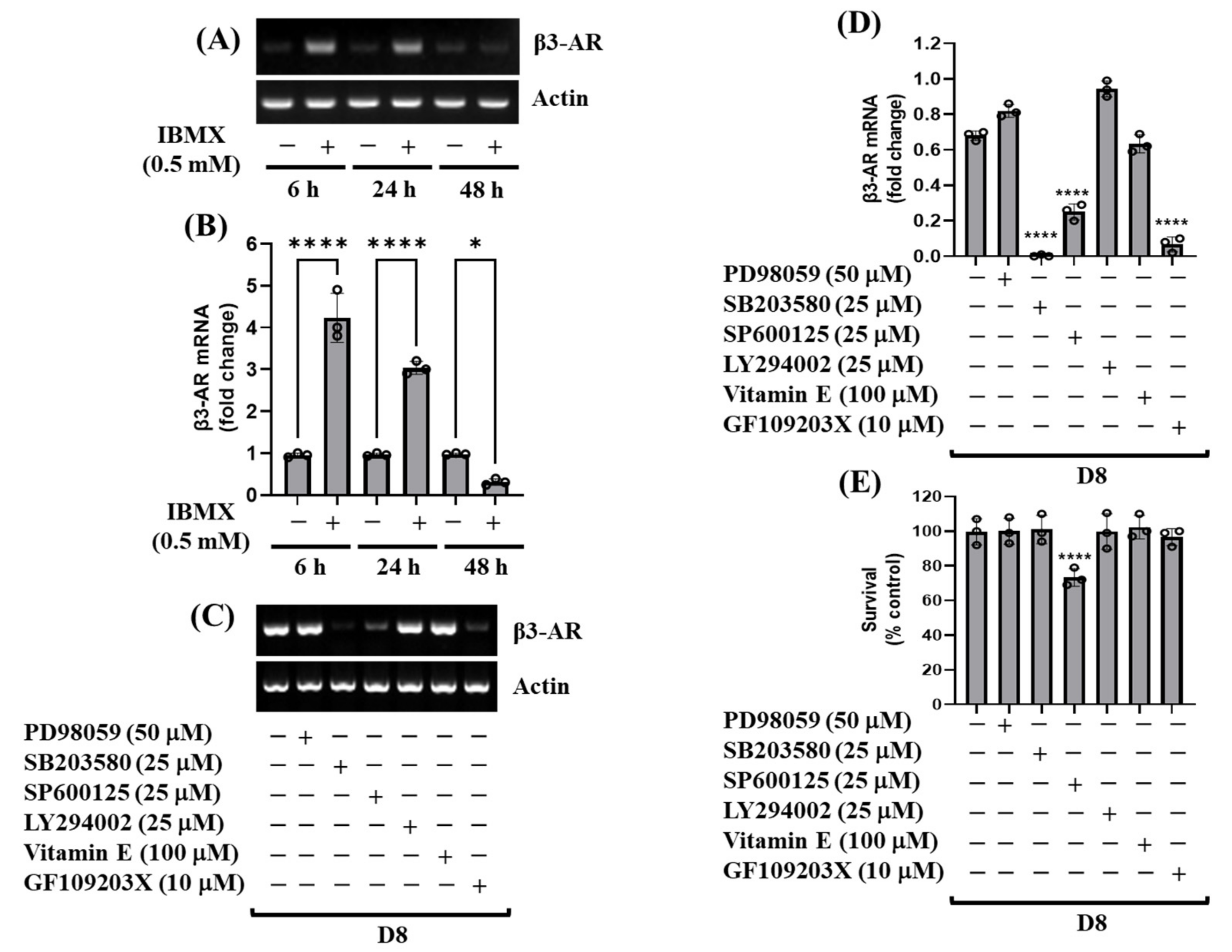
3.1. β3-AR Expression Is Elevated in a Time-Dependent Manner during 3T3-L1 Preadipocyte Differentiation
3.2. cAMP, p38 MAPK, and PKC Are Crucial for the Induction of β3-AR mRNA during 3T3-L1 Preadipocyte Differentiation
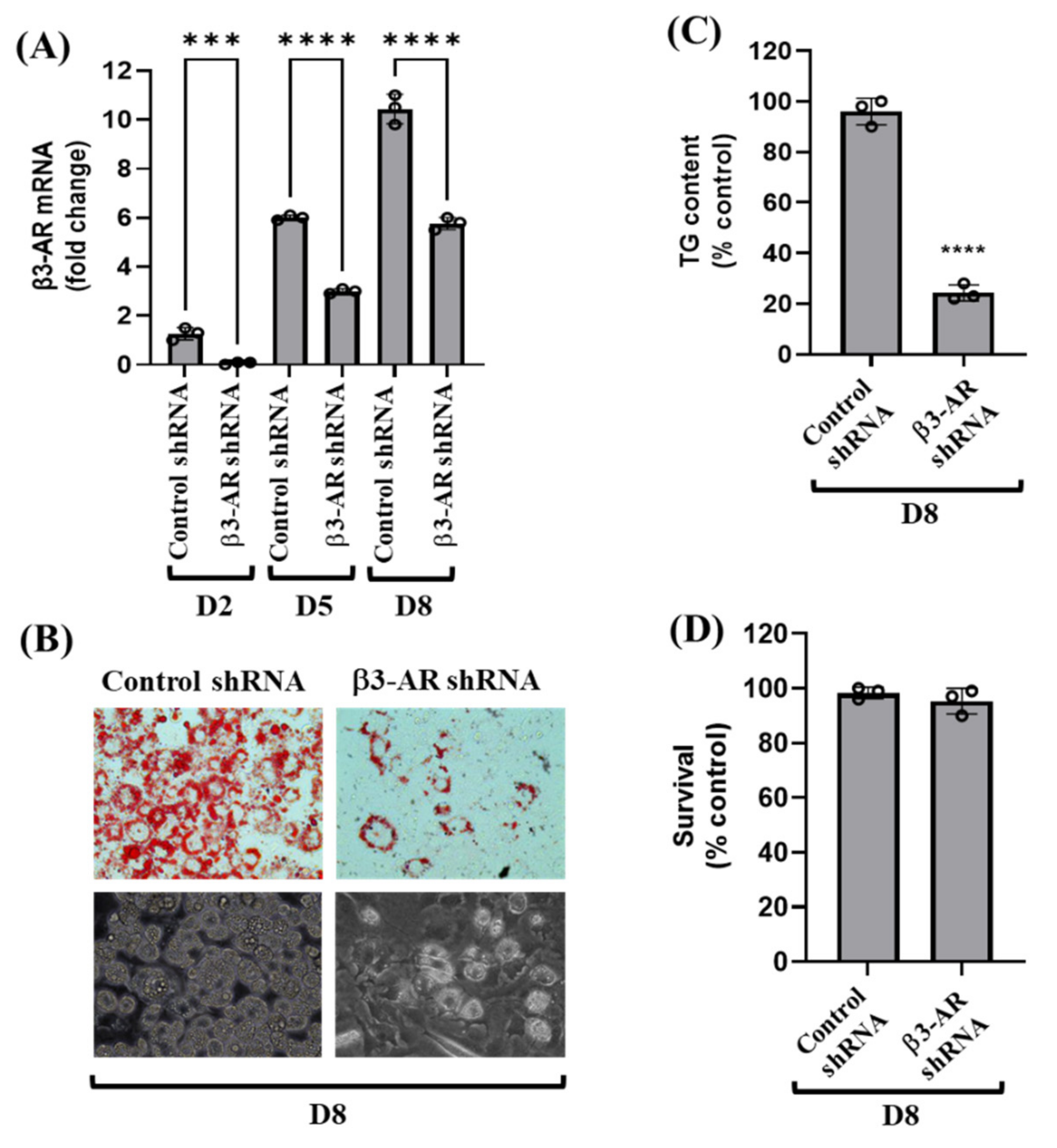
3.3. Knockdown of β3-AR Vastly Reduces Lipid Accumulation and TG Content during 3T3-L1 Preadipocyte Differentiation
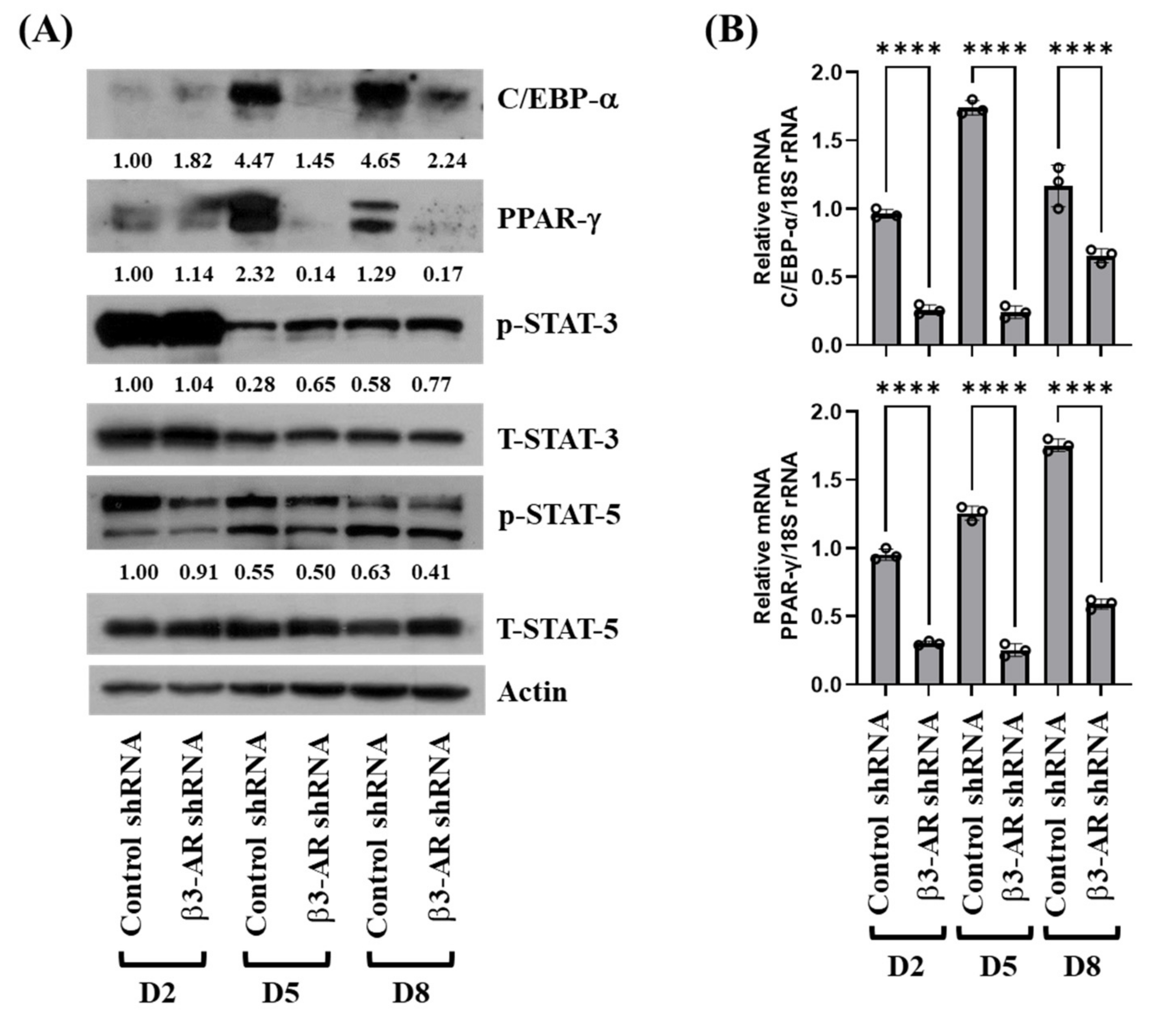
3.4. Knockdown of β3-AR Leads to Decreased Expression and Phosphorylation Levels of C/EBP-α, PPAR-γ, and STAT-5 during 3T3-L1 Preadipocyte Differentiation
3.5. Knockdown of β3-AR Results in Down-Regulation of FASN, Perilipin A, and Leptin Expressions during 3T3-L1 Preadipocyte Differentiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ringel, A.E.; Drijvers, J.M.; Baker, G.J.; Catozzi, A.; García-Cañaveras, J.C.; Gassaway, B.M.; Miller, B.C.; Juneja, V.R.; Nguyen, T.H.; Joshi, S.; et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell 2020, 183, 1848–1866.e26. [Google Scholar] [CrossRef] [PubMed]
- Blaszczak, A.M.; Jalilvand, A.; Hsueh, W.A. Adipocytes, Innate Immunity and Obesity: A Mini-Review. Front. Immunol. 2021, 12, 650768. [Google Scholar] [CrossRef] [PubMed]
- Parra-Peralbo, E.; Talamillo, A.; Barrio, R. Origin and Development of the Adipose Tissue, a Key Organ in Physiology and Disease. Front. Cell Dev. Biol. 2021, 9, 786129. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Z.; Ngandiri, D.A.; Perez, M.L.; Wolf, A.; Wang, Y. The Molecular Brakes of Adipose Tissue Lipolysis. Front. Physiol. 2022, 13, 826314. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Mottillo, E.P. Adipocyte lipolysis: From molecular mechanisms of regulation to disease and therapeutics. Biochem. J. 2020, 477, 985–1008. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.M.; Ahmadian, M.; Keinan, O.; Abu-Odeh, M.; Zhao, P.; Zhou, X.; Keller, M.P.; Gao, H.; Yu, R.T.; Liddle, C.; et al. β3-adrenergic receptor downregulation leads to adipocyte catecholamine resistance in obesity. J. Clin. Investig. 2022, 132, 153357. [Google Scholar] [CrossRef] [PubMed]
- Collins, S. β-Adrenergic Receptors and Adipose Tissue Metabolism: Evolution of an Old Story. Annu. Rev. Physiol. 2022, 84, 1–16. [Google Scholar] [CrossRef]
- Evans, B.A.; Merlin, J.; Bengtsson, T.; Hutchinson, D.S. Adrenoceptors in white, brown, and brite adipocytes. J. Cereb. Blood Flow Metab. 2019, 176, 2416–2432. [Google Scholar] [CrossRef] [PubMed]
- Bel, J.S.; Tai, T.; Khaper, N.; Lees, S.J. Mirabegron: The most promising adipose tissue beiging agent. Physiol. Rep. 2021, 9, e14779. [Google Scholar] [CrossRef]
- Schena, G.; Caplan, M.J. Everything You Always Wanted to Know about β3-AR * (* But Were Afraid to Ask). Cells 2019, 8, 357. [Google Scholar] [CrossRef] [Green Version]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Onal, G.; Kutlu, O.; Gozuacik, D.; Emre, S.D. Lipid Droplets in Health and Disease. Lipids Health Dis. 2017, 16, 128. [Google Scholar] [CrossRef] [Green Version]
- Bahmad, H.F.; Daouk, R.; Azar, J.; Sapudom, J.; Teo, J.C.M.; Abou-Kheir, W.; Al-Sayegh, M. Modeling Adipogenesis: Current and Future Perspective. Cells 2020, 9, 2326. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular Mechanisms of Adipogenesis: The Anti-adipogenic Role of AMP-Activated Protein Kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef]
- Audano, M.; Pedretti, S.; Caruso, D.; Crestani, M.; De Fabiani, E.; Mitro, N. Regulatory mechanisms of the early phase of white adipocyte differentiation: An overview. Cell. Mol. Life Sci. 2022, 79, 139. [Google Scholar] [CrossRef]
- Lage, R.; Diéguez, C.; Vidal-Puig, A.; López, M. AMPK: A metabolic gauge regulating whole-body energy homeostasis. Trends Mol. Med. 2008, 14, 539–549. [Google Scholar] [CrossRef]
- Martini, C.N.; Plaza, M.V.; Vila, M.D.C. PKA-dependent and independent cAMP signaling in 3T3-L1 fibroblasts differentiation. Mol. Cell. Endocrinol. 2009, 298, 42–47. [Google Scholar] [CrossRef]
- Prusty, D.; Park, B.-H.; Davis, K.E.; Farmer, S.R. Activation of MEK/ERK Signaling Promotes Adipogenesis by Enhancing Peroxisome Proliferator-activated Receptor γ (PPARγ) and C/EBPα Gene Expression during the Differentiation of 3T3-L1 Preadipocytes. J. Biol. Chem. 2002, 277, 46226–46232. [Google Scholar] [CrossRef] [Green Version]
- Engelman, J.A.; Lisanti, M.P.; Scherer, P.E. Specific Inhibitors of p38 Mitogen-activated Protein Kinase Block 3T3-L1 Adipogenesis. J. Biol. Chem. 1998, 273, 32111–32120. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Sharma, A.K.; Wolfrum, C. Novel insights into adipose tissue heterogeneity. Rev. Endocr. Metab. Disord. 2022, 23, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Pydi, S.P.; Jain, S.; Tung, W.; Cui, Y.; Zhu, L.; Sakamoto, W.; Jain, S.; Abel, B.S.; Skarulis, M.C.; Liu, J.; et al. Adipocyte β-arrestin-2 is essential for maintaining whole body glucose and energy homeostasis. Nat. Commun. 2019, 10, 2936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spronsen, A.; Nahmias, C.; Krief, S.; Briend-Sutren, M.-M.; Strosberg, A.D.; Emorine, L.J. The promoter and intron/exon structure of the human and mouse beta3-adrenergic-receptor genes. JBIC J. Biol. Inorg. Chem. 1993, 213, 1117–1124. [Google Scholar] [CrossRef]
- Wang, Y.; Voy, B.J.; Urs, S.; Kim, S.; Soltani-Bejnood, M.; Quigley, N.; Heo, Y.-R.; Standridge, M.; Andersen, B.; Dhar, M.; et al. The Human Fatty Acid Synthase Gene and De Novo Lipogenesis Are Coordinately Regulated in Human Adipose Tissue. J. Nutr. 2004, 134, 1032–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, P.A.; Di Gregorio, G.; Lu, T.; Rassouli, N.; Ranganathan, G. Perilipin Expression in Human Adipose Tissue Is Elevated with Obesity. J. Clin. Endocrinol. Metab. 2004, 89, 1352–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavernier, G.; Barbe, P.; Galitzky, J.; Berlan, M.; Caput, D.; Lafontan, M.; Langin, D. Expression of beta3-adrenoceptors with low lipolytic action in human subcutaneous white adipocytes. J. Lipid Res. 1996, 37, 87–97. [Google Scholar] [CrossRef]
- Burl, R.B.; Ramseyer, V.D.; Rondini, E.A.; Pique-Regi, R.; Lee, Y.-H.; Granneman, J.G. Deconstructing Adipogenesis Induced by β3-Adrenergic Receptor Activation with Single-Cell Expression Profiling. Cell Metab. 2018, 28, 300–309.e4. [Google Scholar] [CrossRef] [Green Version]
- Collins, S. β-adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Front. Endocrinol. 2011, 2, 102. [Google Scholar] [CrossRef] [Green Version]
- Lefterova, M.I.; Lazar, M.A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [CrossRef]
- Wolins, N.E.; Brasaemle, D.L.; Bickel, P.E. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006, 580, 5484–5491. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Joseph, F. Adipose Tissue and Adipokines: The Association with and Application of Adipokines in Obesity. Scientifica 2014, 2014, 328592. [Google Scholar] [CrossRef] [PubMed]
- Dalen, K.T.; Schoonjans, K.; Ulven, S.M.; Weedon-Fekjaer, M.S.; Bentzen, T.G.; Koutnikova, H.; Auwerx, J.; Nebb, H.I. Adipose Tissue Expression of the Lipid Droplet–Associating Proteins S3-12 and Perilipin Is Controlled by Peroxisome Proliferator–Activated Receptor-γ. Diabetes 2004, 53, 1243–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roshanzadeh, A.; Yadav, A.K.; Pydi, S.-P.; Kimura, T.; Jang, B.-C. Expression and Role of β3-Adrenergic Receptor during the Differentiation of 3T3-L1 Preadipocytes into Adipocytes. Biology 2022, 11, 772. https://doi.org/10.3390/biology11050772
Roshanzadeh A, Yadav AK, Pydi S-P, Kimura T, Jang B-C. Expression and Role of β3-Adrenergic Receptor during the Differentiation of 3T3-L1 Preadipocytes into Adipocytes. Biology. 2022; 11(5):772. https://doi.org/10.3390/biology11050772
Chicago/Turabian StyleRoshanzadeh, Amir, Anil Kumar Yadav, Sai-Prasad Pydi, Takefumi Kimura, and Byeong-Churl Jang. 2022. "Expression and Role of β3-Adrenergic Receptor during the Differentiation of 3T3-L1 Preadipocytes into Adipocytes" Biology 11, no. 5: 772. https://doi.org/10.3390/biology11050772
APA StyleRoshanzadeh, A., Yadav, A. K., Pydi, S.-P., Kimura, T., & Jang, B.-C. (2022). Expression and Role of β3-Adrenergic Receptor during the Differentiation of 3T3-L1 Preadipocytes into Adipocytes. Biology, 11(5), 772. https://doi.org/10.3390/biology11050772






