The Floral Signals of the Inconspicuous Orchid Malaxis monophyllos: How to Lure Small Pollinators in an Abundant Environment
Abstract
Simple Summary
Abstract
1. Introduction
2. Study Species and Population
3. Plant Material
3.1. Plant Material Collection
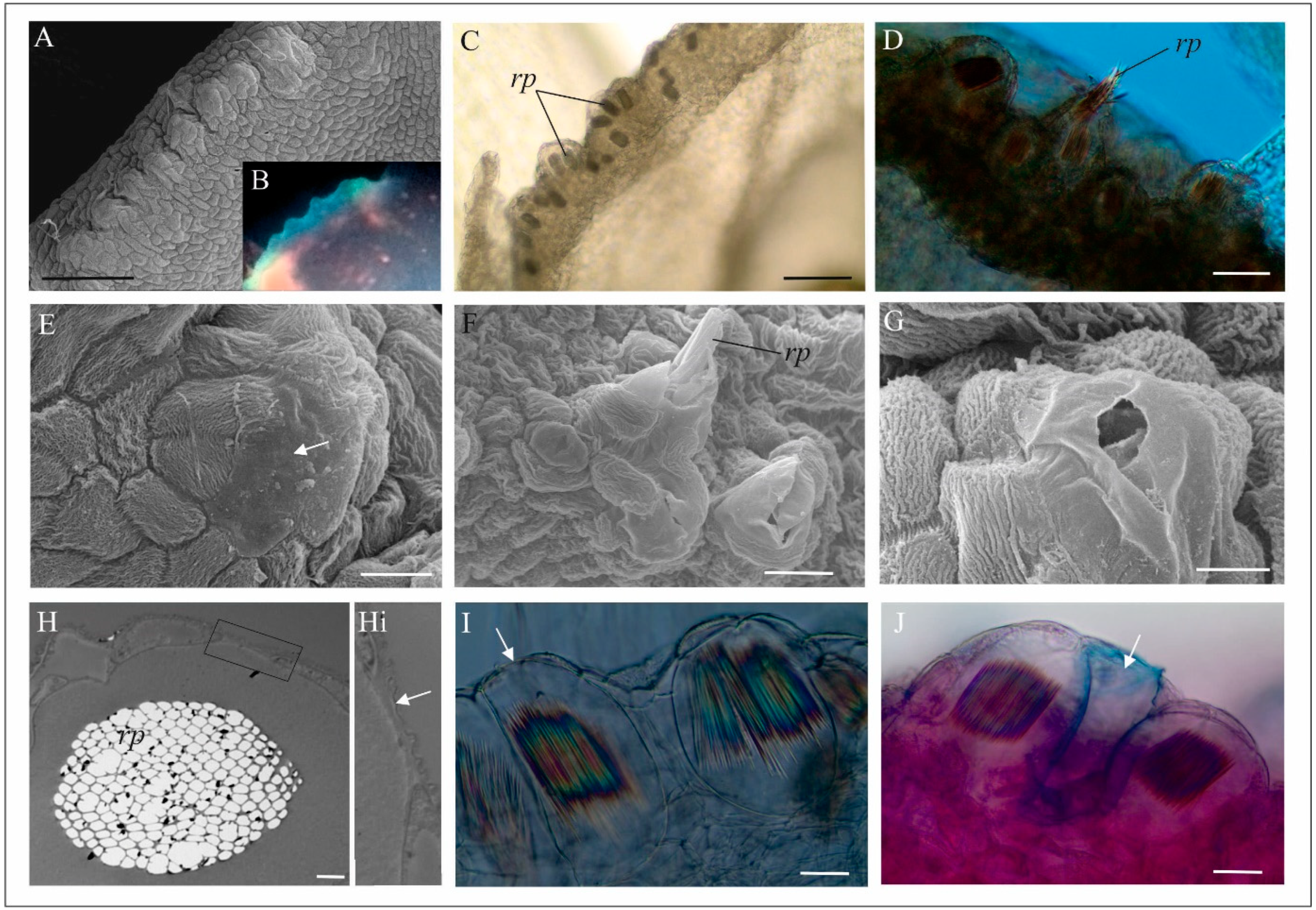
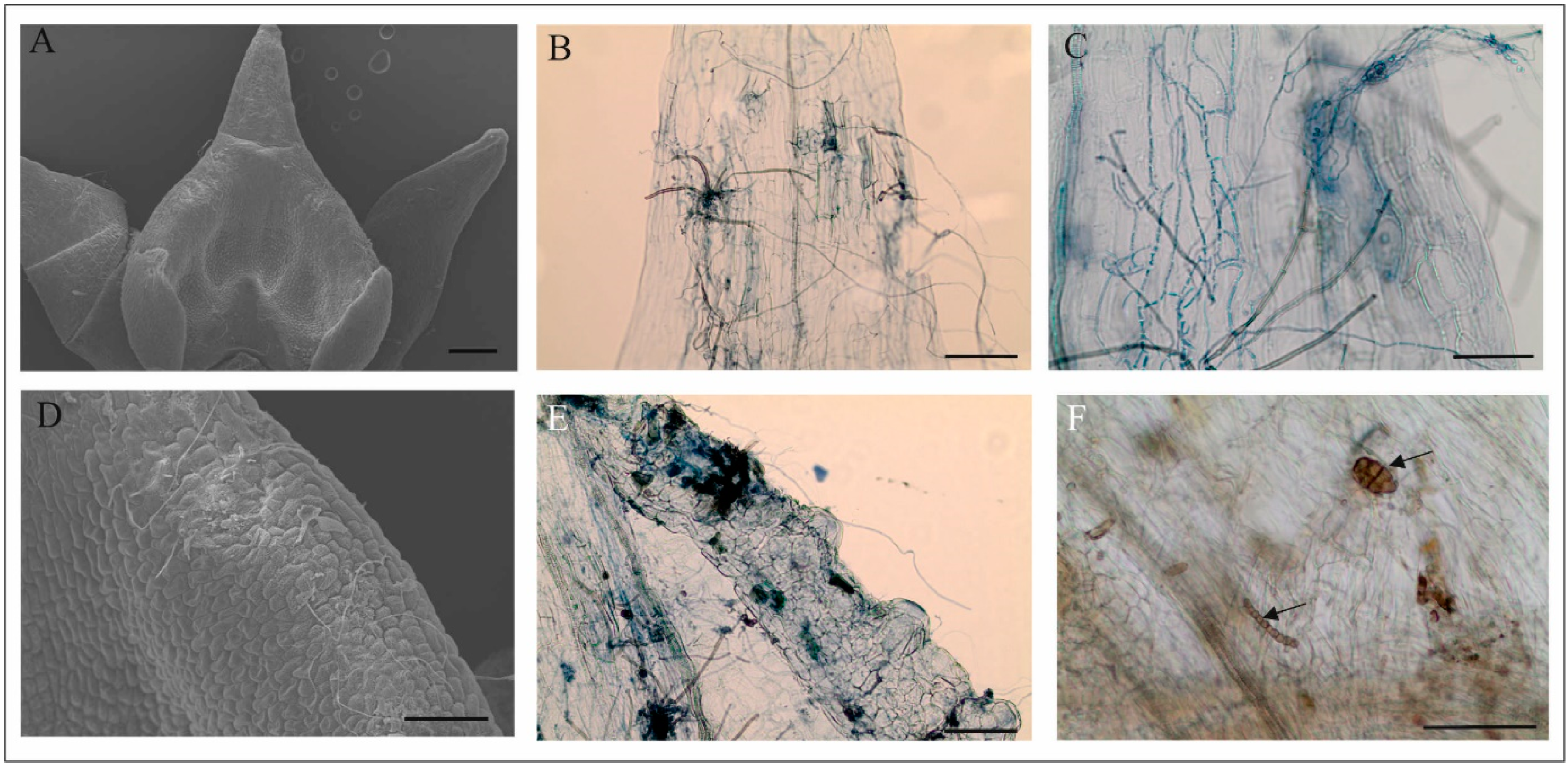
3.2. Anatomical Analyses of Flowers (LM, TEM, SEM)
3.3. Fragrance Collection and Analysis
3.4. The Community of Flower Visitor Insects
4. Results
4.1. Anatomy, Morphology, and Ultrastructure of M. monophyllos Flowers
4.2. Volatile Composition
4.3. Flower Visitor Community
5. Discussion
5.1. Multifactorial Volatile Composition
5.2. Structure and Colour of M. monophyllos Flowers
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dafni, A. Mimicry and deception in pollination. Annu. Rev. Ecol. Evol. Syst. 1984, 15, 259–278. [Google Scholar] [CrossRef]
- Nilsson, L.A. Orchid pollination biology. Trends Ecol. Evol. 1992, 7, 255–259. [Google Scholar] [CrossRef]
- Schiestl, F.P. On the success of a swindle: Pollination by deception in orchids. Sci. Nat. 2005, 92, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Neiland, M.R.M.; Wilcock, C.C. Fruit set, nectar reward, and rarity in the Orchidaceae. Am. J. Bot. 1998, 85, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Jersakova, J.; Johnson, S.; Kindlmann, P. Mechanisms and evolution of deceptive pollination in orchids. Biol. Rev. 2006, 81, 219–235. [Google Scholar] [CrossRef]
- Urru, I.; Stensmyr, M.; Hansson, B. Pollination by brood-site deception. Phytochemistry 2011, 72, 1655–1666. [Google Scholar] [CrossRef]
- Pemberton, R.W. Biotic Resource Needs of Specialist Orchid Pollinators. Bot. Rev. 2010, 76, 275–292. [Google Scholar] [CrossRef]
- Fenster, C.B.; Armbruster, W.S.; Wilson, P.; Dudash, M.R.; Thomson, J.D. Pollination Syndromes and Floral Specialization. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 375–403. [Google Scholar] [CrossRef]
- Leonard, A.S.; Dornhaus, A.; Papaj, D.R. Why are floral signals complex? An outline of functional hypotheses. In Evolution of Plant-Pollinator Relationships; Cambridge University Press: Cambridge, UK, 2012; pp. 279–300. [Google Scholar]
- Schiestl, F.P.; Johnson, S. Pollinator-mediated evolution of floral signals. Trends Ecol. Evol. 2013, 28, 307–315. [Google Scholar] [CrossRef]
- Lemaitre, A.; Pinto, C.F.; Niemeyer, H.M. Generalized pollination system: Are floral traits adapted to different pollinators? Arthropod-Plant Interact. 2014, 8, 261–272. [Google Scholar] [CrossRef]
- Prieto-Benítez, S.; Dötterl, S.; Giménez-Benavides, L. Diel Variation in Flower Scent Reveals Poor Consistency of Diurnal and Nocturnal Pollination Syndromes in Sileneae. J. Chem. Ecol. 2015, 41, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Delle-Vedove, R.; Schatz, B.; Dufay, M. Understanding intraspecific variation of floral scent in light of evolutionary ecology. Ann. Bot. 2017, 120, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Firmage, D.H.; Cole, F.R. Reproductive success and inflorescence size of Calopogon tuberosus (Orchidaceae). Am. J. Bot. 1988, 75, 1371–1377. [Google Scholar] [CrossRef]
- Ohashi, K.; Yahara, T. How Long to Stay on, and How Often to Visit a Flowering Plant? A Model for Foraging Strategy When Floral Displays Vary in Size. Oikos 1999, 86, 386. [Google Scholar] [CrossRef]
- Huda, M.K.; Wilcock, C.C. Impact of floral traits on the reproductive success of epiphytic and terrestrial tropical orchids. Oecologia 2008, 154, 731–741. [Google Scholar] [CrossRef]
- Trunschke, J.; Sletvold, N.; Agren, J. Interaction intensity and pollinator-mediated selection. New Phytol. 2017, 214, 909–912. [Google Scholar] [CrossRef]
- Raguso, R.A. Start making scents: The challenge of integrating chemistry into pollination ecology. Entomol. Exp. Appl. 2008, 128, 196–207. [Google Scholar] [CrossRef]
- Braunschmid, H.; Dötterl, S. Does the Rarity of a Flower’s Scent Phenotype in a Deceptive Orchid Explain Its Pollination Success? Front. Plant Sci. 2020, 11, 1979. [Google Scholar] [CrossRef]
- Wróblewska, A.; Szczepaniak, L.; Bajguz, A.; Jędrzejczyk, I.; Tałałaj, I.; Ostrowiecka, B.; Brzosko, E.; Jermakowicz, E.; Mirski, P. Deceptive strategy in Dactylorhiza orchids: Multidirectional evolution of floral chemistry. Ann. Bot. 2019, 123, 1005–1016. [Google Scholar] [CrossRef]
- Huber, F.K.; Kaiser, R.; Sauter, W.; Schiestl, F.P. Floral scent emission and pollinator attraction in two species of Gymnadenia (Orchidaceae). Oecologia 2005, 142, 564–575. [Google Scholar] [CrossRef]
- Moya, S.; Ackerman, J.D. Variation in the floral fragrance of Epidendrum ciliare (Orchidaceae). Nord. J. Bot. 1993, 13, 41–47. [Google Scholar] [CrossRef]
- Salzmann, C.C.; Cozzolino, S.; Schiestl, F. Floral Scent in Food-Deceptive Orchids: Species Specificity and Sources of Variability. Plant Biol. 2007, 9, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, C.C.; Nardella, A.M.; Cozzolino, S.; Schiestl, F.P. Variability in Floral Scent in Rewarding and Deceptive Orchids: The Signature of Pollinator-imposed Selection? Ann. Bot. 2007, 100, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Margońska, H.B.; Kowalkowska, A.K.; Górniak, M.; Rutkowski, P. Taxonomic Redefinition of Subtribe Malaxidinae (Orchidales, Orchidaceae); Koeltz Scientific Books: Koenigstein, Germany, 2012; pp. 1–606. [Google Scholar]
- Vereecken, N.J.; Schiestl, F.P. On the roles of colour and scent in a specialized floral mimicry system. Ann. Bot. 2009, 104, 1077–1084. [Google Scholar] [CrossRef]
- Ayasse, M.; Stökl, J.; Francke, W. Chemical ecology and pollinator-driven speciation in sexually deceptive orchids. Phytochemistry 2011, 72, 1667–1677. [Google Scholar] [CrossRef]
- Gaskett, A.C. Orchid pollination by sexual deception: Pollinator perspectives. Biol. Rev. 2011, 86, 33–75. [Google Scholar] [CrossRef]
- Aragón, S.; Ackerman, J.D. Density effects on the reproductive success and herbivory of Malaxis massonii. Lindleyana 2001, 16, 3–12. [Google Scholar]
- Reeves, L.M.; Reeves, T. Life history and reproduction of Malaxis paludosa in Minnesota. Amer. Orchid Soc. B 1984, 117, 98–105. [Google Scholar]
- Claessens, J.; Kleynen, J. The Flower of the European Orchid. Form and Function; Jean Claessens & Jacques Kleynen: Voerendaal, The Netherlands, 2011; pp. 137–145. [Google Scholar]
- Bohman, B.; Flematti, G.; A Barrow, R.; Pichersky, E.; Peakall, R. Pollination by sexual deception—It takes chemistry to work. Curr. Opin. Plant Biol. 2016, 32, 37–46. [Google Scholar] [CrossRef]
- Salazar, G.A.; Kite, G.C. Chemical composition of the inflorescence odor of Malaxis rzedowskiana (Orchidaceae). Rev. Mex. Biodivers. 2008, 79, 153–157. [Google Scholar] [CrossRef]
- Vakhrameeva, M.G.; Tatarenko, I.V.; Varlygina, T.I.; Torosyan, G.K.; Zagulski, M.N. Orchids of Russia and Adjacent Countries (with the Border of the Former USSR); A.R.G. Ganter Verlag: Ruggell, Lichtensten, 2008; pp. 417–420, 612. [Google Scholar]
- Jermakowicz, E.; Ostrowiecka, B.; Tałałaj, I.; Pliszko, A.; Kostro-Ambroziak, A. Male and female reproductive success in natural and anthropogenic populations of Malaxis monophyllos (L.) Sw. (Orchidaceae). Biodivers. Res. Conserv. 2015, 39, 37–44. [Google Scholar] [CrossRef]
- Jermakowicz, E.; Brzosko, E. Demographic responses of boreal-montane orchid Malaxis monophyllos (L.) Sw. populations to contrasting environmental conditions. Acta Soc. Bot. Pol. 2016, 85, 1–17. [Google Scholar] [CrossRef]
- Scopece, G.; Cozzolino, S.; Johnson, S.D.; Schiestl, F.P. Pollination Efficiency and the Evolution of Specialized Deceptive Pollination Systems. Am. Nat. 2010, 175, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Vöth, W. Lebensgeschichte und Bestäuber der Orchideen am Beispiel von Niederoösterreich. Stapfia 1999, 65, 1–257. [Google Scholar]
- Larson, B.; Kevan, P.; Inouye, D. Flies and flowers: Taxonomic diversity of anthophiles and pollinators. Can. Entomol. 2001, 133, 439–465. [Google Scholar] [CrossRef]
- Woodcock, T.S.; Larson, B.M.; Kevan, P.G.; Inouye, D.W.; Lunau, K. Flies and Flowers II: Floral Attractants and Rewards. J. Pollinat. Ecol. 2014, 12, 63–94. [Google Scholar] [CrossRef]
- Raguso, R.A. Don’t forget the flies: Dipteran diversity and its consequences for floral ecology and evolution. Appl. Entomol. Zool. 2020, 55, 1–7. [Google Scholar] [CrossRef]
- Stökl, J.; Strutz, A.; Dafni, A.; Svatos, A.; Doubsky, J.; Knaden, M.; Sachse, S.; Hansson, B.S.; Stensmyr, M.C. A Deceptive Pollination System Targeting Drosophilids through Olfactory Mimicry of Yeast. Curr. Biol. 2010, 20, 1846–1852. [Google Scholar] [CrossRef]
- Karremans, A.P.; Pupulin, F.; Grimaldi, D.; Beentjes, K.K.; Butôt, R.; Fazzi, G.E.; Kaspers, K.; Kruizinga, J.; Roessingh, P.; Smets, E.F.; et al. Pollination of, Speckliniaby nectar-feeding, Drosophila: The first reported case of a deceptive syndrome employing aggregation pheromones in Orchidaceae. Ann. Bot. 2015, 116, 437–455. [Google Scholar] [CrossRef]
- Van Der Niet, T.; Hansen, D.; Johnson, S. Carrion mimicry in a South African orchid: Flowers attract a narrow subset of the fly assemblage on animal carcasses. Ann. Bot. 2011, 107, 981–992. [Google Scholar] [CrossRef]
- Kaiser, R. The Scent of Orchids: Olfactory and Chemical Investigations; Elsevier: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Pacini, E.; Hesse, M. Types of Pollen Dispersal Units in Orchids, and their Consequences for Germination and Fertilization. Ann. Bot. 2002, 89, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Boedijn, K.B. Trypan Blue as a stain for fungi. Stain Technol. 1956, 31, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Bronner, R. Simultaneous Demonstration of Lipids and Starch in Plant Tissues. Stain Technol. 1975, 50, 1–4. [Google Scholar] [CrossRef]
- Naczk, A.M.; Kowalkowska, A.K.; Wiśniewska, N.; Haliński, Ł.P.; Kapusta, M.; Czerwicka, M. Floral anatomy, ultrastructure and chemical analysis in Dactylorhiza incarnata/maculata complex (Orchidaceae). Bot. J. Linn. Soc. 2018, 187, 512–536. [Google Scholar] [CrossRef]
- Lipińska, M.M.; Wiśniewska, N.; Gołębiowski, M.; Narajczyk, M.; Kowalkowska, A.K. Floral micromorphology, histochemistry, ultrastructure and chemical composition of floral secretions in three Neotropical Maxillariella species (Orchidaceae). Bot. J. Linn. Soc. 2021, 196, 53–80. [Google Scholar] [CrossRef]
- Loulier, J.; Lefort, F.; Stocki, M.; Asztemborska, M.; Szmigielski, R.; Siwek, K.; Grzywacz, T.; Hsiang, T.; Ślusarski, S.; Oszako, T.; et al. Detection of Fungi and Oomycetes by Volatiles Using E-Nose and SPME-GC/MS Platforms. Molecules 2020, 25, 5749. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska, J.A.; Stocki, M.; Stocka, N.; Ślusarski, S.; Tkaczyk, M.; Caetano, J.M.; Tulik, M.; Hsiang, T.; Oszako, T. Interactions between Phytophthora cactorum, Armillaria gallica and Betula pendula seedlings subjected to defoliation. Forests 2020, 11, 1107. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Stocki, M.; Vetchinnikova, L. Inheritance of specific secondary volatile metabolites in buds of white birch Betula pendula and Betula pubescens hybrids. Trees 2019, 33, 1329–1344. [Google Scholar] [CrossRef]
- Stocki, M.; Banaszczak, P.; Stocka, N.; Borowik, T.; Zapora, E.; Isidorov, V. Taxonomic implications of volatile secondary metabolites emitted from birch (Betula L.) buds. Biochem. Syst. Ecol. 2020, 92, 104132. [Google Scholar] [CrossRef]
- Ostrowiecka, B.; Tałałaj, I.; Brzosko, E.; Jermakowicz, E.; Mirski, P.; Kostro-Ambroziak, A.; Łukasz, M.; Lasoń, A.; Kupryjanowicz, J.; Kotowicz, J.; et al. Pollinators and visitors of the generalized food-deceptive orchid Dactylorhiza majalis in North-Eastern Poland. Biologia 2019, 74, 1247–1257. [Google Scholar] [CrossRef]
- Pérez-Hérnandez, H.; Sánchez-Guillén, D.; Damon, A.A.; Valle-Mora, J. Orchid pollination: Specialization in chance? Bot. J. Linn. Soc. 2011, 165, 251–266. [Google Scholar] [CrossRef]
- Moré, M.; Amorim, F.W.; Benitez-Vieyra, S.; Medina, A.M.; Sazima, M.; Cocucci, A.A. Armament Imbalances: Match and Mismatch in Plant-Pollinator Traits of Highly Specialized Long-Spurred Orchids. PLoS ONE 2012, 7, e41878. [Google Scholar] [CrossRef] [PubMed]
- Steen, R.; Mundal, D. New video registration of Autographa pulchrina (Haworth, 1809) (Lepidoptera, Noctuidae) and Sphinx pinastri L., 1758 (Lepidoptera, Sphingidae) pollinating Platanthera bifolia latiflora (Orchidaceae) in Norway. Nor. J. Entomol. 2013, 60, 57–61. [Google Scholar]
- Chillcott, J.G. A Revision of the Nearctic Species of Fanniinae (Diptera: Muscidae). Mem. Entomol. Soc. Can. 1960, 92, 5–295. [Google Scholar] [CrossRef]
- Shorrocks, B. An ecological classification of European Drosophila species. Oecologia 1977, 26, 335–345. [Google Scholar] [CrossRef]
- Markow, T.A.; O’grady, P. Reproductive ecology of, Drosophila. Funct. Ecol. 2008, 22, 747–759. [Google Scholar] [CrossRef]
- Goldman-Huertas, B.; Mitchell, R.F.; Lapoint, R.T.; Faucher, C.P.; Hildebrand, J.G.; Whiteman, N.K. Evolution of herbivory in Drosophilidae linked to loss of behaviors, antennal responses, odorant receptors, and ancestral diet. Proc. Natl. Acad. Sci. USA 2015, 112, 3026–3031. [Google Scholar] [CrossRef]
- Waser, N.M.; Chittka, L.; Price, M.V.; Williams, N.M.; Ollerton, J. Generalization in Pollination Systems, and Why it Matters. Ecology 1996, 77, 1043–1060. [Google Scholar] [CrossRef]
- Cardoso-Gustavson, P.; de Souza, S.R.; de Barros, F. Floral volatile profile in Pleurothallidinae, an orchid subtribe pollinated by flies: Ecological and phylogenetic considerations. Phytochem. Lett. 2017, 22, 49–55. [Google Scholar] [CrossRef]
- Schiestl, F.P. The evolution of floral scent and insect chemical communication. Ecol. Lett. 2010, 13, 643–656. [Google Scholar] [CrossRef]
- Antoń, S.; Kamińska, M.; Stpiczyńska, M. Comparative structure of the osmophores in the flowers of Stanhopea graveolens Lindley and Cycnoches chlorochilon Klotzsch (Orchidaceae). Acta Agrobot. 2012, 65, 15–24. [Google Scholar] [CrossRef]
- Wiśniewska, N.; Lipińska, M.M.; Gołębiowski, M.; Kowalkowska, A.K. Labellum structure of Bulbophyllum echinolabium J.J. Sm. (section Lepidorhiza Schltr., Bulbophyllinae Schltr., Orchidaceae Juss.). Protoplasma 2019, 256, 1185–1203. [Google Scholar] [CrossRef] [PubMed]
- Skubatz, H.; Kunkel, D.D.; Howald, W.N.; Trenkle, R.; Mookherjee, B. The, Sauromatum guttatumappendix as an osmophore: Excretory pathways, composition of volatiles and attractiveness to insects. New Phytol. 1996, 134, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Venkatshwarlu, U.G.; Chandravadana, M.V.; Tewari, R.P. Volatile flavor compounds of some edible mushrooms (Basidiomycetes). Flavour Fragr. J. 1999, 14, 191–194. [Google Scholar] [CrossRef]
- Pierce, A.M.; Pierce, H.D.; Borden, J.H.; Oehlschlager, A.C. Fungal volatiles: Semiochemicals for stored-product beetles (Coleoptera: Cucujidae). J. Chem. Ecol. 1991, 17, 581–597. [Google Scholar] [CrossRef]
- Combet, E.; Eastwood, D.C.; Burton, K.S.; Henderson, J. Eight-carbon volatiles in mushrooms and fungi: Properties, analysis, and biosynthesis. Mycoscience 2006, 47, 317–326. [Google Scholar] [CrossRef]
- Dobson, H. Relationship between Floral Fragrance Composition and Type of Pollinator. In Biology of Floral Scent; CRC Press: Boca Raton, FL, USA, 2006; pp. 147–198. [Google Scholar]
- Kaiser, R. Flowers and Fungi Use Scents to Mimic Each Other. Science 2006, 311, 806–807. [Google Scholar] [CrossRef]
- Humeau, L.; Micheneau, C.; Jacquemyn, H.; Gauvin-Bialecki, A.; Fournel, J.; Pailler, T. Sapromyiophily in the native orchid, Bulbophyllum variegatum, on Réunion (Mascarene Archipelago, Indian Ocean). J. Trop. Ecol. 2011, 27, 591–599. [Google Scholar] [CrossRef]
- Wong, D.C.J.; Pichersky, E.; Peakall, R. The Biosynthesis of Unusual Floral Volatiles and Blends Involved in Orchid Pollination by Deception: Current Progress and Future Prospects. Front. Plant Sci. 2017, 8, 1955. [Google Scholar] [CrossRef]
- Jürgens, A.; Dötterl, S.; Meve, U. The chemical nature of fetid floral odours in stapeliads (Apocynaceae-Asclepiadoideae-Ceropegieae). New Phytol. 2006, 172, 452–468. [Google Scholar] [CrossRef]
- Jakovlev, J. Fungal hosts of mycetophilids (Diptera: Sciaroidea excluding Sciaridae): A review. Mycology 2012, 3, 11–23. [Google Scholar]
- Martos, F.; Cariou, M.; Pailler, T.; Fournel, J.; Bytebier, B.; Johnson, S. Chemical and morphological filters in a specialized floral mimicry system. New Phytol. 2015, 207, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, P.S.; Humphreys, D.P.; Dyer, K.A. Ongoing hybridization obscures phylogenetic relationships in the Drosophila subquinaria species complex. J. Evol. Biol. 2019, 32, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Borba, E.L.; Semir, J. Pollinator Specificity and Convergence in Fly-pollinated Pleurothallis (Orchidaceae) Species: A Multiple Population Approach. Ann. Bot. 2001, 88, 75–88. [Google Scholar] [CrossRef]
- Policha, T.; Davis, A.; Barnadas, M.; Dentinger, B.T.M.; Raguso, R.A.; Roy, B.A. Disentangling visual and olfactory signals in mushroom-mimicking Dracula orchids using realistic three-dimensional printed flowers. New Phytol. 2016, 210, 1058–1071. [Google Scholar] [CrossRef]
- Endara, L.; Grimaldi, D.; Roy, B. Lord of the flies: Pollination of Dracula orchids. Lankesteriana 2010, 10, 1–11. [Google Scholar] [CrossRef]
- Policha, T.; Grimaldi, D.A.; Manobanda, R.; Troya, A.; Ludden, A.; Dentinger, B.T.M.; Roy, B.A. Dracula orchids exploit guilds of fungus visiting flies: New perspectives on a mushroom mimic. Ecol. Entomol. 2019, 44, 457–470. [Google Scholar] [CrossRef]
- Kelly, M.M.; Gaskett, A.C. UV reflectance but no evidence for colour mimicry in a putative brood-deceptive orchid Corybas cheesemanii. Curr. Zool. 2014, 60, 104–113. [Google Scholar] [CrossRef]
- Buitrago, C.A.D.; Quintero, N.F.A.; Otero, J.T. Nocturnal pollinat, Ion by Fungus gnats of the colombian endemic species, Pleurothallis marthae (orchidaceae: Pleurothallidinae). Lankesteriana 2013, 13, 407–417. [Google Scholar] [CrossRef][Green Version]
- Mochizuki, K.; Kawakita, A. Pollination by fungus gnats and associated floral characteristics in five families of the Japanese flora. Ann. Bot. 2018, 121, 651–663. [Google Scholar] [CrossRef]
- Ackerman, J.D.; Mesler, M.R. Pollination biology of Listera cordata (Orchidaceae). Am. J. Bot. 1979, 66, 820–824. [Google Scholar] [CrossRef]
- Mesler, M.R.; Ackerman, J.D.; Lu, K.L. The Effectiveness of Fungus Gnats as Pollinators. Am. J. Bot. 1980, 67, 564–567. [Google Scholar] [CrossRef]
- Blanco, M.A.; Barboza, G. Pseudocopulatory Pollination in Lepanthes (Orchidaceae: Pleurothallidinae) by Fungus Gnats. Ann. Bot. 2005, 95, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Reiter, N.; Freestone, M.; Brown, G.; Peakall, R. Pollination by sexual deception of fungus gnats (Keroplatidae and Mycetophilidae) in two clades of Pterostylis (Orchidaceae). Bot. J. Linn. Soc. 2019, 190, 101–116. [Google Scholar] [CrossRef]
- Ayasse, M.; Schiestl, F.; Paulus, H.F.; Löfstedt, C.; Hansson, B.; Ibarra, F.; Francke, W. Evolution of reproductive strategies in the sexually deceptive orchid Ophrys sphegodes: How does flower-specific variation of odor signals influence reproductive success? Evolution 2000, 54, 1995–2006. [Google Scholar] [CrossRef]
- Schiestl, F.P.; Ayasse, M.; Paulus, H.F.; Löfstedt, C.; Hansson, B.S.; Ibarra, F.; Francke, W. Sex pheromone mimicry in the early spider orchid (Ophrys sphegodes): Patterns of hydrocarbons as the key mechanism for pollination by sexual deception. J. Comp. Physiol. A 2000, 186, 567–574. [Google Scholar] [CrossRef]
- Snellings, Y.; Herrera, B.; Wildemann, B.; Beelen, M.; Zwarts, L.; Wenseleers, T.; Callaerts, P. The role of cuticular hydrocarbons in mate recognition in Drosophila suzukii. Sci. Rep. 2018, 8, 4996. [Google Scholar] [CrossRef]
- Lahondère, C.; Vinauger, C.; Okubo, R.P.; Wolff, G.H.; Chan, J.K.; Akbari, O.S.; Riffell, J.A. The olfactory basis of orchid pollination by mosquitoes. Proc. Natl. Acad. Sci. USA 2020, 117, 708–716. [Google Scholar] [CrossRef]
- Peach, D.A.H.; Gries, R.; Zhai, H.; Young, N.; Gries, G. Author Correction: Multimodal floral cues guide mosquitoes to tansy inflorescences. Sci. Rep. 2019, 9, 8038. [Google Scholar] [CrossRef]
- Landolt, P.J. Sex Attractant and Aggregation Pheromones of Male Phytophagous Insects. Am. Entomol. 1997, 43, 12–22. [Google Scholar] [CrossRef]
- Mitaka, Y.; Mori, N.; Matsuura, K. Multi-functional roles of a soldier-specific volatile as a worker arrestant, primer pheromone and an antimicrobial agent in a termite. Proc. R. Soc. B Boil. Sci. 2017, 284, 20171134. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, J.W. The Role of Yeast in the Pollination Success of a Neotropical Orchid. Master’s Thesis, University of Oregon, Eugene, OR, USA, 2013. [Google Scholar]
- Koski, M.H.; Ashman, T.-L. Dissecting pollinator responses to a ubiquitous ultraviolet floral pattern in the wild. Funct. Ecol. 2014, 28, 868–877. [Google Scholar] [CrossRef]
- Kelber, A.; Balkenius, A.; Warrant, E.J. Colour Vision in Diurnal and Nocturnal Hawkmoths. Integr. Comp. Biol. 2003, 43, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Peach, D.A.H.; Ko, E.; Blake, A.J.; Gries, G. Ultraviolet inflorescence cues enhance attractiveness of inflorescence odour to Culex pipiens mosquitoes. PLoS ONE 2019, 14, e0217484. [Google Scholar] [CrossRef]
- Sivinski, J.M. Phototropism, Bioluminescence, and the Diptera. Fla. Entomol. 1998, 91, 282. [Google Scholar] [CrossRef]
- Whitney, H.M.; Kolle, M.; Andrew, P.; Chittka, L.; Steiner, U.; Glover, B.J. Floral Iridescence, Produced by Diffractive Optics, Acts As a Cue for Animal Pollinators. Science 2009, 323, 130–133. [Google Scholar] [CrossRef]
- Prychid, C.J.; Rudall, P.J. Calcium Oxalate Crystals in Monocotyledons: A Review of their Structure and Systematics. Ann. Bot. 1999, 84, 725–739. [Google Scholar] [CrossRef]
- Barabé, D.; Lacroix, C.; Chouteau, M.; Gibernau, M. On the presence of extracellular calcium oxalate crystals on the inflorescences of Araceae. Bot. J. Linn. Soc. 2004, 146, 181–190. [Google Scholar] [CrossRef]
- Rudall, P.; Engleman, E.M.; Hanson, L.; Chase, M.W. Embryology, cytology and systematics of, Hemiphylacus, Asparagus and, Anemarrhena (Asparagales). Plant. Syst. Evol. 1998, 211, 181–199. [Google Scholar] [CrossRef]
- Wiśniewska, N.; Kowalkowska, A.K.; Kozieradzka-Kiszkurno, M.; Krawczynska, A.; Bohdanowicz, J. Floral features of two species of Bulbophyllum section Lepidorhiza Schltr.: B. levanae Ames and B. nymphopolitanum Kraenzl. (Bulbophyllinae Schltr., Orchidaceae). Protoplasma 2018, 255, 485–499. [Google Scholar] [CrossRef]
- Kowalkowska, A.; Margońska, H.B. Diversity of labellar micromorphological structures in selected species of Malaxidinae (Orchidales). Acta Soc. Bot. Pol. 2009, 78, 141–150. [Google Scholar] [CrossRef]
- D’arcy, W.G.; Keating, R.C.; Buchmann, S.L. The Calcium Oxalate Package or So-Called Resorption Tissue in Some Angiosperm Anthers. The Anther: Form, Function and Phylogeny; Cambridge University Press: Cambridge, UK, 1996; pp. 159–191. [Google Scholar]
- Chase, M.W.; Peacor, D.R. Crystals of calcium oxalate hydrate on the perianth of Stelis SW. Lindleyana 1987, 2, 91–94. [Google Scholar]
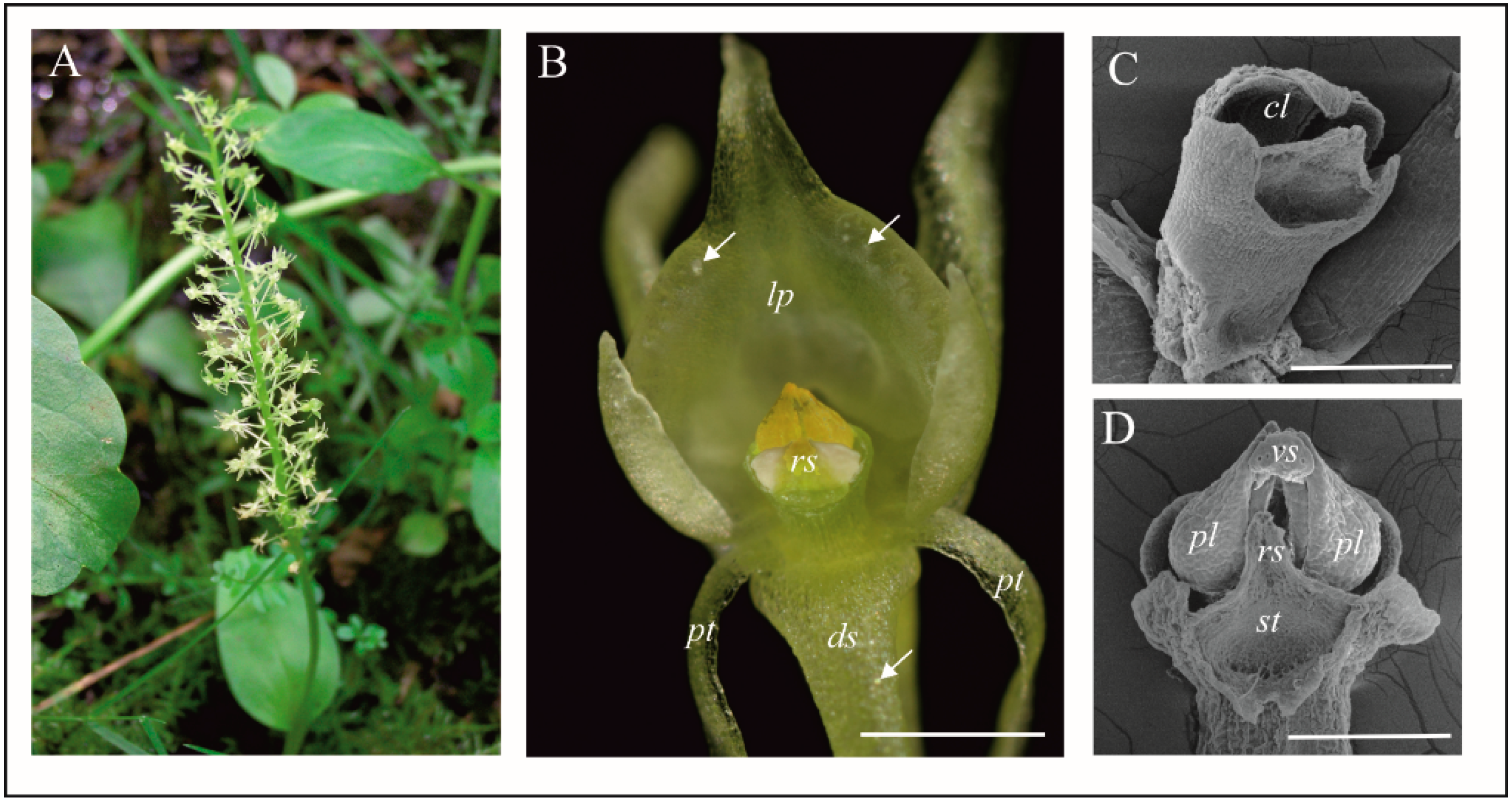
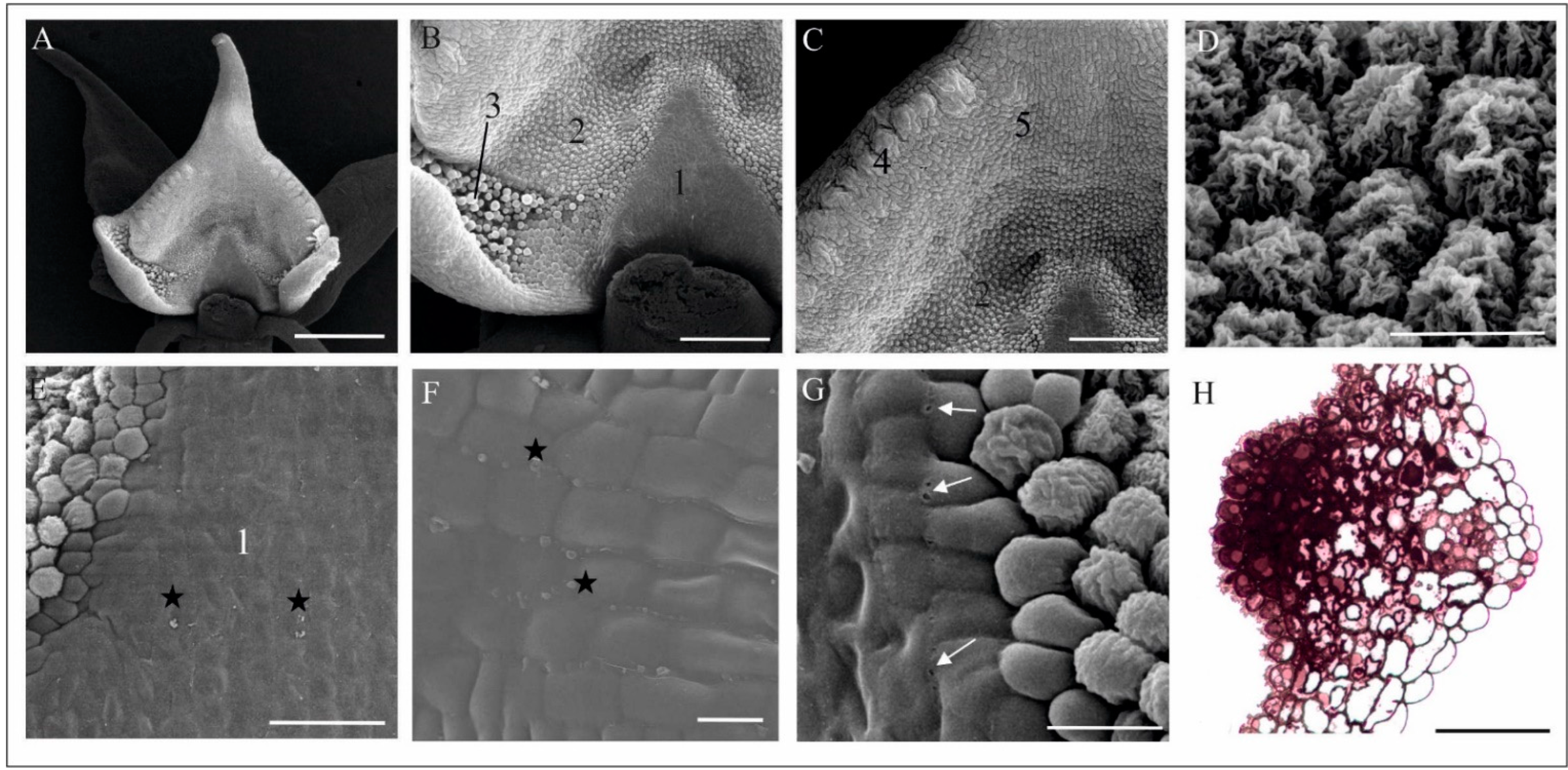
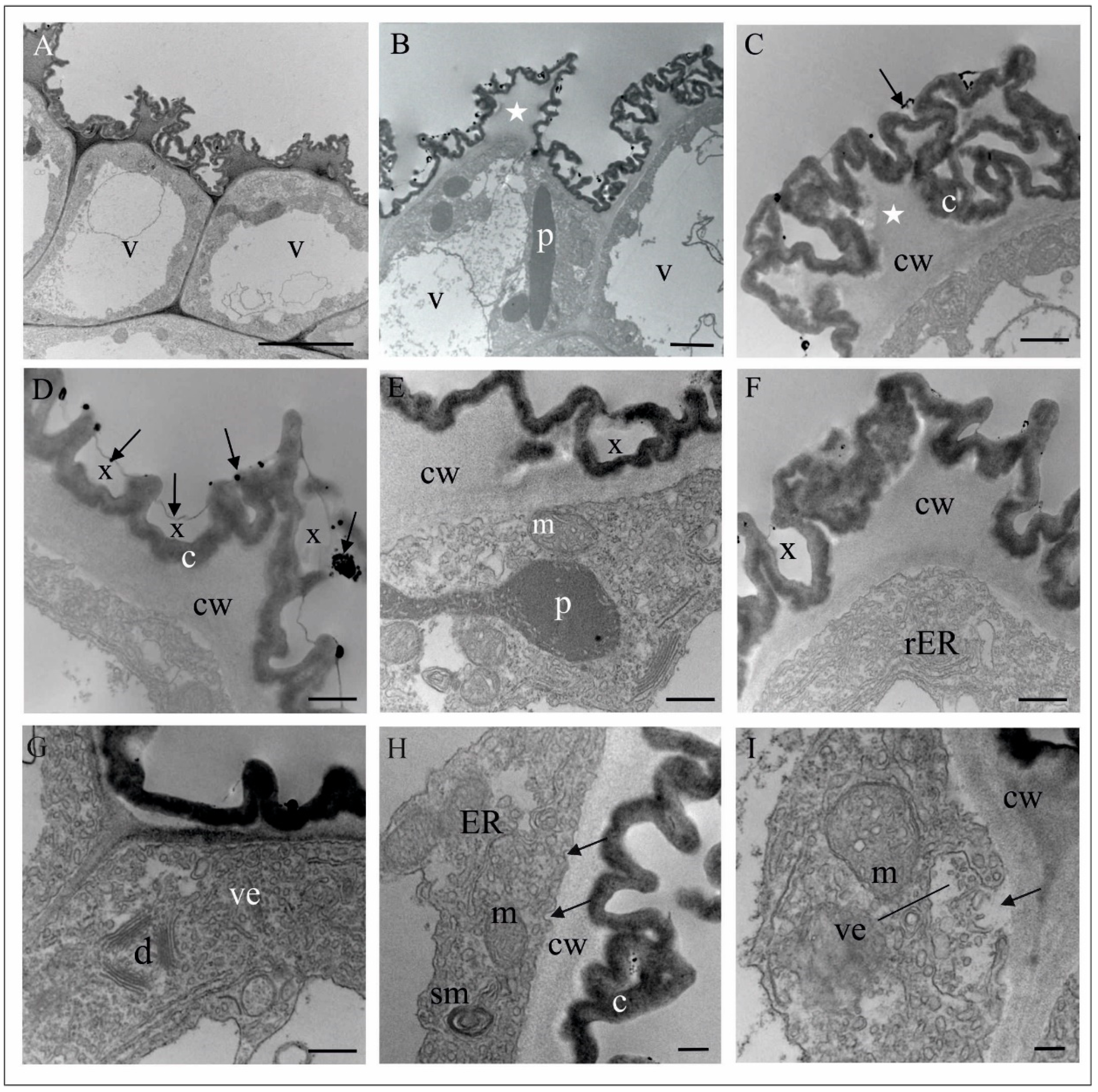

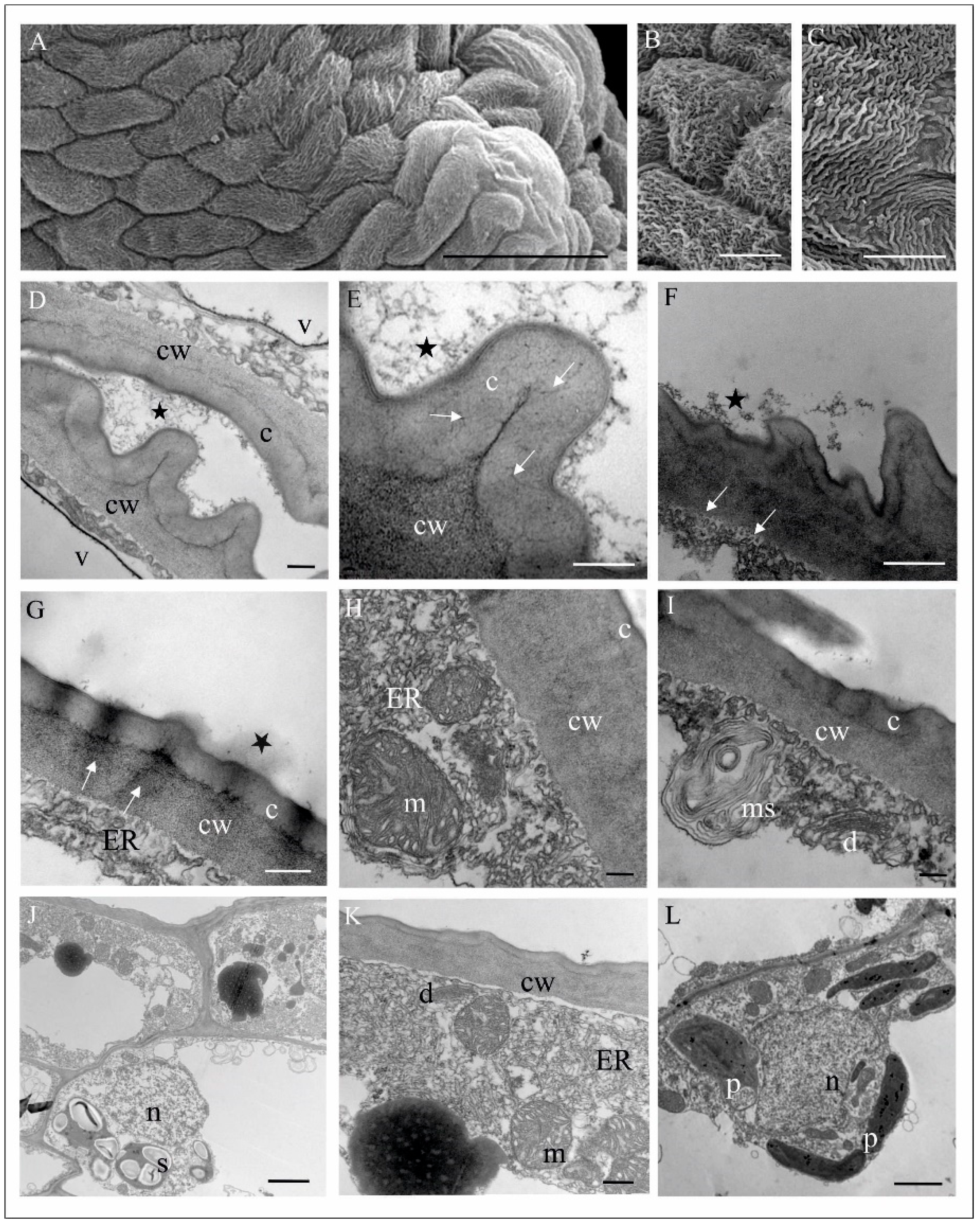
| Order | Family | Species/Genus | N | Nf | Tf (s) |
|---|---|---|---|---|---|
| (Mean ± SD) | |||||
| Diptera | Drosophilidae | Drosophila transversa Fallén, 1823 | 4 | 4.0 * | 66.8 (±122.2) |
| Scaptomyza pallida (Zetterstedt, 1847) or S. graminum (Fallén, 1823) | 3 | 3.7 * | 150.3 (±69.43) | ||
| Sciaroidae | Sciara sp. | 2 | 2.0 * | 52.5 (±67.18) | |
| Mycetophilidae | Unidentified | 2 | 3.0 * | 52.4 (±28.85) | |
| Mycetophilidae or Keroplatidae | Unidentified | 1 | 1 | 5 | |
| Fanniidae | Fannia cf. norvegica Ringdahl, 1934, ♀ | 1 | 3 | 20 | |
| Fannia sp., ♀ | 1 | 1 | >240 | ||
| Culicidae | Unidentified ♀, taxon 1 | 4 | 1.2 * | 10.5 (8.75) | |
| Unidentified ♀, taxon 2 | 2 | 2.0 * | 12.2 (12.97) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jermakowicz, E.; Leśniewska, J.; Stocki, M.; Naczk, A.M.; Kostro-Ambroziak, A.; Pliszko, A. The Floral Signals of the Inconspicuous Orchid Malaxis monophyllos: How to Lure Small Pollinators in an Abundant Environment. Biology 2022, 11, 640. https://doi.org/10.3390/biology11050640
Jermakowicz E, Leśniewska J, Stocki M, Naczk AM, Kostro-Ambroziak A, Pliszko A. The Floral Signals of the Inconspicuous Orchid Malaxis monophyllos: How to Lure Small Pollinators in an Abundant Environment. Biology. 2022; 11(5):640. https://doi.org/10.3390/biology11050640
Chicago/Turabian StyleJermakowicz, Edyta, Joanna Leśniewska, Marcin Stocki, Aleksandra M. Naczk, Agata Kostro-Ambroziak, and Artur Pliszko. 2022. "The Floral Signals of the Inconspicuous Orchid Malaxis monophyllos: How to Lure Small Pollinators in an Abundant Environment" Biology 11, no. 5: 640. https://doi.org/10.3390/biology11050640
APA StyleJermakowicz, E., Leśniewska, J., Stocki, M., Naczk, A. M., Kostro-Ambroziak, A., & Pliszko, A. (2022). The Floral Signals of the Inconspicuous Orchid Malaxis monophyllos: How to Lure Small Pollinators in an Abundant Environment. Biology, 11(5), 640. https://doi.org/10.3390/biology11050640






