Histopathology of Corn Plants Infected by Endophytic Fungi
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Endophytic Fungi
2.2. Seed Inoculation and Corn Growth
2.3. Sample Preparation for Light Microscopy
2.4. Transmission Electron Microscopy
2.5. Data Analysis
3. Results
3.1. Endophytic P. citrinum, F. verticillioides, and F. sacchari in Corn Tissues
3.2. Pathogenicity of Endophytic Fungi
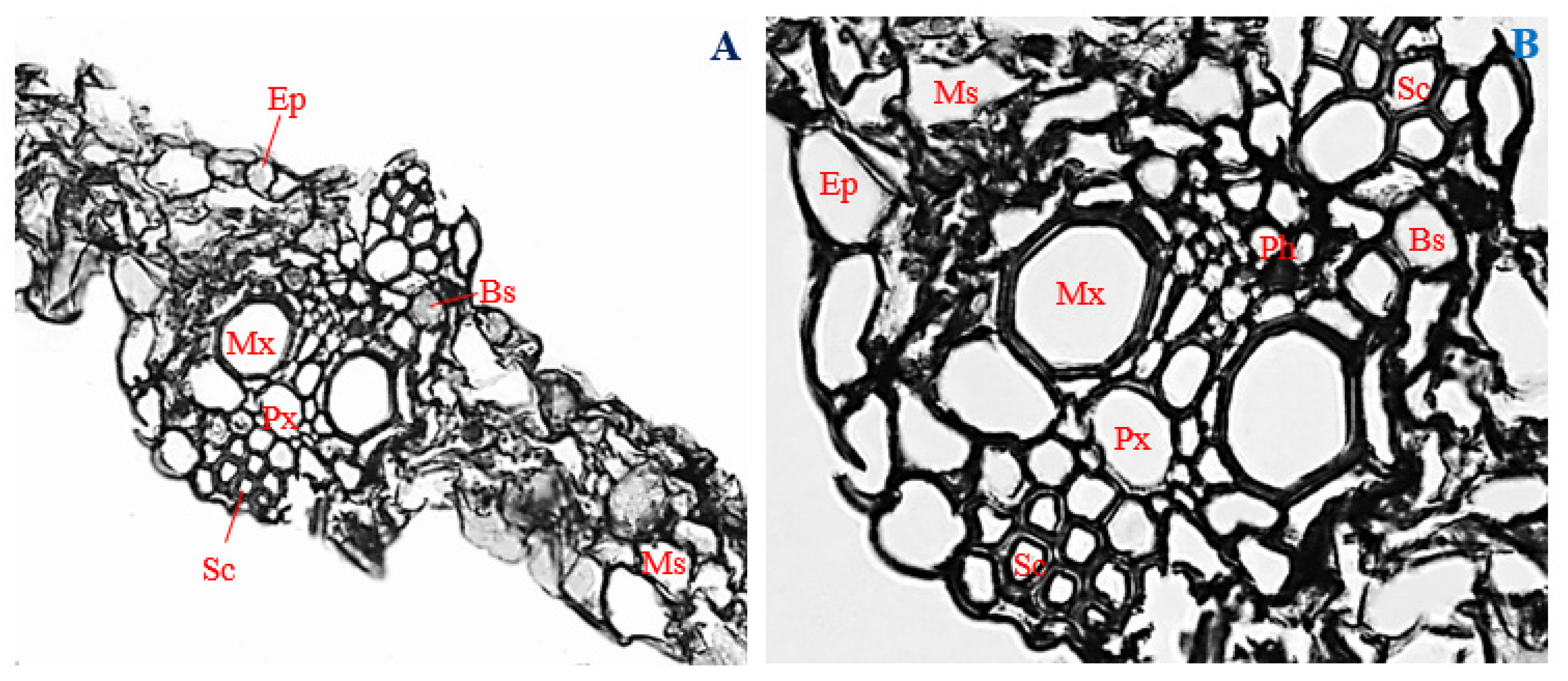
3.3. Histology of Uninfected Corn Roots
3.4. Histology of Corn Roots Infected with Endophytic F. verticillioides
3.5. Histology of Corn Roots Infected with Endophytic F. sacchari
3.6. Histology of Corn Roots Infected with Endophytic P. citrinum
3.7. Histology of Uninfected Corn Stems
3.8. Histology of Corn Stems Infected with Endophytic F. verticillioides
3.9. Histology of Corn Stems Infected with Endophytic F. sacchari
3.10. Histology of Corn Stems Infected with Endophytic P. citrinum
3.11. Histology of Uninfected Corn Leaves
3.12. Histology of Corn Leaves Infected with Endophytic F. verticillioides
3.13. Histology of Corn Leaves Infected with Endophytic P. citrinum
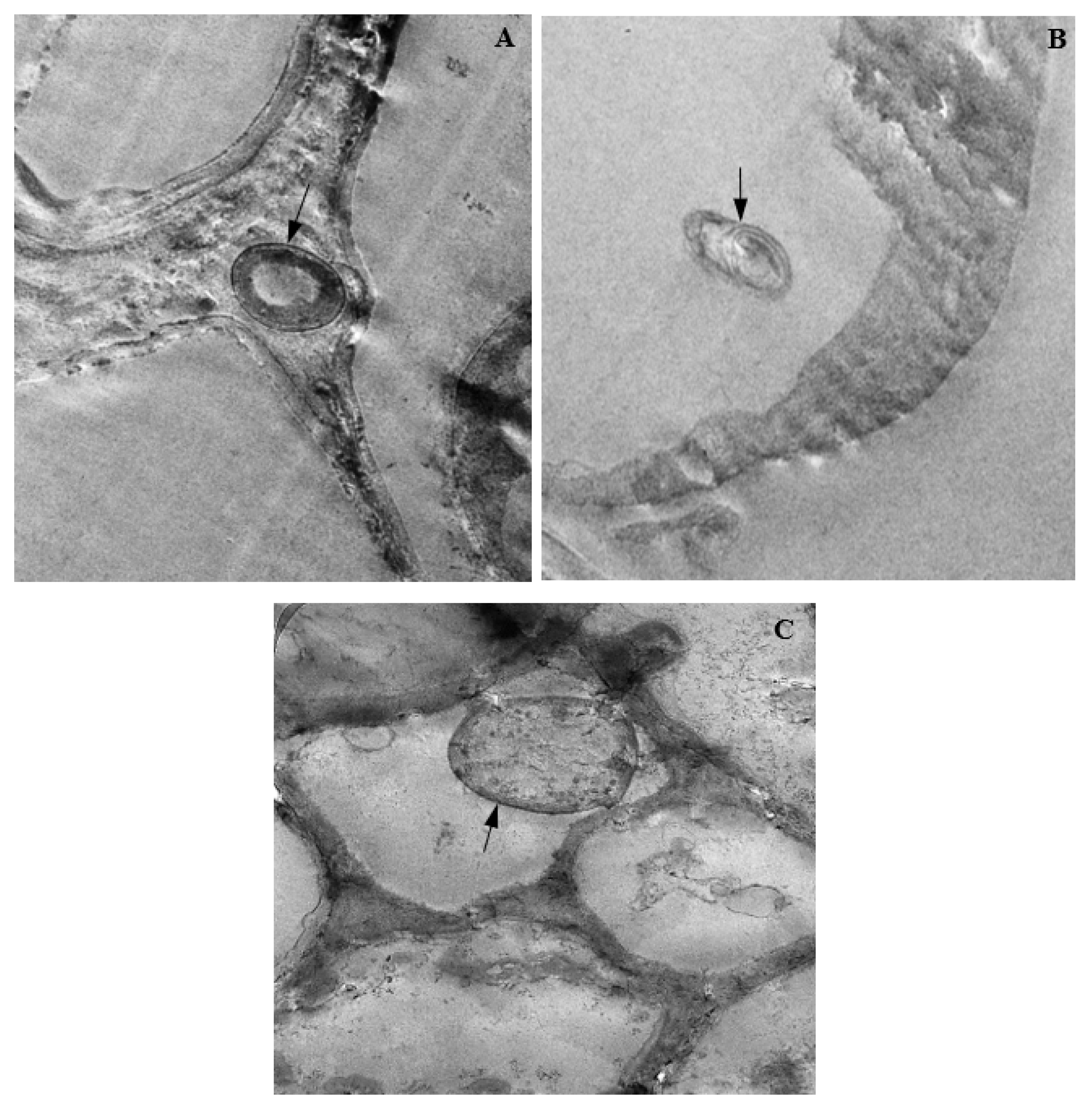
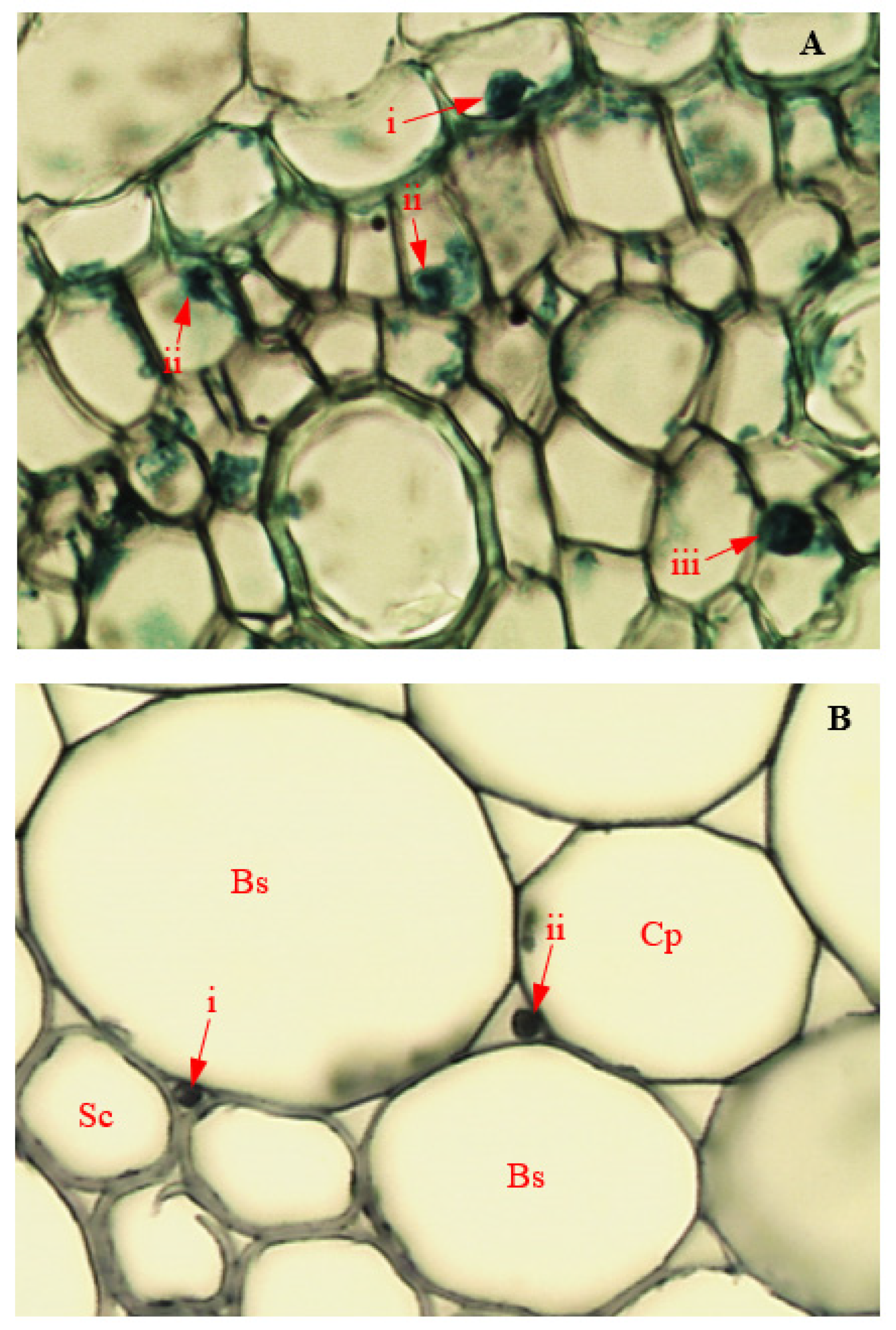
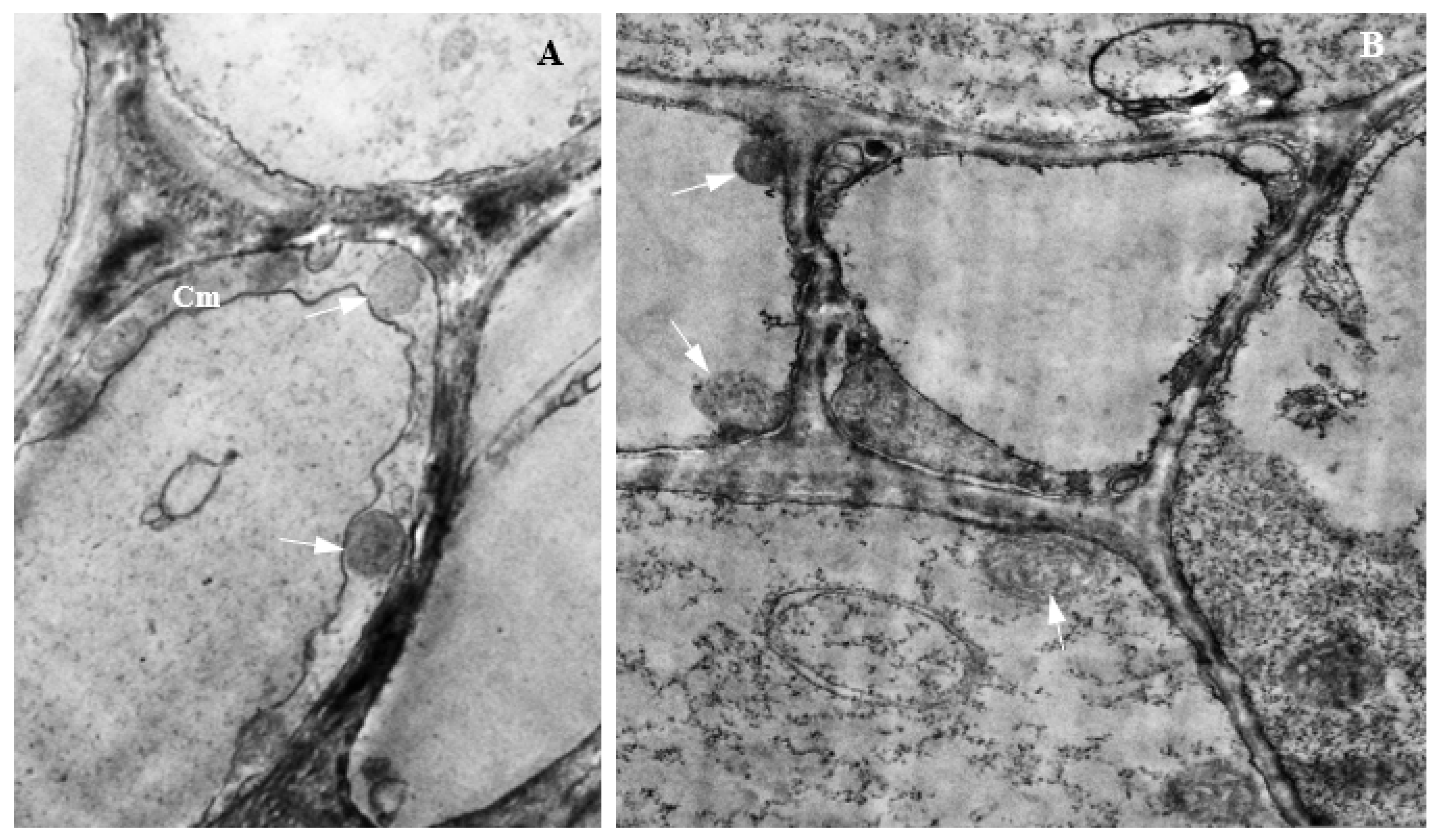
3.14. Colonization of Corn Tissues by Endophytic F. verticillioides
3.15. Colonization of Corn Tissues by Endophytic F. sacchari
3.16. Colonization of Corn Tissues by Endophytic P. citrinum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrini, O. Fungal endophytes of tree leaves. In Microbial Ecology of Leaves; Andrews, J.H., Hirano, S.S., Eds.; Springer: New York, NY, USA, 1991; pp. 179–197. [Google Scholar] [CrossRef]
- Eaton, C.J.; Cox, M.P.; Scott, B. What triggers grass endophytes to switch from mutualism to pathogenism? Plant Sci. 2011, 180, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Yates, I.E.; Bacon, C.W.; Hinton, D.M. Effects of endophytic infection by Fusarium moniliforme on corn growth and cellular morphology. Plant Dis. 1997, 81, 723–728. [Google Scholar] [CrossRef]
- Heng, H.M.; Salleh, B.; Zakaria, L. Molecular identification of Fusarium species in Gibberella fujikuroi species complex from rice, sugarcane and maize from Peninsular Malaysia. Int. J. Mol. Sci. 2011, 12, 6722–6732. [Google Scholar] [CrossRef]
- Renuka, S.; Ramanujam, B. Fungal endophytes from maize (Zea mays L.): Isolation, identification and screening against maize stem borer, Chilo prtellus (Swinhoe). J. Pure Appl. Microbiol. 2016, 10, 523–528. [Google Scholar]
- Lawrence, E.B.; Nelson, P.E.; Ayers, J.E. Histopathology of sweet corn seed and plants infected with Fusarium moniliforme and F. oxysporum. Phytopathology 1981, 71, 379–386. [Google Scholar] [CrossRef]
- Pinto, L.S.R.C.; Azevedo, J.L.; Pereira, J.O.; Vieira, M.L.C.; Labate, C.A. Symptomless infection of banana and maize by endophytic fungi impairs photosynthentic efficiency. New Phytol. 2000, 147, 609–615. [Google Scholar] [CrossRef]
- Hinton, D.M.; Bacon, C.W. The distribution and ultrastructure of the endophyte of toxic tall fescue. Can. J. Bot. 1985, 63, 36–42. [Google Scholar] [CrossRef]
- Kuldau, G.A.; Yates, I.E. Evidence for Fusarium endophytes in cultivated and wild plants. In Microbial Endophytes; Bacon, C.W., White, J.F., Jr., Eds.; Dekker: New York, NY, USA, 2000; pp. 85–117. [Google Scholar] [CrossRef]
- García, A.; Rhoden, S.A.; Rubin Filho, C.J.; Nakamura, C.V.; Pamphile, J.A. Diversity of foliar endophytic fungi from the medicinal plant Sapindus saponaria L. and their localization by scanning electron microscopy. Biol. Res. 2012, 45, 139–148. [Google Scholar] [CrossRef][Green Version]
- White, J.F.; Sharp, L.T.; Martin, T.I.; Glenn, A.E. Endophyte-host associations in grasses. XXI. Studies on the structure and development of Balansia obtecta. Mycologia 1995, 87, 172–181. [Google Scholar] [CrossRef]
- White, J.F.; Reddy, P.V.; Glenn, A.E.; Bacon, C.W. An examination of structural features and relationships in Balansia subgenus Dothichloë. Mycologia 1997, 89, 408–419. [Google Scholar] [CrossRef]
- Bacon, C.W.; Hinton, D.M. Symptomless endophytic colonization of maize by Fusarium moniliforme. Can. J. Bot. 1996, 74, 1195–1202. [Google Scholar] [CrossRef]
- Schulz, B.; Wanke, U.; Draeger, S.; Aust, H.J. Endophytes from herbaceous plants and shrubs: Effectiveness of surface sterilization methods. Mycol. Res. 1993, 97, 1447–1450. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Cigelnik, E.; Nirenberg, H.I. Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 1998, 90, 465–493. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press Inc.: San Diego, CA, USA, 1990; pp. 315–321. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Palencia, E.R. Endophytic Associations of Species in the Aspergillus Section Nigri with Maize (Zea mays) and Peanut (Arachis hypogea) Hosts, and Their Mycotoxins. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2012. [Google Scholar]
- Garcia, J.; Barker, D.G.; Journet, E.P. Seed Storage and Germination; Samuel Roberts Noble Foundation: Admore, OK, USA, 2006; pp. 1–9. [Google Scholar]
- Livingston, D.P., III; Tuong, T.D.; Haigler, C.H.; Avci, U.T.K.U.; Tallury, S.P. Rapid microwave processing of winter cereals for histology allows identification of separate zones of freezing injury in the crown. Crop Sci. 2009, 49, 1837–1842. [Google Scholar] [CrossRef]
- Guzmán, P.; Fernández, V.; Khayet, M.; García, M.L.; Fernández, A.; Gil, L. Ultrastructure of plant leaf cuticles in relation to sample preparation as observed by transmission electron microscopy. Sci. World J. 2014, 2014, 963921. [Google Scholar] [CrossRef]
- Pouzoulet, J.; Scudiero, E.; Schiavon, M.; Rolshausen, P.E. Xylem vessel diameter affects the compartmentalization of the vascular pathogen Phaeomoniella chlamydospora in grapevine. Front. Plant Sci. 2017, 8, 1442. [Google Scholar] [CrossRef]
- Muimba-Kankolongo, A. Food Crop Production by Smallholder Farmers in Southern Africa: Challenges and Opportunities for Improvement; Academic Press: Cambridge, MA, USA, 2018; pp. 1–382. [Google Scholar] [CrossRef]
- Yadeta, K.; Thomma, B. The xylem as battleground for plant hosts and vascular wilt pathogens. Front. Plant Sci. 2013, 4, 97. [Google Scholar] [CrossRef]
- Caprari, C.; Richter, A.; Bergmann, C.; Cicero, S.L.; Salvi, G.; Cervone, F.; De Lorenzo, G. Cloning and characterization of a gene encoding the endopolygalacturonase of Fusarium moniliforme. Mycol. Res. 1993, 97, 497–505. [Google Scholar] [CrossRef]
- Engelsdorf, T.; Hamann, T. An update on receptor-like kinase involvement in the maintenance of plant cell wall integrity. Ann. Bot. 2014, 114, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Kärkönen, A.; Koutaniemi, S. Lignin biosynthesis studies in plant tissue cultures. J. Integr. Plant Biol. 2010, 52, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Ride, J.P. The effect of induced lignification on the resistance of wheat cell walls to fungal degradation. Physiol. Plant Pathol. 1980, 16, 187–196. [Google Scholar] [CrossRef]
- Attia, Z.; Dalal, A.; Moshelion, M. Vascular bundle sheath and mesophyll regulation of leaf water balance in response to chitin. Plant J. 2018, 101, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, K.; Chern, M.; Liu, Y.; Zhu, Z.; Liu, J.; Zhu, X.; Yin, J.; Ran, L.; Xiong, J.; et al. Sclerenchyma cell thickening through enhanced lignification induced by OsMYB30 prevents fungal penetration of rice leaves. New Phytol. 2020, 226, 1850–1863. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Hamayun, M.; Yoon, H.; Kim, H.Y.; Suh, S.J.; Hwang, S.K.; Kim, J.M.; Lee, I.J.; Choo, Y.S.; Yoon, U.H.; et al. Plant growth promotion and Penicillium citrinum. BMC Microbiol. 2008, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Khan, A.L.; Hamayun, M.; Shahzad, R.; Kim, Y.H.; Choi, K.S.; Lee, I.J. Endophytic infection alleviates biotic stress in sunflower through regulation of defence hormones, antioxidants and functional amino acids. Eur. J. Plant Pathol. 2015, 141, 803–824. [Google Scholar] [CrossRef]
- Batista, M.F.; Moscheta, I.S.; Bonato, C.M.; Batista, M.A.; de Almeida, O.J.G.; Inoue, T.T. Aluminum in corn plants: Influence on growth and morpho-anatomy of root and leaf. Rev. Bras. Cienc. Solo 2013, 37, 177–187. [Google Scholar] [CrossRef]
- Farber, D.H.; Mundt, C.C. Effect of plant age and leaf position on susceptibility to wheat stripe rust. Phytopathology 2017, 107, 412–417. [Google Scholar] [CrossRef]
- Marla, S.R.; Chu, K.; Chintamanani, S.; Multani, D.S.; Klempein, A.; DeLeon, A.; Bong-suk, K.; Dunkle, L.D.; Dilkes, B.P.; Johal, G.S. Adult plant resistance in maize to northern leaf spot is a feature of partial loss-of-function alleles of Hm1. PLoS Pathog. 2018, 14, e1007356. [Google Scholar] [CrossRef]
- Raftoyannis, Y.; Dick, M.W. Effects of inoculum density, plant age and temperature on disease severity caused by Pythiaceous fungi on several plants. Phytoparasitica 2002, 30, 67–76. [Google Scholar] [CrossRef]
- Williams, W.P.; Alpe, M.N.; Windham, G.L.; Ozkan, S.; Mylroie, J.E. Comparison of two inoculation methods for evaluating maize for resistance to Aspergillus flavus infection and aflatoxin accumulation. Int. J. Agron. 2013, 2013, 972316. [Google Scholar] [CrossRef]













| Fungal Endophyte | Occurrence (%) | |
|---|---|---|
| Husk | Kernel | |
| P. citrinum | 1.40 | 28.20 |
| F. verticillioides | 8.57 | 3.93 |
| F. sacchari | 8.57 | 0.00 |
| Disease Symptoms | |||
|---|---|---|---|
| Endophyte | Roots | Stems | Leaves |
| F. verticillioides | Reduced root formation | Reduced stem elongation, stem narrowing, and stem rot | Leaf chlorosis and necrosis |
| F. sacchari | Reduced root formation | Stem narrowing, excessive stem elongation, and stem malformation | - |
| P. citrinum | Reduced root formation | Stem narrowing | Leaf chlorosis and necrosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terna, T.P.; Mohamed Nor, N.M.I.; Zakaria, L. Histopathology of Corn Plants Infected by Endophytic Fungi. Biology 2022, 11, 641. https://doi.org/10.3390/biology11050641
Terna TP, Mohamed Nor NMI, Zakaria L. Histopathology of Corn Plants Infected by Endophytic Fungi. Biology. 2022; 11(5):641. https://doi.org/10.3390/biology11050641
Chicago/Turabian StyleTerna, Tersoo P., Nik Mohd Izham Mohamed Nor, and Latiffah Zakaria. 2022. "Histopathology of Corn Plants Infected by Endophytic Fungi" Biology 11, no. 5: 641. https://doi.org/10.3390/biology11050641
APA StyleTerna, T. P., Mohamed Nor, N. M. I., & Zakaria, L. (2022). Histopathology of Corn Plants Infected by Endophytic Fungi. Biology, 11(5), 641. https://doi.org/10.3390/biology11050641






