The BDNF Val66Met Polymorphism Does Not Increase Susceptibility to Activity-Based Anorexia in Rats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Activity-Based Anorexia (ABA)
2.3. Feeding Patterns and Behaviour
2.4. Measurements of UCP1 Protein in Adipose Tissue
2.5. Statistical Analyses
2.5.1. Activity-Based Anorexia
2.5.2. Feeding Patterns and Behaviour
2.5.3. Western Blots
3. Results
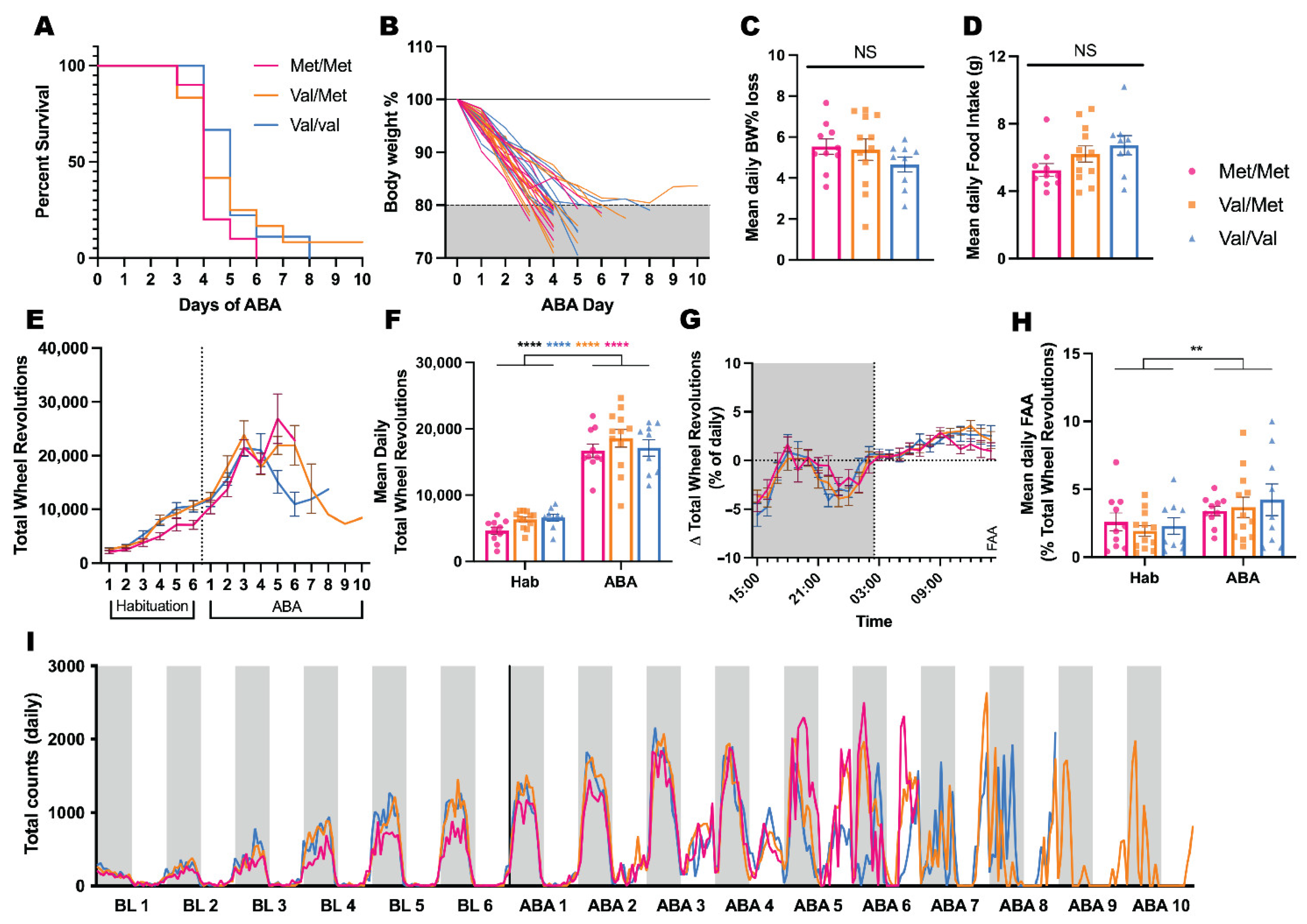
3.1. Effects of BDNF Val68Met on ABA Outcomes
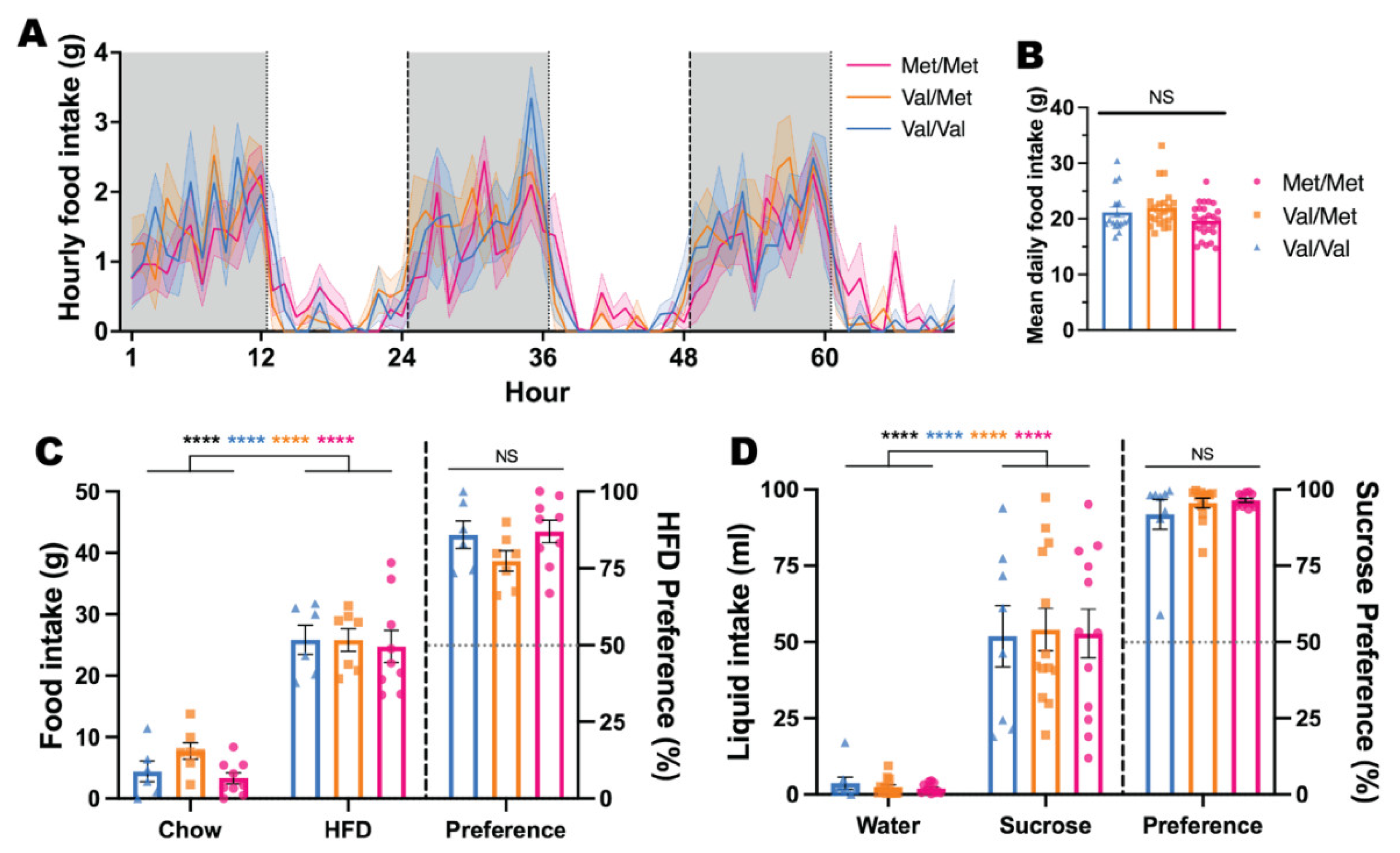
3.2. Effects of BDNF Val68Met on Feeding Patterns and Behaviour
3.3. Effects of BDNF Val68Met on UCP1 Expression in BAT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arcelus, J.; Mitchell, A.J.; Wales, J.; Nielsen, S. Mortality Rates in Patients With Anorexia Nervosa and Other Eating Disorders: A Meta-analysis of 36 Studies. Arch. Gen. Psychiatry 2011, 68, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.J.; Stopyra, M.A.; Friederich, H.C. Neural Processing of Disorder-Related Stimuli in Patients with Anorexia Nervosa: A Narrative Review of Brain Imaging Studies. J. Clin. Med. 2019, 8, 1047. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.; Ramoz, N.; Fladung, A.K.; Gorwood, P. Higher reward value of starvation imagery in anorexia nervosa and association with the Val66Met BDNF polymorphism. Transl. Psychiatry 2016, 6, e829. [Google Scholar] [CrossRef] [PubMed]
- Foldi, C.J.; Milton, L.K.; Oldfield, B.J. The Role of Mesolimbic Reward Neurocircuitry in Prevention and Rescue of the Activity-Based Anorexia (ABA) Phenotype in Rats. Neuropsychopharmacology 2017, 42, 2292. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Aizenstein, H.; Venkatraman, V.K.; Fudge, J.; May, J.C.; Mazurkewicz, L.; Frank, G.K.; Bailer, U.F.; Fischer, L.; Nguyen, V.; et al. Altered Reward Processing in Women Recovered From Anorexia Nervosa. Am. J. Psychiatry 2007, 164, 1842–1849. [Google Scholar] [CrossRef]
- Zastrow, A.; Kaiser, S.; Stippich, C.; Walther, S.; Herzog, W.; Tchanturia, K.; Belger, A.; Weisbrod, M.; Treasure, J.; Friederich, H.-C. Neural Correlates of Impaired Cognitive-Behavioral Flexibility in Anorexia Nervosa. Am. J. Psychiatry 2009, 166, 608–616. [Google Scholar] [CrossRef]
- Ehrlich, S.; Geisler, D.; Ritschel, F.; King, J.A.; Seidel, M.; Boehm, I.; Breier, M.; Clas, S.; Weiss, J.; Marxen, M.; et al. Elevated cognitive control over reward processing in recovered female patients with anorexia nervosa. J. Psychiatry Neurosci. 2015, 40, 307–315. [Google Scholar] [CrossRef]
- Geisler, D.; Ritschel, F.; King, J.A.; Bernardoni, F.; Seidel, M.; Boehm, I.; Runge, F.; Goschke, T.; Roessner, V.; Smolka, M.N.; et al. Increased anterior cingulate cortex response precedes behavioural adaptation in anorexia nervosa. Sci. Rep. 2017, 7, 42066. [Google Scholar] [CrossRef]
- Yilmaz, Z.; Hardaway, J.A.; Bulik, C.M. Genetics and Epigenetics of Eating Disorders. Adv. Genom. Genet. 2015, 5, 131. [Google Scholar]
- Strober, M.; Freeman, R.; Lampert, C.; Diamond, J.; Kaye, W. Controlled Family Study of Anorexia Nervosa and Bulimia Nervosa: Evidence of Shared Liability and Transmission of Partial Syndromes. Am. J. Psychiatry 2000, 157, 393–401. [Google Scholar] [CrossRef]
- Wade, T.D.; Bergin, J.L.; Tiggemann, M.; Bulik, C.M.; Fairburn, C.G. Prevalence and long-term course of lifetime eating disorders in an adult Australian twin cohort. Aust. N. Z. J. Psychiatry 2006, 40, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Bulik, C.M.; Blake, L.; Austin, J. Genetics of Eating Disorders: What the Clinician Needs to Know. Psychiatr. Clin. 2019, 42, 59–73. [Google Scholar] [CrossRef]
- Duncan, L.; Yilmaz, Z.; Gaspar, H.; Walters, R.; Goldstein, J.; Anttila, V.; Bulik-Sullivan, B.; Ripke, S.; Thornton, L.; Hinney, A.; et al. Significant Locus and Metabolic Genetic Correlations Revealed in Genome-Wide Association Study of Anorexia Nervosa. Am. J. Psychiatry 2017, 174, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Thornton, L.M.; Munn-Chernoff, M.A.; Baker, J.H.; Juréus, A.; Parker, R.; Henders, A.K.; Larsen, J.T.; Petersen, L.; Watson, H.J.; Yilmaz, Z.; et al. The Anorexia Nervosa Genetics Initiative (ANGI): Overview and methods. Contemp. Clin. Trials 2018, 74, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Watson, H.J.; Yilmaz, Z.; Thornton, L.M.; Hübel, C.; Coleman, J.R.I.; Gaspar, H.A.; Bryois, J.; Hinney, A.; Leppä, V.M.; Mattheisen, M.; et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 2019, 51, 1207–1214. [Google Scholar] [CrossRef]
- Wei, Z.; Liao, J.; Qi, F.; Meng, Z.; Pan, S. Evidence for the contribution of BDNF-TrkB signal strength in neurogenesis: An organotypic study. Neurosci. Lett. 2015, 606, 48. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164. [Google Scholar] [CrossRef]
- Notaras, M.; van den Buuse, M. Brain-Derived Neurotrophic Factor (BDNF): Novel Insights into Regulation and Genetic Variation. Neuroscientist 2019, 25, 434–454. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Begni, V.; Pariante, C.M.; Riva, M.A. The human BDNF gene: Peripheral gene expression and protein levels as biomarkers for psychiatric disorders. Transl. Psychiatry 2016, 6, e958. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, J.; Chen, D.; Xiu, M.; Yang, F.; Kosten, T.; Kosten, T. Low BDNF is associated with cognitive impairment in chronic patients with schizophrenia. Psychopharmacology 2012, 222, 277–284. [Google Scholar] [CrossRef]
- Kreinin, A.; Lisson, S.; Nesher, E.; Schneider, J.; Bergman, J.; Farhat, K.; Farah, J.; Lejbkowicz, F.; Yadid, G.; Raskin, L.; et al. Blood BDNF Level Is Gender Specific in Severe Depression. PLoS ONE 2015, 10, e0127643. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.L.; Bus, B.A.A.; Spinhoven, P.; Penninx, B.W.J.H.; Prickaerts, J.; Oude Voshaar, R.C.; Elzinga, B.M. Gender specific associations of serum levels of brain-derived neurotrophic factor in anxiety. World J. Biol. Psychiatry 2012, 13, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Brandys, M.K.; Kas, M.J.H.; van Elburg, A.A.; Campbell, I.C.; Adan, R.A.H. A meta-analysis of circulating BDNF concentrations in anorexia nervosa. Neuroscientist 2011, 12, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Watanabe, K.; Hashimoto, E.; Saito, T. Low serum BDNF and food intake regulation: A possible new explanation of the pathophysiology of eating disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.E.; Jimerson, D.C.; Pillai, A.; Wolfe, B.E. Plasma BDNF levels following weight recovery in anorexia nervosa. Physiol. Behav. 2016, 165, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Zwipp, J.; Hass, J.; Schober, I.; Geisler, D.; Ritschel, F.; Seidel, M.; Weiss, J.; Roessner, V.; Hellweg, R.; Ehrlich, S. Serum brain-derived neurotrophic factor and cognitive functioning in underweight, weight-recovered and partially weight-recovered females with anorexia nervosa. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 54, 163–169. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Liao, G.-Y.; Kinney, C.E.; Sahibzada, N.; Xu, B. Discrete BDNF Neurons in the Paraventricular Hypothalamus Control Feeding and Energy Expenditure. Cell Metab. 2015, 22, 175–188. [Google Scholar] [CrossRef]
- Cordeira, J.; Rios, M. Weighing in the Role of BDNF in the Central Control of Eating Behavior. Mol. Neurobiol. 2011, 44, 441–448. [Google Scholar] [CrossRef]
- Rios, M. BDNF and the central control of feeding: Accidental bystander or essential player? Trends Neurosci. 2013, 36, 83–90. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Z.; Kalyani, M.; Janik, J.M.; Shi, H. Effects of energy status and diet on Bdnf expression in the ventromedial hypothalamus of male and female rats. Physiol. Behav. 2014, 130, 99–107. [Google Scholar] [CrossRef]
- Cordeira, J.W.; Frank, L.; Sena-Esteves, M.; Pothos, E.N.; Rios, M. Brain-Derived Neurotrophic Factor Regulates Hedonic Feeding by Acting on the Mesolimbic Dopamine System. J. Neurosci. 2010, 30, 2533. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, M.; Hashimoto, K.; Shimizu, E.; Kumakiri, C.; Koizumi, H.; Okamura, N.; Mitsumori, M.; Komatsu, N.; Iyo, M. Decreased levels of serum brain-derived neurotrophic factor in female patients with eating disorders. Biol. Psychiatry 2003, 54, 485–490. [Google Scholar] [CrossRef]
- Karege, F.; Bondolfi, G.; Gervasoni, N.; Schwald, M.; Aubry, J.-M.; Bertschy, G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol. Psychiatry 2005, 57, 1068. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, H.; Altar, C.A.; Chen, R.; Nakamura, T.; Nakahashi, T.; Kambayashi, J.I.; Sun, B.; Tandon, N.N. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb. Haemost. 2002, 87, 728–734. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, G.; Du, X.; Zhang, Y.; Yin, G.; Dai, J.; He, M.-X.; Soares, J.C.; Li, X.; Zhang, X.Y. Suicide Attempt, Clinical Correlates, and BDNF Val66Met Polymorphism in Chronic Patients With Schizophrenia. Neuropsychology 2018, 32, 199–205. [Google Scholar] [CrossRef]
- Sun, M.-M.; Yang, L.-M.; Wang, Y.; Feng, X.; Cui, K.-Y.; Liu, L.-F.; Chen, Z.-Y. BDNF Val66Met polymorphism and anxiety/depression symptoms in schizophrenia in a Chinese Han population. Psychiatr. Genet. 2013, 23, 124–129. [Google Scholar] [CrossRef]
- Montag, C.; Basten, U.; Stelzel, C.; Fiebach, C.J.; Reuter, M. The BDNF Val66Met polymorphism and anxiety: Support for animal knock-in studies from a genetic association study in humans. Psychiatry Res. 2010, 179, 86–90. [Google Scholar] [CrossRef]
- Notaras, M.; Hill, R.; van den Buuse, M. A role for the BDNF gene Val66Met polymorphism in schizophrenia? A comprehensive review. Neurosci. Biobehav. Rev. 2015, 51, 15–30. [Google Scholar] [CrossRef]
- Hong, C.-J.; Liou, Y.-J.; Tsai, S.-J. Effects of BDNF polymorphisms on brain function and behavior in health and disease. Brain Res. Bull. 2011, 86, 287–297. [Google Scholar] [CrossRef]
- Tsai, S.-J. Critical Issues in BDNF Val66Met Genetic Studies of Neuropsychiatric Disorders. Front. Mol. Neurosci. 2018, 11, 156. [Google Scholar] [CrossRef]
- Ieraci, A.; Madaio, A.I.; Mallei, A.; Lee, F.S.; Popoli, M. Brain-Derived Neurotrophic Factor Val66Met Human Polymorphism Impairs the Beneficial Exercise-Induced Neurobiological Changes in Mice. Neuropsychopharmacology 2016, 41, 3070. [Google Scholar] [CrossRef] [PubMed]
- Ribasés, M.; Gratacòs, M.; Armengol, L.; de Cid, R.; Badía, A.; Jiménez, L.; Solano, R.; Vallejo, J.; Fernández, F.; Estivill, X. Met66 in the brain-derived neurotrophic factor (BDNF) precursor is associated with anorexia nervosa restrictive type. Mol. Psychiatry 2003, 8, 745–751. [Google Scholar] [CrossRef]
- Peciña, M.; Martínez-Jauand, M.; Love, T.; Heffernan, J.; Montoya, P.; Hodgkinson, C.; Stohler, C.S.; Goldman, D.; Zubieta, J.-K. Valence-Specific Effects of BDNF Val66Met Polymorphism on Dopaminergic Stress and Reward Processing in Humans. J. Neurosci. 2014, 34, 5874. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Surgent, O.; Rana, B.S.; Lee, F.; Aoki, C. Variant BDNF-Val66Met Polymorphism is Associated with Layer-Specific Alterations in GABAergic Innervation of Pyramidal Neurons, Elevated Anxiety and Reduced Vulnerability of Adolescent Male Mice to Activity-Based Anorexia. Cereb. Cortex 2016, 27, 3980–3993. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Foldi, C.J.; Milton, L.K.; Oldfield, B.J. A focus on reward in anorexia nervosa through the lens of the activity-based anorexia rodent model. J. Neuroendocrinol. 2017, 29, e12479. [Google Scholar] [CrossRef] [PubMed]
- Gelegen, C.; Van Den Heuvel, J.; Collier, D.A.; Campbell, I.C.; Oppelaar, H.; Hessel, E.; Kas, M.J.H. Dopaminergic and brain-derived neurotrophic factor signalling in inbred mice exposed to a restricted feeding schedule. Genes Brain Behav. 2008, 7, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Madra, M.; Zeltser, L.M. BDNF-Val66Met variant and adolescent stress interact to promote susceptibility to anorexic behavior in mice. Transl. Psychiatry 2016, 6, e776. [Google Scholar] [CrossRef]
- Martha, A.S.; Andreas, S.; Andreas, S. Activity Based Anorexia as an Animal Model for Anorexia Nervosa-A Systematic Review.(Report). Front. Nutr. 2019, 6, 69. [Google Scholar] [CrossRef]
- Ho, E.V.; Klenotich, S.J.; McMurray, M.S.; Dulawa, S.C. Activity-Based Anorexia Alters the Expression of BDNF Transcripts in the Mesocorticolimbic Reward Circuit. PLoS ONE 2016, 11, e0166756. [Google Scholar] [CrossRef]
- Mercado, N.M.; Stancati, J.A.; Sortwell, C.E.; Mueller, R.L.; Boezwinkle, S.A.; Duffy, M.F.; Fischer, D.L.; Sandoval, I.M.; Manfredsson, F.P.; Collier, T.J.; et al. The BDNF Val66Met polymorphism (rs6265) enhances dopamine neuron graft efficacy and side-effect liability in rs6265 knock-in rats. Neurobiol. Dis. 2021, 148, 105175. [Google Scholar] [CrossRef]
- Jaehne, E.J.; Kent, J.N.; Antolasic, E.J.; Wright, B.J.; Spiers, J.G.; Creutzberg, K.C.; De Rosa, F.; Riva, M.A.; Sortwell, C.E.; Collier, T.J.; et al. Behavioral phenotyping of a rat model of the BDNF Val66Met polymorphism reveals selective impairment of fear memory. Transl. Psychiatry 2022, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, N.M.; Stefanidis, A.; Oldfield, B.J. Characterization of the central neural projections to brown, white, and beige adipose tissue. Faseb J. 2017, 31, 4879–4890. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, L.; Ieraci, A.; Amadio, P.; Zarà, M.; Mitro, N.; Lee, F.S.; Tremoli, E.; Barbieri, S.S. Physical Exercise Affects Adipose Tissue Profile and Prevents Arterial Thrombosis in BDNF Val66Met Mice. Cells 2019, 8, 875. [Google Scholar] [CrossRef] [PubMed]
- Fraga, A.; Rial-Pensado, E.; Nogueiras, R.; Fernø, J.; Diéguez, C.; Gutierrez, E.; López, M. Activity-Based Anorexia Induces Browning of Adipose Tissue Independent of Hypothalamic AMPK. Front. Endocrinol. 2021, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Ellenbroek, B.; Youn, J. Rodent models in neuroscience research: Is it a rat race? Dis. Model Mech. 2016, 9, 1079–1087. [Google Scholar] [CrossRef]
- Jankord, R.; Solomon, M.B.; Albertz, J.; Flak, J.N.; Zhang, R.; Herman, J.P. Stress vulnerability during adolescent development in rats. Endocrinology 2011, 152, 629–638. [Google Scholar] [CrossRef]
- Clark, P.J.; Amat, J.; McConnell, S.O.; Ghasem, P.R.; Greenwood, B.N.; Maier, S.F.; Fleshner, M. Running Reduces Uncontrollable Stress-Evoked Serotonin and Potentiates Stress-Evoked Dopamine Concentrations in the Rat Dorsal Striatum. PLoS ONE 2015, 10, e0141898. [Google Scholar] [CrossRef]
- Hare, B.D.; Beierle, J.A.; Toufexis, D.J.; Hammack, S.E.; Falls, W.A. Exercise-associated changes in the corticosterone response to acute restraint stress: Evidence for increased adrenal sensitivity and reduced corticosterone response duration. Neuropsychopharmacology 2014, 39, 1262–1269. [Google Scholar] [CrossRef]
- Notaras, M.; van den Buuse, M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol. Psychiatry 2020, 25, 2251–2274. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrucci, C.L.; Milton, L.K.; Greaves, E.; Stefanidis, A.; van den Buuse, M.; Oldfield, B.J.; Foldi, C.J. The BDNF Val66Met Polymorphism Does Not Increase Susceptibility to Activity-Based Anorexia in Rats. Biology 2022, 11, 623. https://doi.org/10.3390/biology11050623
Pietrucci CL, Milton LK, Greaves E, Stefanidis A, van den Buuse M, Oldfield BJ, Foldi CJ. The BDNF Val66Met Polymorphism Does Not Increase Susceptibility to Activity-Based Anorexia in Rats. Biology. 2022; 11(5):623. https://doi.org/10.3390/biology11050623
Chicago/Turabian StylePietrucci, Carla L., Laura K. Milton, Erika Greaves, Aneta Stefanidis, Maarten van den Buuse, Brian J. Oldfield, and Claire J. Foldi. 2022. "The BDNF Val66Met Polymorphism Does Not Increase Susceptibility to Activity-Based Anorexia in Rats" Biology 11, no. 5: 623. https://doi.org/10.3390/biology11050623
APA StylePietrucci, C. L., Milton, L. K., Greaves, E., Stefanidis, A., van den Buuse, M., Oldfield, B. J., & Foldi, C. J. (2022). The BDNF Val66Met Polymorphism Does Not Increase Susceptibility to Activity-Based Anorexia in Rats. Biology, 11(5), 623. https://doi.org/10.3390/biology11050623





