Impact of Global Climate Change on the Distribution Range and Niche Dynamics of Eleutherodactylus planirostrish in China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Species and Environmental Data
2.2. MaxEnt Model
2.3. Classification of Suitable Habitats
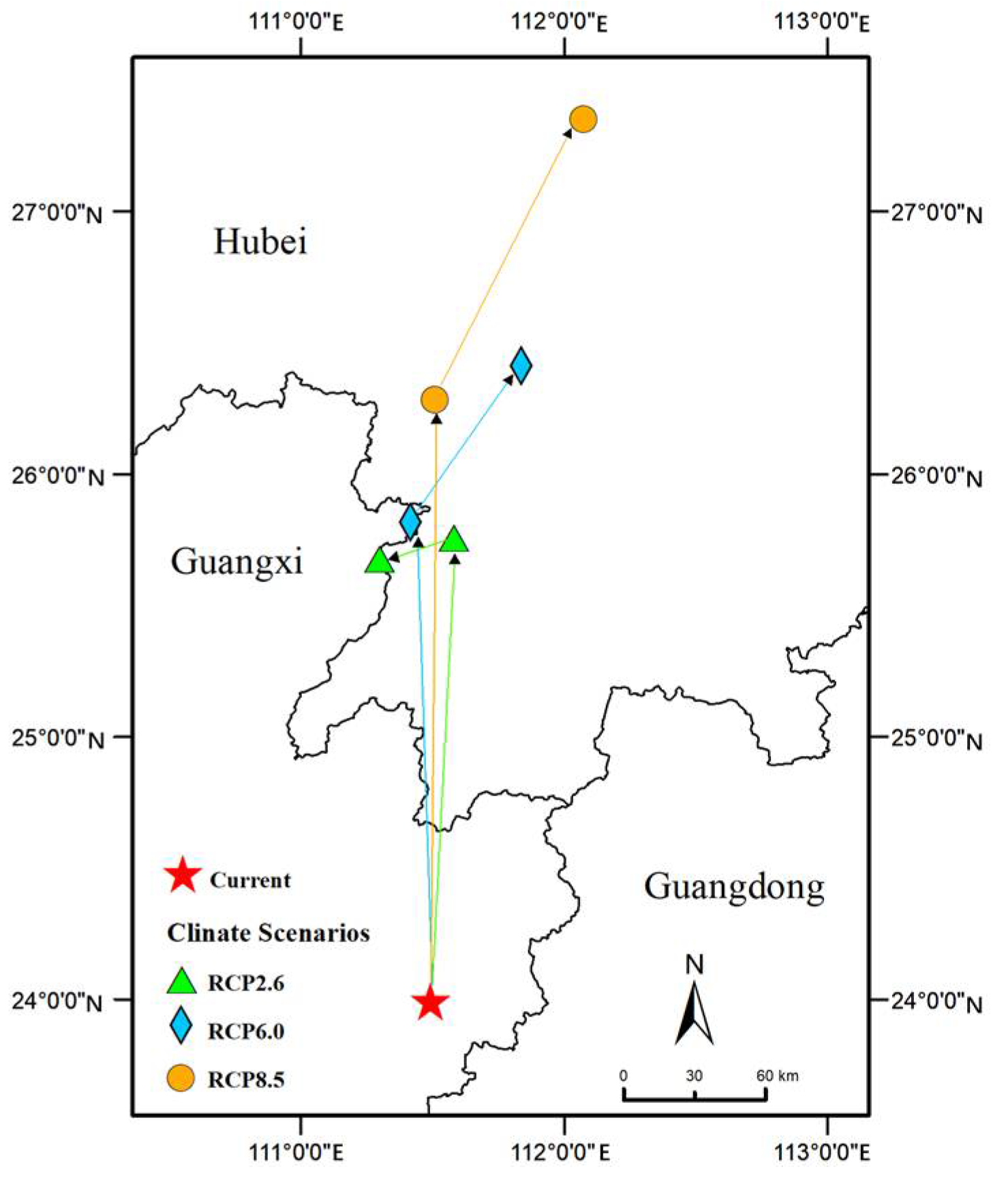
2.4. Changes in Suitable Habitat Area and Centroids
3. Results
3.1. Model Selection and Accuracy Evaluation
3.2. Prediction of Current Distribution of E. planirostris
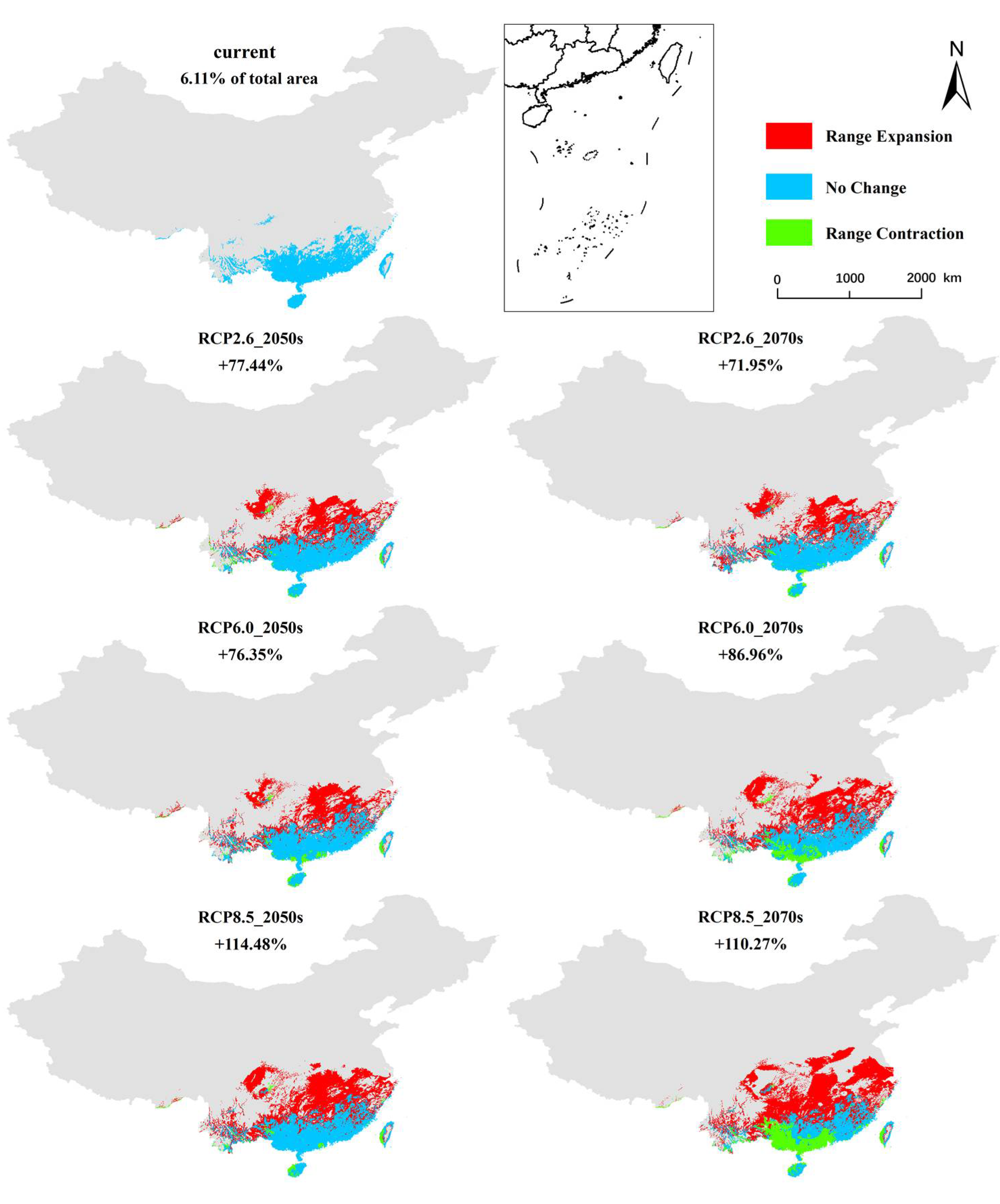
3.3. Prediction of Future E. planirostris Distribution in China
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gama, M.; Crespo, D.; Dolbeth, M.; Anastácio, P.M. Ensemble Forecasting of Corbicula Fluminea Worldwide Distribution: Projections of the Impact of Climate Change. Aquat. Conserv. 2017, 27, 675–684. [Google Scholar] [CrossRef]
- IPCC. The Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Paris, France, 2018. [Google Scholar]
- Duan, R.-Y.; Kong, X.-Q.; Huang, M.-Y.; Varela, S.; Ji, X. The Potential Effects of Climate Change on Amphibian Distribution, Range Fragmentation and Turnover in China. PeerJ 2016, 4, e2185. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Tibatá, J.; Salaman, P.; Graham, C.H. Effects of Climate Change on Species Distribution, Community Structure, and Conservation of Birds in Protected Areas in Colombia. Reg. Environ. Chang. 2013, 13, 235–248. [Google Scholar] [CrossRef]
- Malcolm, J.R.; Liu, C.; Neilson, R.P.; Hansen, L.; Hannah, L.E.E. Global Warming and Extinctions of Endemic Species from Biodiversity Hotspots. Conserv. Biol. 2006, 20, 538–548. [Google Scholar] [CrossRef]
- Slatyer, R.A.; Hirst, M.; Sexton, J.P. Niche Breadth Predicts Geographical Range Size: A General Ecological Pattern. Ecol. Lett. 2013, 16, 1104–1114. [Google Scholar] [CrossRef]
- Urban, M.C.; Tewksbury, J.J.; Sheldon, K.S. On a Collision Course: Competition and Dispersal Differences Create No-Analogue Communities and Cause Extinctions during Climate Change. Proc. R. Soc. B-Biol. Sci. 2012, 279, 2072–2080. [Google Scholar] [CrossRef]
- Hu, L.-L.; Zhang, H.-Y.; Qin, L.; Yan, B.-Q. Current Distribution of Schisandra Chinensis in China and Its Predicted Responses to Climate Change. J. Appl. Ecol. 2012, 23, 2445–2450. [Google Scholar]
- Erasmus, B.F.; Van Jaarsveld, A.S.; Chown, S.L.; Kshatriya, M.; Wessels, K.J. Vulnerability of South African Animal Taxa to Climate Change. Glob. Chang. Biol. 2002, 8, 679–693. [Google Scholar] [CrossRef]
- Guralnick, R. Differential Effects of Past Climate Warming on Mountain and Flatland Species Distributions: A Multispecies North American Mammal Assessment. Glob. Ecol. Biogeogr. 2007, 16, 14–23. [Google Scholar] [CrossRef]
- Hari, R.E.; Livingstone, D.M.; Siber, R.; Burkhardt-Holm, P.; Guettinger, H. Consequences of Climatic Change for Water Temperature and Brown Trout Populations in Alpine Rivers and Streams. Glob. Chang. Biol. 2006, 12, 10–26. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of Global Warming on Wild Animals and Plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological Responses to Recent Climate Change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Ryrholm, N.; Stefanescu, C.; Hill, J.K.; Thomas, C.D.; Descimon, H.; Huntley, B.; Kaila, L.; Kullberg, J.; Tammaru, T. Poleward Shifts in Geographical Ranges of Butterfly Species Associated with Regional Warming. Nature 1999, 399, 579–583. [Google Scholar] [CrossRef]
- Thomas, C.D.; Lennon, J.J. Birds Extend Their Ranges Northwards. Nature 1999, 399, 213. [Google Scholar] [CrossRef]
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate Warming and the Decline of Amphibians and Reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Hersteinsson, P.; Macdonald, D.W. Interspecific Competition and the Geographical Distribution of Red and Arctic Foxes Vulpes Vulpes and Alopex Lagopus. Oikos 1992, 64, 505–515. [Google Scholar] [CrossRef]
- Kiritani, K. Impacts of Global Warming on Nezara Viridula and Its Native Congeneric Species. J. Asia-Pac. Entomol. 2011, 14, 221–226. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.; De Siqueira, M.F.; Grainger, A.; Hannah, L. Extinction Risk from Climate Change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Mittermeier, C.G. Megadiversity: Earth’s Biologically Wealthiest Nations; CEMEX Press: Monterrey, Mexico, 1997; pp. 257–281. [Google Scholar]
- Cope, E.D. On Some New and Little Known American Anura. Proc. Acad. Natl. Sci. Phila. 1862, 14, 151–594. [Google Scholar]
- Heinicke, M.P.; Diaz, L.M.; Hedges, S.B. Origin of Invasive Florida Frogs Traced to Cuba. Biol. Lett. 2011, 7, 407–410. [Google Scholar] [CrossRef]
- Olson, C.A.; Beard, K.H.; Koons, D.N.; Pitt, W.C. Detection Probabilities of Two Introduced Frogs in Hawaii: Implications for Assessing Non-Native Species Distributions. Biol. Invasions 2012, 14, 889–900. [Google Scholar] [CrossRef][Green Version]
- Olson, C.A.; Beard, K.H.; Pitt, W.C. Biology and Impacts of Pacific Island Invasive Species. 8. Eleutherodactylus Planirostris, the Greenhouse Frog (Anura: Eleutherodactylidae) 1. Pac. Sci. 2012, 66, 255–270. [Google Scholar] [CrossRef]
- Lee, W.H.; Lau, M.W.-N.; Lau, A.; Rao, D.-Q.; Sung, Y.-H. Introduction of Eleutherodactylus Planirostris (Amphibia, Anura, Eleutherodactylidae) to Hong Kong. Acta Herpetol. 2016, 11, 85–89. [Google Scholar] [CrossRef]
- Christy, M.T.; Clark, C.S.; Gee, D.E.; Vice, D.; Vice, D.S.; Warner, M.P.; Tyrrell, C.L.; Rodda, G.H.; Savidge, J.A. Recent Records of Alien Anurans on the Pacific Island of Guam. Pac. Sci. 2007, 61, 469–483. [Google Scholar] [CrossRef]
- Kraus, F.; Campbell, E.W.; Allison, A.; Pratt, T. Eleutherodactylus Frog Introduction to Hawaii. Herpetol. Rev. 1999, 30, 21–25. [Google Scholar]
- Bomford, M.; Kraus, F.; Barry, S.C.; Lawrence, E. Predicting Establishment Success for Alien Reptiles and Amphibians: A Role for Climate Matching. Biol. Invasions 2009, 11, 713–724. [Google Scholar] [CrossRef]
- Kraus, F. (Ed.) Alien Reptiles and Amphibians: A Scientific Compendium and Analysis; Springer: Dordrecht, The Netherlands, 2009; Volume 4, pp. 133–369. ISBN 978-1-4020-8945-9. [Google Scholar]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A Toolbox for Comparative Studies of Environmental Niche Models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- van Vuuren, D.P.; Stehfest, E.; den Elzen, M.G.J.; Kram, T.; van Vliet, J.; Deetman, S.; Isaac, M.; Klein Goldewijk, K.; Hof, A.; Mendoza Beltran, A.; et al. RCP2.6: Exploring the Possibility to Keep Global Mean Temperature Increase below 2 °C. Clim. Chang. 2011, 109, 95–116. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent Modeling for Predicting the Potential Geographical Distribution of Two Peony Species under Climate Change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Peterson, A.T.; Soberón, J.; Overton, J.M.; Aragón, P.; Lobo, J.M. Use of Niche Models in Invasive Species Risk Assessments. Biol. Invasions 2011, 13, 2785–2797. [Google Scholar] [CrossRef]
- Jayasinghe, S.L.; Kumar, L. Modeling the Climate Suitability of Tea [Camellia Sinensis(L.) O. Kuntze] in Sri Lanka in Response to Current and Future Climate Change Scenarios. Agr. For. Meteorol. 2019, 272–273, 102–117. [Google Scholar] [CrossRef]
- Steele, K.; Werndl, C. Climate Models, Calibration, and Confirmation. Br. J. Philos Sci. 2013, 64, 609–635. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. Kuenm: An R Package for Detailed Development of Ecological Niche Models Using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A. Insights into the Area under the Receiver Operating Characteristic Curve (AUC) as a Discrimination Measure in Species Distribution Modelling: Insights into the AUC. Glob. Ecol. Biogeogr. 2012, 21, 498–507. [Google Scholar] [CrossRef]
- Liu, B.; Gao, X.; Zheng, K.; Ma, J.; Jiao, Z.; Xiao, J.; Wang, H. The Potential Distribution and Dynamics of Important Vectors Culex Pipiens Pallens and Culex Pipiens Quinquefasciatus in China under Climate Change Scenarios: An Ecological Niche Modelling Approach. Pest. Manag. Sci. 2020, 76, 3096–3107. [Google Scholar] [CrossRef]
- Yan, X.; Wang, S.; Duan, Y.; Han, J.; Huang, D.; Zhou, J. Current and Future Distribution of the Deciduous Shrub Hydrangea Macrophylla in China Estimated by MaxEnt. Ecol. Evol. 2021, 11, 16099–16112. [Google Scholar] [CrossRef]
- Capinha, C.; Larson, E.R.; Tricarico, E.; Olden, J.D.; Gherardi, F. Effects of Climate Change, Invasive Species, and Disease on the Distribution of Native European Crayfishes: Global Change and European Crayfishes. Conserv. Biol. 2013, 27, 731–740. [Google Scholar] [CrossRef]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next Generation Python-Based GIS Toolkit for Landscape Genetic, Biogeographic and Species Distribution Model Analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef]
- Mainali, K.P.; Warren, D.L.; Dhileepan, K.; McConnachie, A.; Strathie, L.; Hassan, G.; Karki, D.; Shrestha, B.B.; Parmesan, C. Projecting Future Expansion of Invasive Species: Comparing and Improving Methodologies for Species Distribution Modeling. Glob. Chang. Biol. 2015, 21, 4464–4480. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wang, J.; Lv, Z.; Liang, P.; Luo, L.; Wang, X.; Wang, Y. First Record of An Alien Invasive Species Eleutherodactylus Planirostris in Mainland China, and Its Population Study. Sichuan J. Zool. 2017, 36, 680–685. [Google Scholar] [CrossRef]
- Garrido, O.H.; Schwartz, A. Anfibios, Reptiles y Aves de La Península de Guanahacabibes; Academia de Ciencias de: Havana, Cuba, 1968; pp. 53–68. [Google Scholar]
- Stewart, M.M.; Martin, G.E. Coconut Husk-Piles-A Unique Habitat for Jamican Terrestrial Frogs. Biotropica 1980, 12, 107–116. [Google Scholar] [CrossRef]
- Goin, C.J. Studies in the Life History of Eleutherodactylus Ricordii Planirostris-Cope-in Florida, Etc. [With Plates.]; University of Florida Press: Gainesville, FL, USA, 1947. [Google Scholar]
- Rödder, D.; Lötters, S. Explanative Power of Variables Used in Species Distribution Modelling: An Issue of General Model Transferability or Niche Shift in the Invasive Greenhouse Frog (Eleutherodactylus Planirostris). Naturwissenschaften 2010, 97, 781–796. [Google Scholar] [CrossRef]
- Yin, Y.; He, Q.; Pan, X.; Liu, Q.; Wu, Y.; Li, X. Predicting Current Potential Distribution and the Range Dynamics of Pomacea Canaliculata in China under Global Climate Change. Biology 2022, 11, 110. [Google Scholar] [CrossRef]
- UNEP Mittermeier. United Nations Environmental Program—Convention on Biological Diversity; UNEP Mittermeier: Montreal, QC, Canada, 2002. [Google Scholar]
- Johovic, I.; Gama, M.; Banha, F.; Tricarico, E.; Anastácio, P.M. A Potential Threat to Amphibians in the European Natura 2000 Network: Forecasting the Distribution of the American Bullfrog Lithobates Catesbeianus. Biol. Conserv. 2020, 245, 108551. [Google Scholar] [CrossRef]
- Pitt, W.C.; Sin, H. Dermal Toxicity of Citric Acid Based Pesticides to Introduced Eleutherodactylus Frogs in Hawaii. In Final Report: QA-992; USDA APHIS WS National Wildlife Research Center: Fort Collins, CO, USA, 2004. [Google Scholar]
- Hara, A.H.; Jacobsen, C.M.; Marr, S.R.; Niino-DuPonte, R.Y. Hot Water as a Potential Disinfestation Treatment for an Invasive Anuran Amphibian, the Coqui Frog, Eleutherodactylus Coqui Thomas (Leptodactylidae), on Potted Plants. Int. J. Pest. Manag. 2010, 56, 255–263. [Google Scholar] [CrossRef]
- Pough, F.H.; Stewart, M.M.; Thomas, R.G. Physiological Basis of Habitat Partitioning in Jamaican Eleutherodactylus. Oecologia 1977, 27, 285–293. [Google Scholar] [CrossRef]

| Environmental Variable | Percentage Contribution (%) |
|---|---|
| Annual Mean Temperature | 27.2 |
| Precipitation of Driest Month | 23 |
| Annual Precipitation | 21.3 |
| Precipitation of Warmest Quarter | 12.4 |
| Isothermality | 7.4 |
| Mean Temperature of Warmest Quarter | 7.2 |
| Mean Diurnal Range | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, C.; Guo, X.; Chen, Y. Impact of Global Climate Change on the Distribution Range and Niche Dynamics of Eleutherodactylus planirostrish in China. Biology 2022, 11, 588. https://doi.org/10.3390/biology11040588
Mu C, Guo X, Chen Y. Impact of Global Climate Change on the Distribution Range and Niche Dynamics of Eleutherodactylus planirostrish in China. Biology. 2022; 11(4):588. https://doi.org/10.3390/biology11040588
Chicago/Turabian StyleMu, Chaosheng, Xuecheng Guo, and Youhua Chen. 2022. "Impact of Global Climate Change on the Distribution Range and Niche Dynamics of Eleutherodactylus planirostrish in China" Biology 11, no. 4: 588. https://doi.org/10.3390/biology11040588
APA StyleMu, C., Guo, X., & Chen, Y. (2022). Impact of Global Climate Change on the Distribution Range and Niche Dynamics of Eleutherodactylus planirostrish in China. Biology, 11(4), 588. https://doi.org/10.3390/biology11040588





