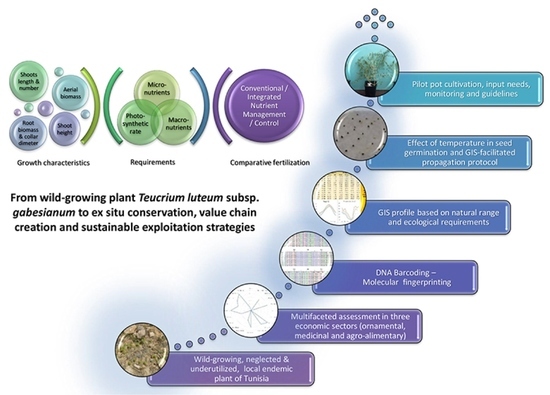
DNA Barcoding, GIS-Facilitated Seed Germination and Pilot Cultivation of Teucrium luteum subsp. gabesianum (Lamiaceae), a Tunisian Local Endemic with Potential Medicinal and Ornamental Value
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
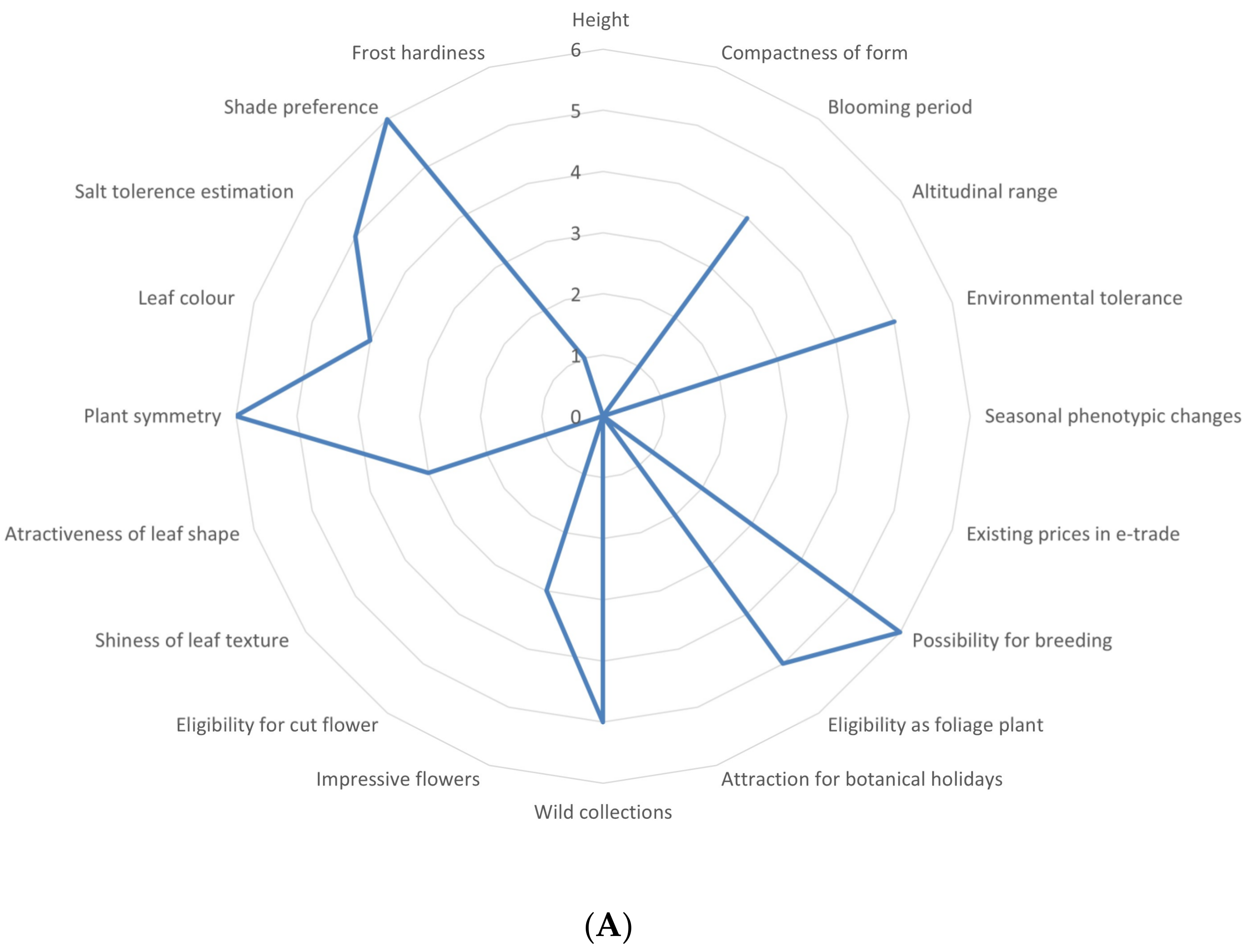
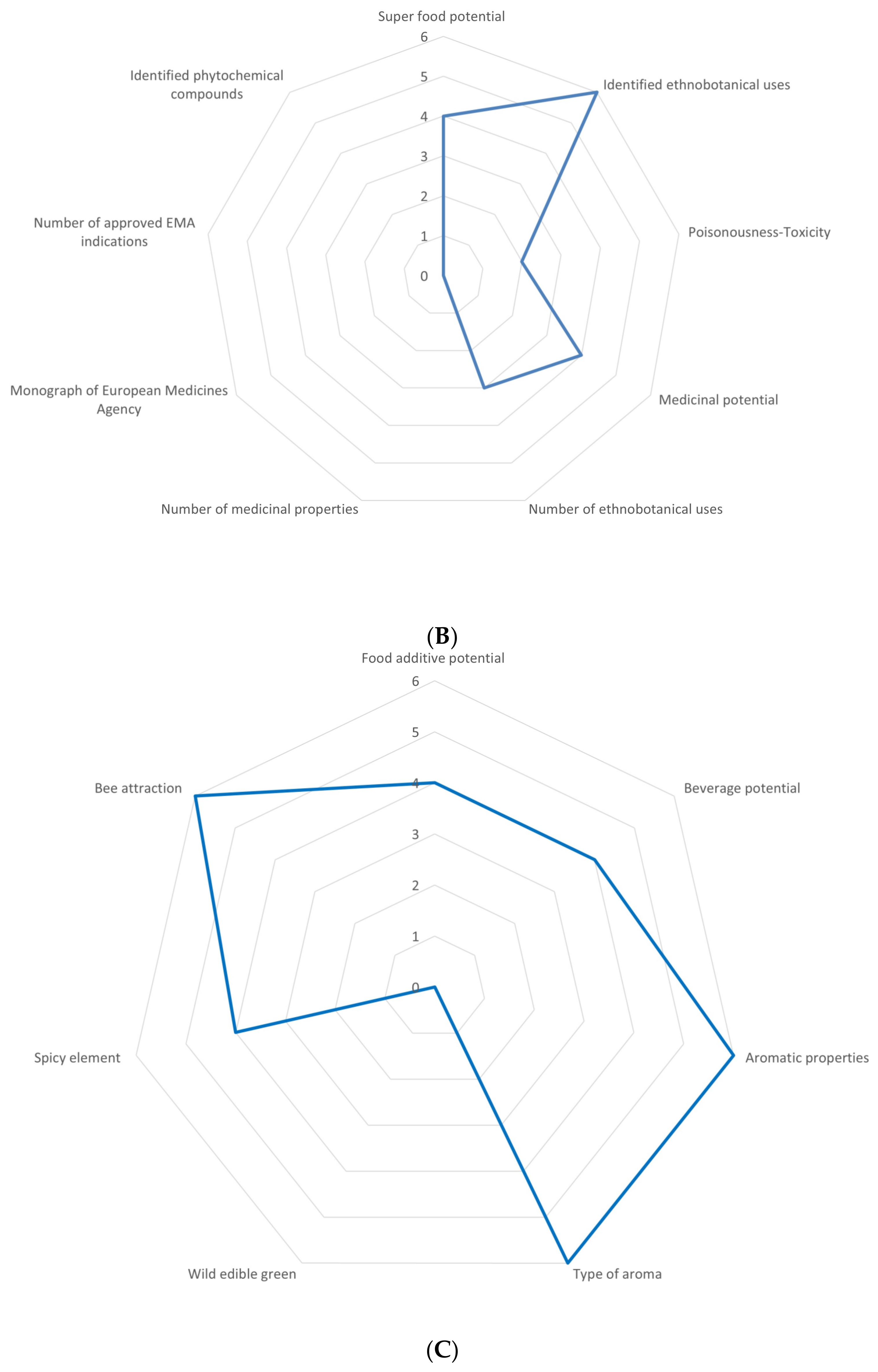
2.1. Multifaceted Evaluation in Different Sectors of Economy
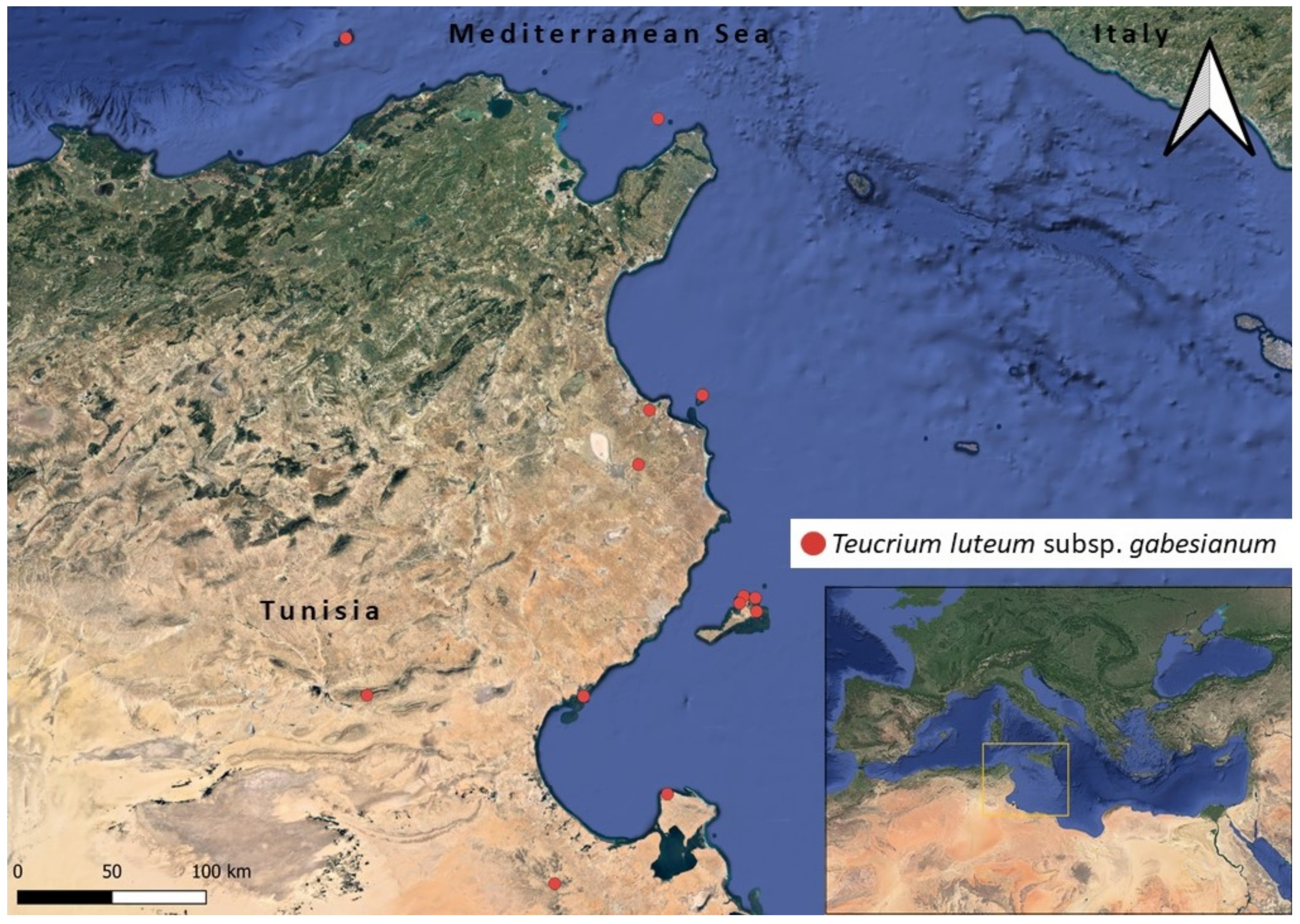
2.2. Distribution Mapping and GIS Ecological Profiling
- (a)
- WorldClim version 2.1 with minimum, maximum, and average temperature (°C), as well as precipitation values (mm) and data for 19 bioclimatic variables for every month derived from 1970–2000 in raster resolution of 1 km2, and
- (b)
2.3. Seed Collection and Storage
2.4. Germination Tests
2.5. Molecular Markers and PCR Procedures
2.6. Pilot Cultivation of Seedlings, Transplantation, and Fertilization Treatments
2.7. Morphological and Physiological Measurements of Seedlings
2.8. Plant Tissue Analyses of Seedlings
2.9. Statistical Analysis
3. Results
3.1. Overview of the Utilization Potential
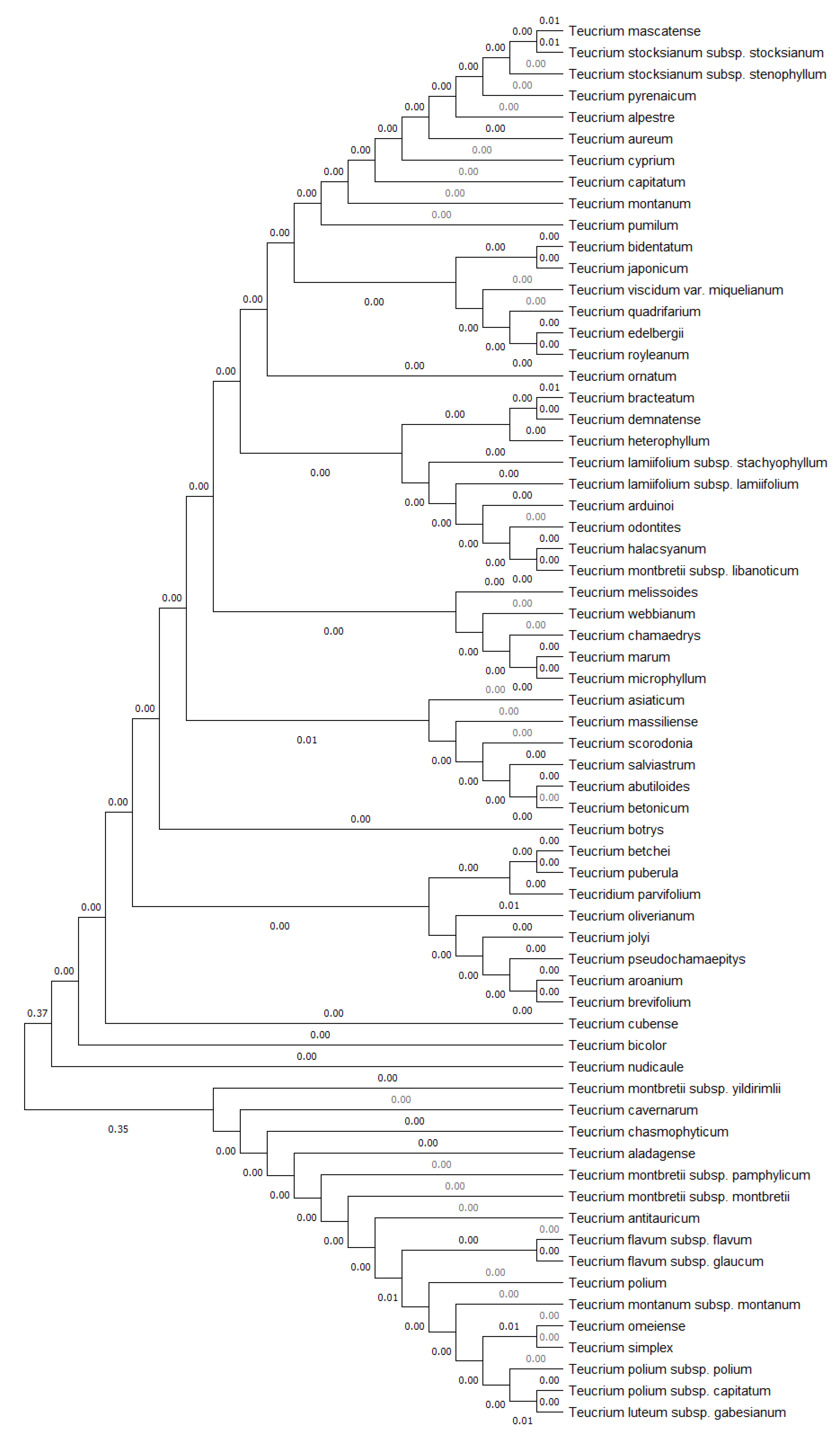
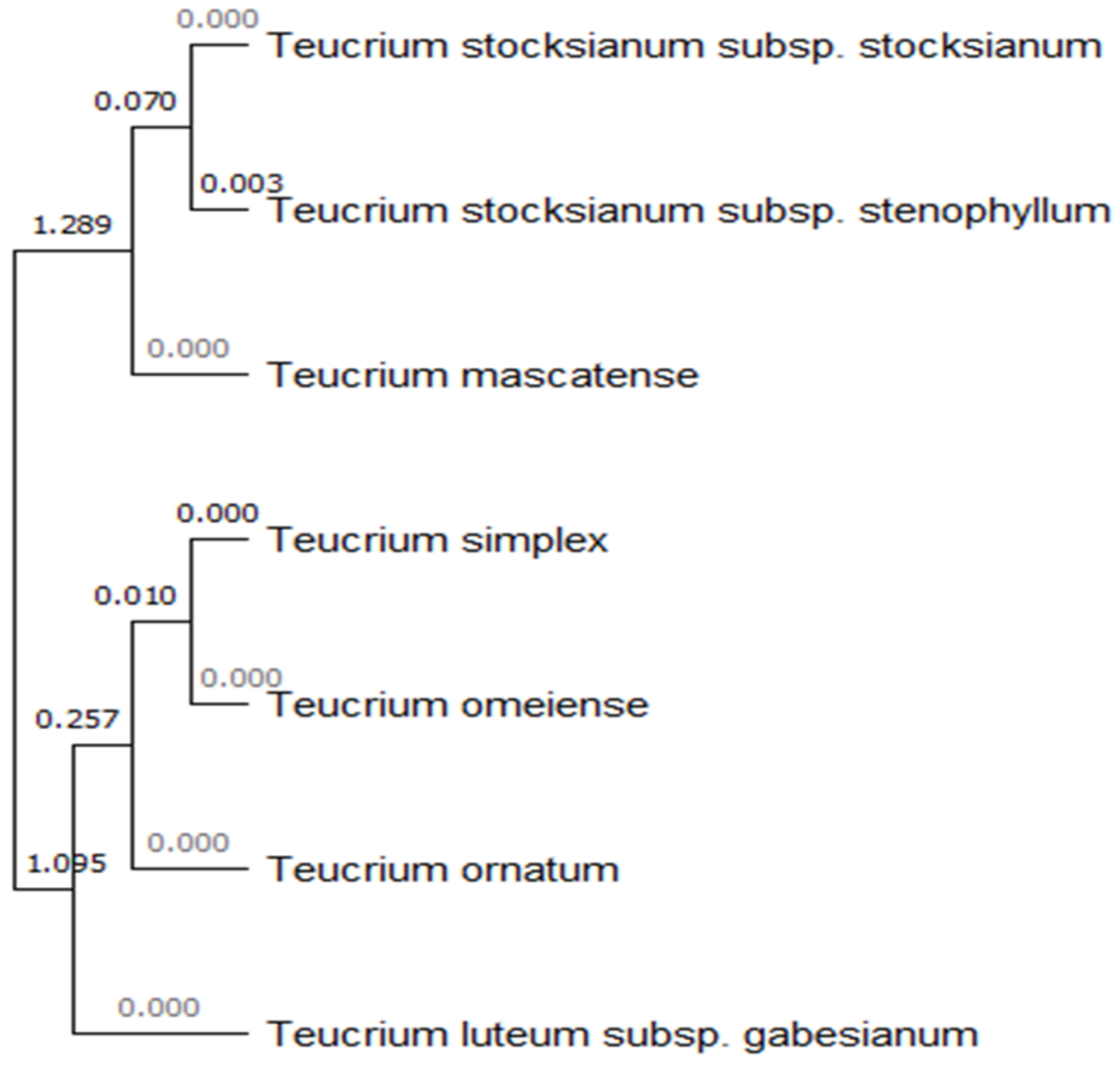
3.2. Molecular Characterization and Annotation in GenBank
3.3. Ecological Profiling
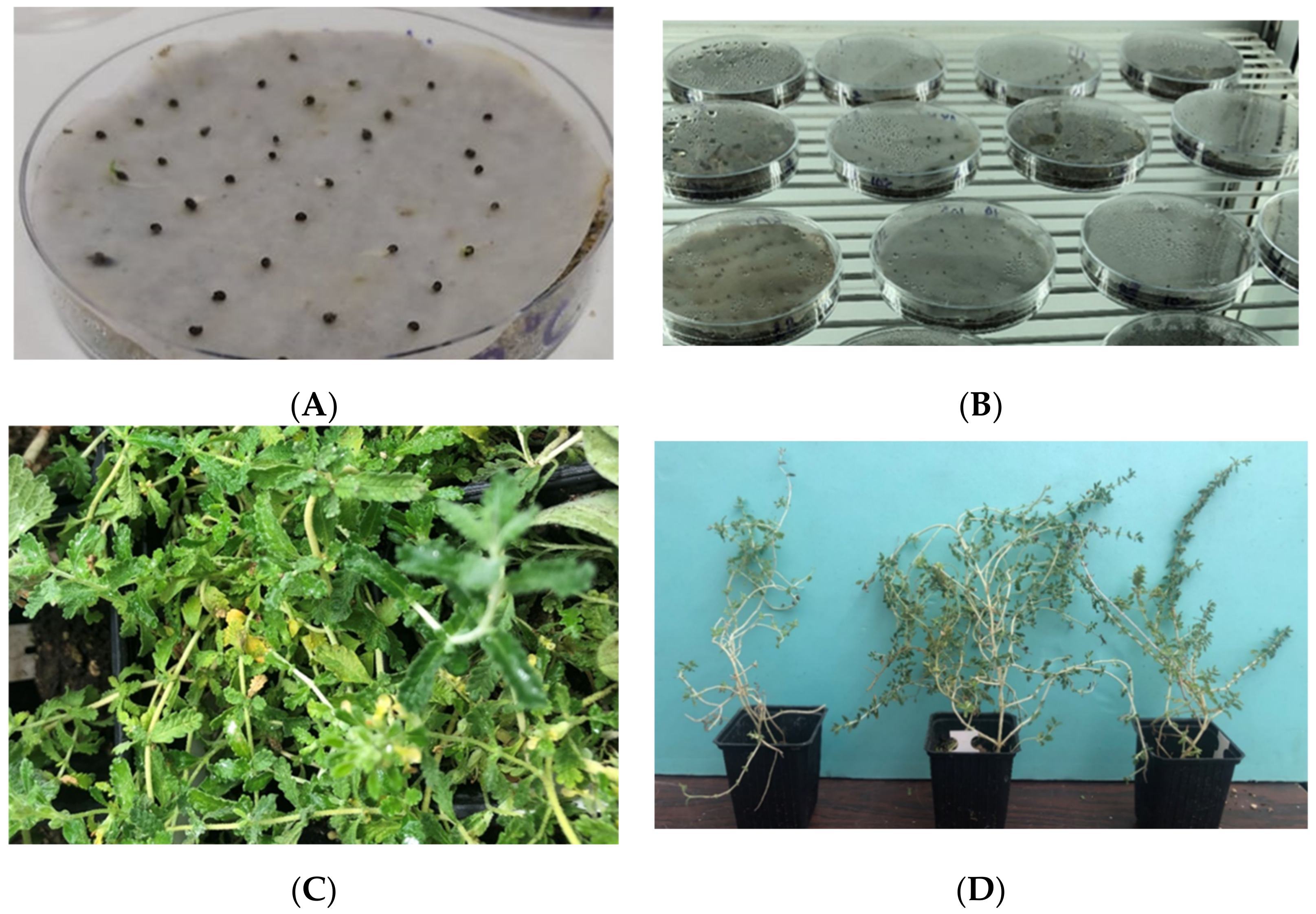
3.4. Seed Germination Tests
3.5. Seedling Growth in Pilot Cultivation
3.6. Macro- and Micronutrient Content
4. Discussion
4.1. Molecular Authentication (DNA Barcoding)
4.2. Seed Germination Facilitated with GIS
4.3. Seedling Growth and Fertilization Response
4.4. Re-Evaluation of Feasibility and Readiness Timescale for Value Chain Creation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Floc’h, E.; Boulos, L.; Véla, E. Catalogue Synonymique Commenté de la Flore de Tunisie, 2nd ed.; Banque Nationale de Gènes de la Tunisie: Tunis, Tunisia, 2010. [Google Scholar]
- Piozzi, F.; Bruno, M.; Rosselli, S.; Maggio, A. Advances in the chemistry of furano-diterpenoids from Teucrium genus. Heterocycles 2005, 65, 1221–1234. [Google Scholar] [CrossRef]
- Ulubelen, A.; Topu, G.; Sönmez, U. Chemical and biological evaluation of genus Teucrium. Stud. Nat. Prod. Chem. 2000, 23, 591–648. [Google Scholar] [CrossRef]
- Candela, R.G.; Rosselli, S.; Bruno, M.; Fontana, G. A review of the phytochemistry, traditional uses and biological activities of the essential oils of genus Teucrium. Planta Med. 2021, 87, 432–479. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, Z.; Yang, J.L.; Venditti, A.; Farimani, M.M. A review of the phytochemistry, ethnopharmacology and biological activities of Teucrium genus (Germander). Nat. Prod. Res. 2022, 1–18, in press. [Google Scholar] [CrossRef] [PubMed]
- Sabzeghabaie, A.; Asgarpanah, J. Essential oil composition of Teucrium polium L. fruits. J. Essent. Oil Res. 2016, 28, 77–80. [Google Scholar] [CrossRef]
- Rahmouni, F.; Saoudi, M.; Rebai, T. Therapeutics studies and biological properties of Teucrium polium (Lamiaceae). Biofactors 2021, 47, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, E. Contribution à Une Étude Ethnobotanique de La Flore Tunisienne; Imprimerie Officielle de la République Tunisienne: Tunis, Tunisia, 2010. [Google Scholar]
- Boukef, K. Médecine Traditionnelle et Pharmacopée: Les Plantes dans La Médecine Traditionnelle Tunisienne; Agence de Cooperation Culturelle et Technique: Paris, France, 1986. [Google Scholar]
- Boukef, K.; Ghileb, G. Contribution à l’Etude des Plantes Utilisées en Médecine Traditionnelle Maghrébine; Médecine Traditionnelle et Pharmacopée Africaine: Paris, France, 1988. [Google Scholar]
- Ben Haj Jilani, I.; Zouaghi, M.; Ghrabi, Z. Ethnobotanical survey of medicinal plants in Northwest Tunisia. J. Med. Anthropol. 2011, 63, 78. [Google Scholar]
- Grigoriadou, K.; Krigas, N.; Lazari, D.; Maloupa, E. Chapter 4—Sustainable use of Mediterranean Medicinal-Aromatic Plants. In Feed Additives: Aromatic Plants and Herds in Animal Nutrition and Health; Florou-Paneri, P.E., Christaki, E., Giannenas, I., Eds.; Elsevier Academic Press: London, UK, 2020; pp. 57–74. Available online: https://doi.org/10.1016/B978-0-12-814700-9.00004-2 (accessed on 13 December 2021).
- Fanourakis, D.; Paschalidis, K.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Liapaki, E.; Juini, M.; Ipsilantis, I.; Maloupa, E.; et al. Pilot cultivation of the local endemic Cretan marjoram Origanum microphyllum (Benth.) Vogel (Lamiaceae): Effect of fertilizers on growth and herbal quality features. Agronomy 2022, 12, 94. [Google Scholar] [CrossRef]
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Kalogiannakis, K.; Debouba, F.J.; Ipsilantis, I.; Tsoktouridis, G.; et al. Pilot cultivation of the vulnerable Cretan endemic Verbascum Arcturus L. (Scrophulariaceae): Effect of fertilization on growth and quality features. Sustainability 2021, 13, 14030. [Google Scholar] [CrossRef]
- Macdonald, B. Practical Woody Plant Propagation for Nursery Growers; Timber Press Inc.: Portland, OR, USA, 2006; p. 669. [Google Scholar]
- Libiad, M.; Khabbach, A.; El Haissoufi, M.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Sharrock, S.; Krigas, N. Ex-situ conservation of single-country endemic plants of Tunisia and northern Morocco (Mediterranean coast and Rif region) in seed banks and botanic gardens worldwide. Kew Bull. 2020, 75, 46. [Google Scholar] [CrossRef]
- Le Houérou, H.N. Bioclimatologie et Biogéographie des Steppes Arides du Nord de l’Afrique: Diversité Biologique, Développement Durable et Désertisation. Options Méditer. Sér. B 1995, 10, 396. [Google Scholar]
- Bourgou, S.; Ben Haj Jilani, I.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V.; et al. Medicinal-cosmetic potential of the local endemic plants of Crete (Greece), Northern Morocco and Tunisia: Priorities for conservation and sustainable exploitation of neglected and underutilized phytogenetic resources. Biology 2021, 10, 1344. [Google Scholar] [CrossRef]
- Libiad, M.; Khabbach, A.; El Haissoufi, M.; Anestis, I.; Lamchouri, F.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Greveniotis, V.; Tsiripidis, I.; et al. Agro-alimentary potential of the neglected and underutilized local endemic plants of Crete (Greece), Rif-Mediterranean coast of Morocco and Tunisia: Perspectives and challenges. Plants 2021, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Krigas, N.; Tsoktouridis, G.; Anestis, I.; Khabbach, A.; Libiad, M.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Lamchouri, F.; Tsiripidis, I.; Tsiafouli, M. Exploring the potential of neglected local endemic plants of three Mediterranean regions in the ornamental sector: Value chain feasibility and readiness timescale for their sustainable exploitation. Sustainability 2021, 13, 2539. [Google Scholar] [CrossRef]
- Krigas, N.; Mouflis, G.; Grigoriadou, K.; Maloupa, E. Conservation of important plants from the Ionian Islands at the Balkan Botanic Garden of Kroussia, N Greece: Using GIS to link the in situ collection data with plant propagation and ex situ cultivation. Biodivers. Conserv. 2010, 19, 3583–3603. [Google Scholar] [CrossRef]
- Krigas, N.; Papadimitriou, K.; Mazaris, A.D. GIS and Ex-Situ Plant Conservation. In Application of Geographic Information Systems; Alam, B.M., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Hatzilazarou, S.; El Haissoufi, M.; Pipinis, E.; Kostas, S.; Libiad, M.; Khabbach, A.; Lamchouri, F.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; et al. GIS-facilitated seed germination and multifaceted evaluation of the endangered Abies marocana Trab. (Pinaceae): Enabling conservation and sustainable exploitation. Plants 2021, 10, 2606. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, P.; Radoglou, K.; Dini-Papanastasi, O.; Spyroglou, G. Enhancing planting stock quality of Italian cypress (Cupressus sempervirens L.) by pre-cultivation in mini-plugs. Ecol. Eng. 2010, 36, 912–919. [Google Scholar] [CrossRef]
- O’Reill, C.; Arnott, J.T.; Owens, J.N. Effects of photoperiod and moisture availability on shoot growth, seedling morphology, and cuticle and epicuticular wax features of container-grown western hemlock seedlings. Can. J. For. Res. 1989, 19, 122–131. [Google Scholar] [CrossRef]
- Radoglou, K.M. Effect of Environmental Stress on Planting Stock and Field Performance. In Proceedings of the National Forestry Conference: Planting Stock of Woody Species, Thessaloniki, Greece, 28–29 January 1999; pp. 15–26. (In Greek). [Google Scholar]
- Buendia Velazquez, M.V.; Lopez Lopez, M.A.; Cetina Alcala, V.M.; Diakite, L. Substrates and nutrient addition rates affect morphology and physiology of Pinus leiophylla seedlings in the nursery stage. iForest 2016, 10, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Oliet, J.; Planelles, R.; Artero, F.; Valverde, R.; Jacobs, D.F.; Segura, M. Field performance of Pinus halepensis planted in Mediterranean arid conditions: Relative influence of seedling morphology and mineral nutrition. New For. 2009, 37, 313–331. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Puertolas, J.; Penuelas, J.L.; Planelles, R. Effect of nitrozen fertilization in the nursery on the drought and frost resistance of Mediterranean forest species. For. Syst. 2005, 14, 408–418. [Google Scholar]
- Grigoriadou, K.; Sarropoulou, V.; Krigas, N.; Maloupa, E.; Tsoktouridis, G. GIS-facilitated effective propagation protocols of the Endangered local endemic of Crete Carlina diae (Rech. f.) Meusel and A. Kástner (Asteraceae): Serving ex-situ conservation needs and its future sustainable exploitation as an ornamental. Plants 2020, 9, 1465. [Google Scholar] [CrossRef] [PubMed]
- Samec, D.; Durgo, K.; Gruz, J.; Kremer, D.; Kosalec, I.; Piljac-Zegarac, J.; Salopek-Sondi, B. Genetic and phytochemical variability of six Teucrium arduini L. populations and their antioxidant/prooxidant behavior examined by biochemical, macromolecule and cell-based approaches. Food Chem. 2015, 186, 298–305. [Google Scholar] [CrossRef]
- Boulila, A.; Bejaoui, A.; Messaoud, C.; Boussaid, M. Genetic diversity and population structure of Teucrium polium (Lamiaceae) in Tunisia. Biochem. Genet. 2010, 48, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Al Rawashdeh, I.M. Genetic variability among and within wild Teucrium polium L. populations at Wadi Shueib area in Jordan. ARPN J. Agric. Biol. Sci. 2015, 10, 267–273. [Google Scholar]
- Tapeh, R.N.G.; Bernousi, I.; Moghadam, A.F.; Mandoulakani, B.A. Genetic diversity and structure of Iranian Teucrium (Teucrium polium L.) populations assessed by ISSR markers. J. Agr. Sci. Technol. 2018, 20, 333–345. [Google Scholar]
- Tabrizi, F.M.; Asri, Y.; Mehregan, I. Population genetic structure and diversity of Teucrium polium in Iran using ISSR markers. Rostaniha 2020, 21, 248–263. [Google Scholar] [CrossRef]
- Sözen, E.; Hilooglu, M.; Kandemír, A. Genetic diversity of local endemic Teucrium leucophyllum Montbret & Aucher ex Bentham. (Lamiaceae) in Turkey. Indian J. Pharm. Educ. Res. 2017, 51, s195–s199. [Google Scholar] [CrossRef] [Green Version]
- Marzouk, R.I.; El-Badak, G. Molecular characterization of Teucrium L. (Lamiaceae) as a prerequisite for its conservation. Asian J. Biol. Sci. 2018, 11, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Kremer, D.; Bolaric, S.; Ballian, D.; Bogunic, F.; Stesevic, D.; Karlovic, K.; Kosalec, I.; Vokurka, A.; Vukovic Rodriguez, J.; Randic, M.; et al. Morphological, genetic and phytochemical variation of the endemic Teucrium arduini L. (Lamiaceae). Phytochemistry 2015, 116, 111–119. [Google Scholar] [CrossRef]
- Djabou, N.; Battesti, M.; Allali, H.; Desjobert, J.; Varesi, L.; Costa, J.; Muselli, A. Chemical and genetic differentiation of Corsican subspecies of Teucrium flavum L. Phytochemistry 2011, 72, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Djabou, N.; Muselli, A.; Allali, H.; Dib, M.; Tabti, B.; Varesi, L.; Costa, J. Chemical and genetic differentiation of two Mediterranean subspecies of Teucrium polium L. Phytochemistry 2012, 83, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Salmaki, Y.; Kattari, S.; Heubl, G.; Brauchler, C. Phylogeny of non-monophyletic Teucrium (Lamiaceae: Ajugoideae): Implications for character evolution and taxonomy. Taxon 2016, 65, 805–822. [Google Scholar] [CrossRef]
- Khan, A.; Asaf, S.; Khan, A.L.; Khan, A.; Al-Harrasi, A.; Al-Sudairy, O.; Kareem, N.M.A.; Al-Saady, N.; Al-Rawahi, A. Complete chloroplast genomes of medicinally important Teucrium species and comparative analyses with related species from Lamiaceae. PeerJ 2019, 7, e7260. [Google Scholar] [CrossRef] [Green Version]
- Padulosi, S.; Thompson, J.; Rudebjer, P. Fighting Poverty, Hunger and Malnutrition with Neglected and Underutilized Species (NUS): Needs, Challenges and the Way Forward; Bioversity International: Rome, Italy, 2013; p. 56. [Google Scholar]
- Pipinis, E.; Hatzilazarou, S.; Kostas, S.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; et al. Facilitating conservation and bridging gaps for the sustainable exploitation of the Tunisian local endemic plant Marrubium aschersonii (Lamiaceae). Sustainability 2022, 14, 1637. [Google Scholar] [CrossRef]
- Puech, S. Contribution à l’étude des Teucrium de la section Polium de Tunisie. Bull. Soc. Bot. Fr. Lett. Bot. 1982, 129, 41–52. [Google Scholar] [CrossRef]
- Pasta, S.; El-Mokni, R.; Médail, F. Flore et Végétation des îles et îlots Satellites de l’Archipel des Kuriates (Tunisie orientale). Bilan de la Biodiversité Végétale Terrestre, Impacts Environnemen—Taux et Recommandations de Gestion; Note Naturaliste PIM: Marseille, France, 2022. [Google Scholar]
- Médail, F.; Charrier, M.; Chaieb, M.; Domina, G.; El Mokni, R.; Pasta, S.; Véla, E. Plantes vasculaires nouvelles ou rares pour la Tunisie présentes sur les îles (Galite, Zembra, Kuriat, Monastir, Kerkennah, Kneiss, Djerba). Flora Mediterr. 2020, 30, 87–112. [Google Scholar] [CrossRef]
- Van den Berghen, C. Liste commentée des plantes vasculaires observées dans l’île de Djerba (Tunisie méridionale). Lejeunia 1981, 105, 1–38. (In French) [Google Scholar]
- Pottier-Alapetite, G. Flore de La Tunisie, Angiospermes—Dicotylédones, 1ère Partie: Gamopétales; Imprimerie Officielle de la République Tunisienne: Tunis, Tunisia, 1981. [Google Scholar]
- Tsoktouridis, G.; Tsiamis, G.; Koutinas, N.; Mantell, S. Molecular detection of bacteria in plant tissues using universal 16S ribosomal DNA degenerated primers. Biotechnol. Biotechnol. Equip. 2014, 28, 583–591. [Google Scholar] [CrossRef]
- Tsoktouridis, G.; Krigas, Ν.; Sarropoulou, V.; Kampouropoulou, S.; Papanastasi, K.; Grigoriadou, Κ.; Menexes, G.; Maloupa, E. Micropropagation and molecular characterization of Thymus sibthorpii Benth. (Lamiaceae), an aromatic-medicinal thyme with ornamental value and conservation concern. In Vitro Cell. Dev. Biol. 2019, 55, 647–658. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Allen, S.E.; Grimshaw, H.M.; Rowland, A.P. Chemical analysis. In Methods in Plant Ecology; Moore, P.D., Chapman, S.B., Eds.; Blackwell Scientific Publication: Oxford, UK, 1986; pp. 285–344. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Bremmer, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of Soil Analysis. Part 2: Chemical and Microbial Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Klockars, A.; Sax, G. Multiple Comparisons; Sage Publications: Newbury Park, CA, USA, 1986; p. 87. [Google Scholar]
- Snedecor, G.W.; Cochran, W.C. Statistical Methods, 7th ed.; The Iowa State University Press: Ames, IA, USA, 1980; p. 507. [Google Scholar]
- El Oualidi, J.; Puech, S. Quelques marqueurs morphologiques des Teucrium section Polium (Lamiaceae) du Maroc. Valeurs diagnostiques à différents niveaux d’intégration. Acta Bot. Malacit. 1993, 18, 163–171. [Google Scholar] [CrossRef]
- El Oualidi, J.; Rascol, J.P.; Martin, A.; Puech, S. Le poliumoside, marqueur chimique de la section Polium du geme Teucrium (Labialae): L’exception du T. midellense, espèce endémique du Maroc. Biochem. Syst. Ecol. 1996, 24, 261–272. [Google Scholar] [CrossRef]
- El Oualidi, J.; Mathez, J.; Navarro, T. Contribution à la connaissance des Teucrium sect. Polium (Labiatae) du Maroc: Le complexe de T. chlorostachyum. Flora Mediterr. 1997, 7, 21–26. [Google Scholar]
- Andary, C.; Rascol, J.P.; Puech, S.; Roussel, J.L.; Privat, G. Les esters de l’acide caféique dans la chimiotaxinomie des Teucrium de la section polium (Lamiaceae). Can. J. Bot. 1988, 66, 1007–1012. [Google Scholar] [CrossRef]
- El Oualidi, J.; Puech, S.; Navarro, T. Geographical variation and successive adaptive radiations of yellow-flowered Teucrium (Labiatae) in the Mediterranean Region. Bot. Rev. 2002, 68, 209–234. [Google Scholar] [CrossRef]
- Navarro, T.; El Oualidi, J. Synopsis of Teucrium, L. (Labiatae) in the Mediterranean region and surrounding areas. Flora Mediterr. 2000, 10, 349–363. [Google Scholar]
- Maccioni, A.; Falconieri, D.; Sanna, C.; Porcedda, S.; Piras, A.; Maxia, A. Characterization of essential oils from different taxa belonging to the genus Teucrium in Sardinia Island, Italy. Plants 2021, 10, 1359. [Google Scholar] [CrossRef]
- Djabou, N.; Allali, H.; Battesti, M.J.; Tabti, B.; Costa, J.; Muselli, A.; Varesi, L. Chemical and genetic differentiation of two Mediterranean subspecies of Teucrium scorodonia L. Phytochemistry 2011, 74, 123–132. [Google Scholar] [CrossRef]
- Benvenuti, S.; Ceccarini, L.; Macchia, M. Germination ecology of Teucrium marum L.: An endemic species of the Tuscany Archipelago. Acta Horticult. 2006, 723, 315–320. [Google Scholar] [CrossRef]
- Nadjafi, F.; Bannayan, M.; Tabrizi, L.; Rastgoo, M. Seed germination and dormancy breaking techniques for Ferula gummosa and Teucrium polium. J. Arid Environ. 2006, 64, 542–547. [Google Scholar] [CrossRef]
- Thanos, C.A.; Kadis, C.C.; Skarou, F. Ecophysiology of germination in the aromatic plants thyme, savory and oregano (Labiatae). Seed Sci. Res. 1995, 5, 161–170. [Google Scholar] [CrossRef]
- Krichen, K.; Mariem, H.B.; Chaieb, M. Ecophysiological requirements on sedd germination of a Mediterranean perennial grass (Stipa tenacissima L.) under controlled temperatures and water stress. S. Afr. J. Bot. 2014, 94, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Gammar, Z.; Nabli, M.; Puech, S. Contribution à l’étude biologique et caryosystématique des Teucrium (Labiatae) de Tunisie. Nat. Monspel. Ser. Bot. Fasc. 1989, 54, 79–92. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Zou, T.X.; Dai, T.B.; Jiang, D. Effects of nitrogen and potassium application levels on flag leaf photosynthetic characteristics after anthesis in winter wheat. Acta Agron. Sin. 2007, 33, 1667–1673. [Google Scholar]
- Zhang, Y.F.; Yu, S.; Li, C.F.; Wang, Y.B.; Diao, Z.F. Response characteristics of plant growth and leaf photochemical activity of sugar beet seedlings to different nitrogen application levels. Acta Agric. Nucl. Sin. 2013, 27, 1391–1400. [Google Scholar]
- Cao, Q.; He, M.R.; Dai, X.L. Effects of interaction between density and nitrogen on grain yield and nitrogen use sufficiency of winter wheat. Plant Nutr. Fert. Sci. 2011, 17, 815–822. [Google Scholar]
- Xiao, W.X.; Xie, F.T.; Zhang, H.J.; Wang, H.Y.; Wang, H. Effect of fertilizer and planting density on photosynthetic characteristics and yield of super-high- yielding soybean cultivar. Chin. J. Oil Crop Sci. 2009, 31, 190–195. [Google Scholar]
- Kunzova, E.; Hejcman, M. Yield development of winter wheat over 50 years of FYM, N, P and K fertilizer application on black earth soil in the Czech Republic. Field Crop. Res. 2009, 111, 226–234. [Google Scholar] [CrossRef]
- Yanjun, Y.; Jinhua, Z.; Linjing, C.; Aiqing, J.; Hongmei, Z.; Pingyi, G. Effects of fertilizer levels and plant density on chlorophyll contents, its fluorescence and grain yield of Setaria italica. Int. J. Agric. Biol. 2018, 20, 737–744. [Google Scholar] [CrossRef]



| pH | Organic Matter (%) | Soluble Salts (mS/cm) | CaCO3 (%) | Mechanical Composition | ||
|---|---|---|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | ||||
| 8.12 | 0.36 | 0.35 | 5.50 | 56.00 | 28.00 | 16.00 |
| Concentration of macronutrient (ppm) | ||||||
| N-No3 | P | K | Mg | Ca | ||
| 8.00 | 8.00 | 104.00 | 842.00 | >2000 | ||
| Concentration of micronutrient (ppm) | ||||||
| Fe | Zn | Mn | Cu | |||
| 4.7 | 2.00 | 7.06 | 0.77 | |||
| Teucrium Taxon | matK | rbcL | trnH/psbA * | 18S-26S | petB/petD | trnL/trnF | rpoC1 | psbK/psbI |
|---|---|---|---|---|---|---|---|---|
| T. betchei | 19 | 10 | - | - | - | 58 | 6 | 68 |
| T. bidentatum | 10 | - | 57 | - | - | 29 | - | - |
| T. brevifolium | - | - | 53 | - | - | 59 | - | - |
| T. canadense | 12 | 6, 6 | 77 | - | - | - | 4 | - |
| T. chamaedrys | 11, 15 | 10 | 37, 43, 43 | - | - | 54 | - | - |
| T. flavum | - | 12 | - | - | - | 55, 56, 58, 58 | - | - |
| T. fruticans | - | 9 | - | - | - | - | - | - |
| T. heterophyllum | - | 5 | - | - | - | 21 | - | - |
| T. divaricatum | 10, 10 | - | 35 | - | - | - | - | - |
| T. junceum | 18 | 10 | - | - | - | - | 6 | 76 |
| T. japonicum | - | - | 52 | - | - | 26 | - | 30 |
| T. marum | 11 | - | - | - | - | 53, 54, 55, 55 | - | - |
| T. mascatense | 9, 9, 3 | 4, 4 | - | - | 51, 51 | 51, 51 | 7, 7 | 75, 75 |
| T. montanum | 2,1 | - | 42, 69 | - | - | 7, 18, 8 | - | - |
| T. nummularifolium | 1 | - | - | - | - | - | - | - |
| T. omeiense | 12 | 7 | 95 | - | 42 | 34 | 4 | 28 |
| T. ornatum | 15, 12, 11, 13 | 6, 6, 6 | 158, 54 | 102 | 41 | 36, 32 | 4 | 26 |
| T. parvifolium | 13 | 8, 9 | - | - | - | 35 | 5 | 31 |
| T. polium | 2, 1 | - | 51, 47 | - | - | 6, 5, 9, 11 | - | - |
| T. quadrifarium | 12 | - | 59 | - | - | 25, 27 | - | - |
| T. racemosum | 20 | 10 | 39 | - | - | - | 5 | 30 |
| T. scordium | 12, 12 | - | 49 | - | - | - | - | - |
| T. scorodonia | 11, 13 | 10, 10 | - | - | - | 40, 41, 42, 39 | - | - |
| T. simplex | 13 | 9 | 95 | - | 42 | 34 | 4 | 28 |
| T. stocksianum subsp. stenophyllum | 5, 3 | 4 | 51 | - | 25 | 38 | 3 | 3 |
| T. stocksianum subsp. stocksianum | 3 | 4 | 50 | - | 25 | 28 | 3 | 3 |
| T. tsinlingense | 12 | - | - | - | - | - | - | - |
| T. veronicoides | - | - | 70 | - | - | - | - | 28, 38 |
| T. viscidum | 9 | 6 | 77, 70, 72 | - | - | 27 | - | 29 |
| Characteristics | Control | INM | ChF |
|---|---|---|---|
| Shoot height (cm) | 26.37 ± 3.92 b | 36.75 ± 4.03 a | 29.50 ± 5.96 b |
| Root collar diameter (mm) | 1.97 ± 0.30 a | 1.86 ± 0.37 a | 1.88 ± 0.19 a |
| Number of apex shoots | 6.37 ± 1.68 c | 12.62 ± 2.67 b | 16.87 ± 2.30 a |
| Length of apex shoots (cm) | 14.87 ± 2.29 ab | 16.50 ± 2.14 a | 12.75 ± 2.25 b |
| Root dry biomass (gr) | 0.20 ± 0.05 a | 0.18 ± 0.04 a | 0.14 ± 0.02 a |
| Above ground dry biomass (gr) | 0.75 ± 0.08 a | 0.82 ± 0.11 a | 0.80 ± 0.16 a |
| Photosynthetic rate (μmol m−2 s−1) | 1.92 ± 0.36 c | 3.80 ± 0.31 b | 4.75 ± 0.48 a |
| Treatment | N (%) | P (mg/gr) | K (mg/gr) | Ca (mg/gr) | Mg (mg/gr) | Na (mg/gr) |
|---|---|---|---|---|---|---|
| Control | 2.14 a | 2.10 a | 13.41 c | 8.37 a | 2.47 a | 4.47 b |
| Inorganic fertilizer | 2.07 a | 2.11 a | 15.39 b | 6.76 b | 2.62 a | 4.28 c |
| INM fertilizers | 2.42 a | 2.28 a | 16.48 a | 7.82 a | 2.44 a | 4.73 a |
| Treatment | Fe (ppm) | Mn (ppm) | Zn (ppm) | Cu (ppm) |
|---|---|---|---|---|
| Control | 453.70 a | 60.95 a | 32.09 a | 4.63 b |
| Inorganic fertilizer | 380.49 b | 53.81 a | 27.51 b | 1.82 c |
| Integrated nutrient management fertilizer | 357.20 b | 62.43 a | 25.65 b | 88.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostas, S.; Hatzilazarou, S.; Pipinis, E.; Bourgou, S.; Ben Haj Jilani, I.; Ben Othman, W.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; et al. DNA Barcoding, GIS-Facilitated Seed Germination and Pilot Cultivation of Teucrium luteum subsp. gabesianum (Lamiaceae), a Tunisian Local Endemic with Potential Medicinal and Ornamental Value. Biology 2022, 11, 462. https://doi.org/10.3390/biology11030462
Kostas S, Hatzilazarou S, Pipinis E, Bourgou S, Ben Haj Jilani I, Ben Othman W, Megdiche-Ksouri W, Ghrabi-Gammar Z, Libiad M, Khabbach A, et al. DNA Barcoding, GIS-Facilitated Seed Germination and Pilot Cultivation of Teucrium luteum subsp. gabesianum (Lamiaceae), a Tunisian Local Endemic with Potential Medicinal and Ornamental Value. Biology. 2022; 11(3):462. https://doi.org/10.3390/biology11030462
Chicago/Turabian StyleKostas, Stefanos, Stefanos Hatzilazarou, Elias Pipinis, Soumaya Bourgou, Imtinen Ben Haj Jilani, Wafa Ben Othman, Wided Megdiche-Ksouri, Zeineb Ghrabi-Gammar, Mohamed Libiad, Abdelmajid Khabbach, and et al. 2022. "DNA Barcoding, GIS-Facilitated Seed Germination and Pilot Cultivation of Teucrium luteum subsp. gabesianum (Lamiaceae), a Tunisian Local Endemic with Potential Medicinal and Ornamental Value" Biology 11, no. 3: 462. https://doi.org/10.3390/biology11030462
APA StyleKostas, S., Hatzilazarou, S., Pipinis, E., Bourgou, S., Ben Haj Jilani, I., Ben Othman, W., Megdiche-Ksouri, W., Ghrabi-Gammar, Z., Libiad, M., Khabbach, A., El Haissoufi, M., Lamchouri, F., Koundourakis, E., Greveniotis, V., Papaioannou, E., Sakellariou, M. A., Anestis, I., Tsoktouridis, G., & Krigas, N. (2022). DNA Barcoding, GIS-Facilitated Seed Germination and Pilot Cultivation of Teucrium luteum subsp. gabesianum (Lamiaceae), a Tunisian Local Endemic with Potential Medicinal and Ornamental Value. Biology, 11(3), 462. https://doi.org/10.3390/biology11030462