Simple Summary
Nanoparticles (Nps), new biotechnological tools, possess unique physical and chemical properties and are increasingly being used in several fields, such as manufacture, medicine and veterinary medicine. In this work, we evaluated the effects of selenium (Se) nanoparticles stabilized with L-Cysteine (Se0Nps/L-Cys) as a nutritional supplement, to modulate immunological, oxidative status, and productive parameters in O. mykiss. The results demonstrated that Se0Nps/L-Cys showed less toxicity and higher antioxidant activity than Se0Nps and Na2SeO3. The Se0Nps/L-Cys, as a dietary supplement, had a significantly better effect on both immunological and physiological parameters, causing improvements at the productive level of O. mykiss when compared with Se0Nps and Na2SeO3. We concluded that Se0Nps sythetised by P. agglomerans, used as dietary supplement, is an environmentally friendly and promising alternative for nutritional supplementation for O. mykiss.
Abstract
The applications of nanoparticles (Nps) as food additives, health enhancers, and antimicrobials in animal production are increasing. The aim of this study was to evaluate the effect of selenium (Se) nanoparticles (Se0Nps) stabilized with L-cysteine (Se0Nps/L-Cys), as a nutritional supplement, on immunological, oxidative status, and productive parameters in O. mykiss. TEM and SEM-EDS showed the accumulation of spherical Se0Nps entirely composed by elemental selenium (Se0) as intracellular and extracellular deposits in Pantoea agglomerans UC-32 strain. The in vitro antioxidant capacity of Se0Nps/L-Cys was significant more efficient ROS scavengers than Se0Nps and Na2SeO3. We also evaluate the effect of Se0Nps/L-Cys on cell viability and oxidative stress in RTgill-W1, RTS-11, or T-PHKM Oncorhynchus mykiss cell lines. Se0Nps/L-Cys showed less toxic and high antioxidant activity than Se0Nps and Na2SeO3. Finally, the dietary Se0Nps/L-Cys had a significant better effect on both plasma lysozyme and respiratory burst activity (innate immune response), on tissular Gpx activity (oxidative status), and on well-being (productive parameter) of O. mykiss when it is compared to Se0Nps and Na2SeO3. Se0Nps/L-Cys is a promising alternative for nutritional supplement for O. mykiss with better performance than Na2SeO3 and Se0Nps, ease to implementation, and reduced environmental impact.
1. Introduction
The rapid increase in the world population and its purchasing power explains the growing demand for food and the consequent rapid development of the aquaculture industry in recent decades [1,2]. Salmon farming is a relatively young industry, which harvested 230 thousand metric tons (mt) in 1990 and reached over 2 million mt in 2018 [3]. Globally, Salmo salar (Atlantic salmon) and Oncorhynchus mykiss (rainbow trout) are among the 15 most traded fish species [3]. The high animal density associated with aquaculture favors the appearance of chronic stress in fish, negatively affecting production [4]. In addition, in rainbow trout, chronic stress may induce oxidative stress (OE), [5] and organic depletion of vitamins and minerals, such as Se [6].
Se is an essential element for animals, and it participates in metabolic processes involved in development, growth, health, and fertility, and it is administered to cultured salmon as a nutritional supplement [7,8]. In addition, Se is a cofactor of multiple proteins (seleno-proteins), including glutathione peroxidase and thioredoxin reductase [9], enzymes which contribute to remove reactive oxygen species (ROS), preventing OE [10]. Kohshahi et al. [11], demonstrated the immune-stimulating effect of different Se chemical forms when included as a nutritional supplement to channel catfish (Ictalurus punctatus).
Hilton et al. [12] reported that the daily requirement of Se for rainbow trout is between 0.15 to 0.38 mg kg−1 dry-matter fed. Rider et al. [13] reported that, under stressful environmental conditions, the requirement could be increased up to 4.0 mg kg−1 (dry mass). Chronic consumption of 13 mg Se kg−1 (dry mass) caused evident signs of toxicity in rainbow trout, resulting, among others, in a decreased growth rate and high mortality [12].
Feeding fish, such as cultured salmonids, with high trophic levels requires the use of fishmeal and fish oil to adequately meet their nutritional needs [14]. Given the reduction in the stock of marine fish [8], food formulas are now including ingredients of vegetal origin to offset the fishmeal price increase [9]. According to Ytrestoyl et al. [14], multiple diets for salmonids include more than 70% of ingredients of plant origin.
Se natural concentration in fishmeal fluctuates between 1.5 and 3.1 mg kg−1 [15] while in vegetal ingredients it varies barely from 0.01 to 0.16 mg kg−1 [16]. Betancor et al. [17] reported that including raw material of vegetable origin to fishmeal could reduce the Se content in salmon fillet, reducing its nutritional value. This outcome may be the consequence of the presence of phytic acid in plants, reducing the availability of Se at the intestinal level of fish [18]. In order to achieve tissue concentrations of Se allowing an adequate development and well-being of farmed salmonid fish, it is necessary to supplement their diet with Se [19]. The chemical species of Se supplemented to fish, either organic (selenomethionine and selenocysteine) or inorganic (Na2SeO3), affects the bioavailability of the micronutrient and has an impact on their metabolism [20]. The inorganic form (Na2SeO3) is less bioavailable and has a greater toxicity than the organic Se species in rainbow trout [21].
Nanoparticles (Nps), new biotechnological tools, possess unique physical and chemical properties and are increasingly being used in several fields, such as imaging, chemical sensors and biosensors, diagnostics, drug delivery, catalysis, energy, photonics, medicine [22], and veterinary medicine [23]. The applications of Nps as food additives, health enhancers, and antimicrobials in animal production are increasing [24,25]. Several authors reported a higher bioavailability and lower toxicity of Se when administered as Nps (Se0Nps) when compared to other chemical forms of Se and also that dietary supplementation with Se0Nps in farmed fish contributes to the improvement of productive indices in intensive aquaculture [8,22,23,24,25,26].
Different chemical and physical methods have been described to produce Se0Nps. In general, these methods involve the use of toxic solvents, the generation of dangerous by-products, and high-energy consumption [27]. On the other hand, since they can grow rapidly and they are easy to manipulate and to culture at a relatively low cost, bacteria are being used as micro-factories capable of biosynthesizing metal Nps [28]. In addition, biogenic Nps, such as Se0Nps, can interact with different substances and the addition of functional chemical groups, or functionalization, (such as thiols, disulphurs, amines, carboxylic acids, phosphine, and other biomolecules) [29]. Functionalization provides Nps with advantages including, among others, inhibiting agglomeration, maintaining particle sizes compatible with metabolic activity, and improving bioavailability [29]. The above considerations encouraged us to produce and characterize functionalized Se0Nps (Se0Nps/L-Cys) and to evaluate if they showed better effects than non-functionalized Se0Nps or inorganic soluble Se (Na2SeO3) on cell viability and oxidative status in three types of cell cultures of O. mykiss. The effects of Se0Nps/L-Cys, Se0Nps, and Na2SeO3 as a nutritional supplement on immunological and oxidative status, and productive parameters for O. mykiss were also compared.
2. Materials and Methods
2.1. Biosynthesis, Purification, and Functionalization of Se0Nps
Pantoea agglomerans UC-32, isolated from the sediments of Camarones river, northern Chile, was reported as a bacterial strain capable to produce Se0Nps [30]. To produce Se0Nps, P. agglomerans UC-32—kept at the culture collection of the Laboratory of Environmental Microbiology, Department of Microbiology, Faculty of Biological Sciences, University of Concepcion, Concepcion, Chile—was cultured overnight under aerobic conditions in trypticase broth (TB) (Merck, Darmstadt, Germany) supplemented with 0.5 mM Na2SeO3 at 30 °C with agitation (100 rpm) [30]. Cultures without Na2SeO3 were used as negative control. The purification of Se0Nps biosynthesized by P. agglomerans UC-32 and its functionalization with L-cysteine were done as described by Chen et al. [25] and Tarrahi et al. [31], respectively. L-cysteine functionalized Se0Nps (Se0Nps/L-Cys) were resuspended in 10 mL Leibovitz’s L-15 medium (Gibco, Waltham, MA, USA) and stored at −80 °C. Non-functionalized Se0Nps were obtained from fresh culture and stored at −80 °C.
2.2. Characterization of Se0Nps Biosynthesized by P. agglomerans UC-32 Strain
The morphology and size of P. agglomerans UC-32 biosynthesized Se0Nps and Se0Nps/L-Cys were evaluated by means of transmission electron microscopy (TEM) as described by Dhanjal and Cameotra [32] using a JEOL JSM 1200EX-II TEM microscope (JEOL, Peabody, MA, USA). Their chemical characterization was done by means of scanning electron microscopy-energy dispersive X-ray Spectroscopy (SEM-EDS), as described by Torres et al. [30], using a JEOL JSM 6380LV SEM microscope (JEOL, Peabody, MA, USA).
2.3. Antioxidant Capacity of Se0Nps/L-Cys
The antioxidant capacity of Se0Nps/L-Cys, Se0Nps, and Na2SeO3 was measured on the basis of their scavenging ROS capacity using three assays: the radical scavenging 2,2′-diphenyl-1-picrylhydrazyl (DPPH) assay, the ferric reducing antioxidant power (FRAP) assay, and the total radical-trapping antioxidant parameter assay (TRAP). The DPPH assay was done following the procedure of Brand-Williams et al. [33]. The IC50 value was calculated to determine the concentration of the sample required to inhibit 50% of the radicals. The lower the IC50 value, the higher the antioxidant activity of samples [34]. The FRAP assay was done as described by Dudonné et al. [35] and the absorbance values obtained were interpolated in a Trolox calibration curve (0–200 mg L−1). The TRAP assay was done according to Romay et al. [36], and the absorbance values were interpolated in a Trolox standard curve (0–120 mg L−1). The absorbances of all three assays were obtained using an Epoch model microplate spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA) adjusted to the appropriate wavelength for each assay. DPPH values were expressed as half-maximal inhibitory concentration (IC50) in mg mL−1. FRAP and TRAP values were expressed in mM Trolox equivalent antioxidant capacity (mM TEAC). Vitamin C (Merck, Darmstadt, Germany), Trolox (Merck, Darmstadt, Germany), and N-acetylcysteine (NAC) (Merck, Darmstadt, Germany) were used as positive controls. Different concentrations of Se0Nps/L-Cys, Se0Nps, or Na2SeO3, in the range of 50–500 μg mL−1 in methanolic solution, were added to DPPH, FRAP, or TRAP solutions.
2.4. Effect of Se0Nps/L-Cys in Rainbow Trout’s Cells Culture (In Vitro Model)
2.4.1. Oncorhynchus Mykiss Cell Lines and Primary Head Kidney Monocyte-like Cells Culture
For in vitro assays, O. mykiss cell lines RTgill-W1 (normal epithelial gill cells; ATCC -CRL2523) and RTS-11 (spleen, monocyte/macrophage-like cells; RRID:CVCL_F835) and primary head kidney monocyte-like (T-PHKM) culture cells were provided by Dr. Luis Mercado (Pontifical Catholic University of Valparaíso, Valparaíso, Chile). RTgill-W1 and RTS-11 cells were cultured in Leibovitz’s L-15 medium (Gibco, Waltham, MA, USA) supplemented with 2% penicillin streptomycin (100 mg mL−1 streptomycin, 100 IU mL−1 penicillin (Gibco, Waltham, MA, USA) and 10% fetal calf serum (FCS) (Gibco, Waltham, MA, USA) for RTgill-W1 cells or 30% FCS for RTS-11 cells. T-PHKM cells were obtained and cultured according to Abarca et al. [37]. The three cell lines were stabilized at 18 °C overnight before been exposed to Se0Nps/L-Cys or Na2SeO3.
2.4.2. In Vitro Analysis of the Toxicity of Se0Nps/L-Cys
RTgill-W1 cells (4 × 104), RTS-11 cells (5 × 104) or T-PHKM cells (5 × 104) in 100 µL Leibovitz’s L-15 medium were placed in each well of 96 wells flat bottom microplates (Merck, Darmstadt, Germany) and cultured at 20 °C. After 18 h of incubation, the culture medium was replaced with fresh medium supplemented with FCS and antibiotics, as indicated above, plus 160, 320, or 640 nM of Se0Nps/L-Cys or Na2SeO3. The stock Se0Nps/L-Cys suspension or selenite solution were prepared in L-15 Leibovitz’s medium. Based on the data reported by Torres et al. [30], three concentrations of either Se0Nps/L-Cys or Na2SeO3 (160, 320, or 640 nM) were used. L-15 Leibovitz’s medium plus RTgill-W1, RTS-11, or T-PHKM cells was used as control in every experiment. After 23 h of culture, 10 µL of 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H—tetrazolium monosodium salt (WST-1) (Roche Applied Science, Indianapolis, IN, USA) were added to each well following the manufacturer’s instructions. Cellular viability was measured at 450 nm using an Epoch microplate spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA). Cytotoxicity of Se0Nps/L-Cys or Na2SeO3 was expressed as percentage of viable cells with respect to the control. All experiments were carried out in triplicate.
2.4.3. In Vitro Effect of Se0Nps/L-Cys against H2O2-Induced Oxidative Stress on Rainbow Trout Cell Cultures
The effect of Se0Nps/L-Cys against H2O2-induced toxicity was evaluated in RTgill-W1, RTS11, and T-PHKM cells measuring cellular ROS concentration according to Singh et al. [38]. Briefly, RTgill-W1 cells (4 × 104 cells), RTS-11 cells (5 × 104 cells) or T-PHKM cells (5 × 104 cells) in 100 µL L-15 Leibovitz’s medium were placed in each well of 96-well flat bottom microplates (Merck, Darmstadt, Germany) and cultured at 20 °C with Se0Nps/L-Cys or Na2SeO3 (160, 320 or 640 nM) for 24 h. Then, L-15 Leibovitz’s medium was carefully extracted and replaced with fresh medium containing 100, 150, or 300 μM hydrogen peroxide (H2O2) as a cellular ROS-inducing agent [39] at 20 °C during 24 h. According to Kling and Olsson [40], H2O2 concentrations chosen were non-lethal for rainbow trout cell lines. After this incubation period, 1 µM of the fluorescent probe 6-carboxy-2′,7′-diclorodihidrofluroresceine diacetate (Carboxy-DCFH-DA) (Molecular Probes/Invitrogen, Waltham, MA, USA) was added and cultures maintained at 20 °C in the dark for additional 30 min. The oxidation of carboxy-DCFH into highly fluorescent 2′,7′-dichlorofluorescein (DCF) by intracellular ROS was evaluated by the fluorescent absorbance value using a microplate reader PR 4100 TSC (Bio-Rad, Hercules, CA, USA). Cells were sampled and fluorescence was measured according to Chen et al. [41]. The ROS effects on cell viability of RTgill-W1, RTS11, and T-PHKM cells were also determined using the same procedure described in Section 2.4.2. The assays were carried out in triplicate.
2.5. Effect of Se0Nps/L-Cys Supplemented Food in Rainbow Trout’s (In Vivo Model)
2.5.1. Feeding Trial Design
All animals used in this study were treated in accordance with the Biosecurity Regulations and Ethical Protocols approved by University of Concepcion Ethics Committee. Apparently healthy 160 rainbow trout having an initial weight of 104.53 ± 8.47 g (mean ± SE) and an initial length of 20.8 ± 3.32 cm (mean ± SE) were obtained from Salmones Pangue (Florida, Chile) and transported to an environmentally controlled semi-closed recirculation system (Laboratory of Pisciculture and Aquatic Pathology (LPAP)), Faculty of Natural Sciences and Oceanography, University of Concepcion, Concepcion, Chile. Trout were kept in fiberglass tanks, at 15.5 ± 0.8 °C and a maximum density of 25 kg fish m−3, containing 8.1 ± 0.08 mg L−1 dissolved oxygen and under a 12:12 light:dark photoperiod [42]. Twenty fish were randomly distributed in each one of 8 tanks. Two tanks were assigned to each one of the below described four different diets assayed, totalling 40 fish per diet group. Fish were acclimated for 21 days, time span in which they were fed an acclimatization diet including the minimum rainbow trout selenium requirement according to the National Research Council (NRC) [19]. The four diets were administered during a 30-day period; one group (control group) received the same acclimatization diet. The three experimental diets were enriched with 5 mg of Se nanoparticles (Se0Nps), of L-cysteine functionalized Se nanoparticles (Se0Nps/L-Cys), or inorganic Se (Na2SeO3) per kg dry food to obtain a non-toxic diet [13,14]. To prepare the diets, the approximate yield of Se0Nps of a 1 L culture of P. agglomerans culture was determined. All diets were prepared weekly, according to Vera [43], by Cargill-Ewos (Coronel, Chile) containing 39–43% crude protein, 10–16% lipid, 3–4% fiber, 9–12% ash, 7–13% moisture, 1–2% calcium, and 1–1.4% phosphate. Fish were fed twice daily (10:00 h and 16:00 h) receiving 2% of their average body weight per day. Eight fish per tank were weighed (BLC 1500 scale, Boeco, Hamburg, Germany) at the beginning of the feeding trial, and subsequently when samples were taken, and the amount of food given adjusted accordingly.
2.5.2. Fish Sampling
On days 0, 15, and 30, six fish from each experimental or control group were carefully captured, sacrificed by an overdose of the anesthetic BZ-20 (50 ppm of sodium pa-ra-aminobenzoate in fresh water; Veterquimica, Santiago, Chile) and then individually weighted (BLC 1500 scale; Boeco, Hamburg, Germany) and measured from the tip of the snout to the rear edge of the fork at the center of the tail fin. Blood was extracted from the caudal vein of each fish, by means of a heparinized 18G needle fitted to a 5 mL syringe and transferred to sterile microtubes containing 0.02 mL of 1000 U mL−1 heparin (Merck, Darm-stadt, Germany). Additionally, samples of liver and dorsal muscle were obtained. Samples were immediately transported at 4 °C to the Laboratory of Environmental Microbiology, University of Concepcion, where plasma was obtained by centrifugation at 5000× g for 10 min, and liver and dorsal muscle were fragmented. Then, plasma, liver and dorsal muscle were stored at −80 °C.
2.5.3. Innate Immune Responses
Plasma lysozyme activity and ROS concentration in white blood cells (WBCs) of six rainbow trout per sampling day and diet were measured. A turbidimetric assay was used to determine plasma lysozyme activity level [44]. Briefly, 950 µL of buffered substrate (0.25 mg of Micrococcus lysodeikticus in 1 mL of buffered 40 mM sodium phosphate pH 6.2) was mixed with 50 µL of fish plasma. The absorbance of the samples was measured at times 0 and 30 min of incubation at room temperature by means of an Epoch spectrophotometer at 450 nm (BioTek Instruments, Inc., Winooski, VT, USA). A 0.001 min−1 absorbance reduction was evaluated as one unit of lysozyme activity [44].
For ROS concentration measurements, an assay evaluating the reduction of nitroblue tetrazolium (NBT) into colored formazan by oxidizing agents was used following the method of Anderson and Siwicki [45].
2.5.4. Activity of the Antioxidant Enzyme Glutathione Peroxidase (Gpx)
The glutathione peroxidase (Gpx) activity was assayed in plasma, according to Lawrence and Burk [46] and liver and dorsal muscle as described by Fontagné-Dicharry et al. [21]. Gpx activity in plasma samples was evaluated immediately after thawing. In the case of liver and muscle, samples were rapidly thawed and homogenized in 10 volumes (w/v) of ice-cold saline for 3 min and centrifuged for 15 min at 4000× g and the supernatants collected to evaluate the activity of GPx. Gpx activity present in the supernatants was measured in a solution of 50 mM phosphate buffer (pH 7.4), 1 mM EDTA (Merck, Darmstadt, Germany), 2 mM sodium azide (Merck, Darmstadt, Germany), 2 mM reduced glutathione (GSH) (Merck, Darmstadt, Germany), 0.1 mM NADPH (Merck, Darmstadt, Germany), and 0.2 mM glutathione reductase (Merck, Darmstadt, Germany) following the reduction of H2O2 (50μM) at 30 °C and 340 nm. One unit of Gpx activity was reported as l mol NADPH consumed per min per mg of plasma protein, using the appropriate molar absorptivity coefficient for NADPH (6220 mol L−1 cm−1). Plasma proteins were measured by the method of Lowry et al. [47].
2.5.5. Effect of Se0Nps/L-Cys on Trout Growth Performance and Survival Rate
The effects of Se0Nps, Se0Nps/L-Cys or Na2SeO3 on productive parameters of the fish were evaluated every five days. Weight and length of each trout and the number of dead fish were recorded to calculate the specific growth rate (SGR), weight gain (WG), condition factor (CF), and survival rate (%), according to Naderi et al. [8] and Lugert et al. [48] using the following Equations (1)–(4):
where w1 = starting weight (g); w2 = final weight (g); days = days in the growth period; w = weight (g); L = length (cm); n1 = initial number of fish; n2 = final number of fish.
When the three different diet and one-control groups were made up, the initial condition factor (ICF) was considered (similar sizes and weights) to make sure that the initial populations of each group were homogeneous with respect to the development stage and the nutritional condition.
2.6. Statistics
One-way analysis of variance (ANOVA) followed by an LSD multiple comparison test was used to determine the statistical significance for multiple comparisons. The Student’s t-test was used for pairwise comparisons. Values of p < 0.05 were considered as statistically significant. All statistical tests were performed using the GraphPad Prism software version 7 for Windows (GraphPad Software, San Diego, CA, USA, www.graphpad.com, accessed on 22 July 2020).
3. Results
3.1. Characterization of Se0Nps Biosynthesized by P. agglomerans UC-32 without and after Functionalization
The size and morphology of the biosynthesized Se0Nps and Se0Nps/L-Cys were analyzed by TEM. TEM observations revealed that both Se0Nps and Se0Nps/L-Cys were sphere-like nanoparticles with sizes between 53 to 170 nm and 32 to 160 nm in diameter, respectively (Figure 1A,C, respectively), which indicated that Se0Nps/L-Cys were significantly smaller than non-functionalized Se0Nps (p < 0.05). SEM-EDS analysis of Se0Nps and Se0Nps/L-Cys showed the presence of peaks corresponding to Se, confirming that the nanoparticles were mainly composed of Se. The presence of C, N, and O signals can be ascribed to cell debris (Figure 1B,D). In the case of Se0Nps/L-Cys, a sulfur (S) peak was, as expected, also observed due to the thiol sidechain of cysteine, confirming their functionalization (Figure 1D).
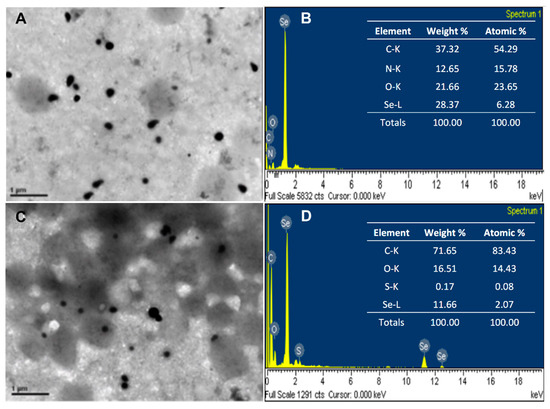
Figure 1.
Selenium nanoparticles produced by Pantoea agglomerans UC-32 strain without functionalization (Se0Nps) and after L-cysteine functionalization (Se0Nps/L-Cys). (A) TEM micrograph of Se0Nps among P. agglomerans UC-32 cell debris; (B) SEM-EDS data obtained from Se0Nps; (C) TEM micrograph of Se0Nps/L-Cys among P. agglomerans UC-32 cell debris; (D) SEM-EDS data obtained from Se0Nps/L-Cys.
3.2. Antioxidant Capacity of Se0Nps/L-Cys
The antioxidant activity of Se0Nps/L-Cys, Se0Nps and Na2SeO3 was evaluated in vitro using the DPPH, FRAP, and TRAP assays (Table 1). Data in Table 1 were obtained by using 500 µg mL−1 of Se0Nps/L-Cys, Se0Nps, or Na2SeO3.

Table 1.
In vitro radical scavenging capacity of 500 µg mL−1 Se0Nps/L-Cys, Se0Nps, and Na2SeO3.
The DPPH assay showed that the ROS scavenger activity of the positive controls (Vit C, Trolox, and NAC) was significantly better than that of the three forms of Se tested. Se0Nps/L-Cys and Se0Nps were more efficient ROS scavengers than Na2SeO3 (p < 0.05). When comparing both types of Nps, the functionalized ones were significantly better (p < 0.05) ROS scavengers than the non-functionalized ones.
Regarding the FRAP assay, the positive Vit C control was a better ROS scavenger than the NAC control and all three Se compounds (p < 0.05). Antioxidant capacity of Se0Nps was higher than Se0Nps/L-Cys (p > 0.05) and Na2SeO3 (p < 0.05). Finally, the TRAP assay showed that Vit C control had the highest antioxidant activity (p < 0.05). Regarding Se compounds, a similar antioxidant activity pattern to DPPH was detected. Se0Nps/L-Cys was a significant better ROS scavenger than Se0Nps and Na2SeO3 (p < 0.05).
3.3. Toxicity of Se0Nps/L-Cys for Cell Lines RTgill-W1 and RTS-11 and Primary Culture T-PHKM
The toxicity of Se0Nps/L-Cys for the cells was expressed in percentage of viable RTgill-W1, RTS-11, or T-PHKM cells when co-cultured with Se0Nps/L-Cys or, for comparison, Na2SeO3 during 24 h (Table 2). The cytotoxicity for both cell lines and the primary culture was dose dependent showing a decreasing cell viability as the concentration of Se0Nps/L-Cys or Na2SeO3 increased. When comparing with the control, the viability of the cells assayed was not significantly reduced (p < 0.05) only when RTgill-W1 (95.64%), RTS-11 (96.39%) or when T-PHKM (96.52%) cells were exposed to either 160 nM Se0Nps/L-Cys or 160 nM Na2SeO3. When comparing the effect of a same Se0Nps/L-Cys or Na2SeO3 concentration, all three cell types showed higher viabilities when exposed to 160, 320, or 640 nM Se0Nps/L-Cys than to Na2SeO3. Results for RTgill-W1 cells showed significant higher viabilities (p < 0.05) when they were exposed to 160, 320, or 640 nM Se0Nps/L-Cys than to Na2SeO3. In the case of RTS-11 cells, viabilities when exposed to 640 nM Se0Nps/L-Cys or Na2SeO3 were 95.67% and 93.74%, respectively, when compared to the control (p < 0.05). On the other hand, similar concentrations of Se0Nps/L-Cys or Na2SeO3 caused no significant differences (p > 0.05) in the survival of T-PHKM cells. Finally, the analysis of cell viability at different concentrations of the same form of Se (Se0Nps/L-Cys or Na2SeO3) showed significant differences (p < 0.05) between 160 nM and 640 nM in the three cellular types, being 640 mM more toxic than 160 mM of both Se sources.

Table 2.
Effect of Se0Nps/L-Cys or Na2SeO3 on the cell viability of cell lines RTgill-W1, RTS-11, and T-PHKM.
3.4. Effect of Se0Nps/L-Cys on H2O2-Induced Oxidative Stress in Cell Lines RTgill-W1 and RTS-11 and T-PHKM Primary Cell Culture
A significant reduction (p < 0.05) in cell viability was observed when the cell viability of all three cell types treated with 100, 150, or 300 µM H2O2 (positive controls) was compared to cells not treated with H2O2 (negative controls), being the highest H2O2 concentration the one causing the largest cell viability reduction in the three cell lines assayed. When the cell viability of the three cell types was compared, RTS cells demonstrated better viabilities than RTgill-W1 or T-PHKM cells when subjected to 100, 150, or 300 µM H2O2 (p < 0.05) (Figure 2).
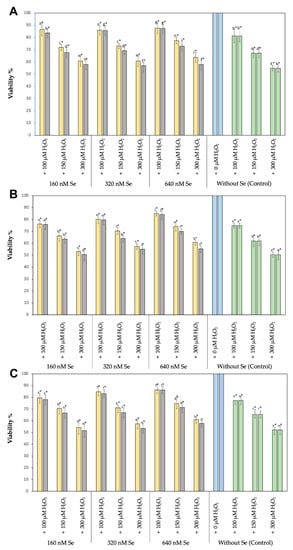
Figure 2.
Cell viability (as percentage of the negative control  ) of cell lines RTS-11 (A), RTgill-W1 (B), and of the primary culture T-PHKM (C) treated with Se0Nps/L-Cys
) of cell lines RTS-11 (A), RTgill-W1 (B), and of the primary culture T-PHKM (C) treated with Se0Nps/L-Cys  or Na2SeO3
or Na2SeO3  and then subjected to H2O2 as a ROS inducing agent. All data is given as mean ± SD. Positive controls
and then subjected to H2O2 as a ROS inducing agent. All data is given as mean ± SD. Positive controls  . Different letters on top of bars indicate significant differences among groups. * Statistically different from the negative control.
. Different letters on top of bars indicate significant differences among groups. * Statistically different from the negative control.
 ) of cell lines RTS-11 (A), RTgill-W1 (B), and of the primary culture T-PHKM (C) treated with Se0Nps/L-Cys
) of cell lines RTS-11 (A), RTgill-W1 (B), and of the primary culture T-PHKM (C) treated with Se0Nps/L-Cys  or Na2SeO3
or Na2SeO3  and then subjected to H2O2 as a ROS inducing agent. All data is given as mean ± SD. Positive controls
and then subjected to H2O2 as a ROS inducing agent. All data is given as mean ± SD. Positive controls  . Different letters on top of bars indicate significant differences among groups. * Statistically different from the negative control.
. Different letters on top of bars indicate significant differences among groups. * Statistically different from the negative control.
RTS-11, RTgill-W1, and T-PHKM cells cultured in the presence of 160, 320, or 640 nM Se0Nps/L-Cys or Na2SeO3 and then subjected to 100, 150, or 300 µM H2O2 showed to better retain their viability when compared to the positive controls in a dose dependent manner, being the best cell viabilities obtained in the cultures containing 640 nM Se0Nps/L-Cys or Na2SeO3. Similarly, as observed in the positive controls, RTS-11 cells showed better viabilities when compared to RTgill-W1 or T-PHKM at all Se0Nps/L-Cys or Na2SeO3 concentrations. RT-gill-W1 was the cell type showing the lowest cell viabilities. Cell viabilities in all experimental groups were significantly less (p < 0.05) than those of the negative controls and significantly better (p < 0.05) than those of positive controls (Figure 2).
Se0Nps/L-Cys (Figure 2) showed to provide a better protection than Na2SeO3 to RTS-11, RTgill-W1, and T-PHKM cells in all the experimental groups exposed to H2O2 (Figure 3). RTS-11 cells cultured in the presence of Se0Nps/L-Cys showed to retain a better cell viability than the one achieved in the presence of Na2SeO3, being it significant in the experimental groups 160 + 100 (86.67% vs. 83.61%, respectively), 160 + 150 (71.85% vs. 67.69%, respectively), 160 + 300 (60.74% vs. 57.99%, respectively), 320 + 150 (73.16% vs. 69.28%, respectively), 320 + 300 (60.81% vs. 57.04%, respectively), 640 + 150 (77.39% vs. 72.93%, respectively), and 640 + 300 (63.76% vs. 58.05%, respectively). On the other hand, RTgill-W1 cells plus Se0Nps/L-Cys showed a significant better viability that the same cell type plus Na2SeO3 in the experimental groups 160 + 300 (53.01% vs. 50.55%, respectively), 320 + 150 (70.37% vs. 64.16%, respectively), 640 + 150 (74.11% vs. 70.02%, respectively), 320 + 300 (57.32% vs. 54.98%, respectively), and 640 + 300 (60.81% vs. 55.45%, respectively). Finally, primary culture T-PHKM cells subjected to Se0Nps/L-Cys showed a viability significantly better than T-PHKM subjected to Na2SeO3 in the groups 160 + 150 (70.51% vs. 66.82%, respectively), 160 + 300 (54.40% vs. 51.73%, respectively), 320 + 150 (71.13% vs. 67.03%, respectively), 320 + 300 (57.61% vs. 53.63%, respectively), 640 + 150 (74.88% vs. 71.49%, respectively), and 640 + 300 (61.14% vs. 57.91%, respectively) (Figure 2).
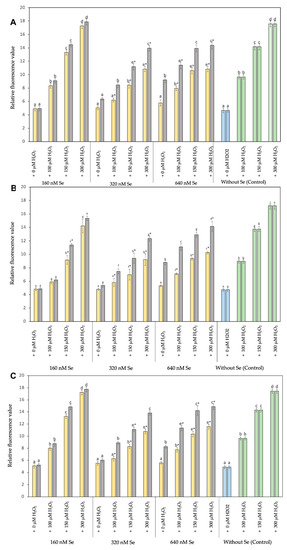
Figure 3.
Level of intracellular ROS on cell lines RTS-11 (A), RTgill-W1 (B), and of the primary culture T-PHKM (C) treated with Se0Nps/L-Cys  or Na2SeO3
or Na2SeO3  and then subjected to H2O2 as a ROS inducing agent. All data is given as mean ± SD. Different letters on top of bars indicate significant differences among groups. * Significant reduction of cellular ROS concentration compared to the positive controls
and then subjected to H2O2 as a ROS inducing agent. All data is given as mean ± SD. Different letters on top of bars indicate significant differences among groups. * Significant reduction of cellular ROS concentration compared to the positive controls  . Negative control
. Negative control  .
.
 or Na2SeO3
or Na2SeO3  and then subjected to H2O2 as a ROS inducing agent. All data is given as mean ± SD. Different letters on top of bars indicate significant differences among groups. * Significant reduction of cellular ROS concentration compared to the positive controls
and then subjected to H2O2 as a ROS inducing agent. All data is given as mean ± SD. Different letters on top of bars indicate significant differences among groups. * Significant reduction of cellular ROS concentration compared to the positive controls  . Negative control
. Negative control  .
.
3.5. In Vitro Effect of Se0Nps/L-Cys on ROS Concentration in Cell Lines RTgill-W1 and RTS-11 and Primary Culture T-PHKM
The effect of Se0Nps/L-Cys on ROS scavenging was evaluated on RTgill-W1, RTS-11, and T-PHKM cells pre-treated with Se0Nps/L-Cys or Na2SeO3 and then subjected to H2O2. The assay used measures the fluorescence emitted by DCF resulting from the oxidation of carboxy-DCFH by intracellular ROS.
As shown in Figure 3, the concentration of cellular ROS in RTgill-W1, RTS-11, and T-PHKM cells co-cultured with Se0Nps/L-Cys or Na2SeO3 (160, 320, or 640 nM) for 24 h was increased respect to each cell type negative control (only cells) in a concentration-dependent manner. T-PHKM cells co-cultured with 320 nM Na2SeO3 and RTgill-W1, RTS-11, and T-PHKM cells co-cultured with 640 nM Na2SeO3 significantly increased the cellular ROS concentration compared to observed in the negative control and three cell types under similar concentration of Se0Nps/L-Cys (p < 0.05).
A markedly increased (p < 0.05) cellular ROS of H2O2-induced RTgill-W1, RTS-11, and T-PHKM positive controls cells (cells plus 100, 150, or 300 µM H2O2) in a dose-dependent manner when compared to the respective negative controls, was observed. RTgill-W1, RTS-11, and T-PHKM cells pre-incubated with 160, 320, or 640 nM Se0Nps/L-Cys significantly reduced the increased cellular ROS concentration induced by 100, 150, or 300 µM H2O2 in a concentration-dependent manner compared to registered in positive controls (Figure 3). A better performance in reducing cellular ROS concentration of Se0Nps/L-Cys than Na2SeO3 in all experimental groups of each cell type, was noted (Figure 3). Se0Nps/L-Cys was a significant (p < 0.05) better cellular ROS concentration reducer than Na2SeO3 in 320 + 100, 320 + 150, 320 + 300, 640 + 100, 640 + 150, and 640 + 300 groups in RTgill-W1, RTS-11, and T-PHKM cells.
3.6. Effect of Se0Nps/L-Cys Supplemented Food in Rainbow Trout (In Vivo Model)
3.6.1. Innate Immune Responses
Plasma lysozyme activity was assessed by its capacity to lyse Micrococcus lysodeikticus and ROS production by leukocytes was assessed by an assay evaluating NBT reduction. Regarding plasma lysozyme activity (Table 3). Since day 15, the plasma lysozyme activity of fish receiving Se0Nps/L-Cys supplemented food was significantly increased when compared to the control group and the group receiving Na2SeO3 supplement food (p > 0.05). On day 30, lysosome activity of the group receiving Se0Nps/L-Cys supplemented food was also significantly higher than the group receiving Se0Nps supplemented food (p > 0.05).

Table 3.
Plasma lysozyme activity (in U mL−1) in rainbow trout fed with 5 mg kg−1 Se dry diet supplemented food for 30 days.
ROS production by peripheral leukocytes was evaluated by the reduction of NBT into the colored compound formazan; therefore, higher absorbances at the wavelength at which formazan absorbs correspond to higher ROS concentrations (Table 4). On the day 15, an increase of cellular ROS of trout receiving Se0Nps (p < 0.05) or Se0Nps/L-Cys (p > 0.05) when compared to the control group, was observed. Samplings on day 30 showed that the group receiving Se0Nps/L-Cys supplemented food significantly increased formazan levels, when compared to the groups whose diet was supplemented with Se0Nps or Na2SeO3 (p < 0.05). On day 30, ROS concentration in the group receiving Se0Nps was also significantly higher than the one in the group receiving Na2SeO3 (p < 0.05).

Table 4.
ROS production by blood leukocytes, evaluated by NBT reduction into formazan, in rainbow trout. Fish were fed with 5 mg kg−1 Se dry diet supplemented food for 30 days.
3.6.2. Activity of the Antioxidant Enzyme Gpx
The activity of the enzyme Gpx in plasma, liver, and dorsal muscle of rainbow trout fed with Se0Nps, Se0Nps/L-Cys, or Na2SeO3 supplemented food for 30 days is shown in Table 5. Significant increases in Gpx activity were observed in plasma, liver, and dorsal muscle in the three groups receiving Se supplemented diet when compared to the control group (p < 0.05). Moreover, the group receiving the diet supplemented with Se0Nps/L-Cys showed a significant higher muscle tissue Gpx activity when compared to the group receiving Se0Nps (p < 0.05) and a significant higher Gpx activity in plasma, liver, and muscle tissue when compared to the group receiving Na2SeO3 (p < 0.05).

Table 5.
Glutathione peroxidase (Gpx) activity in rainbow trout fed with 5 mg kg−1 Se dry diet supplemented food at day 30.
3.6.3. Growth Performance and Survival
Growth performance and survival rate of fish receiving the different dietary treatments during the 30 days of analysis is shown in Table 6. Weight gain (WG) and specific growth rate (SGR) values were not significantly different among groups (p > 0.05). Nevertheless, the final condition factor (FCF) of trout fed food enriched with Se0Nps/L-Cys (1.68%) was significantly higher than FCF of the control group (1.27%), Se0Nps (1.52%), and Na2SeO3 (1.45%) groups (p < 0.05).

Table 6.
Growth performance and survival rate of rainbow trout fed with 5 mg kg−1 Se dry diet supplemented food for 30 days.
4. Discussion
Se is an essential element used by animal organisms, including fish, to carry out physiological processes for an adequate development as required by each species [19]. This chemical element indirectly contributes to remove and prevent oxidative stress, acting as an exogenous antioxidant [48], and plays an integral role in the immune and endocrine systems [49].
Intense fish culture systems maximize the effect of stressors, favoring the rising of diseases along with ensuing important economic losses [50]. According to Baldissera et al. [51], the onset and progression of fish infectious diseases are usually mediated by oxidative stress as well as oxidative damage. Thus, the supplementation of salmonid fish food with Se is necessary to maintain the optimal health and growth of farm-raised fish [52]. Nevertheless, there are conflicting reports on the literature about the effects of different sources of Se supplementation, including inorganic Se and Se nanoparticles, on the physiological parameters of fish species [53].
In the present work, predominantly spherical Se0Nps were produced by the cytoplasmic Na2SeO3 reduction by the bacterium P. agglomerans [54]. The detection of a sulfur (S) peak by SEM-EDS only in L-Cys treated Se0Nps (Se0Nps/L-Cys) confirmed the functionalization of the nanoparticles. L-Cys has proven to be effective as a functionalizing agent for nanoparticles due to the presence of a SH group in its structure [30]. According to Prasanth and Sudarsanakumar [55], Se0Nps functionalization with L-Cys results from the anchoring of the thiol group of cysteine to the surface of the nanoparticles.
TEM results showed that Se0Nps/L-Cys were significantly smaller than non-functionalized Se0Nps. This phenomenon could be associated to the anti-agglomeration property of L-Cys as reported by Perni et al. [56], who indicated L-Cys reduces the surface energy of the silver (Ag) nanoparticles enhancing their separation and preventing further agglomeration. L-Cys has been used as a functionalizing agent not only for Se0Nps but also for other Nps of other chemical composition, such as copper (Cu) [57], zinc (Zn) [58], silver (Ag) [59], and gold (Au) [60]. Several authors have reported the use of L-Cys as a functionalizing agent to obtain smaller Nps [35,61,62].
Our results suggest that Se0Nps/L-Cys were more effective as in vitro ROS scavengers than Na2SeO3. The higher ROS scavenging activity of the functionalized Nps, when compared to non-functionalized Se0Nps, also suggests that the smaller size of the functionalized SeNps and the independent ROS scavenging activity of L-Cys anchored to the surface of the Se0Nps/L-Cys combine their effects to increase the ROS scavenging activity exerted by Se0Nps/L-Cys. With respect the involvement of the size of the Nps on their ROS scavenging capacity, Huang et al. [63] concluded that the ROS scavenging effect, measured by the DPPH assay, is higher as SeNps are smaller. These authors evaluated SeNps of three different sizes and Na2SeO3 as ROS scavengers. In concordance with our findings, Na2SeO3 showed the poorest ROS scavenging activity when compared to SeNps. Matsuura et al. [64] evaluated the effect of L-Cys as a ROS scavenger when integrated to the surface of a 4.44 nm drug carrier L-serine (Ser)-modified polyamidoamine dendrimer. These authors concluded that L-Cys contributed to the ROS- and radical-scavenging efficacy when compared to the dendrimer without L-Cys. Significant antioxidant activity differences among SeNps and inorganic Se forms and positive controls has been previously reported [65,66].
Our results suggest that Se0Nps/L-Cys were more biocompatible than Na2SeO3 in RTgill-W1, RTS-11, and T-PHKM cells. Similar results were reported by Xu et al. [67] comparing cell viability after co-culturing SeNps or Na2SeO3 with human normal colon mucosal epithelial cells (NCM460). These authors reported a significant reduction of the viability of NCM460 cells by ≥0.39 μg/mL Na2SeO3 while the cell toxicity of Lactobacillus casei 393 strain biosynthesized SeNps was observed in the presence of 25 μg mL−1 Na2SeO3. A greater antioxidant activity of Se0Nps/L-Cys than Na2SeO3 has also been demonstrated in a cellular model using human umbilical vein endothelial cells (HUVEC) [30].
The pre-treatment of RTgill-W1, RTS-11, or T-PHKM cells with Se0Nps/L-Cys effectively reduced, exceeding Na2SeO3, the oxidative effect of H2O2 on the two cell lines and the primary cell culture assayed in the present work. Studies support that the exposition of a cell culture to 100 µM H2O2 causes cellular oxidative damage and/or OS [68]. According to Mou et al. [69], metabolic alterations in cells (melanocytes) by the oxidative effect of H2O2 directly influence the rate increase of cell apoptosis. In addition, the pre-treatment with 640 nM Se0Nps/L-Cys was a better attenuator of H2O2-induced oxidative damage than 640 nM Na2SeO3, improving the cell viability and reducing intracellular ROS concentration in the cells studied.
Our results, using RTgill-W1 and RTS-11 cells, suggest that Se0Nps/L-Cys could also contribute to alleviate the effect of oxidative environmental pollutants able to damage gill tissue of rainbow trout. According to Franco et al. [70] and Bopp et al. [71] the greater sensitivity of RTgill-W1 cells to ROS inducing toxins, when compared to some other cell types, could be related to a greater tendency for DNA fragmentation. In this sense, Ucar et al. [72] revealed insecticides, one of the most worldwide common environmental pollutants which negatively affect the health of aquatic organisms, including fish, produce higher genotoxicity and apoptosis in gill cells than in liver cells of rainbow trout due to oxidative damage [73]. Tkachenko et al. [74] assessed the effect of vaccination on the oxidative status of rainbow trout, showing that the activities of GPx, as well as glutathione reductase (GR), were significantly reduced in the muscles and gills of trout vaccinated against furunculosis suggesting that vaccination induced oxidative stress in these organs.
An increase of the activity of plasmatic lysozyme was observed in the rainbow trout receiving Se0Nps/L-Cys in their diet. The increase of plasma lysozyme levels in fish may be associated to an increased proliferation rate of phagocytic cells or to an increase in the number of lysosomes; therefore, assessing the activity of this enzyme seems to be an appropriate marker to evaluate the innate immune response in fish [75]. Kohshahi et al. [11] reported a significant increase of lysozyme activity when rainbow trout food was supplemented with chemically synthetized Se0Nps as compared to a dietary enrichment with Na2SeO3. Harsij et al. [76] also reported a significant increase of plasmatic lysozyme in rainbow trout administered synthetic Se0Nps combined with vitamins C and E. The use of Se0Nps as food supplement in Nile tilapia (Oreochromis niloticus) also significantly increased their plasma lysozyme activity when compared to the control group and to the group receiving Na2SeO3 supplementation [77].
Phagocytes produce respiratory bursts for the purpose of eliminating foreign pathogens during phagocytosis and have been widely used to evaluate the defense against pathogens. Superoxide anion along with hydroxyl radicals and nitric oxides are induced reactive oxygen species, which are related to enhancing microbial killing capacity of macrophages [78,79]. Data from the present study showed that rainbow trout fed with Se0Nps/L-Cys had higher respiratory burst activity (increase in the concentration cytoplasmatic ROS of blood leukocytes) on days 30 of the feeding trial when compared with the other groups. These results agreed with reports by Dawood et al. [80] and Xia et al. [81] who showed an increase in respiratory burst in blood phagocytes of O. niloticus and Danio rerio, respectively, fed for 8 weeks [82] and 9 days [83], respectively, with diets enriched with chemically synthesized Se0Nps.
All organisms have developed a variety of antioxidant defense systems to constantly suppress the production of ROS and remove them in cells of aerobic organisms [50]. Glutathione peroxidases (Gpxs) represent an important enzyme family, which protects living organisms from oxidative damage, catalyzing the reduction of H2O2 and organic hydroperoxides [83]. The Gpx activity in blood (plasma), liver, and muscle suggests that enriching the diet with Se0Nps/L-Cys would induce a better capacity of the antioxidant system to counteract the effect of ROS on the tissues of rainbow trout because it favors a larger Gpx activity, as already reported by Saffari et al. [54]. These authors reported that plasma Gpx was significantly higher in common carps (Cyprinus carpio) fed with Se0Nps than in fish treated with a basal diet (control) or a diet enriched with Na2SeO3. Naderi et al. [26] reported a significantly high Gpx activity in the hepatic tissue of rainbow trout receiving chemically synthetized Se0Nps when compared to the control animals. Khan et al. [50] indicated that the dietary administration of Se0Nps significantly increased Gpx activity in liver and muscle tissues of juvenile Tor putitora when compared to the control.
No relationship was observed between food supplemented with Se0Np, Se0Np/L-Cys, or Na2SeO3 and fish weight. This observation agrees with reports by Naderi et al. [26] who evaluated the effect of dietary supplementation with Se0Nps on SGR and other production parameters in O. mykiss under stress causing conditions. Nevertheless, Harsij et al. [76] reported a significant increase of the growth rate in juvenile rainbow trout chronically exposed to sublethal concentrations of ammonium and fed with food supplemented with a mixture of chemically synthetized Se0Nps and vitamins C and E when compared to the control (only ammonium). The authors postulated that the assayed mixture may have favored the synthesis of the selenoenzyme deiodinase, which is directly involved in the release of the growth hormone from the pituitary gland in vertebrates, including fish [84].
In the present study, the final condition factor (FCF) at the end of the assay, day 30, was better in rainbow trout receiving the Se0Nps/L-Cys supplemented diet. A FCF above 1.00 corresponds to a good health condition or well-being of fish and it correlates with an increase of important production parameters, such as fertility rate, which involves the production of high-quality gametes [85,86]. Our results suggest that supplementation of the diet with Se0Nps/L-Cys, when compared to Na2SeO3, may favor a better efficiency of rainbow trout accumulating energy reserves.
5. Conclusions
Supplementation of rainbow trout diet with Se0Nps/L-Cys had positive effect on fish innate immune response parameters, oxidative status, well-being, and growth. Se0Nps/L-Cys is a promising alternative for nutritional supplementation for rainbow trout with better performance than Na2SeO3, ease of implementation, and reduced environmental impact.
Author Contributions
Conceptualization, F.Y.-L., V.L.C., R.M. and C.T.S.; Methodology, F.Y.-L., V.L.C., L.M., C.J.-G., P.A., A.V. and C.T.S.; Software, P.A., R.M. and K.S.-A.; Validation, F.Y.-L., V.L.C. and C.T.S.; Formal analysis, F.Y.-L., V.L.C., L.M., C.J.-G. and C.T.S.; Investigation, F.Y.-L., V.L.C. and C.T.S.; Resources, V.L.C., L.M., C.J.-G. and C.T.S.; Data curation, F.Y.-L., V.L.C. and C.T.S.; Writing—original draft preparation, F.Y.-L., V.L.C., P.A., K.S.-A., A.G.-C. and C.T.S.; Writing—review and editing, F.Y.-L., V.L.C. and C.T.S.; Visualization, F.Y.-L., V.L.C., P.A. and C.T.S.; Supervision, F.Y.-L., V.L.C. and C.T.S.; Project administration, V.L.C. and C.T.S.; Funding acquisition, F.Y.-L., V.L.C. and C.T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Grant 217.036.045-1.0, VRID, University of Concepcion, Chile. FONDECYT Grant Nº 1191763 (L.M.)
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and under the fish management and care protocol of the psychiculture and aquatic pathology laboratory, protocol approved by the Ethics Committee of the University of Concepcion (protocol code CBB 1084-2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the assistance of the staff of the Electron Microscopy Laboratory, University of Concepcion, Chile.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fouré, J.; Bénassy-Quérét, A.; Fontané, L. Modelling the world economy at the 2050 horizon. Econ. Transit. 2013, 21, 617–654. [Google Scholar] [CrossRef]
- Ellis, T.; Turnbull, J.; Knowles, T.G.; Lines, J.A.; Auchterlonie, N.A. Trends during development of Scottish salmon farming: An example of sustainable intensification? Aquaculture 2016, 458, 82–99. [Google Scholar] [CrossRef]
- Iversen, A.; Asche, F.; Øystein, H.; Nystøyl, R. Production cost and competitiveness in major salmon farming countries 2003–2018. Aquaculture 2020, 522, 735089. [Google Scholar] [CrossRef]
- Yarahmadi, P.; Miandare, H.K.; Fayaz, S.; Caipang, C.M.A. Increased stocking density causes changes in expression of selected stress- and immune-related genes, humoral innate immune parameters and stress responses of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2015, 48, 43–53. [Google Scholar] [CrossRef]
- Trenzado, C.E.; Morales, A.E.; Palma, J.M.; de la Higuera, M. Blood antioxidant defenses and hematological adjustments in crowded/uncrowded rainbow trout (Oncorhynchus mykiss) fed on diets with different levels of antioxidant vitamins and HUFA. Comp. Biochem. Physiol. Part C 2009, 149, 440–447. [Google Scholar] [CrossRef]
- Küçükbay, F.Z.; Yazlak, H.; Karaca, I.; Sahin, N.; Tuzcu, M.; Cakmak, M.N.; Sahin, K. The effects of dietary organic or inorganic selenium in rainbow trout (Oncorhynchus mykiss) under crowding conditions. Aquac. Nutr. 2009, 15, 569–576. [Google Scholar] [CrossRef]
- Takahashi, K.; Suzuki, N.; Ogra, Y. Effect of gut microflora on nutritional availability of selenium. Food Chem. 2020, 319, 126537. [Google Scholar] [CrossRef]
- Mahdi Naderi, M.; Keyvanshokooh, S.; Salati, A.P.; Ghaedi, A. Combined or individual effects of dietary vitamin E and selenium nanoparticles on humoral immune status and serum parameters of rainbow trout (Oncorhynchus mykiss) under high stocking density. Aquaculture 2017, 474, 40–47. [Google Scholar] [CrossRef]
- Kousha, M.; Yeganeh, S.; Amirkolaie, A.K. Synergistic effect of sodium selenite and Pediococcus acidilactici on growth, intestinal bacterial counts, selenium bioavailability, hepatic enzymes and non-specific immune response in rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2019, 26, 74–87. [Google Scholar] [CrossRef]
- Baeverfjord, G.; Prabhu, P.A.; Fjelldal, P.G.; Albrektsen, S.; Hatlen, B.; Denstadli, V.; Ytteborg, E.; Takle, H.; Lock, E.-J.; Berntssen, M.H.G.; et al. Mineral nutrition and bone health in salmonids. Rev. Aquac. 2018, 11, 740–765. [Google Scholar] [CrossRef]
- Kohshahi, A.J.; Sourinejad, I.; Sarkheil, M.; Johari, S.A. Dietary cosupplementation with curcumin and different selenium sources (nanoparticulate, organic, and inorganic selenium): Influence on growth performance, body composition, immune responses, and glutathione peroxidase activity of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2019, 45, 793–804. [Google Scholar] [PubMed]
- Hilton, J.W.; Hodson, P.V.; Slinger, S.J. The requirement and toxicity of selenium in rainbow trout (Salmo gairdneri). J. Nutr. 1980, 110, 2527–2535. [Google Scholar] [CrossRef] [PubMed]
- Rider, S.A.; Davies, S.J.; Jha, A.N.; Fisher, A.A.; Knight, J.; Sweetman, J.W. Supra-nutritional dietary intake of selenite and selenium yeast in normal and stressed rainbow trout (Oncorhynchus mykiss): Implications on selenium status and health responses. Aquaculture 2009, 295, 282–291. [Google Scholar] [CrossRef]
- Ytrestøyl, T.; Aas, T.S.; Åsgård, T. Utilisation of feed resources in production of Atlantic salmon (Salmo salar) in Norway. Aquaculture 2015, 448, 365–374. [Google Scholar] [CrossRef]
- Sanden, M.; Hemre, G.-I.; Måge, A.; Lunestad, B.T.; Espe, M.; Lundebye, A.-K.; Ørnsrud, R. Program for Overvåking av Fiskefôr. Nasjonalt Institutt for Ernærings-og Sjømatforskning (NIFES). 2013. Available online: https://www.mattilsynet.no/dyr_og_dyrehold/for/overvaakingsprogram_fiskefor_2013.16731/binary/Overvåkingsprogram%20fiskefôr%202013 (accessed on 20 September 2021).
- Sanden, M.; Hemre, G.-I.; Måge, A.; Lunestad, B.T.; Espe, M.; Lie, K.K.; Lundebye, A.-K.; Amlund, H.; Waagbø, R.; Ørnsrud, R. Program for Overvåking av Fiskefôr. Nasjonalt Institutt for Ernærings-og Sjømatforskning (NIFES). 2017. Available online: https://www.hi.no/resources/publikasjoner/rapporter-nifes/2017/forrapport2017.pdf (accessed on 20 September 2021).
- Betancor, M.B.; Dam, T.M.C.; Walton, J.; Morken, T.; Campbell, P.J.; Tocher, D.R. Modulation of selenium tissue distribution and selenoprotein expression in Atlantic salmon (Salmo salar L.) fed diets with graded levels of plant ingredients. Br. J. Nutr. 2016, 115, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Marta, S.; Silva, M.S.; Kröckel, S.; Prabhu, P.A.J.; Koppe, W.; Ørnsrud, R.; Waagbø, R.; Araujo, P.; Amlund, H. Apparent availability of zinc, selenium and manganese as inorganic metal salts or organic forms in plant-based diets for Atlantic salmon (Salmo salar). Aquaculture 2019, 503, 562–570. [Google Scholar]
- Committee on the Nutrient Requirements of Fish and Shrimp & National Research Council (NRC). Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Pacitti, D.; Wang, T.; Page, M.M.; Martin, S.A.M.; Sweetman, J.; Feldmann, J.; Secombes, C.J. Characterization of cytosolic glutathione phospholipid-hydroperoxide peroxidase and glutathione peroxidase genes in rainbow trout (Oncorhynchus mykiss) and their modulation by in vitro selenium exposure. Aquat. Toxicol. 2013, 130–131, 97–111. [Google Scholar] [CrossRef]
- Fontagné-Dicharry, S.; Godin, S.; Liu, H.; Prabhu, P.A.J.; Bouyssière, B.; Bueno, M.; Tacon, P.; Médale, F.; Kaushik, S. Influence of the forms and levels of dietary selenium on antioxidant status and oxidative stress-related parameters in rainbow trout (Oncorhynchus mykiss) fry. Br. J. Nutr. 2015, 113, 1876–1887. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Cholewinska, E.; Ognik, K.; Fotschki, B.; Zdunczyk, Z.; Juskiewicz, J. Comparison of the effect of dietary copper nanoparticles and one copper (II) salt on the copper biodistribution and gastrointestinal and hepatic morphology and function in a rat model. PLoS ONE 2018, 13, e0197083. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Elghandour, M.M.; Barbabosa-Pliego, A.; Monroy, J.C.; Mellado, M.; Salem, A.Z. Nanoparticles in Equine Nutrition: Mechanism of Action and Application as Feed Additives. J. Equine Vet. Sci. 2019, 78, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wong, Y.S.; Zheng, W.; Bai, Y.; Huang, L. Selenium nanoparticles fabricated in Undaria pinnatifida polysaccharide solutions induce mitochondria-mediated apoptosis in A375 human melanoma cells. Colloids Surf. B Biointerfaces 2008, 67, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Keyvanshokooh, S.; Salati, A.P.; Ghaedi, A. Proteomic analysis of liver tissue from rainbow trout (Oncorhynchus mykiss) under high rearing density after administration of dietary vitamin E and selenium nanoparticles. Comp. Biochem. Physiol. Part D Genom. Proteom. 2017, 22, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Dobias, J.; Suvorova, E.I.; Bernier-Latmani, R. Role of proteins in controlling selenium nanoparticle size. Nanotechnology 2011, 22, 195605. [Google Scholar] [CrossRef] [PubMed]
- Arshad, A. Bacterial Synthesis and Applications of Nanoparticles. Nano Sci. Nano Technol. 2017, 11, 119. [Google Scholar]
- Subbiah, R.; Veerapandian, M.; Yun, K.S. Nanoparticles: Functionalization and Multifunctional Applications in Biomedical Sciences. Curr. Med. Chem. 2010, 17, 4559–4577. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.K.; Campos, V.L.; León, C.G.; Rodríguez-Llamazares, S.M.; Rojas, S.M.; González, M.; Smith, C.T.; Mondaca, M.A. Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. J. Nanoparticle Res. 2012, 14, 1236. [Google Scholar] [CrossRef]
- Tarrahi, R.; Khataee, A.; Movafeghi, A.; Rezanejad, F.; Gohari, G. Toxicological implications of selenium nanoparticles with different coatings along with Se4+ on Lemna minor. Chemosphere 2017, 181, 655–665. [Google Scholar] [CrossRef]
- Dhanjal, S.; Cameotra, S.S. Aerobic biogenesis of selenium nanospheres by Bacillus cereus isolated from coalmine soil. Microb. Cell Factories 2010, 9, 52. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bensaci, C.; Ghiaba, Z.; Dakmouche, M.; Belfar, A.; Belguidoum, M.; Bentebba, F.Z.; Saidi, M.; Hadjadj, M. In Vitro Evaluation of Antioxidant Potential of Date Palm Collected in Algeria using Electrochemical and Spectrophotometrical Techniques. Korean Chem. Eng. Res. 2021, 59, 153–158. [Google Scholar]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Romay, C.; Pascual, C.E.L.; Lissi, E.A. The reaction between ABTS radical cation and antioxidants and its use to evaluate the antioxidant status of serum samples. Braz. J. Med. Biol. Res. 1996, 29, 175–183. [Google Scholar] [PubMed]
- Abarca, A.; Bethke, J.; Narváez, E.; Flores, R.; Mercado, L. Parameters to evaluate the immunostimulant effect of Zymosan A in head kidney leucocytes (HKL) of salmonids. Lat. Am. J. Aquat. Res. 2012, 40, 545–552. [Google Scholar] [CrossRef]
- Singh, S.P.; Rastogi, R.P.; Hader, D.P.; Sinha, R.P. Temporal dynamics of ROS biogenesis under simulated solar radiation in the cyanobacterium Anabaena variabilis PCC 7937. Protoplasma 2014, 251, 1223–1230. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Kling, P.; Olsson, P.-E. Involvement of differential metallothionein expression in free radical sensitivity of RTG-2 and CHSE-214 Cells. Free Radic. Biol. Med. 2000, 28, 1628–1637. [Google Scholar] [CrossRef]
- Chen, L.-D.; Liu, Z.-H.; Zhang, L.-F.; Yao, J.-N.; Wa, C.-F. Sanggenon C induces apoptosis of colon cancer cells via inhibition of NO production, iNOS expression and ROS activation of the mitochondrial pathway. Oncol. Rep. 2017, 38, 2123–2131. [Google Scholar] [CrossRef]
- Valenzuela, A.; Campos, V.; Yañez, F.; Alveal, K.; Gutiérrez, P.; Rivas, M.; Contreras, N.; Klempau, A.; Fernandez, I.; Oyarzun, C. Application of artificial photoperiod in fish: A factor that increases susceptibility to infectious diseases? Fish Physiol. Biochem. 2012, 38, 943–950. [Google Scholar] [CrossRef]
- Vera, B. Bio-Obtención de Nanopartículas de Selenio y su Potencial Aplicación Como Suplemento Alimentario Inmunoestimulante en Trucha Arcoíris (Oncorhynchus mykiss). Bachelor’s Thesis, University of Concepcion, Concepción, Chile, March 2016. [Google Scholar]
- Takemura, A.; Takano, K. Lysozyme in the ovary of tilapia (Oreochromis mossambicus): Its purification and some biological properties. Fish Physiol. Biochem. 1995, 14, 415–421. [Google Scholar] [CrossRef]
- Anderson, D.; Siwicki, A. Measuring the effects of contaminants on fish by haematological and serological methods. In Modulators of Fish Immune Responses; Stolen, J., Anderson, D., Zelikoff, S., Twerdok, L., Kaattari, S., Eds.; SOS Publications: Fair Haven, NJ, USA, 1993; pp. 95–118. [Google Scholar]
- Lawrence, R.; Burk, R. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Lugert, V.; Thaller, G.; Tetens, J.; Schulz, C.; Krieter, J. A review on fish growth calculation: Multiple functions in fish production and their specific application. Rev. Aquac. 2014, 8, 30–42. [Google Scholar] [CrossRef]
- Mansour, A.T.-E.; Goda, A.A.; Omar, E.A.; SaberKhalil, H.; Esteban, M.-A. Dietary supplementation of organic selenium improves growth, survival, antioxidantand immune status of meagre, Argyrosomus regius, juveniles. Fish Shellfish Immunol. 2017, 68, 516–524. [Google Scholar] [CrossRef]
- Khan, K.U.; Zuberi, A.; Nazir, S.; Fernandes, J.B.K.; Jamil, Z.; Sarwar, H. Effects of dietary selenium nanoparticles on physiological and biochemical aspects of juvenile Tor putitora. Turk. J. Zool. 2016, 40, 704–712. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Parmeggiani, B.; Leipnitz, G.; Verdi, C.M.; Santos, R.V.; Stefani, L.M.; Baldisserotto, B. The disturbance of antioxidant/oxidant balance in fish experimentally infected by Aeromonas caviae: Relationship with disease pathophysiology. Microb. Pathog. 2018, 122, 53–57. [Google Scholar] [CrossRef]
- Farzad, R.; Kuhn, D.; Smith, S.; O’Keefe, S.; Hines, I.; Bushman, T.; Galagarza, O.; Stevens, A. Effects of selenium-enriched prebiotic on the growth performance, innate immune response, oxidative enzyme activity and microbiome of rainbow trout (Oncorhynchus mykiss). Aquaculture 2021, 735980. [Google Scholar] [CrossRef]
- Saffari, S.; Saeed Keyvanshokooh, S.; Zakeri, M.; Johari, S.A.; Pasha-Zanoosi, H.; Mozanzadeh, M.T. Effects of dietary organic, inorganic, and nanoparticulate selenium sources on growth, hemato-immunological, and serum biochemical parameters of common carp (Cyprinus carpio). Fish Physiol. Biochem. 2018, 44, 1087–1097. [Google Scholar] [CrossRef]
- Daza, C.; Campos, V.; Rojas, C.; Rodríguez-Llamazares, S.; Smith, C.; Mondaca, M. Reduction of selenite to elemental Selenium by Pantoea agglomerans. Gayana 2016, 80, 67–74. [Google Scholar] [CrossRef][Green Version]
- Prasanth, S.; Sudarsanakumar, C. Elucidating the interaction of L-cysteine-capped selenium nanoparticles and human serum albumin: Spectroscopic and thermodynamic analysis. New J. Chem. 2017, 41, 9521–9530. [Google Scholar] [CrossRef]
- Perni, S.; Hakala, V.; Prokopovich, P. Biogenic synthesis of antimicrobial silver nanoparticles capped with L-cysteine. Colloids Surf. A 2013, 460, 219–224. [Google Scholar] [CrossRef]
- Prasanth, S.; Rithesh Raj, D.; Vineeshkumar, T.V.; Thomas, R.K.; Sudarsanakumar, C. Exploring the interaction of l-cysteine capped CuS nanoparticles with bovine serum albumin (BSA): A spectroscopic study. RSC Adv. 2016, 6, 58288–58295. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Ding, Y.; Cai, X.; Gu, S.; Cao, Z. Application of l-cysteine capped core–shell CdTe/ZnS nanoparticles as a fluorescence probe for cephalexin. Anal. Methods 2014, 6, 2715–2721. [Google Scholar] [CrossRef]
- Wojnicki, M.; Luty-Błocho, M.; Kotańska, M.; Wytrwal, M.; Tokarski, T.; Krupa, A.; Kołaczkowski, M.; Bucki, A.; Kobielusz, M. Novel and effective synthesis protocol of AgNPs functionalized using L-cysteine as a potential drug carrier. Naunyn-Schmiedebergs Arch. Pharmacol. 2017, 391, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Majzik, A.; Patakfalvi, R.; Hornok, V.; Dékány, I. Growing and stability of gold nanoparticles and their functionalization by cysteine. Gold Bull. 2009, 42, 113–123. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Sun, Y. Size-controlled green synthesis of silver nanoparticles assisted by L-cysteine. Front. Chem. Sci. Eng. 2015, 9, 494–500. [Google Scholar] [CrossRef]
- Chatterjee, A.; Priyam, A.; Das, S.K.; Saha, A. Size tunable synthesis of cysteine-capped CdS nanoparticles by γ-irradiation. J. Colloid Interface Sci. 2006, 294, 334–342. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, J.; Hou, J.; Chen, C. Free radical scavenging efficiency of nano-se in vitro. Free Radic. Biol. Med. 2003, 35, 805–813. [Google Scholar] [CrossRef]
- Matsuura, S.; Katsumi, H.; Suzuki, H.; Hirai, N.; Takashima, R.; Morishita, M.; Sakane, T.; Yamamoto, A. l-Cysteine and l-Serine Modified Dendrimer with Multiple Reduced Thiols as a Kidney-Targeting Reactive Oxygen Species Scavenger to Prevent Renal Ischemia/Reperfusion Injury. Pharmaceutics 2018, 10, 251. [Google Scholar] [CrossRef]
- Bai, K.; Hong, B.; Huang, W.; He, J. Selenium-Nanoparticles-Loaded Chitosan/Chitooligosaccharide Microparticles and Their Antioxidant Potential: A Chemical and in vivo Investigation. Pharmaceutics 2020, 12, 43. [Google Scholar] [CrossRef]
- Forootanfar, H.; Adeli-Sardou, M.; Nikkhoo, M.; Mehrabani, M.; Amir-Heidari, B.; Shahverdi, A.R.; Shakibaie, M. Antioxidant and cytotoxic effect of biologically synthesized selenium nanoparticles in comparison to selenium dioxide. J. Trace Elem. Med. Biol. 2014, 28, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Qiao, L.; Guo, Y.; Ma, L.; Cheng, Y. Preparation, characteristics and antioxidant activity of polysaccharides and proteins-capped selenium nanoparticles synthesized by Lactobacillus casei ATCC 393. Carbohydr. Polym. 2018, 195, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Bettaib, J.; Talarmin, H.; Kalai, F.Z.; Giroux-Metges, M.-A.; Ksouri, R. Limoniastrum guyonianum prevents H2O2-induced oxidative damage in IEC-6 cells by enhancing enzyamtic defense, reducing glutathione depletion and JNK phosphorylation. Biomed. Pharmacother. 2017, 95, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Mou, K.; Pan, W.; Han, D.; Wen, X.; Cao, F.; Miao, Y.; Li, P. Glycyrrhizin protects human melanocytes from H2O2-induced oxidative damage via the Nrf2-dependent induction of HO-1. Int. J. Mol. Med. 2019, 44, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.E.; Sutherland, G.E.; Lavado, R. Xenobiotic metabolism in the fish hepatic cell lines Hepa-E1 and RTH-149, and the gill cell lines RTgill-W1 and G1B: Biomarkers of CYP450 activity and oxidative stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 206–207, 32–40. [Google Scholar] [CrossRef]
- Bopp, S.K.; Abicht, H.K.; Knauer, K. Copper-induced oxidative stress in rainbow trout gill cells. Aquat. Toxicol. 2008, 86, 197–204. [Google Scholar] [CrossRef]
- Uçar, A.; Parlak, V.; Özgeriş, F.B.; Yeltekin, A.Ç.; Alak, G.; Atamanalp, M. Determination of Fipronil toxicity by different biomarkers in gill and liver tissue of rainbow trout (Oncorhynchus mykiss). Vitr. Cell. Dev. Biol.–Anim. 2020, 56, 543–549. [Google Scholar] [CrossRef]
- Banaee, M. Chapter 4. Physiological Dysfunction in Fish after Insecticides Exposure. In Insecticides Development of Safer and More Effective Technologies, 1st ed.; Trdan, S., Ed.; IntechOpen: London, UK, 2013; pp. 103–142. ISBN 978-953-51-5348-1. [Google Scholar]
- Tkachenko, H.; Kurhaluk, N.; Grudniewska, J.; Andriichuk, A. Tissue-specific responses of oxidative stress biomarkers and antioxidant defenses in rainbow trout Oncorhynchus mykiss during a vaccination against furunculosis. Fish Physiol. Biochem. 2014, 40, 1289–1300. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Zommara, M.; Eweedah, N.M.; Helal, A.I.; Aboel-Darag, M.A. The potential role of nano-selenium and vitamin C on the performances of Nile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. 2020, 27, 9843–9852. [Google Scholar] [CrossRef]
- Harsij, M.; Kanani, H.G.; Adineh, H. Effects of antioxidant supplementation (nano-selenium, vitamin C and E) on growth performance, blood biochemistry, immune status and body composition of rainbow trout (Oncorhynchus mykiss) under sub-lethal ammonia exposure. Aquaculture 2020, 521, 734942. [Google Scholar] [CrossRef]
- Neamat-Allah, A.N.F.; Mahmoud, E.A.; Abd El Hakim, Y. Efficacy of dietary Nano-selenium on growth, immune response, antioxidant, transcriptomic profile and resistance of Nile tilapia, Oreochromis niloticus against Streptococcus iniae infection. Fish Shellfish Immunol. 2019, 94, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Dalmo, R.A.; Ingebrigtsen, K.; Bøgwald, J. Non-specific defence mechanisms in fish, with particular reference to the reticuloendothelial system (RES). J. Fish Dis. 1997, 20, 241–273. [Google Scholar] [CrossRef]
- Rodríguez, A.; Esteban, M.Á.; Meseguer, J. Phagocytosis and peroxidase release by seabream (Sparus aurata L.) leucocytes in response to yeast cells. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2003, 272A, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Zommara, M.; Eweedah, N.M.; Helal, A.I. Synergistic Effects of Selenium Nanoparticles and Vitamin E on Growth, Immune-Related Gene Expression, and Regulation of Antioxidant Status of Nile Tilapia (Oreochromis niloticus). Biol. Trace Elem. Res. 2019, 195, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Xia, I.F.; Cheung, J.S.; Wu, M.; Wong, K.-S.; Kong, H.; Zheng, X.; Ka-Hing Wong, K.-H.; Kwok, K.W. Dietary chitosan-selenium nanoparticle (CTS-SeNP) enhance immunity and disease resistance in zebrafish. Fish Shellfish Immunol. 2019, 87, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Longbaf Dezfouli, M.; Ghaedtaheri, A.; Keyvanshokooh, S.; Salati, A.P.; Mousavi, S.M.; Pasha-Zanoosi, H. Combined or individual effects of dietary magnesium and selenium nanoparticles on growth performance, immunity, blood biochemistry and antioxidant status of Asian seabass (Lates calcarifer) reared in freshwater. Aquac. Nutr. 2019, 25, 1422–1430. [Google Scholar] [CrossRef]
- Sattin, G.; Bakiu, R.; Tolomeo, A.M.; Carraro, A.; Coppola, D.; Ferro, D.; Patarnello, T.; Santovito, G. Characterization and expression of a new cytoplasmic glutathione peroxidase 1 gene in the Antarctic fish Trematomus bernacchii. Hydrobiologia 2015, 761, 363–372. [Google Scholar] [CrossRef]
- Tollerz Bratteby, U. Factors Explaining Variation in the Fecundity of Female Baltic Salmon (Salmo salar)–The Influence of Length, Body Condition and Growth Rate at Sea. Master’s Thesis, Swedish University of Agricultural Sciences, Öregrund, Sweden, 2019. [Google Scholar]
- Rautela, A.; Rani, J.; Das, M.D. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: Characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Fang, X.; Wang, Y.; Wang, Z.; Jiang, Z.; Dong, M. Microorganism Assisted Synthesized Nanoparticles for Catalytic Applications. Energies 2019, 12, 190. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).