Pulsed Electro-Magnetic Field (PEMF) Effect on Bone Healing in Animal Models: A Review of Its Efficacy Related to Different Type of Damage
Abstract
Simple Summary
Abstract
1. Introduction
- Bone adapts its shape according to the applied load; this principle is known as Wolff’s law, from the name of the German doctor Julius Wolff who, at the end of the 1800s, described how bone tissue can respond to mechanical load [9].
- In case of fracture, a lesion current can be recorded at the fracture site and the whole biopotential distribution of that bone becomes more negative [2].
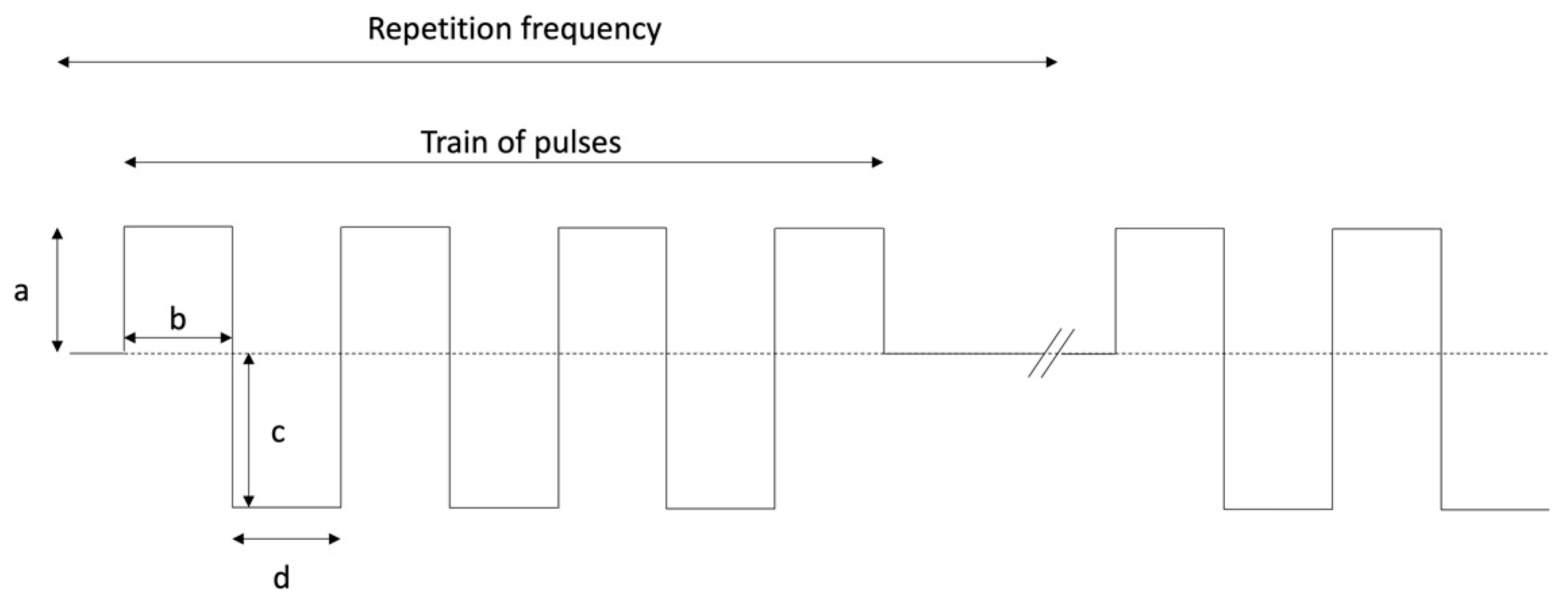
2. PEMF Signal Characteristics
3. Animal Models
4. The Dawn of Electro-Stimulation in Bone Healing and the Effects of Different Signal Characteristics
- ○
- Duration of the signal: 5 ms;
- ○
- Positive width: 250 µs;
- ○
- Positive amplitude: 17 mV;
- ○
- Negative width: 33 μs;
- ○
- Negative amplitude: 150 mV;
- ○
- Repetition frequency: 5 Hz.
5. Main Results in Different Models
5.1. Direct Trauma Models
5.2. Indirect Trauma Models: Metabolic Damage
5.3. Indirect Trauma Models: Disuse Damage
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalfas, I.H. Principles of bone healing. Neurosurg. Focus 2001, 10, 1–4. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Bigham-Sadegh, A.; Moshiri, A. Healing potentials of polymethylmethacrylate bone cement combined with platelet gel in the critical-sized radial bone defect of rats. PLoS ONE 2018, 13, e0194751. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Bahney, C.S.; Zondervan, R.L.; Allison, P.; Theologis, A.; Ashley, J.W.; Ahn, J.; Miclau, T.; Marcucio, R.S.; Hankenson, K.D. Cellular biology of fracture healing. J. Orthop. Res. 2019, 37, 35–50. [Google Scholar] [CrossRef]
- Anesi, A.; Di Bartolomeo, M.; Pellacani, A.; Ferretti, M.; Cavani, F.; Salvatori, R.; Nocini, R.; Palumbo, C.; Chiarini, L. Bone Healing Evaluation Following Different Osteotomic Techniques in Animal Models: A Suitable Method for Clinical Insights. Appl. Sci. 2020, 10, 7165. [Google Scholar] [CrossRef]
- Parenti, S.; Sandoni, L.; Montanari, M.; Zanocco-Marani, T.; Anesi, A.; Iotti, S.; Manfredini, R.; Frassineti, C.; Davalli, P.; Grande, A. Magnesium favors the capacity of vitamin D3 to induce the monocyte differentiation of U937 cells. Magnes. Res. 2021, 34, 114–129. [Google Scholar] [CrossRef]
- Anesi, A.; Generali, L.; Sandoni, L.; Pozzi, S.; Grande, A. From Osteoclast Differentiation to Osteonecrosis of the Jaw: Molecular and Clinical Insights. Int. J. Mol. Sci. 2019, 20, 4925. [Google Scholar] [CrossRef]
- Fukada, E.; Yasuda, I. On the Piezoelectric Effect of Bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Wolff, J. Das Gesetz der Transformation der Knochen; Schattauer: Stuttgart, Germany, 1991; ISBN 9783794514519. [Google Scholar]
- Friedenberg, Z.B.; Brighton, C.T. Bioelectric Potentials in Bone. J. Bone Jt. Surg. 1966, 48, 915–923. [Google Scholar] [CrossRef]
- Rubinacci, A.; Tessari, L. A correlation analysis between bone formation rate and bioelectric potentials in rabbit tibia. Calcif. Tissue Int. 1983, 35, 728–731. [Google Scholar] [CrossRef]
- Gross, D.; Williams, W.S. Streaming potential and the electromechanical response of physiologically-moist bone. J. Biomech. 1982, 15, 277–295. [Google Scholar] [CrossRef]
- Pienkowski, D.; Pollack, S.R. The origin of stress-generated potentials in fluid-saturated bone. J. Orthop. Res. 1983, 1, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.A.; Pawluk, R.J.; Becker, R.O. Effects of electric currents on bone in vivo. Nature 1964, 204, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Norton, L.A. In vivo bone growth in a controlled electric field. Ann. N. Y. Acad. Sci. 1974, 238, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.A.; Pawluk, R.J. Noninvasive methods for stimulating osteogenesis. J. Biomed. Mater. Res. 1975, 9, 371–374. [Google Scholar] [CrossRef]
- Brighton, C.T.; Hozack, W.J.; Brager, M.D.; Windsor, R.E.; Pollack, S.R.; Vreslovic, E.J.; Kotwick, J.E. Fracture healing in the rabbit fibula when subjected to various capacitively coupled electrical fields. J. Orthop. Res. 1985, 3, 331–340. [Google Scholar] [CrossRef]
- Pilla, A.A.; Mont, M.A.; Nasser, P.R.; Khan, S.A.; Figueiredo, M.; Kaufman, J.J.; Siffert, R.S. Non-invasive low-intensity pulsed ultrasound accelerates bone healing in the rabbit. J. Orthop. Trauma 1990, 4, 246–253. [Google Scholar] [CrossRef]
- Tsai, C.L.; Chang, W.H.; Liu, T.K. Preliminary studies of duration and intensity of ultrasonic treatments on fracture repair. Chin. J. Physiol. 1992, 35, 21–26. [Google Scholar]
- Food and Drug Administration Premarket Approval (PMA) of SONIC ACCELERATED FRACTURE HEALING SYSTEM MODEL 2A. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P900009 (accessed on 17 February 2022).
- TRUST Investigators writing group; Busse, J.W.; Bhandari, M.; Einhorn, T.A.; Schemitsch, E.; Heckman, J.D.; Tornetta, P.; Leung, K.-S.; Heels-Ansdell, D.; Makosso-Kallyth, S.; et al. Re-evaluation of low intensity pulsed ultrasound in treatment of tibial fractures (TRUST): Randomized clinical trial. BMJ 2016, 355, i5351. [Google Scholar] [CrossRef][Green Version]
- Barnaba, S.; Papalia, R.; Ruzzini, L.; Sgambato, A.; Maffulli, N.; Denaro, V. Effect of pulsed electromagnetic fields on human osteoblast cultures. Physiother. Res. Int. 2013, 18, 109–114. [Google Scholar] [CrossRef]
- Ongaro, A.; Pellati, A.; Bagheri, L.; Fortini, C.; Setti, S.; De Mattei, M. Pulsed electromagnetic fields stimulate osteogenic differentiation in human bone marrow and adipose tissue derived mesenchymal stem cells. Bioelectromagnetics 2014, 35, 426–436. [Google Scholar] [CrossRef]
- Schwartz, Z.; Simon, B.J.; Duran, M.A.; Barabino, G.; Chaudhri, R.; Boyan, B.D. Pulsed electromagnetic fields enhance BMP-2 dependent osteoblastic differentiation of human mesenchymal stem cells. J. Orthop. Res. 2008, 26, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-Y.; Hsieh, D.-K.; Yu, T.-C.; Chiu, H.-T.; Lu, S.-F.; Luo, G.-H.; Kuo, T.K.; Lee, O.K.; Chiou, T.-W. Effect of pulsed electromagnetic field on the proliferation and differentiation potential of human bone marrow mesenchymal stem cells. Bioelectromagnetics 2009, 30, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Bassett, C.A.; Pawluk, R.J.; Pilla, A.A. Augmentation of bone repair by inductively coupled electromagnetic fields. Science 1974, 184, 575–577. [Google Scholar] [CrossRef]
- Bassett, C.A.; Valdes, M.G.; Hernandez, E. Modification of fracture repair with selected pulsing electromagnetic fields. JBJS J. Bone Jt. Surg. 1982, 64, 888–895. [Google Scholar] [CrossRef]
- De Haas, W.G.; Lazarovici, M.A.; Morrison, D.M. The effect of low frequency magnetic fields on the healing of the osteotomized rabbit radius. Clin. Orthop. Relat. Res. 1979, 245–251. [Google Scholar] [CrossRef]
- Pienkowski, D.; Pollack, S.R.; Brighton, C.T.; Griffith, N.J. Comparison of asymmetrical and symmetrical pulse waveforms in electromagnetic stimulation. J. Orthop. Res. 1992, 10, 247–255. [Google Scholar] [CrossRef]
- Pienkowski, D.; Pollack, S.R.; Brighton, C.T.; Griffith, N.J. Low-power electromagnetic stimulation of osteotomized rabbit fibulae. A randomized, blinded study. J. Bone Jt. Surg. Am. 1994, 76, 489–501. [Google Scholar] [CrossRef]
- Cadossi, R.; Setti, S.; Cadossi, M.; Massari, L. Biophysical stimulation of bone growth in fractures. In Pulsed Electromagnetic Fields for Clinical Applications; Markov, M.S., Ryaby, J.T., Waldorff, E.I., Eds.; CRC Press: Boca Raton, FL, USA, 2020; p. 280. ISBN 9781003001959. [Google Scholar]
- Parry, J.H. Helmholtz Coils and Coil Design. Dev. Solid Earth Geophys. 2013, 3, 551–567. [Google Scholar] [CrossRef]
- Massari, L.; Benazzo, F.; Moretti, B.; Dallari, D.; Perugia, D.; Meani, E.; Cadossi, R. Stimolazione elettrica dell’osteogenesi: Efficacia e tecnologie a confronto. Electrical stimulation of osteogenesis: Efficacy and technologies compared. GIOT Giomale Ital. Ortop. Traumatol. 2011, 37, 272–279. [Google Scholar]
- Anesi, A.; Ferretti, M.; Cavani, F.; Salvatori, R.; Bianchi, M.; Russo, A.; Chiarini, L.; Palumbo, C. Structural and ultrastructural analyses of bone regeneration in rabbit cranial osteotomy: Piezosurgery versus traditional osteotomes. J. Craniomaxillofac. Surg. 2018, 46, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, S.; Zhu, S.; Gao, C.; He, C. Efficacy of Pulsed Electromagnetic Fields on Experimental Osteopenia in Rodents: A Systematic Review. Bioelectromagnetics 2021, 42, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Nunamaker, D.M. Experimental models of fracture repair. Clin. Orthop. Relat. Res. 1998, S56–S65. [Google Scholar] [CrossRef]
- Komori, T. Animal models for osteoporosis. Eur. J. Pharmacol. 2015, 759, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.; Magony, R.; Gortler, H.; Godbout, C.; Schemitsch, E.H.; Nauth, A. Systemically impaired fracture healing in small animal research: A review of fracture repair models. J. Orthop. Res. 2021, 39, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; He, H.; Wang, H.; Gao, C.; Wang, Q.; Wang, D.; Wei, Q.; Yu, X.; He, C. Ovariectomy induced bone loss is antagonized by pulsed electromagnetic fields (PEMFS) and TNF-α and IL-6 gene knockouts in a similar mechanism. Osteoarthr. Cartil. 2018, 26, S90–S91. [Google Scholar] [CrossRef]
- Lei, T.; Liang, Z.; Li, F.; Tang, C.; Xie, K.; Wang, P.; Dong, X.; Shan, S.; Jiang, M.; Xu, Q.; et al. Pulsed electromagnetic fields (PEMF) attenuate changes in vertebral bone mass, architecture and strength in ovariectomized mice. Bone 2018, 108, 10–19. [Google Scholar] [CrossRef]
- Cai, J.; Shao, X.; Yang, Q.; Yang, Y.; Yan, Z.; Luo, E.; Feng, X.; Jing, D. Pulsed electromagnetic fields modify the adverse effects of glucocorticoids on bone architecture, bone strength and porous implant osseointegration by rescuing bone-anabolic actions. Bone 2020, 133, 115266. [Google Scholar] [CrossRef] [PubMed]
- Brent, M.B.; Brüel, A.; Thomsen, J.S. A Systematic Review of Animal Models of Disuse-Induced Bone Loss. Calcif. Tissue Int. 2021, 108, 561–575. [Google Scholar] [CrossRef]
- Caliogna, L.; Medetti, M.; Bina, V.; Brancato, A.M.; Castelli, A.; Jannelli, E.; Ivone, A.; Gastaldi, G.; Annunziata, S.; Mosconi, M.; et al. Pulsed Electromagnetic Fields in Bone Healing: Molecular Pathways and Clinical Applications. Int. J. Mol. Sci. 2021, 22, 7403. [Google Scholar] [CrossRef]
- Canè, V.; Botti, P.; Remaggi, F.; Marotti, G. Differences in the rate of closure of standard holes made in different regions of long bones. Calcif. Tissue Int. 1987, 41, 67. [Google Scholar]
- Canè, V.; Botti, P.; Soana, S. Pulsed magnetic fields improve osteoblast activity during the repair of an experimental osseous defect. J. Orthop. Res. 1993, 11, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Canè, V.; Zaffe, D.; Cavani, F.; Botti, P.; Soana, S. Pulsed Electromagnetic Fields Modulate Enzymatic Actmty During the Early Stages of Bone Repair. Electro Magn. 1997, 16, 143–152. [Google Scholar] [CrossRef]
- Yang, H.J.; Kim, R.Y.; Hwang, S.J. Pulsed Electromagnetic Fields Enhance Bone Morphogenetic Protein-2 Dependent-Bone Regeneration. Tissue Eng. A 2015, 21, 2629–2637. [Google Scholar] [CrossRef]
- Midura, R.J.; Ibiwoye, M.O.; Powell, K.A.; Sakai, Y.; Doehring, T.; Grabiner, M.D.; Patterson, T.E.; Zborowski, M.; Wolfman, A. Pulsed electromagnetic field treatments enhance the healing of fibular osteotomies. J. Orthop. Res. 2005, 23, 1035–1046. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, L.; Jiang, L.; Li, H. Therapeutic effect of pulsed electromagnetic field on bone wound healing in rats. Electromagn. Biol. Med. 2021, 40, 26–32. [Google Scholar] [CrossRef]
- Yonemori, K.; Matsunaga, S.; Ishidou, Y.; Maeda, S.; Yoshida, H. Early effects of electrical stimulation on osteogenesis. Bone 1996, 19, 173–180. [Google Scholar] [CrossRef]
- Streit, A.; Watson, B.C.; Granata, J.D.; Philbin, T.M.; Lin, H.-N.; O’Connor, J.P.; Lin, S. Effect on Clinical Outcome and Growth Factor Synthesis With Adjunctive Use of Pulsed Electromagnetic Fields for Fifth Metatarsal Nonunion Fracture: A Double-Blind Randomized Study. Foot Ankle Int. 2016, 37, 919–923. [Google Scholar] [CrossRef]
- Al-Awar, A.; Kupai, K.; Veszelka, M.; Szűcs, G.; Attieh, Z.; Murlasits, Z.; Török, S.; Pósa, A.; Varga, C. Experimental Diabetes Mellitus in Different Animal Models. J. Diabetes Res. 2016, 2016, 9051426. [Google Scholar] [CrossRef]
- Rees, D.A.; Alcolado, J.C. Animal models of diabetes mellitus. Diabet. Med. 2005, 22, 359–370. [Google Scholar] [CrossRef]
- Murray, C.E.; Coleman, C.M. Impact of Diabetes Mellitus on Bone Health. Int. J. Mol. Sci. 2019, 20, 4873. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and Its Effect on Bone and Fracture Healing. Curr. Osteoporos. Rep. 2015, 13, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Cortet, B. Bone repair in osteoporotic bone: Postmenopausal and cortisone-induced osteoporosis. Osteoporos. Int. 2011, 22, 2007–2010. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.H.; Miclau, T.; Chow, S.K.-H.; Yang, F.F.; Alt, V. Fracture healing in osteoporotic bone. Injury 2016, 47 (Suppl. 2), S21–S26. [Google Scholar] [CrossRef]
- Jackson, R.D.; Mysiw, W.J. Insights into the epidemiology of postmenopausal osteoporosis: The Women’s Health Initiative. Semin. Reprod. Med. 2014, 32, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Fortier, M.; Menopause and Osteoporosis Working Group. Osteoporosis in menopause. J. Obstet. Gynaecol. Can. 2014, 36, 839–840. [Google Scholar] [CrossRef]
- Jing, D.; Li, F.; Jiang, M.; Cai, J.; Wu, Y.; Xie, K.; Wu, X.; Tang, C.; Liu, J.; Guo, W.; et al. Pulsed electromagnetic fields improve bone microstructure and strength in ovariectomized rats through a Wnt/Lrp5/β-catenin signaling-associated mechanism. PLoS ONE 2013, 8, e79377. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, S.; Guo, H.; Xia, L.; Liu, H.; Qin, Y.; He, C. Pulsed electromagnetic field stimulates osteoprotegerin and reduces RANKL expression in ovariectomized rats. Rheumatol. Int. 2013, 33, 1135–1141. [Google Scholar] [CrossRef]
- Zhou, J.; He, H.; Yang, L.; Chen, S.; Guo, H.; Xia, L.; Liu, H.; Qin, Y.; Liu, C.; Wei, X.; et al. Effects of pulsed electromagnetic fields on bone mass and Wnt/β-catenin signaling pathway in ovariectomized rats. Arch. Med. Res. 2012, 43, 274–282. [Google Scholar] [CrossRef]
- Chang, K.; Chang, W.H.-S. Pulsed electromagnetic fields prevent osteoporosis in an ovariectomized female rat model: A prostaglandin E2-associated process. Bioelectromagnetics 2003, 24, 189–198. [Google Scholar] [CrossRef]
- Sert, C.; Mustafa, D.; Düz, M.Z.; Akşen, F.; Kaya, A. The preventive effect on bone loss of 50-Hz, 1-mT electromagnetic field in ovariectomized rats. J. Bone Miner. Metab. 2002, 20, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Van der Jagt, O.P.; van der Linden, J.C.; Waarsing, J.H.; Verhaar, J.A.N.; Weinans, H. Electromagnetic fields do not affect bone micro-architecture in osteoporotic rats. Bone Jt. Res. 2014, 3, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Mishima, S. The effect of long-term pulsing electromagnetic field stimulation on experimental osteoporosis of rats. J. UOEH 1988, 10, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, L.-Q.; Xia, Q.-J.; He, C.-Q. Effects of pulsed electromagnetic fields on the mRNA expression of CAII and RANK in ovariectomized rats. Rheumatol. Int. 2012, 32, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Camargo, W.A.; de Vries, R.; van Luijk, J.; Hoekstra, J.W.; Bronkhorst, E.M.; Jansen, J.A.; van den Beucken, J.J.J.P. Diabetes Mellitus and Bone Regeneration: A Systematic Review and Meta-Analysis of Animal Studies. Tissue Eng. B. Rev. 2017, 23, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Cai, J.; Shen, G.; Huang, J.; Li, F.; Li, J.; Lu, L.; Luo, E.; Xu, Q. The preventive effects of pulsed electromagnetic fields on diabetic bone loss in streptozotocin-treated rats. Osteoporos. Int. 2011, 22, 1885–1895. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, Z.; Zhao, Y.; Jing, D.; Tang, C.; Ding, Y.; Feng, X. Effects of low-intensity pulsed electromagnetic fields on bone microarchitecture, mechanical strength and bone turnover in type 2 diabetic db/db mice. Sci. Rep. 2017, 7, 10834. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Liao, Y.; Feng, W.; Fu, C.; Guo, X. Pulsed electromagnetic fields inhibit bone loss in streptozotocin-induced diabetic rats. Endocrine 2015, 49, 258–266. [Google Scholar] [CrossRef]
- Liu, Y.-Z.; Akhter, M.P.; Gao, X.; Wang, X.-Y.; Wang, X.-B.; Zhao, G.; Wei, X.; Wu, H.-J.; Chen, H.; Wang, D.; et al. Glucocorticoid-induced delayed fracture healing and impaired bone biomechanical properties in mice. Clin. Interv. Aging 2018, 13, 1465–1474. [Google Scholar] [CrossRef]
- Hachemi, Y.; Rapp, A.E.; Picke, A.-K.; Weidinger, G.; Ignatius, A.; Tuckermann, J. Molecular mechanisms of glucocorticoids on skeleton and bone regeneration after fracture. J. Mol. Endocrinol. 2018, 61, R75–R90. [Google Scholar] [CrossRef]
- Ding, S.; Peng, H.; Fang, H.-S.; Zhou, J.-L.; Wang, Z. Pulsed electromagnetic fields stimulation prevents steroid-induced osteonecrosis in rats. BMC Musculoskelet. Disord. 2011, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gou, H.; Wang, S.; Zhu, J.; Tian, S.; Yu, L. Effect of Pulsed Electromagnetic Field on Bone Formation and Lipid Metabolism of Glucocorticoid-Induced Osteoporosis Rats through Canonical Wnt Signaling Pathway. Evid.-Based. Complement. Alternat. Med. 2016, 2016, 4927035. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-P.; Chen, S.; Peng, H.; Zhou, J.-L.; Fang, H.-S. Pulsed electromagnetic fields protect the balance between adipogenesis and osteogenesis on steroid-induced osteonecrosis of femoral head at the pre-collapse stage in rats. Bioelectromagnetics 2014, 35, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Skerry, T.M. The response of bone to mechanical loading and disuse: Fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch. Biochem. Biophys. 2008, 473, 117–123. [Google Scholar] [CrossRef]
- Yang, P.-F.; Nie, X.-T.; Wang, Z.; Al-Qudsy, L.H.H.; Ren, L.; Xu, H.-Y.; Rittweger, J.; Shang, P. Disuse Impairs the Mechanical Competence of Bone by Regulating the Characterizations of Mineralized Collagen Fibrils in Cortical Bone. Front. Physiol. 2019, 10, 775. [Google Scholar] [CrossRef]
- Dadwal, U.C.; Maupin, K.A.; Zamarioli, A.; Tucker, A.; Harris, J.S.; Fischer, J.P.; Rytlewski, J.D.; Scofield, D.C.; Wininger, A.E.; Bhatti, F.U.R.; et al. The effects of spaceflight and fracture healing on distant skeletal sites. Sci. Rep. 2019, 9, 11419. [Google Scholar] [CrossRef]
- Garg, P.; Strigini, M.; Peurière, L.; Vico, L.; Iandolo, D. The Skeletal Cellular and Molecular Underpinning of the Murine Hindlimb Unloading Model. Front. Physiol. 2021, 12, 749464. [Google Scholar] [CrossRef]
- Uebelhart, D.; Demiaux-Domenech, B.; Roth, M.; Chantraine, A. Bone metabolism in spinal cord injured individuals and in others who have prolonged immobilisation. A review. Paraplegia 1995, 33, 669–673. [Google Scholar] [CrossRef]
- Stein, T.P. Weight, muscle and bone loss during space flight: Another perspective. Eur. J. Appl. Physiol. 2013, 113, 2171–2181. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chang, Y.-T.; Lin, R.-W.; Chang, C.-W.; Wang, G.-J.; Lai, K.-A. Single pulsed electromagnetic field restores bone mass and microarchitecture in denervation/disuse osteopenic mice. Med. Eng. Phys. 2020, 80, 52–59. [Google Scholar] [CrossRef]
- Li, B.; Bi, J.; Li, W.; Huang, S.; Zhang, S.; Zhao, J.; Meng, Q.; Fei, J. Effects of pulsed electromagnetic fields on histomorphometry and osteocalcin in disuse osteoporosis rats. Technol. Health Care 2017, 25, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Cai, J.; Wu, Y.; Shen, G.; Li, F.; Xu, Q.; Xie, K.; Tang, C.; Liu, J.; Guo, W.; et al. Pulsed electromagnetic fields partially preserve bone mass, microarchitecture, and strength by promoting bone formation in hindlimb-suspended rats. J. Bone Miner. Res. 2014, 29, 2250–2261. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-W.; Zhao, J.-H. Pulsed electromagnetic fields stimulation affects BMD and local factor production of rats with disuse osteoporosis. Bioelectromagnetics 2010, 31, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Massari, L.; Benazzo, F.; Falez, F.; Perugia, D.; Pietrogrande, L.; Setti, S.; Osti, R.; Vaienti, E.; Ruosi, C.; Cadossi, R. Biophysical stimulation of bone and cartilage: State of the art and future perspectives. Int. Orthop. 2019, 43, 539–551. [Google Scholar] [CrossRef]
- Vadalà, M.; Morales-Medina, J.C.; Vallelunga, A.; Palmieri, B.; Laurino, C.; Iannitti, T. Mechanisms and therapeutic effectiveness of pulsed electromagnetic field therapy in oncology. Cancer Med. 2016, 5, 3128–3139. [Google Scholar] [CrossRef]
- Varani, K.; Vincenzi, F.; Pasquini, S.; Blo, I.; Salati, S.; Cadossi, M.; De Mattei, M. Pulsed Electromagnetic Field Stimulation in Osteogenesis and Chondrogenesis: Signaling Pathways and Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 809. [Google Scholar] [CrossRef]
- Daish, C.; Blanchard, R.; Fox, K.; Pivonka, P.; Pirogova, E. The Application of Pulsed Electromagnetic Fields (PEMFs) for Bone Fracture Repair: Past and Perspective Findings. Ann. Biomed. Eng. 2018, 46, 525–542. [Google Scholar] [CrossRef]
- Androjna, C.; Fort, B.; Zborowski, M.; Midura, R.J. Pulsed electromagnetic field treatment enhances healing callus biomechanical properties in an animal model of osteoporotic fracture. Bioelectromagnetics 2014, 35, 396–405. [Google Scholar] [CrossRef]
- Chalidis, B.; Sachinis, N.; Assiotis, A.; Maccauro, G. Stimulation of bone formation and fracture healing with pulsed electromagnetic fields: Biologic responses and clinical implications. Int. J. Immunopathol. Pharmacol. 2011, 24, 17–20. [Google Scholar] [CrossRef]
- GBD 2019 Fracture Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990-2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet. Heal. Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Hannemann, P.F.W.; Göttgens, K.W.A.; van Wely, B.J.; Kolkman, K.A.; Werre, A.J.; Poeze, M.; Brink, P.R.G. The clinical and radiological outcome of pulsed electromagnetic field treatment for acute scaphoid fractures: A randomised double-blind placebo-controlled multicentre trial. J. Bone Jt. Surg. Br. 2012, 94, 1403–1408. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martinez-Rondanelli, A.; Martinez, J.P.; Moncada, M.E.; Manzi, E.; Pinedo, C.R.; Cadavid, H. Electromagnetic stimulation as coadjuvant in the healing of diaphyseal femoral fractures: A randomized controlled trial. Colomb. Med. 2014, 45, 67–71. [Google Scholar] [CrossRef]
- Hannemann, P.F.W.; Mommers, E.H.H.; Schots, J.P.M.; Brink, P.R.G.; Poeze, M. The effects of low-intensity pulsed ultrasound and pulsed electromagnetic fields bone growth stimulation in acute fractures: A systematic review and meta-analysis of randomized controlled trials. Arch. Orthop. Trauma Surg. 2014, 134, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Assiotis, A.; Sachinis, N.P.; Chalidis, B.E. Pulsed electromagnetic fields for the treatment of tibial delayed unions and nonunions. A prospective clinical study and review of the literature. J. Orthop. Surg. Res. 2012, 7, 24. [Google Scholar] [CrossRef]
- Shi, H.; Xiong, J.; Chen, Y.; Wang, J.; Qiu, X.; Wang, Y.; Qiu, Y. Early application of pulsed electromagnetic field in the treatment of postoperative delayed union of long-bone fractures: A prospective randomized controlled study. BMC Musculoskelet. Disord. 2013, 14, 35. [Google Scholar] [CrossRef]
| Study Object | Abbreviation Used | Clinical Meaning |
|---|---|---|
| Soluble adenylyl cyclase (sAC), cyclic adenosine monophosphate (cAMP), protein kinase A (PKA), and cAMP response element-binding protein (CREB) signaling pathways | sAC/cAMP/PKA/CREB pathway | Pathway promotes bone formation |
| Wingless-related integration site pathway | Wnt pathway | Pathway promotes bone formation |
| LDL receptor related protein 5 | LRP5 | Enhances Wnt pathway activation |
| Dickkopf1 | DKK1 | Antagonize Wnt pathway activation |
| Sclerostin | Sost | |
| Alkaline phosphatase | ALP | Indirect evaluation of osteoblastic differentiation, proliferation and activity |
| Collagen type I alpha 1 chain | Col1a1 | |
| Osteocalcin | OCN | |
| Procollagen type 1 n-terminal propeptide | P1NP | Indirect evaluation of osteoclastic differentiation, proliferation and activity |
| Cathepsin K | CTSK | |
| Matrix metalloproteinase 9 | MMP9 | |
| Tartrate resistant acic phosphatase | TRAP | |
| CCAAT/enhancer-binding protein alpha | C/EBP-alpha | |
| Peroxisome proliferator-activated receptor gamma | PPAR-gamma | |
| Receptor activator of nuclear factor kappa-Β | RANK | |
| Receptor activator of nuclear factor kappa-Β ligand | RANKL | |
| TNF Receptor Associated Factor 6 | TRAF-6 | Antagonize osteoclastic differentiation and activity |
| Osteoprotegerin | OPG | |
| Bone morphogenetic protein-2 | BMP-2 | Enhance osteoblastic differentiation |
| Fibroblast growth factor | FGF | |
| recombinant human Bone Morphogenetic Protein-2 | rhBMP-2 | |
| Runt-related transcription factor 2 | Runx2 | |
| Transforming Growth Factor Beta 1 | TGF-beta 1 | |
| Placental Growth Factor | PlGF | Play a major role in angiogenesis and vasculogenesis, which are key to bone formation |
| Vascular endothelial growth factor | VEGF | |
| Angiopoietin-2 | Ang | |
| Brain-derived neurotrophic factor | BDNF | |
| Tunica interna endothelial cell kinase-2 | Tie-2 | |
| Bone Surface/Bone Volume | BS/BV | Morphometric parameters linked to bone formation and evaluable by microCT analysis |
| Bone Mineral Density | BMD | |
| Bone Volume | BV | |
| Bone Volume/Total Volume | BV/TV | |
| Connectivity density | Conn.D | |
| Mean trabecular thickness | MTT | |
| Structure model index | SMI | |
| Trabecular area | Tb.Ar | |
| Trabecular number | Tb.N | |
| Trabecular separation | Tb.Sp | |
| Trabecular thickness | Tb.Th |
| Magnetic Field (Range) | Frequency of the Trains of Pulses (Range) | Duration of Each Session (Range) | Overall Treatment Duration (Range) | |
|---|---|---|---|---|
| Osteotomic damage | 0.2–2.8 mT | 10–75 Hz | 2 h–24 h | 2–21 weeks |
| Ovariectomy induced osteoporosis | 0.96–3.82 mT | 7.5–50 Hz | 40 m–8 h | 4–8 weeks |
| Glucocorticoid induced osteoporosis | 1.2–4 mT | 8–50 Hz | 40 m–4 h | 4–12 weeks |
| Diabetes induced osteopenia | 2–3.8 mT | 8–15 Hz | 40 m–8 h | 8–12 weeks |
| Disuse osteopenia | 0.6–3.8 mT | 10–50 Hz | 40 m–8 h | 1–12 weeks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bartolomeo, M.; Cavani, F.; Pellacani, A.; Grande, A.; Salvatori, R.; Chiarini, L.; Nocini, R.; Anesi, A. Pulsed Electro-Magnetic Field (PEMF) Effect on Bone Healing in Animal Models: A Review of Its Efficacy Related to Different Type of Damage. Biology 2022, 11, 402. https://doi.org/10.3390/biology11030402
Di Bartolomeo M, Cavani F, Pellacani A, Grande A, Salvatori R, Chiarini L, Nocini R, Anesi A. Pulsed Electro-Magnetic Field (PEMF) Effect on Bone Healing in Animal Models: A Review of Its Efficacy Related to Different Type of Damage. Biology. 2022; 11(3):402. https://doi.org/10.3390/biology11030402
Chicago/Turabian StyleDi Bartolomeo, Mattia, Francesco Cavani, Arrigo Pellacani, Alexis Grande, Roberta Salvatori, Luigi Chiarini, Riccardo Nocini, and Alexandre Anesi. 2022. "Pulsed Electro-Magnetic Field (PEMF) Effect on Bone Healing in Animal Models: A Review of Its Efficacy Related to Different Type of Damage" Biology 11, no. 3: 402. https://doi.org/10.3390/biology11030402
APA StyleDi Bartolomeo, M., Cavani, F., Pellacani, A., Grande, A., Salvatori, R., Chiarini, L., Nocini, R., & Anesi, A. (2022). Pulsed Electro-Magnetic Field (PEMF) Effect on Bone Healing in Animal Models: A Review of Its Efficacy Related to Different Type of Damage. Biology, 11(3), 402. https://doi.org/10.3390/biology11030402










