RETRACTED: Advantage of Species Diversification to Facilitate Sustainable Development of Aquaculture Sector
Abstract
:Simple Summary
Abstract
1. Introduction
2. Species Diversification in Aquaculture: Systemic Approach for Sustainable Food Production
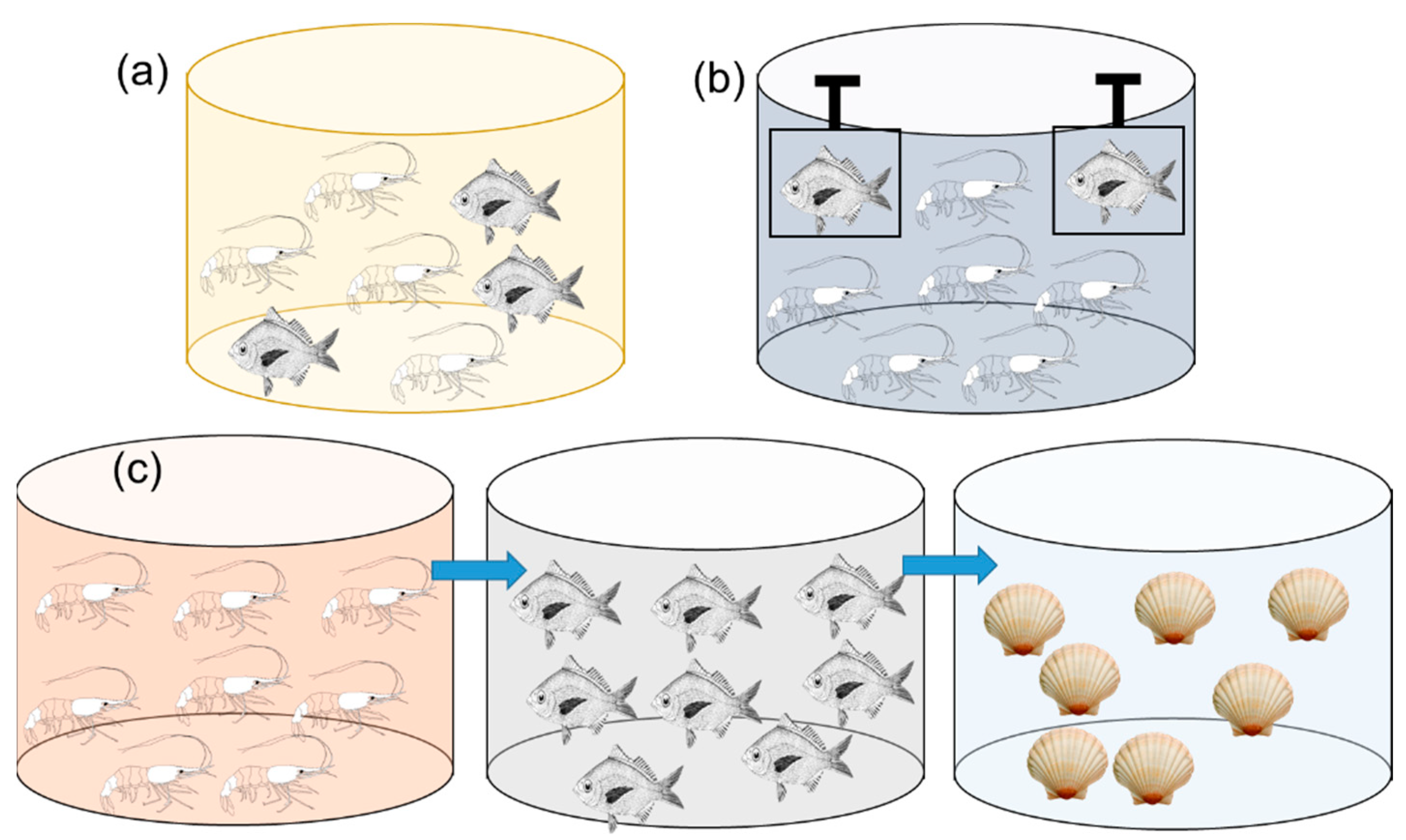
2.1. Species Compatibility: Principle of Polyculture
2.2. Species Complementarity: Benefits of Polyculture
2.2.1. Basic Complementarity
2.2.2. Enhanced Complementarity via Trophic Interactions
2.2.3. Enhanced Complementarity via Mutualism or Commensalism
3. Shrimp, Crab Polyculture
4. Future Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bongaarts, J. Development: Slow down population growth. Nature 2016, 530, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Pingali, P.L. Green Revolution: Impacts, limits, and the path ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.P.; Swinton, S.M. Reconciling agricultural productivity and environmental integrity: A grand challenge for agriculture. Front. Ecol. Environ. 2005, 3, 38–46. [Google Scholar] [CrossRef]
- Letourneau, D.K.; Armbrecht, I.; Rivera, B.S.; Lerma, J.M.; Carmona, E.J.; Daza, M.C.; Escobar, S.; Galindo, V.; Gutiérrez, C.; López, S.D.; et al. Does plant diversity benefit agroecosystems? A synthetic review. Ecol. Appl. 2011, 21, 9–21. [Google Scholar] [CrossRef]
- Isbell, F.; Adler, P.R.; Eisenhauer, N.; Fornara, D.; Kimmel, K.; Kremen, C.; Letourneau, D.K.; Liebman, M.; Polley, H.W.; Quijas, S.; et al. Benefits of increasing plant diversity in sustainable agroecosystems. J. Ecol. 2017, 105, 871–879. [Google Scholar] [CrossRef]
- Valenti, W.C.; Kimpara, J.M.; Preto, B.d.L.; Moraes-Valenti, P. Indicators of sustainability to assess aquaculture systems. Ecol. Indic. 2018, 88, 402–413. [Google Scholar] [CrossRef]
- Rice, J.C.; Garcia, S.M. Fisheries, food security, climate change, and biodiversity: Characteristics of the sector and perspectives on emerging issues. ICES J. Mar. Sci. 2011, 68, 1343–1353. [Google Scholar] [CrossRef]
- Polimeno, L.; Lisanti, M.T.; Rossini, M.; Giacovazzo, E.; Polimeno, L.; Debellis, L.; Ballini, A.; Topi, S.; Santacroce, L. Anisakis allergy: Is aquacultured fish a safe and alternative food to wild-capture fisheries for anisakis simplex-sensitized patients? Biology 2021, 10, 106. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Bell, M.V.; Little, D.C.; Yakupitiyage, A.; Rakshit, S.K. Polyunsaturated fatty acid content of wild and farmed tilapias in thailand: effect of aquaculture practices and implications for human nutrition. J. Agric. Food Chem. 2006, 54, 4304–4310. [Google Scholar] [CrossRef] [PubMed]
- Jennings, S.; Stentiford, G.D.; Leocadio, A.M.; Jeffery, K.R.; Metcalfe, J.D.; Katsiadaki, I.; Auchterlonie, N.A.; Mangi, S.C.; Pinnegar, J.K.; Ellis, T.; et al. Aquatic food security: Insights into challenges and solutions from an analysis of interactions between fisheries, aquaculture, food safety, human health, fish and human welfare, economy and environment. Fish Fish. 2016, 17, 893–938. [Google Scholar] [CrossRef]
- Decker, E.A.; Crum, A.D.; Mims, S.D.; Tidwell, J.H. Processing yields and composition of paddlefish (Polyodon spathula), a potential aquaculture species. J. Agric. Food Chem. 1991, 39, 686–688. [Google Scholar] [CrossRef]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso Jr, J.R.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Medeiros, M.V.; Aubin, J.; Camargo, A.F.M. Life cycle assessment of fish and prawn production: Comparison of monoculture and polyculture freshwater systems in Brazil. J. Clean. Prod. 2017, 156, 528–537. [Google Scholar] [CrossRef]
- Aubin, J.; Callier, M.; Rey-Valette, H.; Mathé, S.; Wilfart, A.; Legendre, M.; Slembrouck, J.; Caruso, D.; Chia, E.; Masson, G.; et al. Implementing ecological intensification in fish farming: Definition and principles from contrasting experiences. Rev. Aquac. 2019, 11, 149–167. [Google Scholar] [CrossRef]
- Garlock, T.; Asche, F.; Anderson, J.; Bjørndal, T.; Kumar, G.; Lorenzen, K.; Ropicki, A.; Smith, M.D.; Tveterås, R. A global blue revolution: Aquaculture growth across regions, species, and countries. Rev. Fish. Sci. Aquac. 2020, 28, 107–116. [Google Scholar] [CrossRef]
- Valderrama, D.; Hishamunda, N.; Zhou, X. Estimating employment in world aquaculture. FAO Aquac. Newsl. 2010, 45, 24–25. [Google Scholar]
- Humphries, F.; Benzie, J.; Morrison, C. A systematic quantitative literature review of aquaculture genetic resource access and benefit sharing. Rev. Aquac. 2018, 11, 1133–1147. [Google Scholar] [CrossRef]
- Thorvaldsen, T.; Kongsvik, T.; Holmen, I.M.; Størkersen, K.; Salomonsen, C.; Sandsund, M.; Bjelland, H.V. Occupational health, safety and work environments in Norwegian fish farming—Employee perspective. Aquaculture 2020, 524, 735238. [Google Scholar] [CrossRef]
- Huntingford, F. Animal Welfare in Aquaculture. Aquac. Innov. Soc. Transform. 2009, 17, 21–33. [Google Scholar]
- Carrera, M.; Piñeiro, C.; Martinez, I. Proteomic Strategies to Evaluate the Impact of Farming Conditions on Food Quality and Safety in Aquaculture Products. Foods 2020, 9, 1050. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Mairesse, G.; Gardeur, J.-N.; Brun-Bellut, J. Concept and Determinism of Quality in Percid Fishes. In Biology and Culture of Percid Fishes: Principles and Practices; Kestemont, P., Dabrowski, K., Summerfelt, R.C., Eds.; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Metian, M.; Troell, M.; Christensen, V.; Steenbeek, J.; Pouil, S. Mapping diversity of species in global aquaculture. Rev. Aquac. 2020, 12, 1090–1100. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I.; Henao, A.; Lana, M.A. Agroecology and the design of climate change-resilient farming systems. Agron. Sustain. Dev. 2015, 35, 869–890. [Google Scholar] [CrossRef]
- Rahman, M.M.; Nagelkerke, L.A.J.; Verdegem, M.C.J.; Wahab, M.A.; Verreth, J.A.J. Relationships among water quality, food resources, fish diet and fish growth in polyculture ponds: A multivariate approach. Aquaculture 2008, 275, 108–115. [Google Scholar] [CrossRef]
- Samuel-Fitwi, B.; Wuertz, S.; Schroeder, J.P.; Schulz, C. Sustainability assessment tools to support aquaculture development. J. Clean. Prod. 2012, 32, 183–192. [Google Scholar] [CrossRef]
- Bracken, M.E.S. Monocultures versus polycultures. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Oxford, UK, 2008; pp. 2446–2449. [Google Scholar]
- Zimmermann, S.; Nair, C.; New, M. Chapter 11—Grow-Out Systems—Polyculture and Integrated Culture. In Freshwater Prawns: Biology and Farming; Wiley-Blackwell: Oxford, UK, 2009. [Google Scholar]
- Nicholls, C.; Altieri, M. Agroecology: Principles for the conversion and redesign of farming systems. J. Ecosyst. Ecography 2016, 1, 1–8. [Google Scholar]
- Altieri, M.A.; Nicholls, C.I.; Montalba, R. Technological approaches to sustainable agriculture at a crossroads: An agroecological perspective. Sustainability 2017, 9, 349. [Google Scholar] [CrossRef]
- Freed, S.; Barman, B.; Dubois, M.; Flor, R.J.; Funge-Smith, S.; Gregory, R.; Hadi, B.A.R.; Halwart, M.; Haque, M.; Jagadish, S.V.K.; et al. Maintaining diversity of integrated rice and fish production confers adaptability of food systems to global change. Front. Sustain. Food Syst. 2020, 4, 576179. [Google Scholar] [CrossRef]
- Dumont, B.; Fortun-Lamothe, L.; Jouven, M.; Thomas, M.; Tichit, M. Prospects from agroecology and industrial ecology for animal production in the 21st century. Animal 2013, 7, 1028–1043. [Google Scholar] [CrossRef] [PubMed]
- Danish, M.S.S.; Senjyu, T.; Sabory, N.R.; Khosravy, M.; Grilli, M.L.; Mikhaylov, A.; Majidi, H. A Forefront framework for sustainable aquaponics modeling and design. Sustainability 2021, 13, 9313. [Google Scholar] [CrossRef]
- Lobillo-Eguíbar, J.; Fernández-Cabanás, V.M.; Bermejo, L.A.; Pérez-Urrestarazu, L. Economic sustainability of small-scale aquaponic systems for food self-production. Agronomy 2020, 10, 1468. [Google Scholar] [CrossRef]
- Henriksson, P.J.G.; Rico, A.; Zhang, W.; Ahmad-Al-Nahid, S.; Newton, R.; Phan, L.T.; Zhang, Z.; Jaithiang, J.; Dao, H.M.; Phu, T.M.; et al. Comparison of asian aquaculture products by use of statistically supported life cycle assessment. Environ. Sci. Technol. 2015, 49, 14176–14183. [Google Scholar] [CrossRef]
- Wilting, H.C.; Schipper, A.M.; Bakkenes, M.; Meijer, J.R.; Huijbregts, M.A.J. Quantifying biodiversity losses due to human consumption: A global-scale footprint analysis. Environ. Sci. Technol. 2017, 51, 3298–3306. [Google Scholar] [CrossRef]
- Silknetter, S.; Creed, R.P.; Brown, B.L.; Frimpong, E.A.; Skelton, J.; Peoples, B.K. Positive biotic interactions in freshwaters: A review and research directive. Freshw. Biol. 2020, 65, 811–832. [Google Scholar] [CrossRef]
- Liu, X.-g.; Xu, H.; Liu, C. Ecological engineering technologies for optimizing freshwater pond aquaculture. In Aquaculture in China: Success Stories and Modern Trends; Wiley: Hoboken, NJ, USA, 2018; pp. 555–576. [Google Scholar]
- Kautsky, N.; Berg, H.; Folke, C.; Larsson, J.; Troell, M. Ecological footprint for assessment of resource use and development limitations in shrimp and tilapia aquaculture. Aquac. Res. 2008, 28, 753–766. [Google Scholar] [CrossRef]
- Altieri, M.A. Agriculture, Traditional. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Academic Press: Waltham, MA, USA, 2001; pp. 119–125. [Google Scholar]
- Weitzman, J. Applying the ecosystem services concept to aquaculture: A review of approaches, definitions, and uses. Ecosyst. Serv. 2019, 35, 194–206. [Google Scholar] [CrossRef]
- Zélé, F.; Magalhães, S.; Kéfi, S.; Duncan, A.B. Ecology and evolution of facilitation among symbionts. Nat. Commun. 2018, 9, 4869. [Google Scholar] [CrossRef]
- Ahmed, N.; Ward, J.D.; Saint, C.P. Can integrated aquaculture-agriculture (IAA) produce “more crop per drop”? Food Secur. 2014, 6, 767–779. [Google Scholar] [CrossRef]
- Li, M.; Callier, M.D.; Blancheton, J.-P.; Galès, A.; Nahon, S.; Triplet, S.; Geoffroy, T.; Menniti, C.; Fouilland, E.; Roque d’orbcastel, E. Bioremediation of fishpond effluent and production of microalgae for an oyster farm in an innovative recirculating integrated multi-trophic aquaculture system. Aquaculture 2019, 504, 314–325. [Google Scholar] [CrossRef]
- Andrén, O.; Kätterer, T. Agriculture systems. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Oxford, UK, 2008; pp. 96–101. [Google Scholar]
- Stickney, R.R. Polyculture in aquaculture. In Sustainable Food Production; Christou, P., Savin, R., Costa-Pierce, B.A., Misztal, I., Whitelaw, C.B.A., Eds.; Springer: New York, NY, USA, 2013; pp. 1366–1368. [Google Scholar]
- Asiri, F.; Chu, K.-H. A novel recirculating aquaculture system for sustainable aquaculture: Enabling wastewater reuse and conversion of waste-to-immune-stimulating fish feed. ACS Sustain. Chem. Eng. 2020, 8, 18094–18105. [Google Scholar] [CrossRef]
- Bergman, K.; Henriksson, P.J.G.; Hornborg, S.; Troell, M.; Borthwick, L.; Jonell, M.; Philis, G.; Ziegler, F. Recirculating aquaculture is possible without major energy tradeoff: Life cycle assessment of warmwater fish farming in sweden. Environ. Sci. Technol. 2020, 54, 16062–16070. [Google Scholar] [CrossRef] [PubMed]
- Bricknell, I.R.; Birkel, S.D.; Brawley, S.H.; Van Kirk, T.; Hamlin, H.J.; Capistrant-Fossa, K.; Huguenard, K.; Van Walsum, G.P.; Liu, Z.L.; Zhu, L.H.; et al. Resilience of cold water aquaculture: A review of likely scenarios as climate changes in the Gulf of Maine. Rev. Aquac. 2021, 13, 460–503. [Google Scholar] [CrossRef]
- Thomas, M.; Pasquet, A.; Aubin, J.; Nahon, S.; Lecocq, T. When more is more: Taking advantage of species diversity to move towards sustainable aquaculture. Biol. Rev. 2021, 96, 767–784. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pierce, B. The ‘Blue revolution’—Aquaculture must go green. World Aquac. 2002, 33, 4–5. [Google Scholar]
- Couture, J.L.; Froehlich, H.E.; Buck, B.H.; Jeffery, K.R.; Krause, G.; Morris Jr, J.A.; Pérez, M.; Stentiford, G.D.; Vehviläinen, H.; Halpern, B.S. Scenario analysis can guide aquaculture planning to meet sustainable future production goals. ICES J. Mar. Sci. 2021, 78, 821–831. [Google Scholar] [CrossRef]
- Nhan, D.K.; Phong, L.T.; Verdegem, M.J.C.; Duong, L.T.; Bosma, R.H.; Little, D.C. Integrated freshwater aquaculture, crop and livestock production in the Mekong delta, Vietnam: Determinants and the role of the pond. Agric. Syst. 2007, 94, 445–458. [Google Scholar] [CrossRef]
- Bostock, J.; McAndrew, B.; Richards, R.; Jauncey, K.; Telfer, T.; Lorenzen, K.; Little, D.; Ross, L.; Handisyde, N.; Gatward, I.; et al. Aquaculture: Global status and trends. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2897–2912. [Google Scholar] [CrossRef]
- Zajdband, A.D. Integrated Agri-Aquaculture Systems. In Genetics, Biofuels and Local Farming Systems; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 87–127. [Google Scholar] [CrossRef]
- Tian, X.; Li, D.; Dong, S.; Yan, X.; Qi, Z.; Liu, G.; Lu, J. An experimental study on closed-polyculture of penaeid shrimp with tilapia and constricted tagelus. Aquaculture 2001, 202, 57–71. [Google Scholar] [CrossRef]
- Buck, B.H.; Troell, M.F.; Krause, G.; Angel, D.L.; Grote, B.; Chopin, T. State of the art and challenges for offshore integrated multi-trophic aquaculture (IMTA). Front. Mar. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- Clark, M.R.; Althaus, F.; Schlacher, T.A.; Williams, A.; Bowden, D.A.; Rowden, A.A. The impacts of deep-sea fisheries on benthic communities: A review. ICES J. Mar. Sci. 2015, 73, i51–i69. [Google Scholar] [CrossRef]
- Ortuño Crespo, G.; Dunn, D.C. A review of the impacts of fisheries on open-ocean ecosystems. ICES J. Mar. Sci. 2017, 74, 2283–2297. [Google Scholar] [CrossRef]
- Henne, J.P.; Romero, M.M.; Carmichael, G.J. Polyculture of endangered bonytails and razorback suckers in recirculated water. N. Am. J. Aquac. 2007, 69, 388–394. [Google Scholar] [CrossRef]
- Kozłowski, M.; Szczepkowski, M.; Wunderlich, K.; Szczepkowska, B.; Piotrowska, I. Polyculture of juvenile pikeperch (Sander lucioperca (L.)) and sterlet (Acipenser ruthenus L.) in a recirculating system. Fish. Aquat. Life 2015, 22, 237–242. [Google Scholar] [CrossRef]
- Dannheim, J.; Bergström, L.; Birchenough, S.N.R.; Brzana, R.; Boon, A.R.; Coolen, J.W.P.; Dauvin, J.-C.; De Mesel, I.; Derweduwen, J.; Gill, A.B.; et al. Benthic effects of offshore renewables: Identification of knowledge gaps and urgently needed research. ICES J. Mar. Sci. 2019, 77, 1092–1108. [Google Scholar] [CrossRef]
- Sonay, F.D.; Bascinar, N. An investigation on the effects of juvenile rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelinus fontinalis) monoculture and duo-culture farming in freshwater and seawater on growth performance. Iran. J. Fish. Sci. 2017, 16, 38–49. [Google Scholar]
- Guragain, P.; Tkachov, M.; Båtnes, A.S.; Olsen, Y.; Winge, P.; Bones, A.M. Principles and methods of counteracting harmful salmon–arthropod interactions in salmon farming: Addressing possibilities, limitations, and future options. Front. Mar. Sci. 2021, 8, 701793. [Google Scholar] [CrossRef]
- Monwar, M.; Ruhul, A.; Sarker, A.; Das, N. Polyculture of seabass with tilapia for the utilization of brown fields in the coastal areas of Cox’s Bazar, Bangladesh. Inte. J. Fish. Aqua. 2013, 5, 104–109. [Google Scholar]
- Ibrahem, M.; Shaheed, I.; Abo, H.; Yazeed, E.; Korani, H. Assessment of the susceptibility of polyculture reared African Catfish and Nile tilapia to Edwardsiella tarda. J. Am. Sci. 2011, 7, 779–786. [Google Scholar]
- Borremans, B.; Faust, C.; Manlove, K.R.; Sokolow, S.H.; Lloyd-Smith, J.O. Cross-species pathogen spillover across ecosystem boundaries: Mechanisms and theory. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180344. [Google Scholar] [CrossRef] [PubMed]
- Teixeira Alves, M.; Taylor, N.G.H. Models suggest pathogen risks to wild fish can be mitigated by acquired immunity in freshwater aquaculture systems. Sci. Rep. 2020, 10, 7513. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.L.; Williams, S.L.; Strong, D.R. Aquaculture-a gateway for exotic species. Science 2001, 294, 1655–1656. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cheng, L.; Liu, J.; Li, Z.; Xie, S.; De Silva, S.S. Freshwater aquaculture in PR China: Trends and prospects. Rev. Aquac. 2015, 7, 283–302. [Google Scholar] [CrossRef]
- Martínez-Porchas, M.; Martínez-Córdova, L.R.; Porchas-Cornejo, M.A.; López-Elías, J.A. Shrimp polyculture: A potentially profitable, sustainable, but uncommon aquacultural practice. Rev. Aquac. 2010, 2, 73–85. [Google Scholar] [CrossRef]
- Barrington, K.; Chopin, T.; Robinson, S. Integrated multi-trophic aquaculture (IMTA) in marine temperate waters. Integr. Maric. A Glob. Rev. 2009, 529, 7–46. [Google Scholar]
- Pascoe, S.D.; Plagányi, É.E.; Dichmont, C.M. Modelling multiple management objectives in fisheries: Australian experiences. ICES J. Mar. Sci. 2016, 74, 464–474. [Google Scholar] [CrossRef]
- Thomas, M.; Lecocq, T.; Abregal, C.; Nahon, S.; Aubin, J.; Jaeger, C.; Wilfart, A.; Schaeffer, L.; Ledoré, Y.; Puillet, L.; et al. The effects of polyculture on behaviour and production of pikeperch in recirculation systems. Aquac. Rep. 2020, 17, 100333. [Google Scholar] [CrossRef]
- David, F.S.; Proença, D.C.; Valenti, W.C. Phosphorus budget in integrated multitrophic aquaculture systems with nile tilapia, Oreochromis niloticus, and Amazon river prawn, Macrobrachium amazonicum. J. World Aquac. Soc. 2017, 48, 402–414. [Google Scholar] [CrossRef]
- Franchini, A.C.; Costa, G.A.; Pereira, S.A.; Valenti, W.C.; Moraes-Valenti, P. Improving production and diet assimilation in fish-prawn integrated aquaculture, using iliophagus species. Aquaculture 2020, 521, 735048. [Google Scholar] [CrossRef]
- Gao, X.; Ke, C.; Zhang, M.; Li, X.; Wu, F.; Liu, Y. N and P budgets of Haliotis discus hanai, Apostichopus japonicas, and Sebastes schlegeli in a polyculture system. Aquac. Res. 2019, 50, 2398–2409. [Google Scholar] [CrossRef]
- Xie, C.; Li, J.; Li, D.; Shen, Y.; Gao, Y.; Zhang, Z. Grass Carp: The Fish that Feeds Half of China. In Aquaculture in China: Success Stories and Modern Trends; Wiley: Hoboken, NJ, USA, 2018; pp. 93–115. [Google Scholar]
- Kumaresan, A.; Pathak, K.A.; Bujarbaruah, K.M.; Vinod, K. Analysis of integrated animal-fish production system under subtropical hill agro ecosystem in India: Growth performance of animals, total biomass production and monetary benefit. Trop. Anim. Health Prod. 2009, 41, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Boock, M.V.; Marques, H.L.d.A.; Mallasen, M.; Barros, H.P.; Moraes-Valenti, P.; Valenti, W.C. Effects of prawn stocking density and feeding management on rice-prawn culture. Aquaculture 2016, 451, 480–487. [Google Scholar] [CrossRef]
- Rahman, M.M.; Verdegem, M.C.J.; Nagelkerke, L.A.J.; Wahab, M.A.; Milstein, A.; Verreth, J.A.J. Growth, production and food preference of rohu Labeo rohita (H.) in monoculture and in polyculture with common carp Cyprinus carpio (L.) under fed and non-fed ponds. Aquaculture 2006, 257, 359–372. [Google Scholar] [CrossRef]
- Dey, B.K.; Dugassa, G.H.; Hinzano, S.M.; Bossier, P. Causative agent, diagnosis and management of white spot disease in shrimp: A review. Rev. Aquac. 2020, 12, 822–865. [Google Scholar] [CrossRef]
- Kumar, M.R.; Aravind, R.; Raj, J.; Artheeswaran, N.; Pandey, A.P. Commensalism between jellyfish and juveniles of carangids in coral reef habitats of Palk Bay, India. Ecol. Environ. Conserv. 2014, 20, 139–141. [Google Scholar]
- Hosseini Aghuzbeni, S.H.; Hajirezaee, S.; Khara, H. Polyculture of western white shrimp, Litopenaeus vannamei Boone, 1931 with Grey mullet, Mugil cephalus Linnaeus, 1758 controls external parasites of western white shrimp. Aquac. Res. 2016, 47, 2983–2988. [Google Scholar] [CrossRef]
- Vaughan, D.B.; Grutter, A.S.; Hutson, K.S. Cleaner shrimp are a sustainable option to treat parasitic disease in farmed fish. Sci. Rep. 2018, 8, 13959. [Google Scholar] [CrossRef]
- Correia, M.; Azevedo, I.C.; Peres, H.; Magalhães, R.; Oliva-Teles, A.; Almeida, C.M.R.; Guimarães, L. Integrated multi-trophic aquaculture: A laboratory and hands-on experimental activity to promote environmental sustainability awareness and value of aquaculture products. Front. Mar. Sci. 2020, 7, 156. [Google Scholar] [CrossRef]
- Yi, S. Contingent valuation of sustainable integrated agriculture–aquaculture products: The case of rice–fish farming systems in South Korea. Agronomy 2019, 9, 601. [Google Scholar] [CrossRef]
- Chopin, T. Integrated multi-trophic aquaculture. What it is and why you should care… and don’t confuse it with polyculture. North. Aquac. 2006, 12, 4. [Google Scholar]
- Chopin, T.; Sawhney, M. Seaweeds and their mariculture. In Encyclopedia of Ocean Sciences; Academic Press: New York, NY, USA, 2009; pp. 317–326. [Google Scholar]
- Troell, M.; Joyce, A.; Chopin, T.; Neori, A.; Buschmann, A.H.; Fang, J.-G. Ecological engineering in aquaculture—Potential for integrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture 2009, 297, 1–9. [Google Scholar] [CrossRef]
- Neori, A.; Chopin, T.; Troell, M.; Buschmann, A.H.; Kraemer, G.P.; Halling, C.; Shpigel, M.; Yarish, C. Integrated aquaculture: Rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 2004, 231, 361–391. [Google Scholar] [CrossRef]
- Rossi, L.; Bibbiani, C.; Fierro-Sañudo, J.F.; Maibam, C.; Incrocci, L.; Pardossi, A.; Fronte, B. Selection of marine fish for integrated multi-trophic aquaponic production in the Mediterranean area using DEXi multi-criteria analysis. Aquaculture 2021, 535, 736402. [Google Scholar] [CrossRef]
- Chopin, T. Aquaculture, Integrated multi-trophic (imta)aquacultureintegrated multi-trophic (IMTA). In Sustainable Food Production; Christou, P., Savin, R., Costa-Pierce, B.A., Misztal, I., Whitelaw, C.B.A., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Sousa, A.M.M.; Rocha, C.M.R.; Gonçalves, M.P. Chapter 24—Agar. In Handbook of Hydrocolloids, 3rd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 731–765. [Google Scholar]
- Camelo-Guarín, S.; Molinet, C.; Soto, D. Recommendations for implementing integrated multitrophic aquaculture in commercial farms at the landscape scale in southern Chile. Aquaculture 2021, 544, 737116. [Google Scholar] [CrossRef]
- Gunning, D.; Maguire, J.; Burnell, G. The Development of sustainable saltwater-based food production systems: A review of established and novel concepts. Water 2016, 8, 598. [Google Scholar] [CrossRef]
- Tyson, R.V.; Treadwell, D.D.; Simonne, E.H. Opportunities and challenges to sustainability in aquaponic systems. Hort Technol. 2011, 21, 6. [Google Scholar] [CrossRef]
- Goddek, S.; Delaide, B.; Mankasingh, U.; Ragnarsdottir, K.V.; Jijakli, H.; Thorarinsdottir, R. Challenges of sustainable and commercial aquaponics. Sustainability 2015, 7, 4199–4224. [Google Scholar] [CrossRef]
- Love, D.C.; Fry, J.P.; Genello, L.; Hill, E.S.; Frederick, J.A.; Li, X.; Semmens, K. An international survey of aquaponics practitioners. PLoS ONE 2014, 9, e102662. [Google Scholar] [CrossRef]
- Jaeger, C.; Foucard, P.; Tocqueville, A.; Nahon, S.; Aubin, J. Mass balanced based LCA of a common carp-lettuce aquaponics system. Aquac. Eng. 2019, 84, 29–41. [Google Scholar] [CrossRef]
- Cahya, M.D.; Yustiati, A.; Andriani, Y. Polyculture and integrated multi trophic aquaculture (IMTA) in Indonesia: A review. Torani J. Fish. Mar. Sci. 2021, 4, 72–85. [Google Scholar]
- Barrington, K.; Ridler, N.; Chopin, T.; Robinson, S.; Robinson, R. Social aspects of the sustainability of integrated multi-tropic aquaculture. Auac. Inter. 2010, 18, 201–211. [Google Scholar]
- Carballeira Braña, C.B.; Cerbule, K.; Senff, P.; Stolz, I.K. Towards environmental sustainability in marine finfish aquaculture. Front. Mar. Sci. 2021, 8, 666662. [Google Scholar] [CrossRef]
- Fernandes-Salvador, J.A.; Davidson, K.; Sourisseau, M.; Revilla, M.; Schmidt, W.; Clarke, D.; Miller, P.I.; Arce, P.; Fernández, R.; Maman, L.; et al. Current status of forecasting toxic harmful algae for the north-east atlantic shellfish aquaculture industry. Front. Mar. Sci. 2021, 8, 666583. [Google Scholar] [CrossRef]
- Angel, D.L.; Eden, N.; Breitstein, S.; Yurman, A.; Katz, T.; Spanier, E. In situ biofiltration: A means to limit the dispersal of effluents from marine finfish cage aquaculture. Hydrobiologia 2002, 469, 1–10. [Google Scholar] [CrossRef]
- Chopin, T. Aquaculture, integrated multi-trophic (IMTA). In Encyclopedia of Sustainability Science and Technology; Springer: Dordrecht, The Netherland, 2013; Volume 12, pp. 542–564. [Google Scholar]
- Nelson, E.J.; MacDonald, B.A.; Robinson, S.M.C. The absorption efficiency of the suspension-feeding sea cucumber, Cucumaria frondosa, and its potential as an extractive integrated multi-trophic aquaculture (IMTA) species. Aquaculture 2012, 370–371, 19–25. [Google Scholar] [CrossRef]
- Mungkung, R.; Aubin, J.; Prihadi, T.H.; Slembrouck, J.; van der Werf, H.M.G.; Legendre, M. Life cycle assessment for environmentally sustainable aquaculture management: A case study of combined aquaculture systems for carp and tilapia. J. Clean. Prod. 2013, 57, 249–256. [Google Scholar] [CrossRef]
- Navarrete-Mier, F.; Sanz-Lázaro, C.; Marín, A. Does bivalve mollusc polyculture reduce marine fin fish farming environmental impact? Aquaculture 2010, 306, 101–107. [Google Scholar] [CrossRef]
- Busch, D.S.; Griffis, R.; Link, J.; Abrams, K.; Baker, J.; Brainard, R.E.; Ford, M.; Hare, J.A.; Himes-Cornell, A.; Hollowed, A.; et al. Climate science strategy of the US National Marine Fisheries Service. Mar. Policy 2016, 74, 58–67. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Ma, C.-H.; Lee, H.-T.; Hsu, T.-H. Polyculture of juvenile dog conch Laevistrombus canarium reveals high potentiality in integrated multitrophic aquaculture (IMTA). Biology 2021, 10, 812. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Ronza, P.; Garcia-Oliveira, P.; Pereira, A.G.; Losada, A.P.; Prieto, M.A.; Quiroga, M.I.; Simal-Gandara, J. Aquaculture as a circular bio-economy model with Galicia as a study case: How to transform waste into revalorized by-products. Trends Food Sci. Technol. 2022, 119, 23–35. [Google Scholar] [CrossRef]
- Deudero, S.; Tor, A.; Alomar, C.; Jose, V.; Sarriera, P.; Blanco, A. Integrated multitrophic aquaculture: Filter feeders bivalves as efficient reducers of wastes derived from coastal aquaculture assessed with stable isotope analyses. In Aquaculture and the Environment—A Shared Destiny; IntechOpen: London, UK, 2011. [Google Scholar]
- Jerónimo, D.; LillebØ, A.I.; Santos, A.; Cremades, J.; Calado, R. Performance of polychaete assisted sand filters under contrasting nutrient loads in an integrated multi-trophic aquaculture (IMTA) system. Sci. Rep. 2020, 10, 20871. [Google Scholar] [CrossRef] [PubMed]
- Chopin, T.; MacDonald, B.; Robinson, S.; Cross, S.; Pearce, C.; Knowler, D.; Noce, A.; Reid, G.K.; Cooper, J.; Speare, D.; et al. The Canadian integrated multi-trophic aquaculture network (CIMTAN)—A network for a new era of ecosystem responsible aquaculture. Fisheries 2013, 38, 297–308. [Google Scholar] [CrossRef]
- Chopin, T. Marine Aquaculture in Canada: Well-established monocultures of finfish and shellfish and an emerging integrated multi-trophic aquaculture (IMTA) approach including seaweeds, other invertebrates, and microbial communities. Fisheries 2015, 40, 28–31. [Google Scholar] [CrossRef]
- Giangrande, A.; Pierri, C.; Arduini, D.; Borghese, J.; Licciano, M.; Trani, R.; Corriero, G.; Basile, G.; Cecere, E.; Petrocelli, A.; et al. An Innovative IMTA System: Polychaetes, sponges and macroalgae co-cultured in a southern italian in-shore mariculture plant (Ionian Sea). J. Mar. Sci. Eng. 2020, 8, 733. [Google Scholar] [CrossRef]
- Chopin, T.; Buschmann, A.H.; Halling, C.; Troell, M.; Kautsky, N.; Neori, A.; Kraemer, G.P.; Zertuche-González, J.A.; Yarish, C.; Neefus, C. Integrating seaweeds into marine aquaculture systems: A key toward sustainability. J. Phycol. 2001, 37, 975–986. [Google Scholar] [CrossRef]
- Buchholz, C.M.; Krause, G.; Buck, B.H. Seaweed andman. In Seaweed Biology: Novel Insights into Ecophysiology, Ecology and Utilization; Wiencke, C., Bischof, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 471–493. [Google Scholar]
- Chopin, T.; Robinson, S.; Troell, M.; Neori, A.; Buschmann, A.; Fang, J. Multitrophic Integration for Sustainable Marine Aquaculture. In The Encyclopedia of Ecology, Ecological Engineering; Elsevier: Oxford, UK, 2008; Volume 3, pp. 2463–2475. [Google Scholar]
- Chopin, T. Integrated multi-trophic aquaculture (IMTA): Responsibly farming our waters by taking advantage of ecosystem services within a circular economy approach. In Networking Fridays Seminar Series at the AIR Centre; Atlantic International Research Centre: Azores Islands, Portugal, 2021. [Google Scholar]
- Brown, N.; Eddy, S.; Plaud, S. Utilization of waste from a marine recirculating fish culture system as a feed source for the polychaete worm, Nereis virens. Aquaculture 2011, 322–323, 177–183. [Google Scholar] [CrossRef]
- Prein, M. Integration of aquaculture into crop—Animal systems in Asia. Agric. Syst. 2002, 71, 127–146. [Google Scholar] [CrossRef]
- Pant, J.; Demaine, H.; Edwards, P. Assessment of the aquaculture subsystem in integrated agriculture-aquaculture systems in Northeast Thailand. Aquac. Res. 2004, 35, 289–298. [Google Scholar] [CrossRef]
- Maucieri, C.; Nicoletto, C.; Schmautz, Z.; Sambo, P.; Komives, T.; Borin, M.; Junge, R. Vegetable intercropping in a small-scale aquaponic system. Agronomy 2017, 7, 63. [Google Scholar] [CrossRef]
- Devendra, C.; Thomas, D. Crop–animal interactions in mixed farming systems in Asia. Agric. Syst. 2002, 71, 27–40. [Google Scholar] [CrossRef]
- Huong, N.V.; Cuong, T.H.; Thu, T.T.N.; Lebailly, P. Efficiency of Different integrated agriculture aquaculture systems in the red river delta of Vietnam. Sustainability. 2018, 10, 493. [Google Scholar] [CrossRef]
- Goddek, S.; Schmautz, Z.; Scott, B.; Delaide, B.; Keesman, K.J.; Wuertz, S.; Junge, R. The effect of anaerobic and aerobic fish sludge supernatant on hydroponic lettuce. Agronomy 2016, 6, 37. [Google Scholar] [CrossRef]
- de Sousa, A.M.B.; Santos, R.R.S.; Moraes, F.H.R.; Gehring, C. Exploring the potential for sustainable weed control with integrated rice–fish culture for smallholder irrigated rice agriculture in the Maranhão Lowlands of Amazonia. Renew. Agric. Food Syst. 2012, 27, 107–114. [Google Scholar] [CrossRef]
- Clausen, J.H.; Madsen, H.; Van, P.T.; Dalsgaard, A.; Murrell, K.D. Integrated parasite management: Path to sustainable control of fishborne trematodes in aquaculture. Trends Parasitol. 2015, 31, 8–15. [Google Scholar] [CrossRef]
- van Dam, A.; Beveridge, M.; Azim, E.; Verdegem, M. The potential of fish production based on periphyton. Rev. Fish Biol. Fish. 2002, 12, 1–31. [Google Scholar] [CrossRef]
- Michielsens, C.G.J.; Lorenzen, K.; Phillips, M.J.; Gauthier, R. Asian carp farming systems: Towards a typology and increased resource use efficiency. Aquac. Res. 2002, 33, 403–413. [Google Scholar] [CrossRef]
- Bruno, J.F.; Stachowicz, J.J.; Bertness, M.D. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 2003, 18, 119–125. [Google Scholar] [CrossRef]
- McNeely, D.; Bertness, M.; Gaines, S.; Hay, M. Marine Community Ecology. Ecology 2001, 82, 2968–2969. [Google Scholar] [CrossRef]
- Gallien, L.; Carboni, M. The community ecology of invasive species: Where are we and what’s next? Ecography 2017, 40, 335–352. [Google Scholar] [CrossRef]
- Frid, C.L.J.; Paramor, O.A.L. Feeding the world: What role for fisheries? ICES J. Mar. Sci. 2012, 69, 145–150. [Google Scholar] [CrossRef]
- Daskalov, G.M.; Grishin, A.N.; Rodionov, S.; Mihneva, V. Trophic cascades triggered by overfishing reveal possible mechanisms of ecosystem regime shifts. Proc. Natl. Acad. Sci. USA 2007, 104, 10518–10523. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Velásquez, V.; Navia, A.F.; Rosa, R.S.; Guimarães, P.R., Jr.; Raimundo, R.L.G. Resource partitioning between fisheries and endangered sharks in a tropical marine food web. ICES J. Mar. Sci. 2021, 78, 2518–2527. [Google Scholar] [CrossRef]
- Glencross, J.S.; Lavers, J.L.; Woehler, E.J. A proposed framework for reporting mass mortality (wreck) events of seabirds. ICES J. Mar. Sci. 2021, 78, 1935–1942. [Google Scholar] [CrossRef]
- Ferriss, B.E.; Reum, J.C.P.; McDonald, P.S.; Farrell, D.M.; Harvey, C.J. Evaluating trophic and non-trophic effects of shellfish aquaculture in a coastal estuarine foodweb. ICES J. Mar. Sci. 2015, 73, 429–440. [Google Scholar] [CrossRef]
- Boucher, D.H.; James, S.; Keeler, K.H. The Ecology of Mutualism. Annu. Rev. Ecol. Syst. 1982, 13, 315–347. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Stanley, E.H.; Zanden, M.J.V. State of the world’s freshwater ecosystems: Physical, chemical, and biological changes. Annu. Rev. Environ. Resour. 2011, 36, 75–99. [Google Scholar] [CrossRef]
- Wang, J.; Beusen, A.H.W.; Liu, X.; Bouwman, A.F. Aquaculture production is a large, spatially concentrated source of nutrients in chinese freshwater and coastal seas. Environ. Sci. Technol. 2020, 54, 1464–1474. [Google Scholar] [CrossRef]
- van der Heide, T.; Angelini, C.; de Fouw, J.; Eklöf, J.S. Facultative mutualisms: A double-edged sword for foundation species in the face of anthropogenic global change. Ecol. Evol. 2021, 11, 29–44. [Google Scholar] [CrossRef]
- Rahman, M. Role of common carp (Cyprinus carpio) in aquaculture production systems. Front. Life Sci. 2015, 8, 399–410. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, J.; Ren, W.; Guo, L.; Cheng, Y.; Li, J.; Li, K.; Zhu, Z.; Zhang, J.; Luo, S.; et al. Can the co-cultivation of rice and fish help sustain rice production? Sci. Rep. 2016, 6, 28728. [Google Scholar] [CrossRef] [PubMed]
- Brooker, A.J.; Papadopoulou, A.; Gutierrez, C.; Rey, S.; Davie, A.; Migaud, H. Sustainable production and use of cleaner fish for the biological control of sea lice: Recent advances and current challenges. Vet. Rec. 2018, 183, 383. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, E.; Davie, A.; Migaud, H. Delousing efficiency of farmed ballan wrasse (Labrus bergylta) against Lepeophtheirus salmonis infecting Atlantic salmon (Salmo salar) post-smolts. Pest Manag. Sci. 2014, 70, 1274–1282. [Google Scholar] [CrossRef]
- Skiftesvik, A.B.; Bjelland, R.M.; Durif, C.M.F.; Johansen, I.S.; Browman, H.I. Delousing of Atlantic salmon (Salmo salar) by cultured vs. wild ballan wrasse (Labrus bergylta). Aquaculture 2013, 402–403, 113–118. [Google Scholar] [CrossRef]
- Barrett, L.T.; Oveton, K.; Stien, L.H.; Oppedal, F.; Dempster, T. Effect of cleaner fish on sea lice in Norwegian salmon aquaculture: A national scale data analysis. Int. J. Parasitol. 2020, 50, 787–796. [Google Scholar] [CrossRef]
- Stachowicz, J. Mutualism, Facilitation, and the Structure of Ecological Communities. BioScience 2001, 51, 235–245. [Google Scholar] [CrossRef]
- Hay, M.; Parker, J.; Burkepile, D.; Caudill, C.; Wilson, A.; Hallinan, Z.; Chequer, A. Mutualisms and aquatic community structure: The enemy of my enemy is my friend. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 175–197. [Google Scholar] [CrossRef]
- Gliessman, S.R. Species interactions and community ecology in low external-input agriculture. Am. J. Altern. Agric. 1987, 2, 160–165. [Google Scholar] [CrossRef]
- Bertness, M.; Callaway, R. Positive interactions in communities. Trends Ecol. Evol. 1994, 9, 191–193. [Google Scholar] [CrossRef]
- Bshary, R.; Côté, I. New perspectives on marine cleaning mutualism. In Fish Behav; CRC Press: Boca Raton, FL, USA, 2008; pp. 563–592. [Google Scholar]
- Harden-Davies, H. The next wave of science diplomacy: Marine biodiversity beyond national jurisdiction. ICES J. Mar. Sci. 2017, 75, 426–434. [Google Scholar] [CrossRef]
- Bosma, R.H.; Nguyen, T.H.; Siahainenia, A.J.; Tran, H.T.P.; Tran, H.N. Shrimp-based livelihoods in mangrove silvo-aquaculture farming systems. Rev. Aquac. 2016, 8, 43–60. [Google Scholar] [CrossRef]
- Wang, A.; Ma, X.; Xu, J.; Lu, W. Methane and nitrous oxide emissions in rice-crab culture systems of northeast China. Aquac. Fish. 2019, 4, 134–141. [Google Scholar] [CrossRef]
- N’souvi, K.; Sun, C.; Che, B. Aquaculture technology adoption and profitability of the polyculture system practiced by prawn and crab farmers: Case study of Anhui province in China. Aquac. Rep. 2021, 21, 100896. [Google Scholar] [CrossRef]
- Hisano, H.; Barbosa, P.T.L.; Hayd, L.A.; Mattioli, C.C. Evaluation of Nile tilapia in monoculture and polyculture with giant freshwater prawn in biofloc technology system and in recirculation aquaculture system. Int. Aquat. Res. 2019, 11, 335–346. [Google Scholar] [CrossRef]
- Muangkeow, B.; Ikejima, K.; Powtongsook, S.; Yi, Y. Effects of white shrimp, Litopenaeus vannamei (Boone), and Nile tilapia, Oreochromis niloticus L., stocking density on growth, nutrient conversion rate and economic return in integrated closed recirculation system. Aquaculture 2007, 269, 363–376. [Google Scholar] [CrossRef]
- Tuan, T.; Li, S. Potential role of prebiotics and probiotics in conferring health benefits in economically important crabs. Fish Shellfish Immunol. Rep. 2022, 3, 100041. [Google Scholar]
- Wang, Y.; Yang, H.; Ye, C.; Chen, X.; Zhang, J.; Xu, M. Effects of plant species on soil microbial processes and CH4 emission from constructed wetlands. Environ. Pollut. 2013, 174C, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Flegel, T.W. A future vision for disease control in shrimp aquaculture. J. World Aquac. Soc. 2019, 50, 249–266. [Google Scholar] [CrossRef]
- Maynou, F. Sale price flexibilities of Mediterranean hake and red shrimp. Mar. Policy 2022, 136, 104904. [Google Scholar] [CrossRef]
- Nguyen, T.; Momtaz, S.; Zimmerman, K. Wastewater from Shrimp Farming and Water Pollution in Vietnam. In Proceedings of the 3rd International Conference on Enviornmental Science and Technology, Houston, TX, USA, 6–9 August 2007. [Google Scholar]
- Páez-Osuna, F.; Guerrero-Galván, S.R.; Ruiz-Fernández, A.C. The environmental impact of shrimp aquaculture and the coastal pollution in Mexico. Mar. Pollut. Bull. 1998, 36, 65–75. [Google Scholar] [CrossRef]
- do Nascimento Vieira, F.; Pedrotti, F.; Buglione Neto, C.; Mouriño, J.L.; Beltrame, E.; Martins, M.; Ramirez, C.; Vinatea, L. Lactic-acid bacteria increase the survival of marine shrimp, Litopenaeus vannamei, after infection with Vibrio harveyi. Braz. J. Oceanogr. 2007, 55, 251–255. [Google Scholar]
- Nguyen Thi Truc, L.; Nguyen Thanh, T.; Tran Thi Hong, T.; Pham Van, D.; Vo Thi Tuyet, M.; Nguyen Trong, N.; Phan Cong, M.; Cao Ngoc, D.; Truong Quoc, P. Effects of feed mixed with lactic acid bacteria and carbon, nitrogen, phosphorus supplied to the water on the growth and survival rate of white leg shrimp (Penaeus vannamei) infected with acute hepatopancreatic necrosis disease caused by Vibrio parahaemolyticus. Biology 2021, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.; Gräslund, S.; Wahlström, A.; Poungshompoo, S.; Bengtsson, B.-E.; Kautsky, N. Antibiotic use in shrimp farming and implications for environmental impacts and human health. Int. J. Food Sci. Technol. 2003, 38, 255–266. [Google Scholar] [CrossRef]
- Luu, Q.H.; Nguyen, T.B.T.; Nguyen, T.L.A.; Do, T.T.T.; Dao, T.H.T.; Padungtod, P. Antibiotics use in fish and shrimp farms in Vietnam. Aquac. Rep. 2021, 20, 100711. [Google Scholar] [CrossRef]
- Otoshi, C.A.; Arce, S.M.; Moss, S.M. Growth and reproductive performance of broodstock shrimp reared in a biosecure recirculating aquaculture system versus a flow-through pond. Aquac. Eng. 2003, 29, 93–107. [Google Scholar] [CrossRef]
- Kaya, D.; Genc, E.; Genc, M.A.; Aktas, M.; Eroldogan, O.T.; Guroy, D. Biofloc technology in recirculating aquaculture system as a culture model for green tiger shrimp, Penaeus semisulcatus: Effects of different feeding rates and stocking densities. Aquaculture 2020, 528, 735526. [Google Scholar] [CrossRef]
- Krishnani, K.K.; Parimala, V.; Gupta, B.P.; Azad, I.S.; Meng, X.; Abraham, M. Bagasse-assisted bioremediation of ammonia from shrimp farm wastewater. Water Environ. Res. Res. Publ. Water Environ. Fed. 2006, 78, 938–950. [Google Scholar] [CrossRef]
- Brito, L.O.; Cardoso Junior, L.d.O.; Lavander, H.D.; Abreu, J.L.d.; Severi, W.; Gálvez, A.O. Bioremediation of shrimp biofloc wastewater using clam, seaweed and fish. Chem. Ecol. 2018, 34, 901–913. [Google Scholar] [CrossRef]
- Eldani, A.; Primavera, J.H. Effect of different stocking combinations on growth, production and survival of milkfish (Chanos chanos Forskal) and prawn (Penaeus monodon Fabricius) in polyculture in brackishwater ponds. Aquaculture 1981, 23, 59–72. [Google Scholar] [CrossRef]
- Martinez-Cordova, L.R.; Martinez-Porchas, M. Polyculture of Pacific white shrimp, Litopenaeus vannamei, giant oyster, Crassostrea gigas and black clam, Chione fluctifraga in ponds in Sonora, Mexico. Aquaculture 2006, 258, 321–326. [Google Scholar] [CrossRef]
- Lalramchhani, C.; Balasubramanian, C.P.; Panigrahi, A.; Ghoshal, T.K.; Das, S.; Shyne Anand, P.S.; Vijayan, K.K. Polyculture of Indian white shrimp (Penaeus indicus) with Milkfish (Chanos chanos) and its effect on growth performances, water quality and microbial load in brackishwater pond. J. Coast. Res. 2019, 86, 43–48. [Google Scholar] [CrossRef]
- Bunting, S.W. Horizontally integrated aquaculture development: Exploring consensus on constraints and opportunities with a stakeholder Delphi. Aquac. Int. 2008, 16, 153–169. [Google Scholar] [CrossRef]
- Irz, X.; McKenzie, V. Profitability and technical efficiency of aquaculture systems in pampaanga, philippines. Aquac. Econ. Manag. 2003, 7, 195–211. [Google Scholar] [CrossRef]
- Rahman, M.M. Food Web Interactions and Nutrients Dynamics in Polyculture Ponds. Ph.D. Thesis, Wageningen University and Research, Ann Arbor, MI, USA, 2006. [Google Scholar]
- Wang, J.-Q.; Li, D.; Dong, S.; Wang, K.; Tian, X. Experimental studies on polyculture in closed shrimp ponds: I. Intensive polyculture of Chinese shrimp (Penaeus chinensis) with tilapia hybrids. Aquaculture 1998, 163, 11–27. [Google Scholar] [CrossRef]
- Nguyen, N.A.; Vinh, N.; An, B.; Lan, L.M.; Hai, T. Polyculture culture of black tiger shrimp Penaeus monodon and red seaweed Gracilaria tenuistipitata under different densities: Effects on water quality, post-larvae performance and their resistance against Vibrio parahaemolyticus. J. Appl. Phycol. 2020, 32, 4333–4345. [Google Scholar]
- Athithan, S.; Ramanathan, N.; Thommai, F.; Ramadhas, V. Polyculture of tiger shrimp and carps in hardwater seas onal ponds. Indian J. Fish. 2005, 52, 339–343. [Google Scholar]
- Tendencia, E.A.; Fermin, A.C.; dela Peña, M.R.; Choresca, C.H. Effect of Epinephelus coioides, Chanos chanos, and GIFT tilapia in polyculture with Penaeus monodon on the growth of the luminous bacteria Vibrio harveyi. Aquaculture 2006, 253, 48–56. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, X.; Yang, X.; Hines, A.H. Current trends in hatchery techniques and stock enhancement for chinese mitten crab, Eriocheir japonica sinensis. Rev. Fish. Sci. 2008, 16, 377–384. [Google Scholar] [CrossRef]
- Xianliang, Z.; Fang, X.; Shumin, L.; Xinzhong, L.; Xu, H.; Kiayong, J.; Honglang, H. China Fishery Statistical Yearbook 2018; China Agriculture Press: Beijing, China, 2018; pp. 24–35. [Google Scholar]
- Romana-Eguia, M.R.R.; Rutaqquio, M.P.; Gutierrez, R.C.; Salayou, N.D. Assesment of tilapia-freshwater prawn co-culture schemes in tanks and lakes-based cages for increased farm production. Sustainability 2021, 13, 13574. [Google Scholar] [CrossRef]
- Douglass, J.G.; Duffy, J.E.; Bruno, J.F. Herbivore and predator diversity interactively affect ecosystem properties in an experimental marine community. Ecol. Lett. 2008, 11, 598–608. [Google Scholar] [CrossRef]
- Bolivar, G.; Fitzsimmons, K. New dimensions in farmed tilapia. In Proceedings of the 6th International Symposium on Tilapia in Aquaculture (ISTA), Manila, Philippines, 12–16 September 2004. [Google Scholar]
- Calado, R.; Mota, V.C.; Madeira, D.; Leal, M.C. Summer is coming! tackling ocean warming in atlantic salmon cage farming. Animals 2021, 11, 1800. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.-H.; Tsai, W.-S. Integrated pond culture: A type of spatially sequential polyculture. J. World Maric. Soc. 1985, 16, 429–436. [Google Scholar] [CrossRef]
- McIntosh, D.; Fitzsimmons, K. Characterization of effluent from an inland, low-salinity shrimp farm: What contribution could this water make if used for irrigation. Aquac. Eng. 2003, 27, 147–156. [Google Scholar] [CrossRef]
- Martínez-Córdova, L.R.; Peña-Messina, E. Biotic communities and feeding habits of Litopenaeus vannamei (Boone 1931) and Litopenaeus stylirostris (Stimpson 1974) in monoculture and polyculture semi-intensive ponds. Aquac. Res. 2005, 36, 1075–1084. [Google Scholar] [CrossRef]
- Hamel, J.-F.; Conand, C.; Pawson, D.L.; Mercier, A. The sea cucumber Holothuria scabra (Holothuroidea: Echinodermata): Its biology and exploitation as Beche-de-mer. In Advances in Marine Biology; Academic Press: Cambridge, MA, USA, 2001; Volume 41, pp. 129–223. [Google Scholar]
- Garcia-Pérez, A.; Alston, D.E.; Cortés-Maldonado, R. Growth, survival, yield, and size distributions of freshwater prawn Macrobrachium rosenbergii and Tilapia Oreochromis niloticus in polyculture and monoculture systems in Puerto Rico. J. World Aquac. Soc. 2000, 31, 446–451. [Google Scholar] [CrossRef]
- Hellio, C.; Pons, A.M.; Beaupoil, C.; Bourgougnon, N.; Gal, Y.L. Antibacterial, antifungal and cytotoxic activities of extracts from fish epidermis and epidermal mucus. Int. J. Antimicrob. Agents 2002, 20, 214–219. [Google Scholar] [CrossRef]
- Tendencia, E.A.; dela Peña, M.R.; Fermin, A.C.; Lio-Po, G.; Choresca, C.H.; Inui, Y. Antibacterial activity of tilapia Tilapia hornorum against Vibrio harveyi. Aquaculture 2004, 232, 145–152. [Google Scholar] [CrossRef]
- Shirota, K.; Sato, T.; Sekiguchi, J.; Miyauchi, K.; Mochizuki, A.; Matsumiya, M. Purification and characterization of chitinase isozymes from a red algae, Chondrus verrucosus. Biosci. Biotechnol. Biochem. 2008, 72, 3091–3099. [Google Scholar] [CrossRef]
- Hoang, M.N.; Nguyen, P.N.; Bossier, A.; Bossier, P. Effect of shrimp-fish polyculture on immune parameters, disease resistance of white shrimp and the prevalence of Vibrio spp. Aquac. Res. 2022, 53, 1316–1326. [Google Scholar] [CrossRef]
- Hosseini Aghuzbeni, S.H.; Hajirezaee, S.; Matinfar, A.; Khara, H.; Ghobadi, M. A preliminary study on polyculture of western white shrimp (Litopenaeus vannamei) with mullet (Mugil cephalus): An assessment of water quality, growth parameters, feed intake efficiency and survival. J. Appl. Anim. Res. 2017, 45, 247–251. [Google Scholar] [CrossRef]
- Wang, B.; Tian, J.S.; Zhou, Z.C. Food web in jellyfish-shrimp-shellfish polyculture pond. J. Appl. Ecol. 2021, 32, 2028–2034. [Google Scholar]
- Lombardi, J.V.; de Almeida Marques, H.L.; Pereira, R.T.L.; Barreto, O.J.S.; de Paula, E.J. Cage polyculture of the Pacific white shrimp Litopenaeus vannamei and the Philippines seaweed Kappaphycus alvarezii. Aquaculture 2006, 258, 412–415. [Google Scholar] [CrossRef]
- Ruenglertpanyakul, W.; Attasat, S.; Wanichpongpan, P. Nutrient removal from shrimp farm effluent by aquatic plants. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2004, 50, 321–330. [Google Scholar] [CrossRef]
- Copertino, M.d.S.; Tormena, T.; Seeliger, U. Biofiltering efficiency, uptake and assimilation rates of Ulva clathrata (Roth) J. Agardh (Clorophyceae) cultivated in shrimp aquaculture waste water. J. Appl. Phycol. 2009, 21, 31–45. [Google Scholar] [CrossRef]
- Lindsey Zemke-White, W.; Ohno, M. World seaweed utilisation: An end-of-century summary. J. Appl. Phycol. 1999, 11, 369–376. [Google Scholar] [CrossRef]
- Rasha, R.K.; Rahman, M.R.; Huq, A.S.M.A.; Jalil, G.M.A. Productivity and resource use efficiency of tiger shrimp farming in some selected areas of bagerhat district in Bangladesh. Asia-Pac. J. Rural Dev. 2019, 29, 7–19. [Google Scholar] [CrossRef]
- Dhar, A.R.; Uddin, M.T.; Roy, M.K. Assessment of organic shrimp farming sustainability from economic and environmental viewpoints in Bangladesh. Environ. Res. 2020, 180, 108879. [Google Scholar] [CrossRef]
- Zhang, K.; Tian, X.-L.; Dong, S.-L.; Feng, J.; He, R.-P. An experimental study on the budget of organic carbon in polyculture systems of swimming crab with white shrimp and short-necked clam. Aquaculture 2015, 451, 58–64. [Google Scholar] [CrossRef]
- Ray, S.; Mondal, P.; Paul, A.K.; Iqbal, S.; Atique, U.; Islam, M.S.; Mahboob, S.; Al-Ghanim, K.A.; Al-Misned, F.; Begum, S. Role of shrimp farming in socio-economic elevation and professional satisfaction in coastal communities. Aquac. Rep. 2021, 20, 100708. [Google Scholar] [CrossRef]
- Mirera, D.; Ochiewo, J.; Munyi, F. Social and economic implications of small-scale mud crab (Scylla serrata) aquaculture: The case of organised community groups. Aquac. Int. 2014, 22, 1499–1514. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, X.; Li, J. Chinese mitten crab culture: Current status and recent progress towards sustainable development. In Aquaculture in China: Success Stories and Modern Trends; Wiley: Hoboken, NJ, USA, 2018; pp. 197–217. [Google Scholar]
- Sui, L.; Zhang, F.; Wang, X.; Bossier, P.; Sorgeloos, P.; Hänfling, B. Genetic diversity and population structure of the Chinese mitten crab Eriocheir sinensis in its native range. Mar. Biol. 2009, 156, 1573–1583. [Google Scholar] [CrossRef]
- Wang, S.; He, Y.; Wang, Y.; Tao, N.; Wu, X.; Wang, X.; Qiu, W.; Ma, M. Comparison of flavour qualities of three sourced Eriocheir sinensis. Food Chem. 2016, 200, 24–31. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Z.; Wang, C.; Li, E.; Qin, J.G.; Chen, L. Dietary supplementation of selenium yeast enhances the antioxidant capacity and immune response of juvenile Eriocheir Sinensis under nitrite stress. Fish Shellfish Immunol. 2019, 87, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Wei, D.; Hou, D.; Wang, H.; Liu, J.; Weng, S.; He, J.; Huang, Z. Sediment microbiota in polyculture of shrimp and fish pattern is distinctive from those in monoculture intensive shrimp or fish ponds. Sci. Total Environ. 2021, 787, 147594. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.-N.; Dong, Y.-X. Construction of multivariate composite calculation model of soil organic carbon content in plough horizon based on geodetector. Trop. Geogr. 2018, 38, 546–556. [Google Scholar]
- Mohan Dey, M.; Javien Paraguas, F.; Srichantuk, N.; Xinhua, Y.; Bhatta, R.; Thi Chau Dung, L. Technical efficiency of freshwater pond polyculture production in selected asian countries: Estimation and implication. Aquac. Econ. Manag. 2005, 9, 39–63. [Google Scholar] [CrossRef]
- Quyen, N.T.K.; Berg, H.; Gallardo, W.; Da, C.T. Stakeholders’ perceptions of ecosystem services and Pangasius catfish farming development along the Hau River in the Mekong Delta, Vietnam. Ecosyst. Serv. 2017, 25, 2–14. [Google Scholar] [CrossRef]
- Wan, N.-F.; Chen, J.; Ji, X.-Y.; Chacón-Labella, J.; Zhang, H.; Fan, N.-N.; Jiang, J.-X.; Li, B. Co-culture of multiple aquatic species enhances vegetable production in coastal Shanghai. J. Clean. Prod. 2019, 241, 118419. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, W.; Su, Y.; Huang, M.; Wang, X.; Yan, B.; Ma, S. Production of pacific white shrimp polycultured with swimming crab at different densities, and nutrient budget in the enclosure system. J. Ocean Univ. China 2022, 21, 171–178. [Google Scholar] [CrossRef]
- Dube, K.; Chanu, T. Organic Aquaculture: Way to sustainable production. In Advances in Fish Research; Narendra Publishing House: New Delhi, India, 2012; pp. 219–229. [Google Scholar]
- Shyne Anand, P.S.; Balasubramanian, C.P.; Lalramchhani, C.; Panigrahi, A.; Gopal, C.; Ghoshal, T.K.; Vijayan, K.K. Comparison of mudcrab-based brackishwater polyculture systems with different finfish species combinations in Sundarban, India. Aquac. Res. 2018, 49, 2965–2976. [Google Scholar] [CrossRef]
- Li, J.Y.; Chang, D.; Li, B.N.; Wu, X.G.; Zhu, Z.W.; Cheng, Y.X. Benefit-cost analysis of different rice-based production systems. J. Fish China 2014, 38, 1431–1438. [Google Scholar]
- Lai, Q.T.; Tuan, V.A.; Thuy, N.T.B.; Huynh, L.D.; Duc, N.M. A closer look into shrimp yields and mangrove coverage ratio in integrated mangrove-shrimp farming systems in Ca Mau, Vietnam. Aquac. Int. 2022. [Google Scholar] [CrossRef]
- Swapan, M.S.H.; Gavin, M. A desert in the delta: Participatory assessment of changing livelihoods induced by commercial shrimp farming in Southwest Bangladesh. Ocean Coast. Manag. 2011, 54, 45–54. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin III, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Balvanera, P.; Pfisterer, A.B.; Buchmann, N.; He, J.-S.; Nakashizuka, T.; Raffaelli, D.; Schmid, B. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 2006, 9, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- BELTON, B.; LITTLE, D. The development of aquaculture in central thailand: Domestic demand versus export-led production. J. Agrar. Change 2008, 8, 123–143. [Google Scholar] [CrossRef]
- Purcell, S.W.; Patrois, J.; Fraisse, N. Experimental evaluation of co-culture of juvenile sea cucumbers, Holothuria scabra (Jaeger), with juvenile blue shrimp, Litopenaeus stylirostris (Stimpson). Aquac. Res. 2006, 37, 515–522. [Google Scholar] [CrossRef]
- Nilsson, J.A.; Johnson, C.R.; Fulton, E.A.; Haward, M. Fisheries sustainability relies on biological understanding, evidence-based management, and conducive industry conditions. ICES J. Mar. Sci. 2019, 76, 1436–1452. [Google Scholar] [CrossRef]
- Costa-Pierce, B.A.; Page, G.G. Aquaculture, Sustainability Science in. In Sustainable Food Production; Christou, P., Savin, R., Costa-Pierce, B.A., Misztal, I., Whitelaw, C.B.A., Eds.; Springer: New York, NY, USA, 2013; pp. 206–222. [Google Scholar]
- Saraiva, J.L.; Arechavala-Lopez, P.; Castanheira, M.F.; Volstorf, J.; Heinzpeter Studer, B. A global assessment of welfare in farmed fishes: The FishEthoBase. Fishes 2019, 4, 30. [Google Scholar] [CrossRef]
- Yusoff, F.M.; Abdullah, A.F.; Aris, A.Z.; Umi, W.A.D. Impacts of COVID-19 on the Aquatic Environment and Implications on Aquatic Food Production. Sustainability 2021, 13, 11281. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hossain, M.Y.; Tanjin, S.; Mawa, Z.; Hasan, M.R.; Jasmine, S. Effects of COVID-19 pandemic on Baor (Oxbow lake) fisheries: Decreased economic livelihoods and food security. Lakes Reserv 2021, 26, e12374. [Google Scholar] [CrossRef]
- Tey, Y.S.; Li, E.; Bruwer, J.; Abdullah, A.M.; Brindal, M.; Radam, A.; Ismail, M.M.; Darham, S. The relative importance of factors influencing the adoption of sustainable agricultural practices: A factor approach for Malaysian vegetable farmers. Sustain. Sci. 2014, 9, 17–29. [Google Scholar] [CrossRef]
- Gao, F. Evolution trend and internal mechanism of regional total factor productivity in Chinese agriculture. J. Quant. Tech. Econ. 2015, 32, 3–19. [Google Scholar]
- Yang, G.; Yang, M.Y. The empirical analysis of correlative effect among China’s agricultural total factor productivity: Based on static and dynamic spatial panel model. Econ. Geogr. 2013, 11, 122–129. [Google Scholar]
- Stieglitz, J.D.; Hoenig, R.H.; Baggett, J.K.; Tudela, C.E.; Mathur, S.K.; Benetti, D.D. Advancing production of marine fish in the United States: Olive flounder, Paralichthys olivaceus, aquaculture. J. World Aquac. Soc. 2021, 52, 566–581. [Google Scholar] [CrossRef]
- Tal, Y.; Schreier, H.J.; Sowers, K.R.; Stubblefield, J.D.; Place, A.R.; Zohar, Y. Environmentally sustainable land-based marine aquaculture. Aquaculture 2009, 286, 28–35. [Google Scholar] [CrossRef]
- Suantika, G.; Situmorang, M.L.; Kurniawan, J.B.; Pratiwi, S.A.; Aditiawati, P.; Astuti, D.I.; Azizah, F.F.N.; Djohan, Y.A.; Zuhri, U.; Simatupang, T.M. Development of a zero water discharge (ZWD)—Recirculating aquaculture system (RAS) hybrid system for super intensive white shrimp (Litopenaeus vannamei) culture under low salinity conditions and its industrial trial in commercial shrimp urban farming in Gresik, East Java, Indonesia. Aquac. Eng. 2018, 82, 12–24. [Google Scholar]
- Zhang, S.-Y.; Li, G.; Wu, H.-B.; Liu, X.-G.; Yao, Y.-H.; Tao, L.; Liu, H. An integrated recirculating aquaculture system (RAS) for land-based fish farming: The effects on water quality and fish production. Aquac. Eng. 2011, 45, 93–102. [Google Scholar] [CrossRef]
- Summerfelt, S. Emerging trends in Salmonid ras part I. closed-containment culture advances. Glob. Aquac. Advocate 2015, 18, 64–65. [Google Scholar]
- Martin, S.J.; Mather, C.; Knott, C.; Bavington, D. ‘Landing’ salmon aquaculture: Ecologies, infrastructures and the promise of sustainability. Geoforum 2021, 123, 47–55. [Google Scholar] [CrossRef]
- Rice, J. Evolution of international commitments for fisheries sustainability. ICES J. Mar. Sci. 2013, 71, 157–165. [Google Scholar] [CrossRef]
- Scanlon, Z. The art of “not undermining”: Possibilities within existing architecture to improve environmental protections in areas beyond national jurisdiction. ICES J. Mar. Sci. 2017, 75, 405–416. [Google Scholar] [CrossRef]
- Thi Da, C.; Anh Tu, P.; Livsey, J.; Tang, V.T.; Berg, H.; Manzoni, S. Improving productivity in integrated fish-vegetable farming systems with recycled fish pond sediments. Agronomy 2020, 10, 1025. [Google Scholar] [CrossRef]
| Concept | Description | Reference |
|---|---|---|
| Agrochemicals | Chemicals used in agriculture including insecticides, fungicides, weedicides, growth promoters, and fertilizers. Agrochemical-based monoculture of aquatic species must be heavily opposed and stopped in the interest of human health and environmental protection. | [33] |
| Agroecology | The application of ecological principles to improve agricultural systems and practices leads to new farming methods that increase yield while reducing environmental impact. | [34] |
| Aquaponics | An aquaculture system that recycles waste produced by combined aquatic animals to provide nutrients for hydroponically grown vegetables and simultaneously purify the water. | [35,36] |
| Aquaculture | The breeding, rearing, and harvesting of aquatic animals or cultivation of aquatic plants for food; essentially, it means farming in water. | [37] |
| Biodiversity | A measure of variation at the genetic, taxonomic, species, and ecosystem levels. | [38] |
| Commensalism | A biological association between two animals, wherein one derives benefits and the other derives neither advantage nor harm. | [39] |
| Ecological engineering | The deliberate design of ecosystems to mutually benefit humans and nature. | [40] |
| Ecological footprint | The impact of an individual human or community directly on the environment; it is expressed as the amount of land area necessary to sustain given resources. | [41] |
| Ecological functions | Various organisms perform different positive ecological functions in a given ecosystem. | [42] |
| Ecological intensification | A knowledge-intensive procedure that involves the optimum functioning of environmental functions and biodiversity aspects to improve the overall performance of the agricultural system and eventually the farmers’ livelihoods. | [16] |
| Ecosystem services | The multitude of benefits that the ecosystem provides to human society. Biodiversity is essential to enforce ecosystem functions and the delivery of ecosystem services. | [43] |
| Facilitation | An ecological process categorized under commensalism interactions that refers to positive species interactions benefitting at least one species and causing harm to neither. | [44] |
| Integrated agriculture–aquaculture (IAA) | Combining two or more activities that involve agriculture, aquaculture, and household components. | [45] |
| Integrated multi-trophic aquaculture (IMTA) | A concept that harnesses the food chain, wherein the waste generated by one species nourishes another. This farming approach involves culturing a fed species in combination with an extractive species that can use the waste from the former as nourishment for their growth. | [46] |
| Monoculture | Farming a single species at any intensity, either in water or land. | [47] |
| Mutualism | A common type of positive ecological interaction that can be categorized into either facultative or obligate interactions, wherein both combined species derive overall benefits. | [39] |
| Polyculture | Sustainable agriculture approach that involves the simultaneous culture of several species in one location based on the ecological principle of species diversity designed by imitating natural ecosystems. | [48] |
| Recirculated aquaculture system (RAS) | An indoor aquaculture system that purifies and recirculates the same water. It has a low footprint and allows safe year-round farming under controlled conditions. | [49,50] |
| Resilience | In an ecological context, resilience is the ability of an ecosystem to overcome disturbances or stresses by reorganizing to maintain normal functioning while undergoing changes. | [51] |
| Species diversity | Overview of the number of different species that coexist in an ecosystem and their relative abundances. | [24] |
| Species compatibility | A phenomenon wherein combined taxa can co-exist in the same ecosystem without negative or detrimental interactions (predation, parasitism) or competition for resources (space, food, or shelter). | [52] |
| Species complementarity | The capacity of a combined species to utilize different parts of accessible resources that include by-products of combined species, or their capacity to exhibit positive interactions (either mutualistic/commensal) and contribute to the sustainability of the aquaculture systems. | [52] |
| Sustainability | Achieving economic, ecological, social, and human needs without compromising the loss of essential resources required for future generations. | [14] |
| Aquaculture Case Studies | Consequences for Sustainability | Process Implications | References |
|---|---|---|---|
| Basic Complementarity | |||
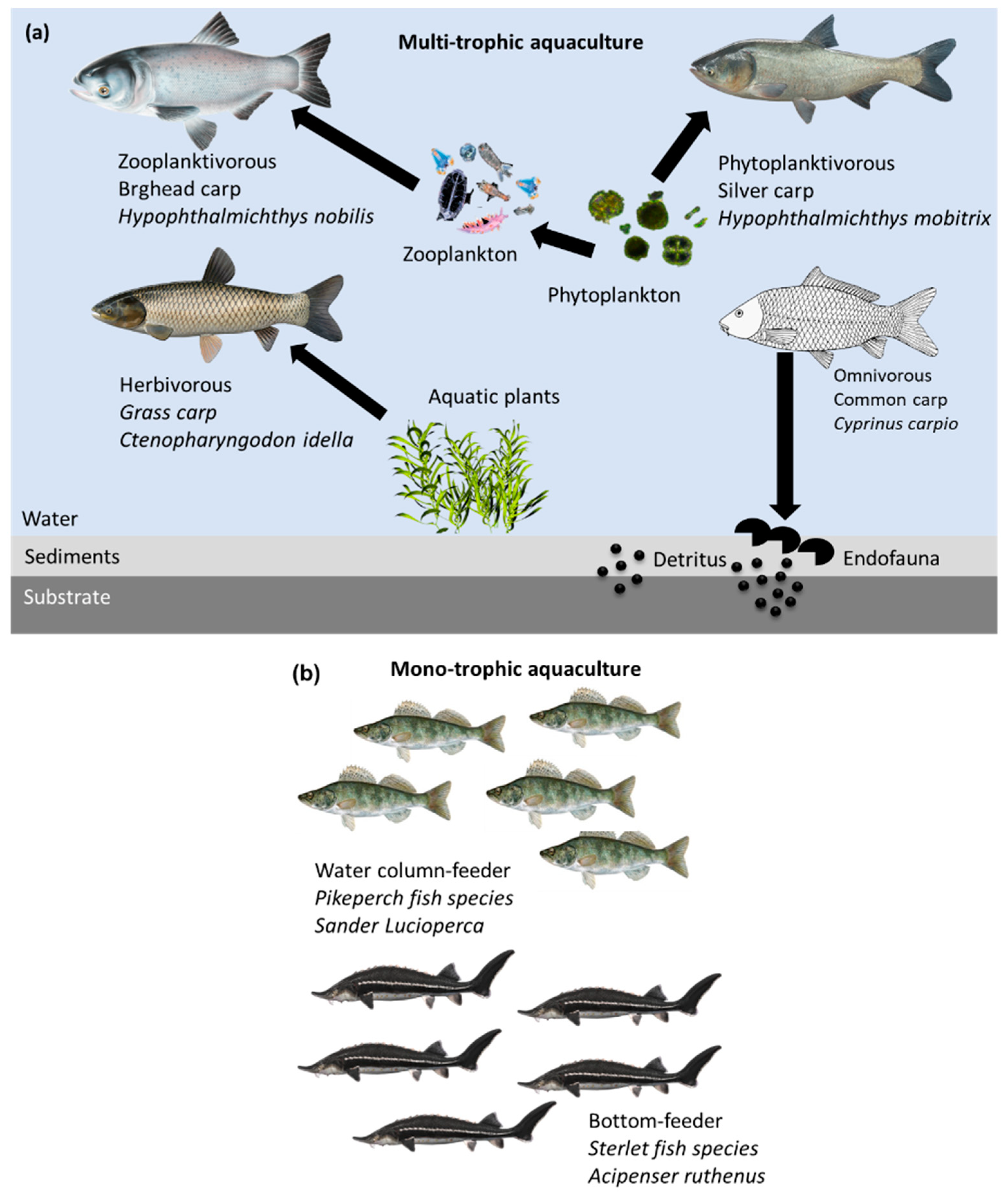
| Polyculture of juvenile pikeperch fish and sterlet fish in an RAS. | Requires less labor than that required to grow the same species in monoculture systems. | Benthic trophic behavior of starlet fish allows them to use food that accumulates at the bottom. | [63] |
| Polyculture system study effect of behavior on the production of pikeperch in an RAS. | Upgrades production performance, increasing mass from 25 to 51% compared to that in a monoculture system. | Improves spatial resource use and decreases competition for trophic resources among combined fish species. | [76] |
| Freshwater aquaculture of fish species in cage systems ensures the consumption of non-consumed food dispersed in the internal cages. | Maintains water quality without risk of the fish escaping. Suitable for exotic farm species. | Polyculture system for fish in multi-layered cages, suitable for high-value sturgeon (Acipenser sp.) in the internal cage area and cyprinids (silver carp (Hypophthalmichthys molitrix) and bighead carp (Hypophthalmichthys nobilis), and in the external cages considering crucian carp (Carassius auratus)). | [72] |
| Enhanced Complementarity | |||
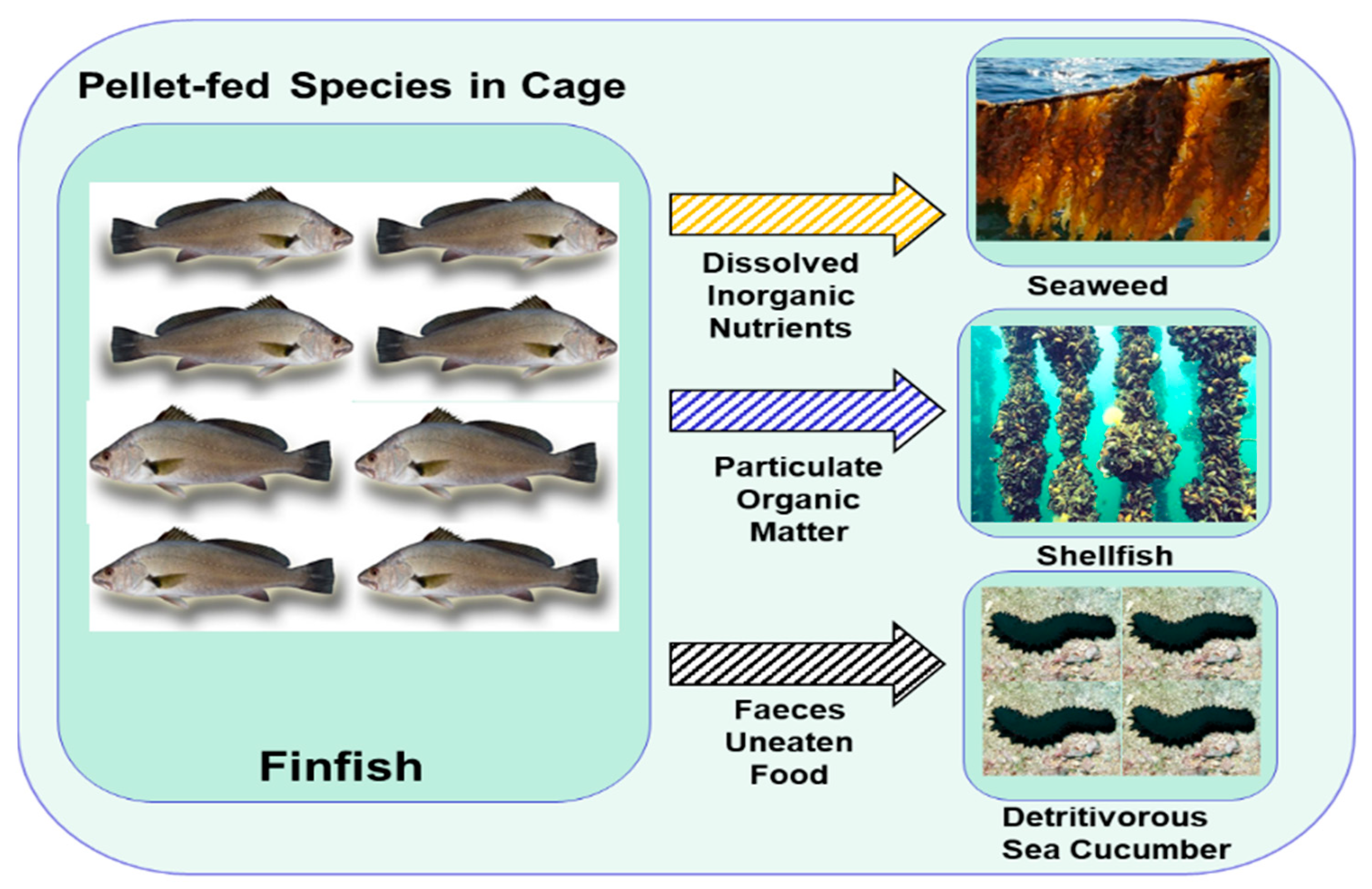
| The IMTA system: farming of pellet-fed aquaculture species, e.g., shrimp or fish species whose extracts dissolve organic or particulate matter, e.g., fish, mollusks, and echinoderms; extracts or dissolved organic–inorganic matter is feed for species e.g., microorganisms, macrophytes, and macro-algae. | Maintains water quality and improves nutrient cycling within IMTA systems. Improves diversification of production: About 50% of feed proteins are converted into consumable protein. | Combines several species based on ecological functionality. | [46] |
| IMTA system: intensive and semi-intensive aquaculture system: prawn (Macrobrachium amazonicum) and tilapia (Oreochromis niloticus). | Helps to improve nutrient cycling and diversification of aquatic food products. | Combines several species based on ecological functionality that ensures that co-products of one species function as a feed source for the other. | [77] |
| IMTA system: iliophagus (Prochilodus lineatus) integrated with benthic shrimp (Macrobrachium amazonicum) and pelagic fish (Colossoma macropomum). | Helps improve nutrient cycling in aquaculture ponds because the waste of one species is a valuable feed for the other species. It also helps improve diversification production by increasing total species yields by about 35%. | Combining fed fish species with two other fish species fed on the wasted feed and waste of another species. | [78] |
| IMTA system: involves the aquaculture of fish (Sebastes schlegeli), shellfish (Haliotis discus hannai), and sea cucumber (Apostichopus japonicas) in an RAS. | This system helps improve nutrient cycling. Aquaculture of shellfish and sea cucumber is also known to improve nitrogen and phosphate budgets. It improves diversification and increases the growth rate compared to those of monoculture systems. | Combining different species in aquaculture based on their diverse feeding habits and ecological niches, e.g., pellet-fed species with deposit-fed species. | [79] |
| IMTA system: a combination of several plant species, e.g., water lettuce (Pistia spp.), water convolvulus (Ipomea aquatica), and water hyacinth (Eichhornia crassipes) along with combined fish, e.g., grass carp (Ctenopharyngodon idella) or shellfish species. | Helps maintain the water quality. The plant species are involved in removing total nitrogen and total phosphorus while decreasing chemical oxygen demand. This system increases the yield of grass carp, improves survival rate, and reduces drug use compared to conventional aquaculture systems run without aquatic plant species. Production of value-added products (salads, vegetables). | In addition to their enhanced water purification efficiency, plants consume nutrients released by fish or shellfish species. Microbes that grow on the roots of plants degrade pollutants and reduce excess nutrients dissolved in water (N and P). Plants also provide natural habitats for fish and shellfish species. | [40,80] |
| Integrated Agriculture–Aquaculture (IAA) | |||
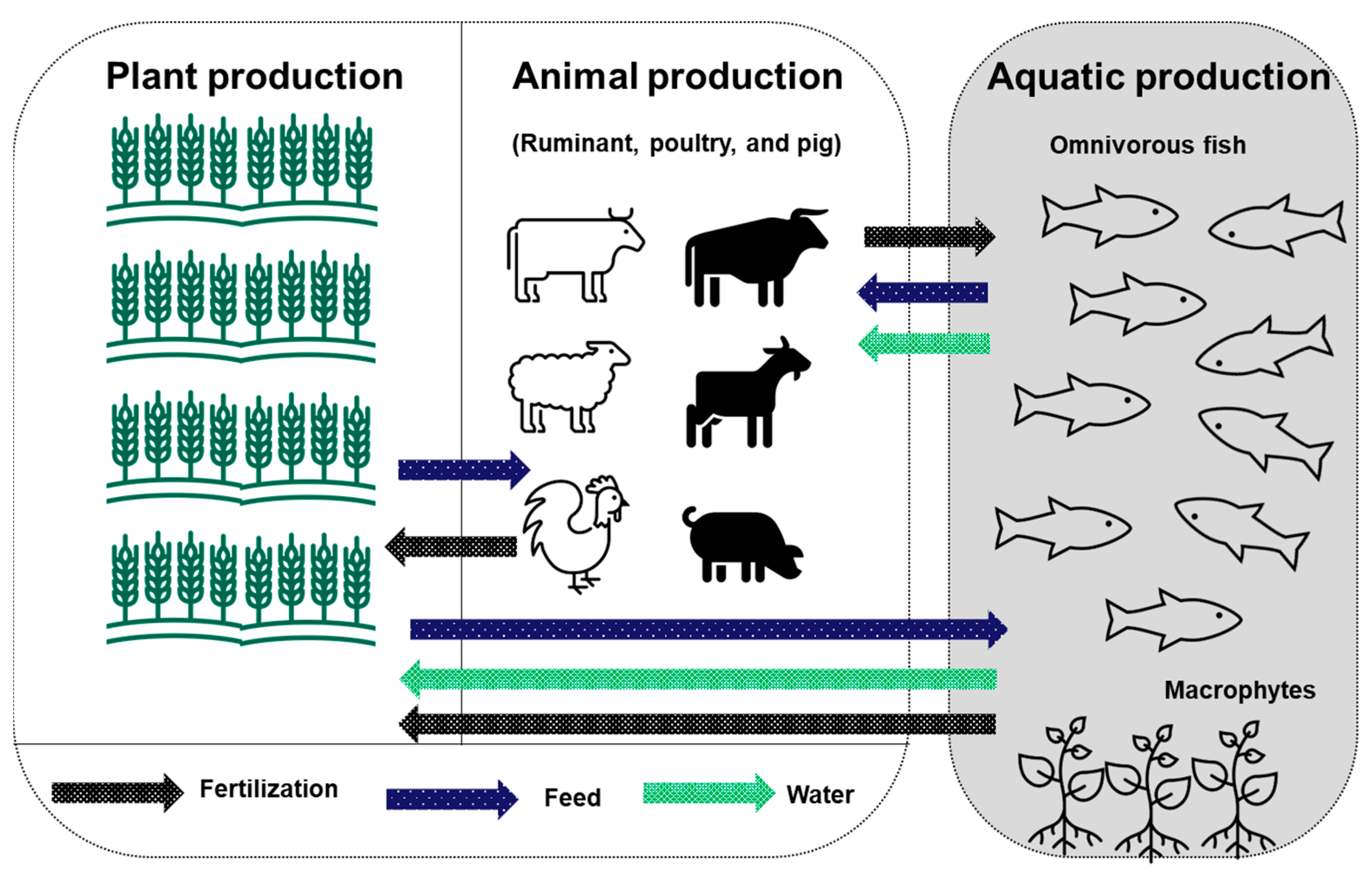
| IAA system: maintaining the diversity of integrated rice and fish production. | Improves diversification of farm products and increases economic feasibility. | Combines several species and maintains diversity based on ecological functioning. | [33] |
| IAA system: using waste from livestock and other agricultural practices to fertilize fishponds. Applying fishpond sediments to fertilize agricultural land. Using by-products of the crop to feed livestock (cattle, poultry, and pigs) and combined fish species. | Helps recycle nutrients from terrestrial agricultural systems to aquaculture systems and vice versa, either directly or indirectly. Helps increase the yield of livestock and aquaculture. | Combines several species based on their ecological functions. | [55,81] |
| IAA system: integrating terrestrial rice farming with freshwater prawn (Macrobrachium rosenbergii). | Improves diversification of production and increases economic feasibility. | The IAA system is multi-spatial and utilizes both water and soil resource utilization more efficiently than monoculture systems. | [82] |
| Commensal Relationship | |||
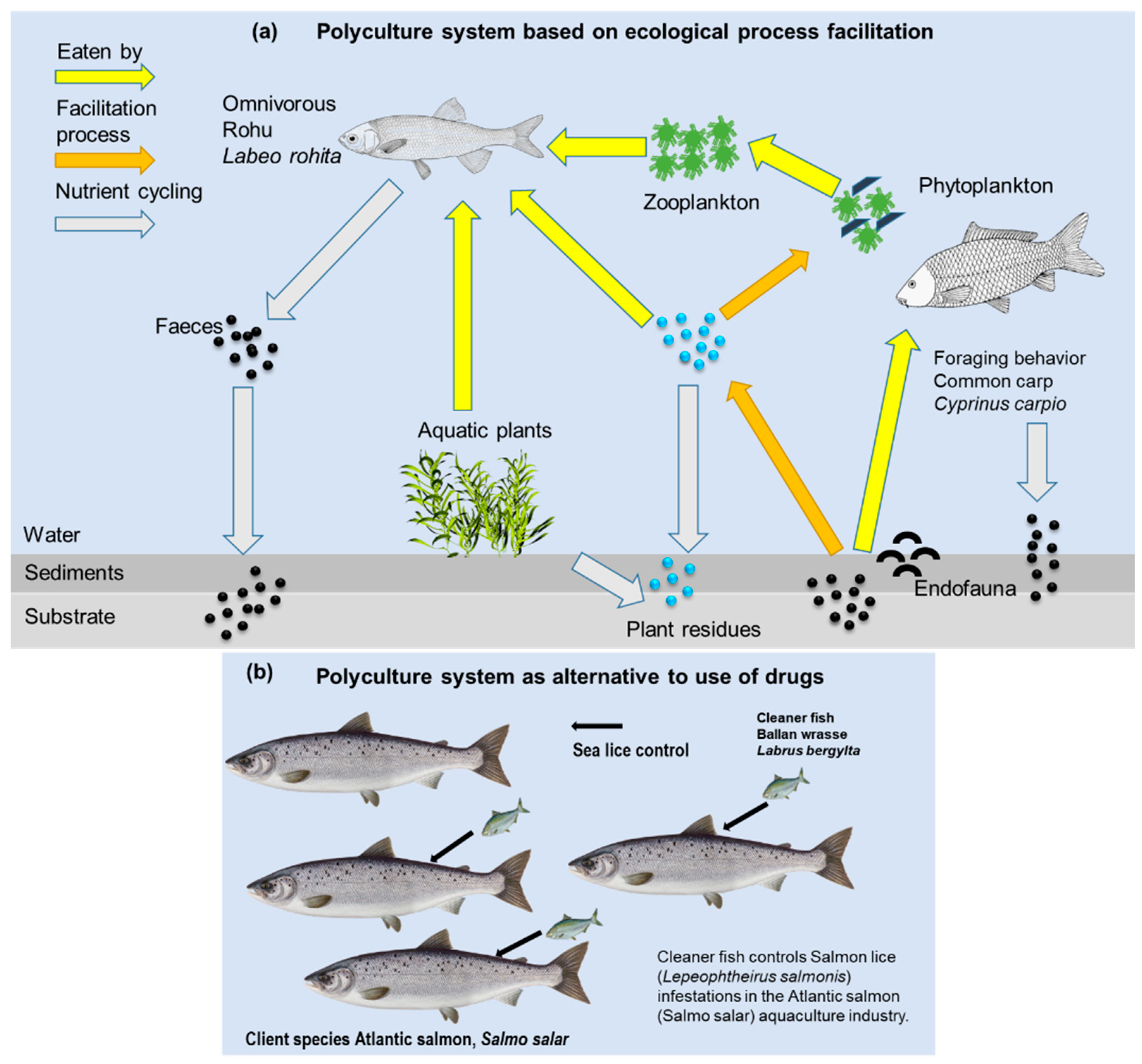
| Commensal relationship: Combines carp (Cyprinus carpio) and rohu (Labeo rohita) in a pond. | Increases productivity by approximately 40% compared to rohu monoculture. | Facilitation relationship: redispersion of feed by the carp species. | [83] |
| Commensal relationship: polyculture of omnivorous fish (Takifugu obscurus) and white shrimp (Litopenaeus vannamei). | Increases productivity owing to better animal welfare and health conditions. Improves the resistance of shrimp species and protects them against diseases. Improves the survival rate of shrimp species. | Antibacterial and antifungal properties of fish mucus improve the survival rate of white shrimp species. | [84] |
| Commensal relationship: Between jellyfish and juvenile carangids in coral reef habitats. | Protects juvenile carangids from predators because they hide in the vicinity of jellyfish tentacles that are used as a shield against potential predators. | The coral reef habitat facilitates the commensal relationship between juvenile carangid and jellyfish, allowing carangid fish to sustain their stock. | [85] |
| Commensal relationship: polyculture of grey mullet (Mugil cephalus) with white shrimp (Litopenaeus vannamei). | Improves farm performance and water quality. Decreases parasite attacks in polyculture because of low amounts of total organic matter and sediments in the water. | Involves species that improve the quality of the farm environment due to their commensal feeding behavior and diet habits. | [86] |
| Mutualism: polyculture system for cleaner shrimp (Lysmata vittata) and orange-spotted grouper (Epinephelus coioides). | Ensures environmental protection because combining these species in culture is a safer alternative than using chemical treatments for parasitic diseases. It also helps to improve diversification of production, thereby increasing economic feasibility. Cleaner shrimp species have high-value ornamental purposes. This species feeds on fish parasites. | Involves species that act as natural predators by feeding on parasites and other pathogens. It provides a safe alternative to chemical treatment. | [87] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-Y.; Shinde, S.K.; Kadam, A.A.; Saratale, R.G.; Saratale, G.D.; Kumar, M.; Syed, A.; Bahkali, A.H.; Ghodake, G.S. RETRACTED: Advantage of Species Diversification to Facilitate Sustainable Development of Aquaculture Sector. Biology 2022, 11, 368. https://doi.org/10.3390/biology11030368
Kim D-Y, Shinde SK, Kadam AA, Saratale RG, Saratale GD, Kumar M, Syed A, Bahkali AH, Ghodake GS. RETRACTED: Advantage of Species Diversification to Facilitate Sustainable Development of Aquaculture Sector. Biology. 2022; 11(3):368. https://doi.org/10.3390/biology11030368
Chicago/Turabian StyleKim, Dae-Young, Surendra Krushna Shinde, Avinash Ashok Kadam, Rijuta Ganesh Saratale, Ganesh Dattatraya Saratale, Manu Kumar, Asad Syed, Ali H. Bahkali, and Gajanan Sampatrao Ghodake. 2022. "RETRACTED: Advantage of Species Diversification to Facilitate Sustainable Development of Aquaculture Sector" Biology 11, no. 3: 368. https://doi.org/10.3390/biology11030368
APA StyleKim, D.-Y., Shinde, S. K., Kadam, A. A., Saratale, R. G., Saratale, G. D., Kumar, M., Syed, A., Bahkali, A. H., & Ghodake, G. S. (2022). RETRACTED: Advantage of Species Diversification to Facilitate Sustainable Development of Aquaculture Sector. Biology, 11(3), 368. https://doi.org/10.3390/biology11030368








