Bray-Call Sequences in the Mediterranean Common Bottlenose Dolphin (Tursiops truncatus) Acoustic Repertoire
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Acoustic Analysis
2.4. Descriptive and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kershenbaum, A.; Blumstein, D.T.; Roch, M.A.; Akçay, Ç.; Backus, G.; Bee, M.A.; Bohn, K.; Cao, Y.; Carter, G.; Caesar, C.; et al. Acoustic sequences in non-human animals: A tutorial review and prospectus. Biol. Rev. 2016, 91, 13–52. [Google Scholar] [CrossRef]
- Gerhardt, H.C.; Huber, F. Acoustic Communication in Insects and Anurans: Common Problems and Diverse Solutions; Chicago University Press: Chicago, IL, USA, 2002. [Google Scholar]
- Gentner, T.Q.; Hulse, S.H. Perceptual mechanisms for individual vocal recognition in European starlings, Sturnus vulgaris. Anim. Behav. 1998, 56, 579–594. [Google Scholar] [CrossRef]
- Root-Gutteridge, H.; Bencsik, M.; Chebli, M.; Gentle, L.K.; Terrell-Nield, C.; Bourit, A.; Yarnell, R.W. Identifying individual wild Eastern grey wolves (Canis lupus lycaon) using fundamental frequency and amplitude of howls. Bioacoustics 2014, 23, 55–66. [Google Scholar] [CrossRef]
- Sayigh, L.S.; Esch, H.C.; Wells, R.S.; Janik, V.M. Facts about signature whistles of bottlenose dolphins, Tursiops truncatus. Anim. Behav. 2007, 74, 1631–1642. [Google Scholar] [CrossRef]
- Courts, R.; Erbe, C.; Wellard, R.; Boisseau, O.; Jenner, K.C.; Jenner, M.N. Australian long-finned pilot whales (Globicephala melas) emit stereotypical, variable, biphonic, multi-component, and sequenced vocalisations, similar to those recorded in the northern hemisphere. Sci. Rep. 2020, 10, 20609. [Google Scholar] [CrossRef] [PubMed]
- Slocombe, K.E.; Zuberbuhler, K. Food-associated calls in chimpanzees: Responses to food types or food preferences? Anim. Behav. 2006, 72, 989–999. [Google Scholar] [CrossRef]
- Blumstein, D.T. The evolution, function, and meaning of marmot alarm communication. Adv. Study Behav. 2007, 37, 371–401. [Google Scholar]
- Schel, A.M.; Tranquilli, S.; Zuberbühler, K. The alarm call system of two species of black-and-white colobus monkeys (Colobus polykomos and Colobus guereza). J. Comp. Psychol. 2009, 123, 136–150. [Google Scholar] [CrossRef]
- Gero, S.; Whitehead, H.; Rendell, L. Individual, unit and vocal clan level identity cues in sperm whale codas. R. Soc. Open Sci. 2016, 3, 150372. [Google Scholar] [CrossRef] [PubMed]
- Pace, D.S.; Lanfredi, C.; Airoldi, S.; Giacomini, G.; Silvestri, M.; Pavan, G.; Ardizzone, D. Trumpet sounds emitted by male sperm whales in the Mediterranean Sea. Sci. Rep. 2021, 11, 5867. [Google Scholar] [CrossRef]
- Ford, J.K. Vocal traditions among resident killer whales (Orcinus orca) in coastal waters of British Columbia. Can. J. Zool. 1991, 69, 1454–1483. [Google Scholar] [CrossRef]
- Payne, R.S.; Mcvay, S. Songs of humpback whales. Science 1971, 173, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Zwamborn, E.M.J.; Whitehead, H. Repeated call sequences and behavioural context in long-finned pilot whales off Cape Breton, Nova Scotia, Canada. Bioacoustics 2017, 26, 169–183. [Google Scholar] [CrossRef]
- Caldwell, M.C.; Caldwell, D.K. Individualized whistle contours in bottlenose dolphins (Tursiops truncatus). Nature 1965, 207, 434–435. [Google Scholar] [CrossRef]
- Janik, V.M.; King, S.L.; Sayigh, L.S.; Wells, R.S. Identifying signature whistles from recordings of groups of unrestrained bottlenose dolphins (Tursiops truncatus). Mar. Mamm. Sci. 2013, 29, 109–122. [Google Scholar] [CrossRef]
- Luís, A.R.; Alves, I.S.; Sobreira, F.V.; Couchinho, M.N.; dos Santos, M.E. Brays and bits: Information theory applied to acoustic communication sequences of bottlenose dolphins. Bioacoustics 2019, 28, 286–296. [Google Scholar] [CrossRef]
- Jones, B.; Zapetis, M.; Samuelson, M.M.; Ridgway, S. Sounds produced by bottlenose dolphins (Tursiops truncatus): A review of the defining characteristics and acoustic criteria of the dolphin vocal repertoire. Bioacoustics 2019, 29, 399–440. [Google Scholar] [CrossRef]
- Dos Santos, M.E.; Caporin, G.; Moreira, H.O.; Ferreira, A.J.; Coelho, J.L.B. Acoustic behavior in a local population of bottlenose dolphins. In Sensory Abilities of Cetaceans; Thomas, J.A., Kastelein, R.A., Eds.; Springer: Boston, MA, USA, 1990; pp. 585–598. [Google Scholar]
- Dos Santos, M.E.; Ferreira, A.J.; Harzen, S. Rhythmic sound sequences emitted by aroused bottlenose dolphins in the Sado Estuary, Portugal. In Sensory Systems of Aquatic Mammals; Evans, P.G.H., Nice, H., Eds.; De Spil Publishers: Woerden, The Netherlands, 1995; pp. 325–334. [Google Scholar]
- López, B.; Shirai, J. Mediterranean common bottlenose dolphin’s repertoire and communication use. In Dolphins: Anatomy, Behavior, and Threats; Pierce, L., Correa, A.G., Eds.; Nova Science Publishers: New York, NY, USA, 2009; pp. 129–148. [Google Scholar]
- Gridley, T.; Nastasi, A.; Kriesell, H.J.; Elwen, S.H. The acoustic repertoire of wild common bottlenose dolphins (Tursiops truncatus) in Walvis Bay, Namibia. Bioacoustics 2015, 24, 153–174. [Google Scholar] [CrossRef]
- Herzing, D.L. Synchronous and rhythmic vocalizations and correlated underwater behavior of free-ranging atlantic spotted dolphins (Stenella frontalis) and bottlenose dolphins (Tursiops truncatus) in the Bahamas. Anim. Behav. Cogn. 2015, 2, 14–29. [Google Scholar] [CrossRef]
- Janik, V.M. Food-related bray calls in wild bottlenose dolphins (Tursiops truncatus). Proc. R. Soc. B 2000, 267, 923–927. [Google Scholar] [CrossRef] [PubMed]
- King, S.L.; Janik, V.M. Come dine with me: Food-associated social signalling in wild bottlenose dolphins (Tursiops truncatus). Anim Cogn. 2015, 18, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Pace, D.S.; Di Marco, C.; Giacomini, G.; Ferri, S.; Silvestri, M.; Papale, E.; Casoli, E.; Ventura, D.; Mingione, M.; Alaimo Di Loro, P.; et al. Capitoline dolphins: Residency patterns and abundance estimate of Tursiops truncatus at the Tiber River estuary (Mediterranean Sea). Biology 2021, 10, 275. [Google Scholar] [CrossRef]
- Pace, D.S.; Giacomini, G.; Campana, I.; Paraboschi, M.; Pellegrino, G.; Silvestri, M.; Alessi, J.; Angeletti, D.; Cafaro, V.; Pavan, G.; et al. An integrated approach for cetacean knowledge and conservation in the central Mediterranean Sea using research and social media data sources. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1302–1323. [Google Scholar] [CrossRef]
- Triossi, F.; Willis, T.J.; Pace, D.S. Occurrence of bottlenose dolphins Tursiops truncatus in natural gas fields of the northwestern Adriatic S ea. Mar. Ecol. 2013, 34, 373–379. [Google Scholar] [CrossRef]
- Martino, S.; Pace, D.S.; Moro, S.; Casoli, E.; Ventura, D.; Frachea, A.; Silvestri, M.; Arcangeli, A.; Giacomini, G.; Ardizzone, G.D.; et al. Integration of presence-only data from several data sources. A case study on dolphins’ spatial distribution. Ecography 2021, 44, 1533–1543. [Google Scholar] [CrossRef]
- Papale, E.; Ceraulo, M.; Giardino, G.; Buffa, G.; Filiciotto, F.; Grammauta, R.; Maccarone, V.; Mazzola, S.; Buscaino, G. Association patterns and population dynamics of bottlenose dolphins in the Strait of Sicily (Central Mediterranean Sea) implication for management. Soc. Popul. Ecol. 2016, 59, 55–64. [Google Scholar] [CrossRef]
- Papale, E.; Alonge, G.; Grammauta, R.; Ceraulo, M.; Giacoma, C.; Mazzola, S.; Buscaino, G. Year-round acoustic patterns of dolphins and interaction with anthropogenic activities in the Sicily Strait, central Mediterranean Sea. Ocean Coast. Manag. 2020, 197, 105320. [Google Scholar] [CrossRef]
- Parra, G.J. Resource partitioning in sympatric delphinids: Space use and habitat preferences of Australian snubfin and Indo-Pacific humpback dolphins. J. Anim. Ecol. 2006, 75, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Mann, J. Behavioral sampling methods for cetaceans: A review and critique. Mar. Mamm. Sci. 1999, 15, 102–122. [Google Scholar] [CrossRef]
- Mariani, M.; Miragliuolo, A.; Mussi, B.; Russo, G.F.; Ardizzone, G.; Pace, D.S. Analysis of the natural markings of Risso’s dolphins (Grampus griseus) in the central Mediterranean Sea. J. Mammal. 2016, 97, 1512–1524. [Google Scholar] [CrossRef]
- Corkeron, P.J.; Minton, G.; Collins, T.; Findlay, K.; Willson, A.; Baldwin, R. Spatial models of sparse data to inform cetacean conservation planning: An example from Oman. Endang. Species Res. 2011, 15, 39–52. [Google Scholar] [CrossRef][Green Version]
- Papale, E.; Alonge, G.; Caruso, F.; Grammauta, R.; Mazzola, S.; Mussi, B.; Pace, D.S.; Buscaino, G. The higher, the closer, the better? Influence of sampling frequency and distance on the acoustic properties of short-beaked common dolphins burst pulses in the Mediterranean Sea. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 51–60. [Google Scholar] [CrossRef]
- Center for Conservation Bioacoustics. Raven Pro: Interactive Sound Analysis Software, Version 2.0; Cornell Laboratory of Ornithology: Ithaca, NY, USA, 2019; Available online: https://ravensoundsoftware.com/ (accessed on 18 December 2021).
- Connor, R.C.; Smolker, R.A. Pop’ goes the dolphin: A vocalization male bottlenose dolphin produced during courtships. Behaviour 1996, 133, 643–662. [Google Scholar]
- Gottman, J.M.; Roy, A.K. Sequential Analysis; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Shannon, C.E. 1948. A mathematical theory of communication. Bell. Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Fedurek, P.; Zuberbühler, K.; Semple, S. Trade-offs in the production of animal vocal sequences: Insights from the structure of wild chimpanzee pant hoots. Front. Zool. 2017, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Luís, A.R.; Couchinho, M.N.; dos Santos, M.E. A quantitative analysis of pulsed signals emitted by wild bottlenose dolphins. PLoS ONE 2016, 11, e0157781. [Google Scholar] [CrossRef] [PubMed]
- Simard, P.; Lace, N.; Gowans, S.; Quintana-Rizzo, E.; Kuczaj, A.S.; Wells, S.R.; Mann, A.D. Low frequency narrow-band calls in bottlenose dolphins (Tursiops truncatus): Signal properties, function, and conservation implications. J. Acoust. Soc. Am. 2011, 130, 3068–3076. [Google Scholar] [CrossRef]
- Barbosa, M.; Bittencourt, L.; Bisi, T.L.; Lailson-Brito, J.; Azevedo, A.F. Characterisation and classification method of burst pulses produced by Guiana dolphins (Sotalia guianensis). Bioacoustics 2022, 31, 69–80. [Google Scholar] [CrossRef]
- Fischer, J.; Hammerschmidt, K. Functional referents and acoustic similarity revisited: The case of Barbary macaque alarm calls. Anim Cogn. 2001, 4, 29–35. [Google Scholar] [CrossRef]
- Schehka, S.; Esser, K.H.; Zimmermann, E. Acoustical expression of arousal in conflict situations in tree shrews (Tupaia belangeri). J. Comp. Physiol. A 2007, 193, 845–852. [Google Scholar] [CrossRef]
- Wiley, R.H. Errors, exaggeration, and deception in animal communication. In Behavioral Mechanisms in Evolutionary Ecology; Real, L., Ed.; University of Chicago Press: Chicago, IL, USA, 1994; pp. 157–189. [Google Scholar]
- Payne, R.J.H.; Pagel, M. Why do animals repeat displays? Anim. Behav. 1997, 54, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Manser, M.B. The acoustic structure of suricates’ alarm calls varies with predator type and the level of response urgency. Proc. R. Soc. B 2001, 268, 2315–2324. [Google Scholar] [CrossRef] [PubMed]
- Schel, A.M.; Candiotti, A.; Zuberbühler, K. Predator-deterring alarm call sequences in Guereza colobus monkeys are meaningful to conspecifics. Anim. Behav. 2010, 80, 799–808. [Google Scholar] [CrossRef]
- Lemasson, A.; Ouattara, K.; Bouchet, H.; Zuberbühler, K. Speed of call delivery is related to context and caller identity in Campbell’s monkey males. Sci. Nat. 2010, 97, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Macedonia, J.M. What is communicated in the antipredator calls of lemurs: Evidence from playback experiments with ring tailed and ruffed lemurs. Ethology 1990, 86, 177–190. [Google Scholar] [CrossRef]
- Guo, D.; Ding, J.; Liu, H.; Zhou, L.; Feng, J.; Luo, B.; Liu, Y. Social calls influence the foraging behavior in wild big-footed myotis. Front. Zool. 2021, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.J.O.; Biassoni, N.; Samuels, A.; Tyack, P.L. Whale songs lengthen in response to sonar. Nature 2000, 405, 903. [Google Scholar] [CrossRef] [PubMed]
- Foote, A.D.; Osborne, R.W.; Hoelzel, A.R. Whale-call response to masking boat noise. Nature 2004, 428, 910. [Google Scholar] [CrossRef] [PubMed]
- Budenz, T.; Heib, S.; Kusch, J. Functions of bat social calls: The influence of local abundance, interspecific interactions and season on the production of pipistrelle (Pipistrellus pipistrellus) type D social calls. Acta Chiropt. 2009, 11, 173–182. [Google Scholar] [CrossRef]
- Ouattara, K.; Lemasson, A.; Zuberbühler, K. Campbell’s monkeys concatenate vocalizations into context-specific call sequences. Proc. Natl. Acad. Sci. USA 2009, 106, 22026–22031. [Google Scholar] [CrossRef] [PubMed]
- Searcy, W.A. Song repertoire and mate choice in birds. Am. Zool. 1992, 32, 71–80. [Google Scholar] [CrossRef]
- Luís, A.R.; May-Collado, L.J.; Rako-Gospić, N.; Gridley, T.; Papale, E.; Azevedo, A.; Silva, M.A.; Buscaino, G.; Herzing, D.; dos Santos, M.E. Vocal universals and geographic variations in the acoustic repertoire of the common bottlenose dolphin. Sci. Rep. 2021, 11, 11847. [Google Scholar] [CrossRef]
- Pace, D.S.; Arcangeli, A.; Mussi, B.; Vivaldi, C.; Ledon, C.; Lagorio, S.; Giacomini, G.; Pavan, G.; Ardizzone, G. Habitat suitability modeling in different sperm whale social groups. J. Wildl. Manag. 2018, 82, 1062–1073. [Google Scholar] [CrossRef]
- Azzolin, M.; Gannier, A.; Papale, E.; Papale, B.G.; Mussi, B.; Ardizzone, G.; Giacoma, C.; Pace, D.S. Whistle variability of the Mediterranean short beak common dolphin. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 36–50. [Google Scholar] [CrossRef]
- Gregorietti, M.; Papale, E.; Ceraulo, M.; de Vita, C.; Pace, D.S.; Tranchida, G.; Mazzola, S.; Buscaino, G. Acoustic Presence of Dolphins through Whistles Detection in Mediterranean Shallow Waters. J. Mar. Sci. Eng. 2021, 9, 78. [Google Scholar] [CrossRef]
- Connor, R.C.; Wells, R.S.; Mann, J.; Read, A.J. The bottlenose dolphin: Social relationships in a fission–fusion society. In Cetacean Societies: Field Studies of Dolphins and Whales; Mann, J., Connor, R.C., Tyack, P.L., Whitehead, H., Eds.; University of Chicago Press: Chicago, IL, USA, 2000; pp. 91–126. [Google Scholar]
- Fury, C.A.; Harrison, P.L. Abundance, site fidelity and range patterns of Indo-Pacific bottlenose dolphins (Tursiops aduncus) in two Australian subtropical estuaries. Mar. Freshw. Res. 2008, 59, 1015–1027. [Google Scholar] [CrossRef]
- Rossman, S.; Ostrom, P.H.; Stolen, M.; Barros, N.B.; Gandhi, H.; Stricker, C.A.; Wells, R.S. Individual specialization in the foraging habits of female bottlenose dolphins living in a trophically diverse and habitat rich estuary. Oecologia 2015, 178, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Janik, V.M.; Slater, P.J.B. The different roles of social learning in vocal communication. Anim. Behav. 2000, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, E.R. Geographic variations in the whistles of bottlenose dolphins (Tursiops truncatus) along the east and west coasts of Australia. J. Acoust. Soc. Am. 2010, 128, 924–935. [Google Scholar] [CrossRef]
- La Manna, G.; Gospić, R.N.; Manghi, M.; Picciulin, M.; Sarà, G. Assessing geographical variation on whistle acoustic structure of three Mediterranean populations of common bottlenose dolphin (Tursiops truncatus). Behaviour 2017, 154, 583–607. [Google Scholar] [CrossRef]
- Papale, E.; Gamba, M.; Perez-Gil, M.; Martin, V.M.; Giacoma, C. Dolphins adjust species-specific frequency parameters to compensate for increasing background noise. PLoS ONE 2015, 10, e0121711. [Google Scholar]
- Wang, D.W.; Würsig, B.; Evans, W.E. Whistles of bottlenose dolphins: Comparisons among populations. Aquat. Mamm. 1995, 21, 65–77. [Google Scholar]
- Méndez-Cárdenas, M.; Randrianambinina, B.; Rabesandratana, B.; Rasoloharijaona, S.; Zimmermann, E. Geographic variation in loud calls of sportive lemurs (Lepilemur ssp.) and their implications for conservation. Am. J. Primatol. 2008, 70, 828–838. [Google Scholar] [CrossRef]
- Pace, D.S.; Mussi, B.; Gordon, J.C.; Wurtz, M. Foreword. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 24, 1–3. [Google Scholar] [CrossRef]
- Pedrazzi, G.; Giacomini, G.; Pace, D.S. First Report of Epimeletic and Acoustic Behavior in Mediterranean Common Bottlenose Dolphins (Tursiops truncatus) Carrying Dead Calves. Biology 2022, 11, 337. [Google Scholar] [CrossRef]

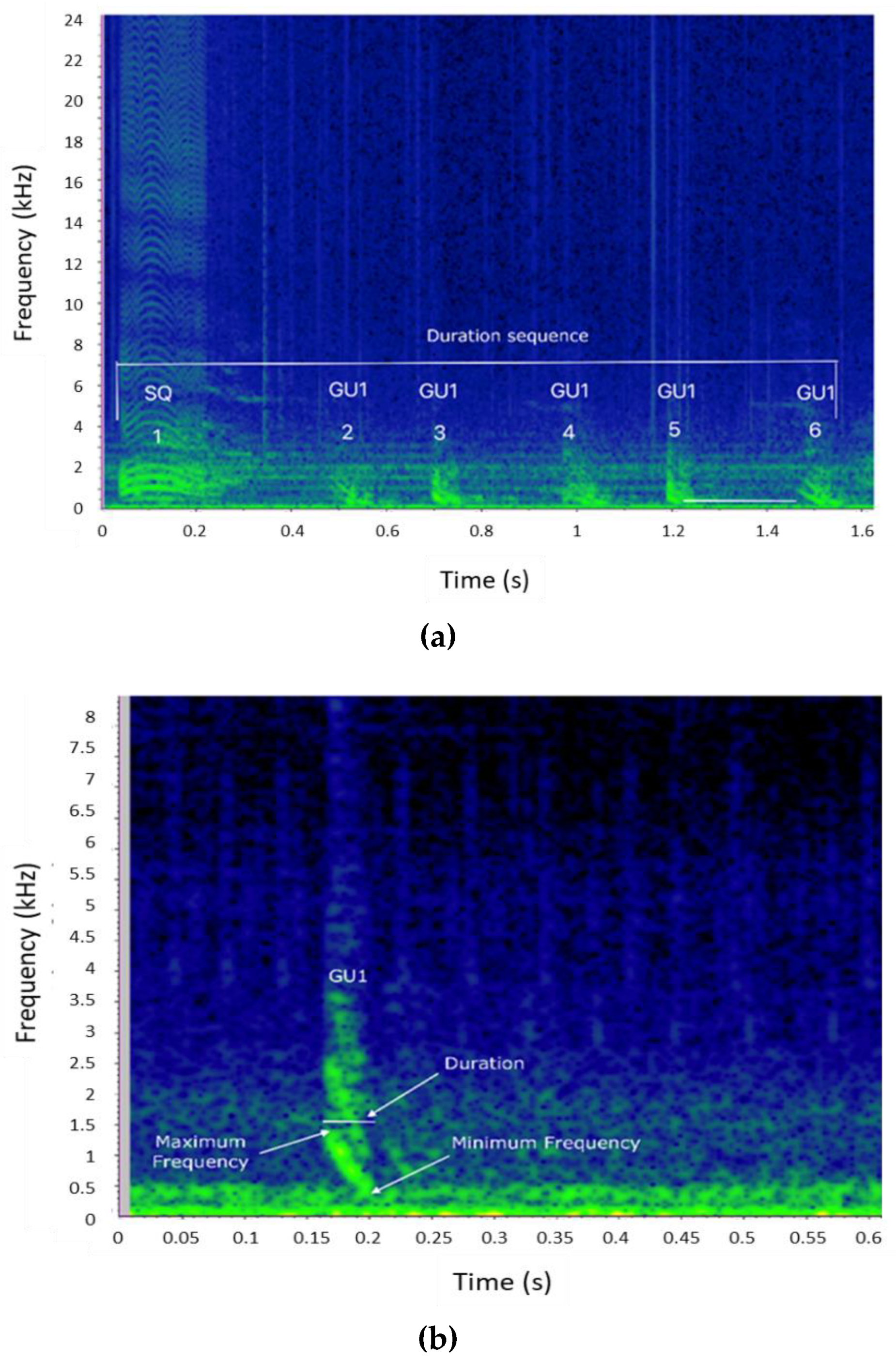

| Site | Platform and Survey Methods | Acoustic Equipment | Recording Effort | Year |
|---|---|---|---|---|
| Strait of Sicily | Boat-based survey using a motorboat powered by a four-stroke 100 HP outboard engine, in suitable weather conditions (sea state < 4 Douglas, wind force Beaufort < 4, no rain, no fog), at a steady speed of 6–8 kn. Both non-systematic haphazard (sensu [35]) and systematic sampling procedure. More details in [30,31]. | One omnidirectional hydrophone Bruel e Kjer (Nærum, Denmark) model 8104 (sensitivity -205.6 dB re 1 V/1 μPa ± 4.0 dB), with a bandwidth < 0.1 Hz to >80 kHz) One digital sound card Avisoft Bioacoustics USGH 416HB (data format 16–24-bit WAV, sampling rate 44.1, 48 and 96 ks/s [36]). | 3.8 h, resulting in 422 .wav files | 2012–2015 |
| Tyrrhenian Sea | Boat-based survey using a sailing vessel Beneteau Oceanis 41.1 powered by a 55 hp Volvo diesel engine, in suitable weather conditions (sea state < 3 Douglas, wind force Beaufort < 3, no rain, no fog), at a steady speed of 4–6 kn. Non-systematic haphazard sampling procedure (sensu [35]). More details in [26]. | 2017–2018: Two Colmar omnidirectional hydrophones (La Spezia, Italia) model GP0280 provided by CIBRA-Pavia University (sensitivity -168.8 dB re 1 V/μPa@ 5 kHz, flat frequency response from 1 to 30 kHz ± 5 dB), with a bandwidth 5 Hz–90 kHz 2019–2020: One towed hydrophone Aquarian Audio (Anacortes, WA, USA) model H1c-2018 provided by Nauta srl (sensitivity -199 dB re 1 V/μPa, flat frequency response from 20 Hz to 4 kHz ± 4 dB), with a bandwidth < 0.1 to >100 kHz. Digital sound card Roland Quad Capture UA55 (data format 16–24-bit WAV, sampling rate 44.1, 48 and 96 ks/s [36]). | 115.3 h, resulting in 871 .wav files | 2017–2020 |
| Parameter | Description |
|---|---|
| Element type | Type of acoustic element composing the sequence |
| Acoustic elements in the sequence (N) | Number of detectable elements composing each sequence |
| Sequence duration (s) | Time interval between the beginning of the first and the end of the last element |
| Element duration (s) | Duration of each element in the sequence |
| Inter-element interval (s) | Time interval between the end of an element and the beginning of the following one |
| Minimum Frequency (Hz) | The lower frequency of each element composing the sequence |
| Maximum Frequency (Hz) | The higher frequency of each element composing the sequence |
| Peak frequency (Hz) | The frequency with maximum amplitude in the spectrum (for POP and CR element types) |
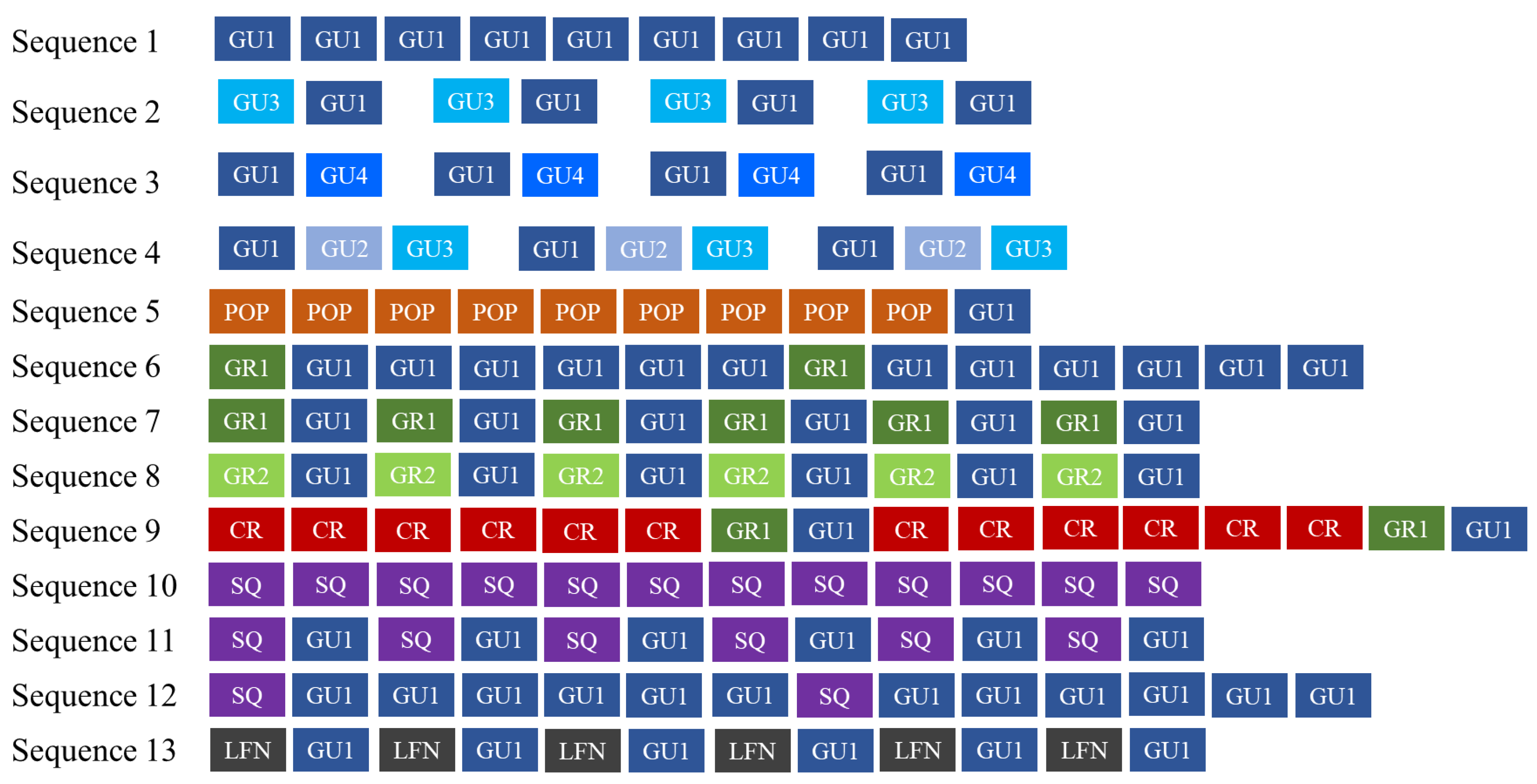
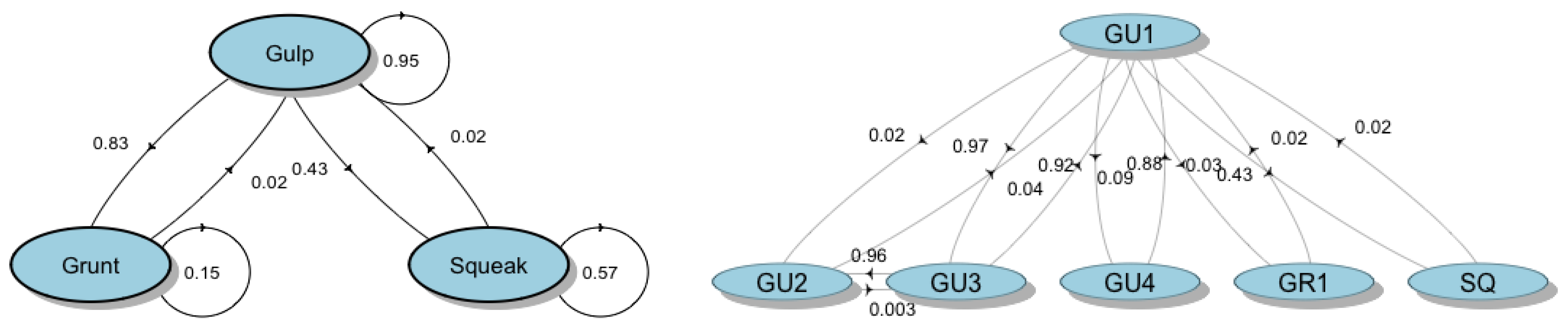
| Element Type | Sequence Id Number | ID Element Type/Subtype (N of Elements) | Description |
|---|---|---|---|
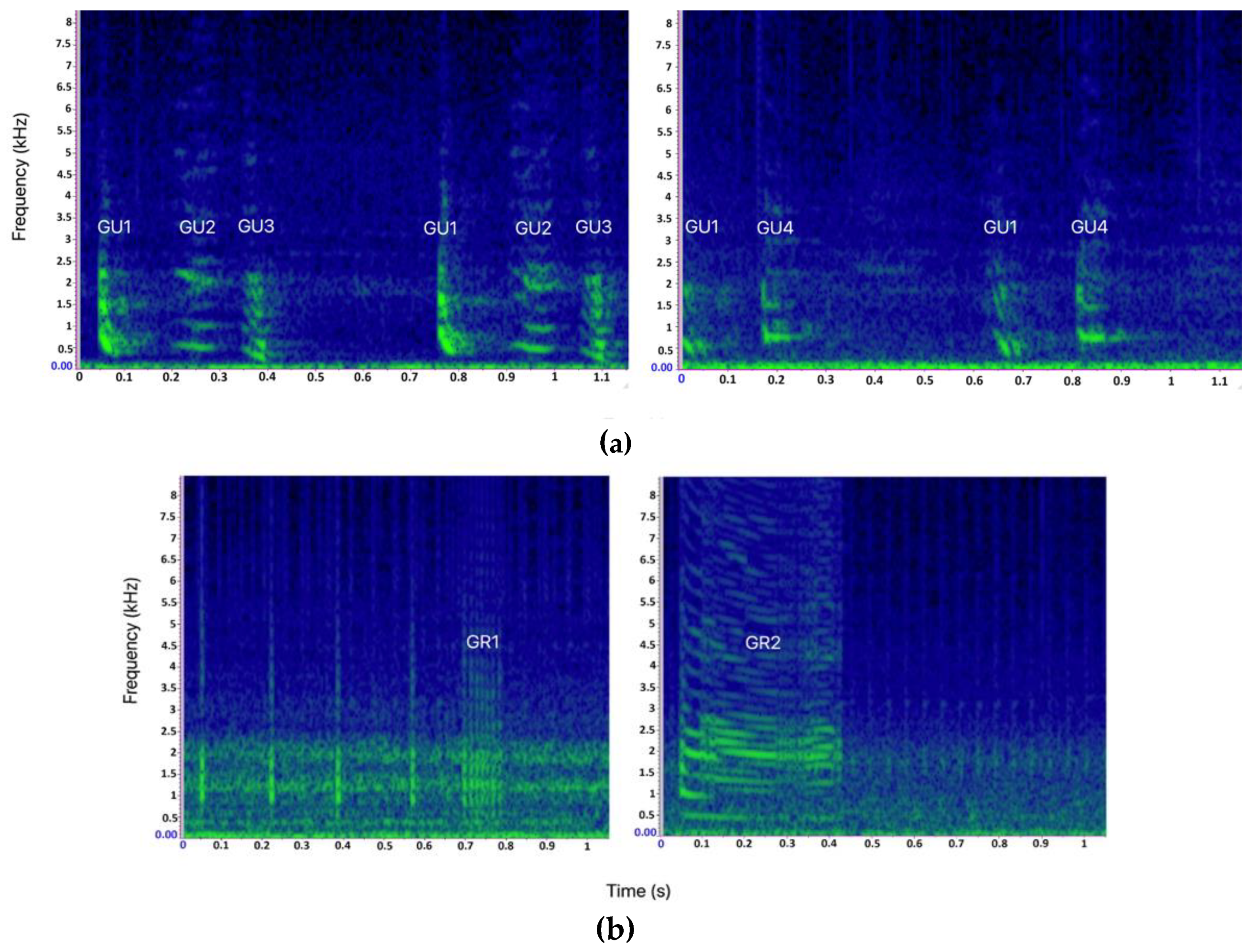
| Gulp (GU) | 1 | GU1 (1711) | A single-element sequence composed of Gulp (GU1) repeated in series of 3 to 105 elements |
| 2 | GU3 (233)–GU1 (229) | A multi-element sequence composed of 4 to 32 elements containing the repetition of Gulp pairs (GU3 and GU1) | |
| 3 | GU1 (97)–GU4 (93) | A multi-element sequence containing the repetition of Gulp pairs (GU1 and GU4), with 8 to 11 GU1 and 3 to 23 GU4 elements per sequence | |
| 4 | GU1 (108)–GU2 (97)–GU3 (107) | A multi-element sequence containing the repetition of Gulp triplets (GU1, GU2 and GU3), with the repetition of 2 to 22 GU1, 2 to 20 GU2 and 2 to 22 GU3 elements per sequence | |
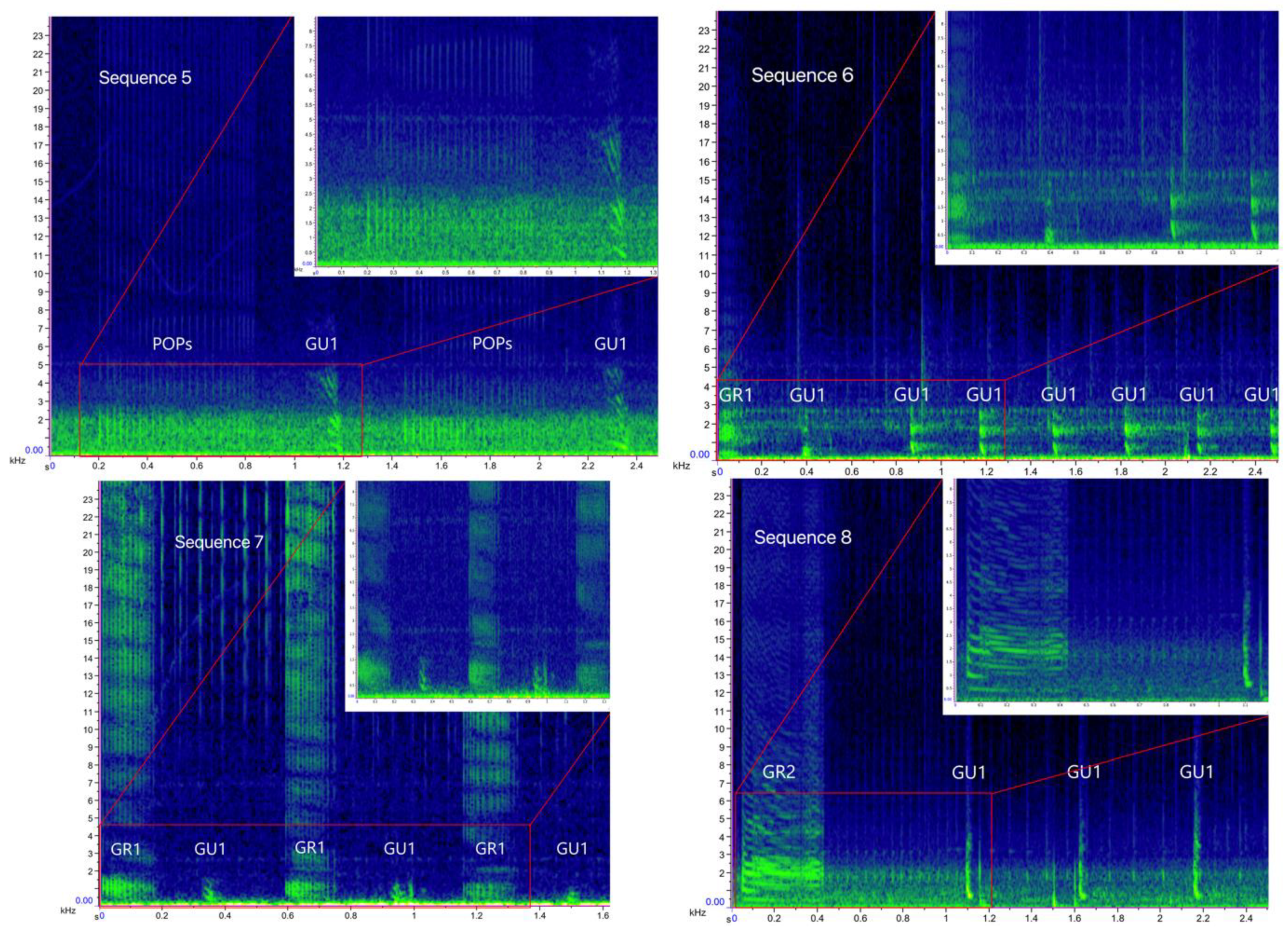
| Gulp (GU) and Pop (POP) | 5 | GU1 (32)–POP (412) | A multi-element sequence containing a series of POPs, alternated with 1 to 8 Gulp (GU1). The number of POP ranged from 9 to 27, while the number of POP series within the sequence varied from 1 to 16 |
| Grunt (GR) and Gulp (GU) | 6 | GR1 (5)–GU1 (94) | A multi-element sequence containing a Grunt (GR1) followed by a series of 6 to 35 Gulp (GU1) |
| 7 | GR1 (45)–GU1 (46) | A multi-element sequence containing a Grunt (GR1) and a Gulp (GU1) alternated, with 3–9 GR1 and 3 to 11 GU1 elements per sequence | |
| 8 | GR2 (20)–GU1 (142) | A multi-element sequence containing a Grunt (GR2) followed by a series of 3 to 26 Gulp (GU1) | |
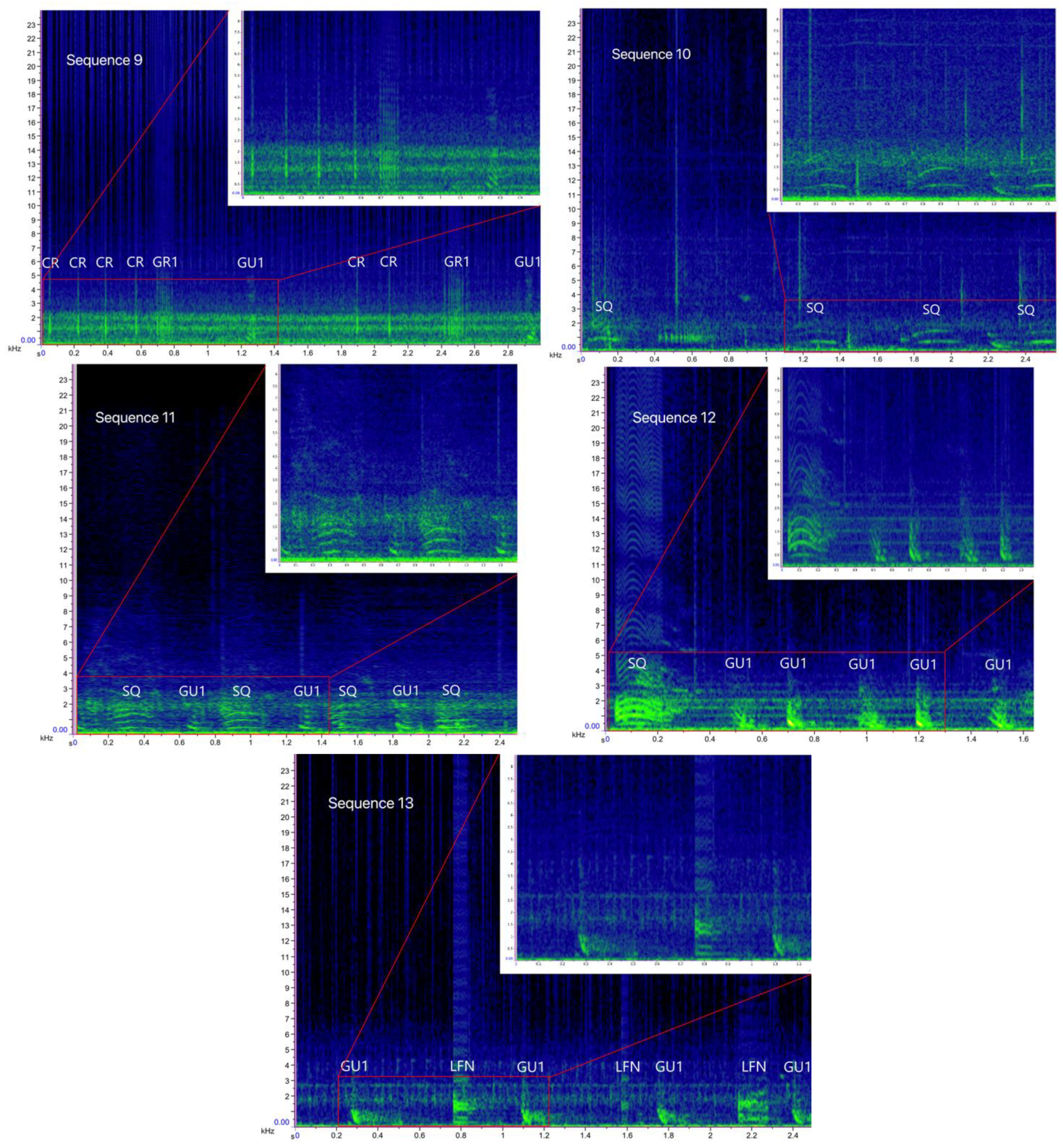
| Crack (CR), Grunt (GR) and Gulp (GU) | 9 | CR (157)–GR1 (34)–GU1 (34) | A multi-element sequence containing a series of 2–11 Cracks (CR) followed by a Grunt (GR1) and a Gulp (GU1) |
| Squeak (SQ) | 10 | SQ (61) | A single-element sequence composed of Squeaks (SQ) repeated in series of 2 to 14 elements |
| Squeak (SQ) and Gulp (GU) | 11 | SQ (47)–GU1 (41) | A multi-element sequence containing a Squeak (SQ) and a Gulp (GU1) alternated, with 2 to 13 SQ and 2 to 8 GU1 elements per sequence |
| 12 | SQ (14)–GU1 (119) | A multi-element sequence containing a Squeak (SQ) followed by a series of 3 to 27 Gulps (GU1) | |
| Low-Frequency Narrowband sounds (LNF) and Gulp (GU) | 13 | LFN (19)–GU1 (34) | A multi-element sequence containing a LFN and a Gulp (GU1) alternated, with 4–6 LFN and 3 to 17 GU1 elements per sequence |
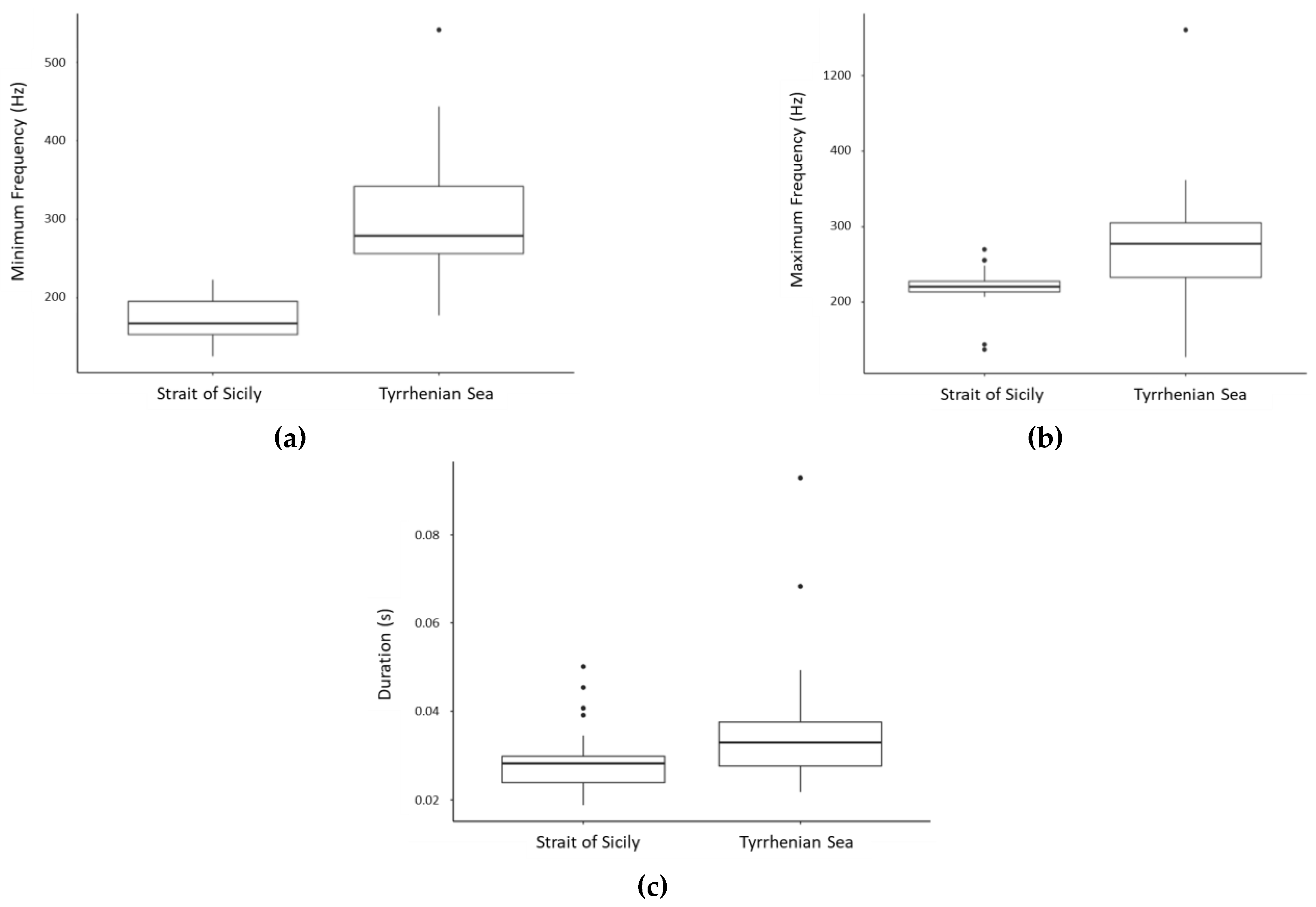
| Sequence Id Number | Sequence Typology | Element Type/Subtype | Sequence Duration (s) | Inter Element Interval (s) | Pair/Triplet Duration (s) | Inter-Pair /Triplet Interval (s) | Element Minimun Frequency (Hz) | Element Maximum Frequency (Hz) | Element Peak Frequency (Hz) | Element Duration (s) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Single element | GU1 | 6.3 ± 8.2 (0.15–115) | 0.4 ± 0.3 | - | - | 293 ± 90 | 768 ± 154 | - | 0.039 ± 0.01 |
| 2 | Multi-element organized in pairs | GU1 | 9.5 ± 10.4 (0.6–68) | 0.11 ± 0 | 0.17 ± 0.05 | 0.44 ± 0.16 | 262 ± 49 | 753 ± 105 | - | 0.036 ± 0.01 |
| GU3 | 223 ± 49 | 572 ± 74 | - | 0.041 ± 0.01 | ||||||
| 3 | Multi-element organized in pairs | GU1 | 7.9 ± 8.1 (1.4–36.4) | 0.14 ± 0 | 0.25 ± 0.08 | 0.40 ± 0.10 | 290 ± 58 | 685 ± 76 | - | 0.038 ± 0.02 |
| GU4 | 592 ± 71 | 868 ± 86 | - | 0.070 ± 0.06 | ||||||
| 4 | Multi-element organized in triplets | GU1 | 9.8 ± 8.3 (1.4–34.5) | 0.13 ± 0.1 | 0.4 ± 0.11 | 0.23 ± 0.1 | 278 ± 40 | 775 ± 95 | - | 0.036 ± 0.01 |
| GU2 | 361 ± 83 | 584 ± 70 | - | 0.036 ± 0.01 | ||||||
| GU3 | 204 ± 35 | 491 ± 54 | - | 0.039 ± 0.00 | ||||||
| 5 | Multi-element alternated | GU1 | 8.9 ± 7.4 (2–30) | 0.2 ± 0 * | - | - | 281 ± 52 | 842 ± 162 | - | 0.038 ± 0.01 |
| POP | - | - | - | 813 ± 158 | - | |||||
| 6 | Multi-element | GR1 | 10.9 ± 8.4 (2–24) | 0.45 ± 0.3 ** | - | - | - | - | - | 0.11 ± 0.03 |
| GU1 | 342 ± 76 | 874 ± 112 | - | 0.032 ± 0.00 | ||||||
| 7 | Multi-element alternated | GR1 | 5.0 ± 4.6 (1.6–24) | 0.17 ± 0.0 | - | - | - | - | - | 0.18 ± 0.10 |
| GU1 | 278 ± 64 | 700 ± 129 | - | 0.043 ± 0.01 | ||||||
| 8 | Multi-element | GR2 | 4.7 ± 3.6 (1–18) | 0.59 ± 0.4 ˟ | - | - | - | - | - | 0.14 ± 0.09 |
| GU1 | 324 ± 116 | 874 ± 234 | - | 0.043 ± 0.01 | ||||||
| 9 | Multi-element | CR | 4.9 ± 5.7 (0.6–26) | 0.13 ± 0 | - | - | - | - | 1237 ± 279 | - |
| GR1 | 1.12 ± 0.4 | - | - | - | 0.14 ± 0.09 | |||||
| GU1 | 1.25 ± 0.5 | 395 ± 243 | 750 ± 238 | - | 0.030 ± 0.01 | |||||
| 10 | Single element | SQ | 3.7 ± 1.9 (0.6–8.3) | 0.5 ± 0.3 | - | - | 535 ± 204 | 892 ± 303 | - | 0.17 ± 0.07 |
| 11 | Multi-element alternated | SQ | 5.5 ± 3.9 (1.4–15.9) | 0.65 ± 0.2 | - | - | 472 ± 120 | 772 ± 172 | - | 0.13 ± 0.07 |
| GU1 | 306 ± 114 | 705 ± 114 | - | 0.044 ± 0.01 | ||||||
| 12 | Multi-element | SQ | 5.5 ± 3.9 (1.4–15.9) | 0.3 ± 0.1 ˟˟ | - | - | 303 ± 39 | 621 ± 99 | - | 0.20 ± 0.08 |
| GU1 | 326 ± 136 | 829 ± 220 | - | 0.039 ± 0.01 | ||||||
| 13 | Multi-element alternated | LFN | 4.4 ± 2.7 (1.6–7.9) | 0.3 ± 0.2 | - | - | 256 ± 64 | 650 ± 97 | - | 0.084 ± 0.04 |
| GU1 | 246 ± 58 | 533 ± 151 | - | 0.035 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pace, D.S.; Tumino, C.; Silvestri, M.; Giacomini, G.; Pedrazzi, G.; Pavan, G.; Papale, E.; Ceraulo, M.; Buscaino, G.; Ardizzone, G. Bray-Call Sequences in the Mediterranean Common Bottlenose Dolphin (Tursiops truncatus) Acoustic Repertoire. Biology 2022, 11, 367. https://doi.org/10.3390/biology11030367
Pace DS, Tumino C, Silvestri M, Giacomini G, Pedrazzi G, Pavan G, Papale E, Ceraulo M, Buscaino G, Ardizzone G. Bray-Call Sequences in the Mediterranean Common Bottlenose Dolphin (Tursiops truncatus) Acoustic Repertoire. Biology. 2022; 11(3):367. https://doi.org/10.3390/biology11030367
Chicago/Turabian StylePace, Daniela Silvia, Carla Tumino, Margherita Silvestri, Giancarlo Giacomini, Giulia Pedrazzi, Gianni Pavan, Elena Papale, Maria Ceraulo, Giuseppa Buscaino, and Giandomenico Ardizzone. 2022. "Bray-Call Sequences in the Mediterranean Common Bottlenose Dolphin (Tursiops truncatus) Acoustic Repertoire" Biology 11, no. 3: 367. https://doi.org/10.3390/biology11030367
APA StylePace, D. S., Tumino, C., Silvestri, M., Giacomini, G., Pedrazzi, G., Pavan, G., Papale, E., Ceraulo, M., Buscaino, G., & Ardizzone, G. (2022). Bray-Call Sequences in the Mediterranean Common Bottlenose Dolphin (Tursiops truncatus) Acoustic Repertoire. Biology, 11(3), 367. https://doi.org/10.3390/biology11030367










