Ischemic Cardiomyopathy versus Non-Ischemic Dilated Cardiomyopathy in Patients with Reduced Ejection Fraction— Clinical Characteristics and Prognosis Depending on Heart Failure Etiology (Data from European Society of Cardiology Heart Failure Registries)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Study Endpoints
2.3. Statistical Analysis
3. Results
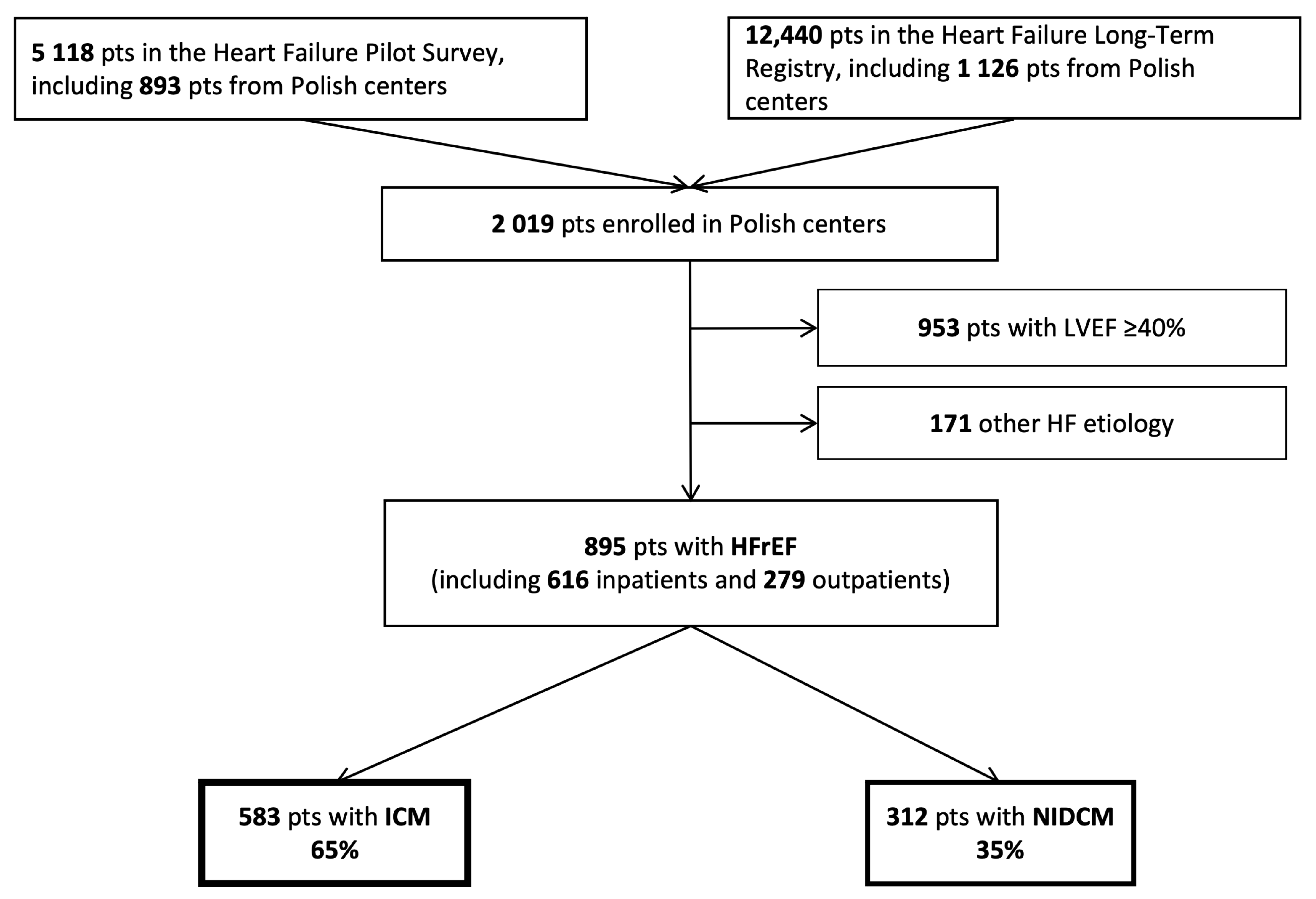
3.1. Study Group Selection
3.2. Clinical Characteristics
3.3. One-Year Outcomes and Clinical Predictors
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Parén, P.; Schaufelberger, M.; Björck, L.; Lappas, G.; Fu, M.; Rosengren, A. Trends in prevalence from 1990 to 2007 of patients hospitalized with heart failure in Sweden. Eur. J. Heart Fail. 2014, 16, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Gerber, Y.; Weston, S.A.; Redfield, M.M.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Killian, J.M.; Roger, V.L. A Contemporary Appraisal of the Heart Failure Epidemic in Olmsted County, Minnesota, 2000–2010. JAMA Intern. Med. 2015, 175, 996–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tymińska, A.; Kapłon-Cieślicka, A.; Ozierański, K.; Peller, M.; Balsam, P.; Marchel, M.; Crespo-Leiro, M.G.; Maggioni, A.P.; Jankowska, E.A.; Drożdż, J.; et al. Anemia at Hospital Admission and Its Relation to Outcomes in Patients With Heart Failure (from the Polish Cohort of 2 European Society of Cardiology Heart Failure Registries). Am. J. Cardiol. 2017, 119, 2021–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wideqvist, M.; Cui, X.; Magnusson, C.; Schaufelberger, M.; Fu, M. Hospital readmissions of patients with heart failure from real world: Timing and associated risk factors. ESC Heart Fail. 2021, 8, 1388–1397. [Google Scholar] [CrossRef]
- Pecini, R.; Møller, D.V.; Torp-Pedersen, C.; Hassager, C.; Køber, L. Heart failure etiology impacts survival of patients with heart failure. Int. J. Cardiol. 2011, 149, 211–215. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Tilz, R.; Boveda, S.; Dobreanu, D.; Sciaraffia, E.; Mansourati, J.; Papiashvili, G.; Dagres, N. Implantable cardioverter defibrillator use for primary prevention in ischaemic and non-ischaemic heart disease—indications in the post-DANISH trial era: Results of the European Heart Rhythm Association survey. EP Eur. 2017, 19, 660–664. [Google Scholar] [CrossRef]
- Lourenço, C.; Saraiva, F.; Martins, H.; Baptista, R.; Costa, S.; Coelho, L.; Vieira, H.; Monteiro, P.; Franco, F.; Gonçalves, L.; et al. Ischemic versus non-ischemic cardiomyopathy--are there differences in prognosis? Experience of an advanced heart failure center. Rev. Port. Cardiol. 2011, 30, 181–197. [Google Scholar]
- Martínez-Sellés, M.; Doughty, R.N.; Poppe, K.; Whalley, G.A.; Earle, N.; Tribouilloy, C.; McMurray, J.J.V.; Swedberg, K.; Køber, L.; Berry, C.; et al. Gender and survival in patients with heart failure: Interactions with diabetes and aetiology. Results from the MAGGIC individual patient meta-analysis. Eur. J. Heart Fail. 2012, 14, 473–479. [Google Scholar] [CrossRef]
- Frazier, C.G.; Alexander, K.P.; Newby, L.K.; Anderson, S.; Iverson, E.; Packer, M.; Cohn, J.; Goldstein, S.; Douglas, P.S. Associations of gender and etiology with outcomes in heart failure with systolic dysfunction: A pooled analysis of 5 randomized control trials. J. Am. Coll. Cardiol. 2007, 49, 1450–1458. [Google Scholar] [CrossRef] [Green Version]
- Beiert, T.; Straesser, S.; Malotki, R.; Stöckigt, F.; Schrickel, J.W.; Andrié, R.P. Increased mortality and ICD therapies in ischemic versus non-ischemic dilated cardiomyopathy patients with cardiac resynchronization having survived until first device replacement. Arch. Med. Sci. 2019, 15, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Higgins, A.Y.; Bjerre, J.; Parzynski, C.S.; Minges, K.E.; Ahmad, T.; Desai, N.R.; Enriquez, A.; Spatz, E.S.; Friedman, D.J.; Curtis, J.P.; et al. Comparison of Mortality and Readmission in Non-Ischemic Versus Ischemic Cardiomyopathy After Implantable Cardioverter-Defibrillator Implantation. Am. J. Cardiol. 2020, 133, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, A.P.; Dahlström, U.; Filippatos, G.; Chioncel, O.; Leiro, M.C.; Drozdz, J.; Fruhwald, F.; Gullestad, L.; Logeart, D.; Metra, M.; et al. EURObservational Research Programme: The Heart Failure Pilot Survey (ESC-HF Pilot). Eur. J. Heart Fail. 2010, 12, 1076–1084. [Google Scholar] [CrossRef] [Green Version]
- Crespo-Leiro, M.G.; Anker, S.D.; Maggioni, A.P.; Coats, A.J.; Filippatos, G.; Ruschitzka, F.; Ferrari, R.; Piepoli, M.F.; Delgado Jimenez, J.F.; Metra, M.; et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur. J. Heart Fail. 2016, 18, 613–625. [Google Scholar] [CrossRef] [Green Version]
- Kadish, A.; Dyer, A.; Daubert, J.P.; Quigg, R.; Estes, N.A.M.; Anderson, K.P.; Calkins, H.; Hoch, D.; Goldberger, J.; Shalaby, A.; et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N. Engl. J. Med. 2004, 350, 2151–2158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strickberger, S.A.; Hummel, J.D.; Bartlett, T.G.; Frumin, H.I.; Schuger, C.D.; Beau, S.L.; Bitar, C.; Morady, F. Amiodarone versus implantable cardioverter-defibrillator:randomized trial in patients with nonischemicdilated cardiomyopathy and asymptomaticnonsustained ventricular tachycardia—AMIOVIRT. J. Am. Coll. Cardiol. 2003, 41, 1707–1712. [Google Scholar] [CrossRef] [Green Version]
- Bänsch, D.; Antz, M.; Boczor, S.; Volkmer, M.; Tebbenjohanns, J.; Seidl, K.; Block, M.; Gietzen, F.; Berger, J.; Kuck, K.H. Primary Prevention of Sudden Cardiac Death in Idiopathic Dilated Cardiomyopathy. Circulation 2002, 105, 1453–1458. [Google Scholar] [CrossRef] [Green Version]
- Køber, L.; Thune, J.J.; Nielsen, J.C.; Haarbo, J.; Videbæk, L.; Korup, E.; Jensen, G.; Hildebrandt, P.; Steffensen, F.H.; Bruun, N.E.; et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N. Engl. J. Med. 2016, 375, 1221–1230. [Google Scholar] [CrossRef] [Green Version]
- Bart, B.A.; Shaw, L.K.; McCants, C.B.; Fortin, D.F.; Lee, K.L.; Califf, R.M.; O’Connor, C.M. Clinical Determinants of Mortality in Patients with Angiographically Diagnosed Ischemic or Nonischemic Cardiomyopathy. J. Am. Coll. Cardiol. 1997, 30, 1002–1008. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, L.W.; Tillisch, J.H.; Hamilton, M.; Luu, M.; Chelimsky-Fallick, C.; Moriguchi, J.; Kobashigawa, J.; Walden, J. Importance of hemodynamic response to therapy in predicting survival with ejection fraction ≤ 20% secondary to ischemic or nonischemic dilated cardiomyopathy. Am. J. Cardiol. 1990, 66, 1348–1354. [Google Scholar] [CrossRef]
- Levy, W.C.; Mozaffarian, D.; Linker, D.T.; Sutradhar, S.C.; Anker, S.D.; Cropp, A.B.; Anand, I.; Maggioni, A.; Burton, P.; Sullivan, M.D.; et al. The Seattle Heart Failure Model. Circulation 2006, 113, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Tymińska, A.; Balsam, P.; Ozierański, K.; Peller, M.; Kapłon-Cieślicka, A.; Wancerz, A.; Galas, M.; Marchel, M.; Crespo-Leiro, M.G.; Maggioni, A.P.; et al. Heart failure patients with a previous coronary revascularization: Results from the ESC-HF Registry. Kardiol. Pol. 2018, 76, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tymińska, A.; Ozierański, K.; Caforio, A.L.P.; Marcolongo, R.; Marchel, M.; Kapłon-Cieślicka, A.; Baritussio, A.; Filipiak, K.J.; Opolski, G.; Grabowski, M. Myocarditis and inflammatory cardiomyopathy in 2021: An update. Pol. Arch. Intern. Med. 2021, 131, 594–606. [Google Scholar] [CrossRef]
- Balmforth, C.; Simpson, J.; Shen, L.; Jhund, P.S.; Lefkowitz, M.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.; Solomon, S.D.; Swedberg, K.; et al. Outcomes and Effect of Treatment According to Etiology in HFrEF: An Analysis of PARADIGM-HF. JACC Heart Fail. 2019, 7, 457–465. [Google Scholar] [CrossRef]
- Komajda, M.; Schöpe, J.; Wagenpfeil, S.; Tavazzi, L.; Böhm, M.; Ponikowski, P.; Anker, S.D.; Filippatos, G.S.; Cowie, M.R.; On behalf of the Qualify Investigators. Physicians’ guideline adherence is associated with long-term heart failure mortality in outpatients with heart failure with reduced ejection fraction: The QUALIFY international registry. Eur. J. Heart Fail. 2019, 21, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Gajanana, D.; Shah, M.; Junpapart, P.; Romero-Corral, A.; Figueredo, V.M.; Bozorgnia, B. Mortality in systolic heart failure revisited: Ischemic versus non-ischemic cardiomyopathy. Int. J. Cardiol. 2016, 224, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Opolski, G.; Ozierański, K.; Lelonek, M.; Balsam, P.; Wilkins, A.; Ponikowski, P.; On Behalf of the Polish Qualify Investigators. Adherence to the guidelines on the management of systolic heart failure in ambulatory care in Poland. Data from the international QUALIFY survey. Pol. Arch. Intern. Med. 2017, 127, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejczak, M.; Andreotti, F.; Kowalewski, M.; Buffon, A.; Ciccone, M.M.; Parati, G.; Scicchitano, P.; Umińska, J.M.; De Servi, S.; Bliden, K.P.; et al. Implantable Cardioverter-Defibrillators for Primary Prevention in Patients With Ischemic or Nonischemic Cardiomyopathy. Ann. Intern. Med. 2017, 167, 103–111. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Mareev, Y.; Linde, C. Reflections on EchoCRT: Sound guidance on QRS duration and morphology for CRT? Eur. Heart J. 2015, 36, 1948–1951. [Google Scholar] [CrossRef] [Green Version]
- Linde, C.; Abraham, W.T.; Gold, M.R.; St John Sutton, M.; Ghio, S.; Daubert, C. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J. Am. Coll. Cardiol. 2008, 52, 1834–1843. [Google Scholar] [CrossRef] [Green Version]
| HFrEF Patients (n = 895) | p-Value | ||
|---|---|---|---|
| ICM (n = 583) | NIDCM (n = 312) | ||
| Baseline characteristics | |||
| Age, years | 66.5 (58.7–75.2) | 58.2 (49.3–65.2) | <0.001 |
| Male | 468 (80.3%) | 255 (81.7%) | 0.66 |
| Previous hospitalization | 350 (61.2%); n = 572 | 179 (57.9%); n = 309 | 0.35 |
| BMI, kg/m2 | 27.30 (24.70–30.10); n = 554 | 27.80 (25.00–31.80); n = 310 | 0.01 |
| Current LVEF, % | 28 (20–33) | 25 (20–30) | 0.01 |
| Previous HF hospitalization | 433 (74.3%) | 183 (58.7%) | <0.001 |
| Prior PCI or CABG | 424 (72.7%) | 0 (0.0%) | <0.001 |
| Moderate or severe mitral regurgitation | 302 (57.7%); n = 523 | 149 (54.2%); n = 275 | 0.37 |
| Moderate or severe aortic stenosis | 16 (3.1%); n = 523 | 7 (2.6%); n = 272 | 0.83 |
| Moderate or severe aortic regurgitation | 41 (7.8%); n = 524 | 13 (4.8%); n = 272 | 0.13 |
| Moderate or severe tricuspid regurgitation | 182 (34.8%); n = 523 | 109 (40.1%); n = 272 | 0.16 |
| LVEDD, mm | 63.0 (58.0–70.0); n = 506 | 67.0 (60.5–75.0); n = 267 | <0.001 |
| LBBB | 89 (17.0%); n = 524 | 59 (21.8%); n = 271 | 0.10 |
| QRS, ms | 114.5 (100.0–139.2); n = 496 | 118.0 (100.0–141.0); n = 255 | 0.48 |
| Hypertension | 400 (68.7%); n = 582 | 112 (36.1%); n = 310 | <0.001 |
| History of atrial fibrillation | 201 (34.5%) | 130 (41.8%); n = 311 | 0.04 |
| Peripheral artery disease | 97 (16.7%); n = 582 | 13 (4.2%) | <0.001 |
| Diabetes | 233 (40.0%) | 82 (26.3%) | <0.001 |
| Chronic kidney disease | 140 (24.0%) | 42 (13.5%) | <0.001 |
| COPD | 121 (20.8%) | 40 (12.8%) | 0.01 |
| Prior stroke or TIA | 78 (13.4%) | 24 (7.7%) | 0.01 |
| Current or former smoking | 406 (70.7%); n = 574 | 198 (63.7%); n = 311 | 0.03 |
| Alcohol usage | 331 (60.5%); n = 547 | 210 (70.7%); n = 297 | 0.01 |
| Pacemaker | 30 (5.1%) | 9 (2.9%) | 0.13 |
| ICD | 168 (28.8%) | 102 (32.7%) | 0.25 |
| CRT | 61 (10.5%) | 36 (11.5%) | 0.65 |
| Clinical status and laboratory findings | |||
| Heart rate, b.p.m. | 75.0 (67.0–92.0); n = 581 | 80.0 (70.0–97.8) | 0.04 |
| SBP, mmHg | 115 (105–125); n = 582 | 115 (105–130) | 0.82 |
| DBP, mmHg | 70 (60–80); n = 581 | 70 (70–80) | <0.001 |
| NYHA class | n = 579 | n = 312 | 0.01 |
| I | 11 (1.9%) | 14 (4.5%) | - |
| II | 177 (30.6%) | 119 (38.1%) | - |
| III | 254 (43.9%) | 117 (37.5%) | - |
| IV | 137 (23.7%) | 62 (19.9%) | - |
| Hemoglobin, g/dL | 13.4 (12.2–14.7); n = 535 | 14.2 (13.0–15.2); n = 259 | <0.001 |
| Serum creatinine, mg/dL | 1.1 (0.9–1.4); n = 550 | 1.1 (0.9–1.3); n = 268 | 0.01 |
| eGFR, mL/min/1.73 m2 | 68.9 (48.1–93.8); n = 550 | 86.3 (60.0–113.7); n = 268 | 0.01 |
| Serum sodium, mmol/L | 138.8 (136.0–141.0); n = 547 | 139.0 (136.0–141.0); n = 263 | 0.803 |
| NT-proBNP | 3566.0 (1575.0–7654.2); n = 170 | 2724.0 (793.0–5227.0); n = 97 | 0.014 |
| Pharmacotherapy (at discharge) | |||
| Diuretics | 508 (87.3%) n = 582 | 281 (90.4%) | 0.19 |
| Aldosterone antagonist | 429 (73.7%); n = 582 | 254 (81.7%) | 0.01 |
| ACE-I | 450 (77.3%); n = 582 | 254 (81.4%) | 0.17 |
| ARB | 54 (9.3%); n = 582 | 35 (11.3%) | 0.35 |
| β-blocker | 540 (92.8%); n = 582 | 294 (94.2%) | 0.48 |
| Statins | 492 (84.5%); n = 582 | 154 (49.4%) | <0.001 |
| Anticoagulants | 213 (36.7%); n = 581 | 146 (46.8%) | 0.01 |
| Antiplatelets | 466 (80.1%); n = 582 | 125 (40.1%) | <0.001 |
| Digitalis | 135 (23.2%); n = 582 | 112 (35.9%) | <0.001 |
| Amiodarone | 74 (12.7%); n = 582 | 50 (16.0%) | 0.18 |
| Antiarrhytmics | 38 (6.5%); n = 582 | 13 (4.2%) | 0.17 |
| CCB | 50 (8.6%); n = 582 | 9 (2.9%) | 0.001 |
| One-year outcome | |||
| NYHA | n = 447 | n = 254 | 0.14 |
| I | 37 (8.3%) | 35 (13.8%) | - |
| II | 251 (56.2%) | 137 (53.9%) | - |
| III | 137 (30.6%) | 72 (28.3%) | - |
| IV | 22 (4.9%) | 10 (3.9%) | - |
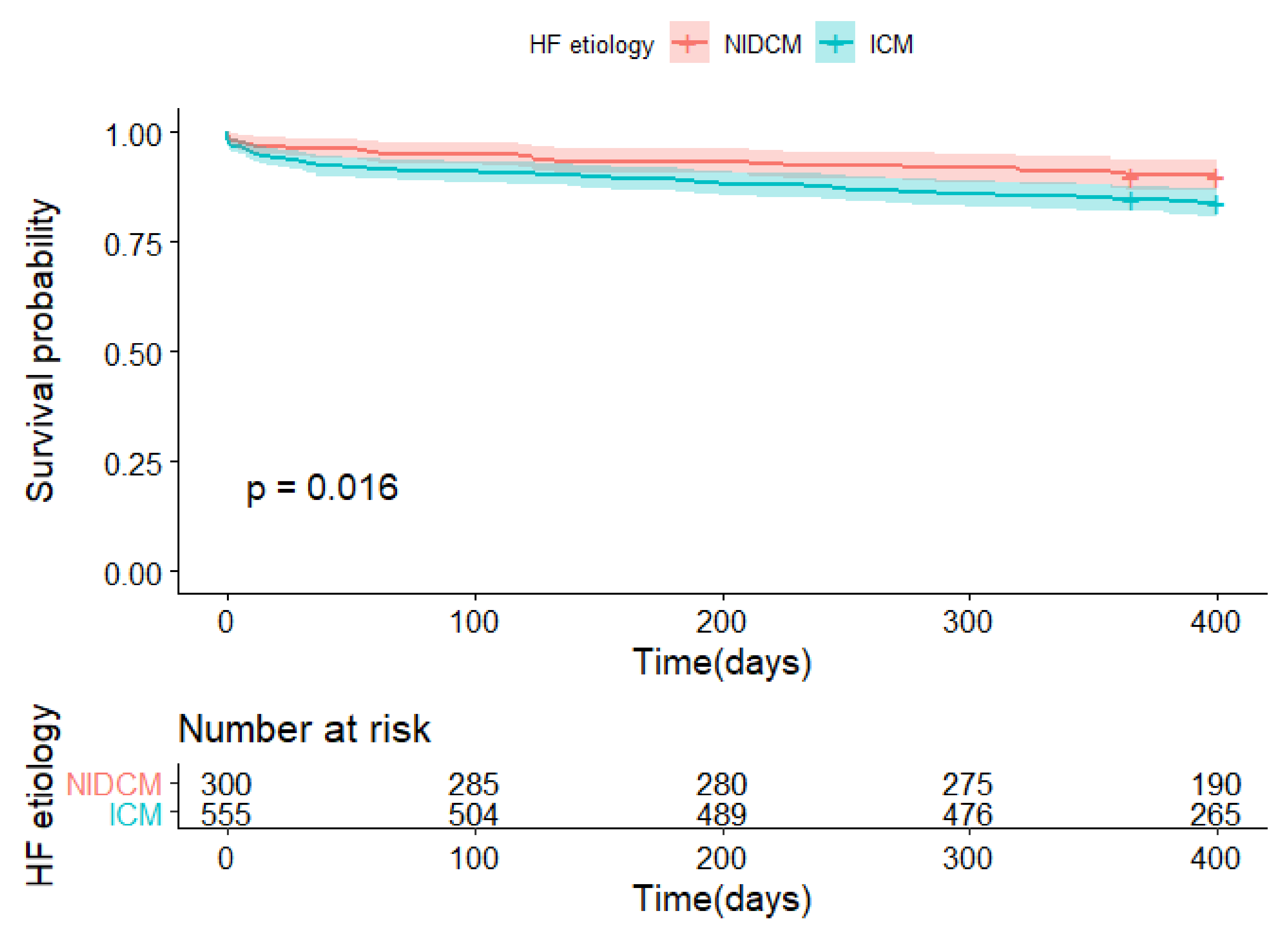
| Death | 88 (15.9%); n = 555 | 30 (10.0%); n = 301 | 0.02 |
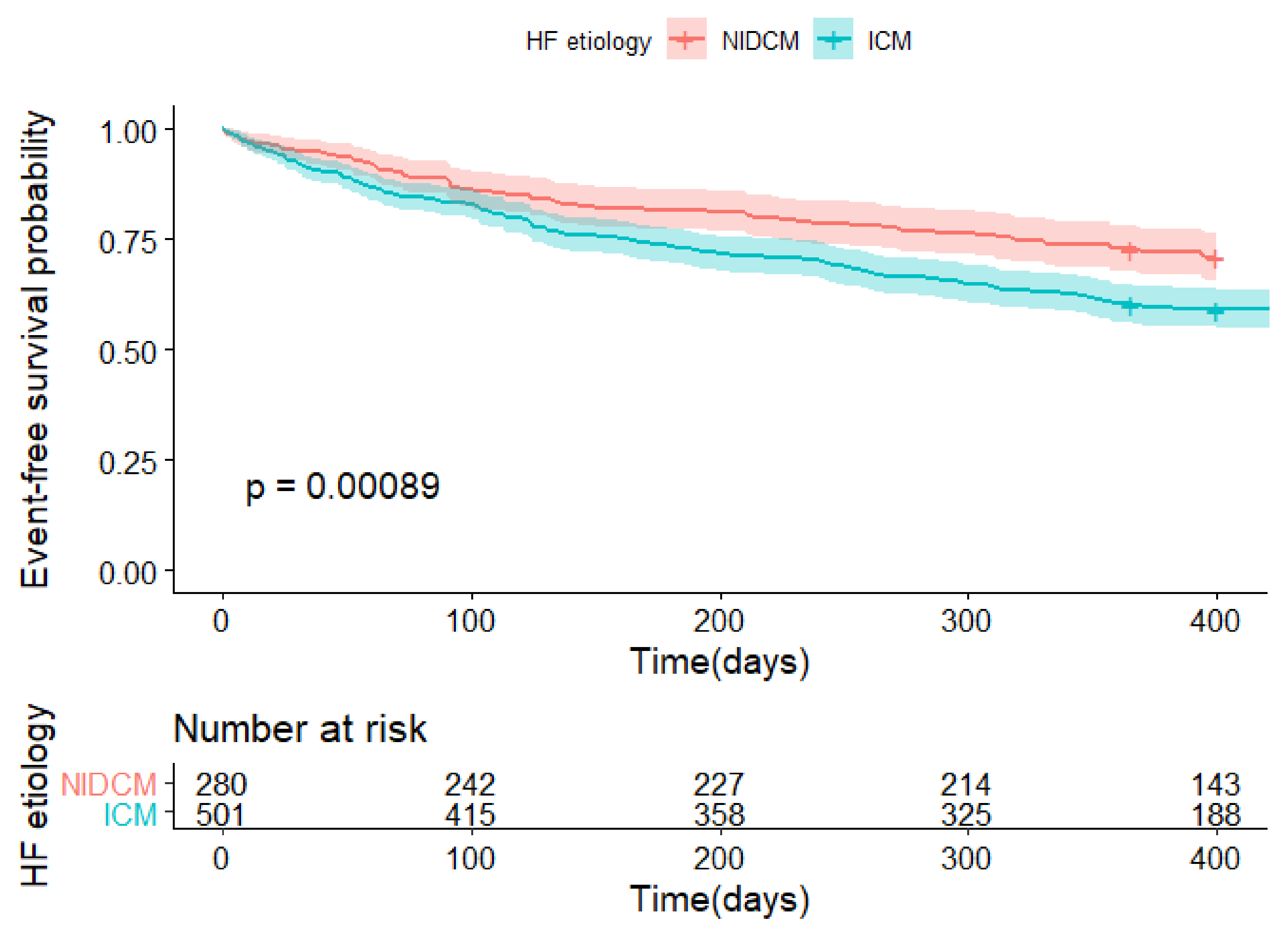
| Death or rehospitalization | 205 (40.9%); n = 501 | 80 (28.6%); n = 280 | 0.001 |
| HFrEF Patients (n = 616) | p-Value | ||
|---|---|---|---|
| ICM (n = 433) | NIDCM (n = 183) | ||
| Clinical status at hospital admission | |||
| Cardiogenic shock | 13/427 (3.1%); n = 417 | 9/175 (5.1%); n = 175 | 0.24 |
| Heart rate, b.p.m. | 80.0 (70.0–100.0); n = 432 | 86.0 (73.5–105.0) | 0.01 |
| SBP, mmHg | 120.0 (110.0–140.0); n = 432 | 120.0 (109.5–133.5) | 0.04 |
| DBP, mmHg | 80 (70–84); n = 431 | 76 (70–80) | 0.43 |
| NYHA | n = 429 | n = 183 | 0.96 |
| I | 3 (0.7%) | 1 (0.5%) | - |
| II | 92 (21.4%) | 39 (21.3%) | - |
| III | 201 (46.9%) | 83 (45.4%) | - |
| IV | 133 (31.0%) | 60 (32.8%) | - |
| Pacemaker | 25 (5.8%) | 7 (3.8%) | 0.43 |
| CRT | 36 (8.3%) | 17 (9.3%) | 0.75 |
| ICD | 112 (25.9%) | 52 (28.4%) | 0.55 |
| Laboratory findings at admission | |||
| Hemoglobin, g/dL | 13.3 (12.1–14.7); n = 425 | 13.9 (12.8–15.1); n = 181 | <0.001 |
| Serum creatinine, mg/dL | 1.1 (0.9–1.5); n = 428 | 1.1 (0.9–1.3); n = 181 | 0.02 |
| eGFR, mL/min/1.73 m2 | 64.6 (45.6–89.2); n = 428 | 80.5 (58.2–110.7); n = 181 | <0.001 |
| Serum sodium, mmol/L | 138.0 (136.0–141.0); n = 431 | 138.0 (136.0–140.5); n = 182 | 0.88 |
| Serum potassium, mmol/L | 4.4 (4.1–4.8); n = 430 | 4.5 (4.2–4.8); n = 182 | 0.22 |
| Management during index hospitalization | |||
| Inotropic support | 65 (15.0%) | 40 (22.0%) | 0.046 |
| Diuretic i.v. | 300 (69.8%); n = 430 | 120 (65.6%) | 0.34 |
| Nitrates i.v. | 63 (14.6%); n = 431 | 26 (14.2%) | 1.0 |
| Clinical status and laboratory findings at discharge | |||
| Heart rate, b.p.m. | 70 (65–78); n = 420 | 72 (68–80) n = 173 | 0.001 |
| SBP, mmHg | 115.0 (105.0–120.0); n = 423 | 115.0 (100.0–120.8); n = 176 | 0.81 |
| DBP, mmHg | 70 (60–80); n = 422 | 70 (65–80); n = 176 | 0.13 |
| NYHA | n = 424 | n = 176 | 0.04 |
| I | 27 (6.4%) | 6 (3.4%) | - |
| II | 219 (51.7%) | 111 (63.1%) | - |
| III | 167 (39.4%) | 53 (30.1%) | - |
| IV | 11 (2.6%) | 6 (3.4%) | - |
| Hemoglobin, g/dL | 12.9 (11.4–14.3); n = 279 | 13.5 (12.6–14.8); n = 107 | 0.001 |
| Serum creatinine, mg/dL | 1.2 (0.9–1.5); n = 329 | 1.1 (0.9–1.3); n = 130 | 0.07 |
| Serum sodium, mmol/L | 138 (136–141); n = 351 | 138 (135–140); n = 140 | 0.34 |
| Serum potassium, mmol/L | 4.4 (4.1–4.7); n = 352 | 4.5 (4.2–4.8); n = 142 | 0.13 |
| In-hospital outcomes | |||
| Hospitalization length, days | 8 (4–12) | 7 (4–12) | 0.49 |
| Time in ICCU, days | 1 (0–5); n = 418 | 0 (0–3.2); n = 176 | 0.04 |
| Death during hospitalization | 14 (3.2%) | 7 (3.8%) | 0.81 |
| Primary Endpoint | Secondary Endpoint | |||||
|---|---|---|---|---|---|---|
| Variable | HR | CI | p-Value | HR | CI | p-Value |
| HF etiology as ICM (NIDCM as reference) | 1.46 | 0.87–2.47 | 0.16 | 1.56 | 1.16–2.11 | 0.003 |
| Age, years | 1.04 | 1.02–1.06 | <0.001 | 1.00 | 0.99–1.01 | 0.72 |
| LVEF, % | 0.96 | 0.93–0.98 | 0.003 | 0.97 | 0.95–0.99 | <0.001 |
| NYHA class, * class IV or III vs. II or I | 1.66 | 1.08–2.54 | 0.02 | 1.72 | 1.33–2.22 | <0.001 |
| NIDCM | ICM | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary Endpoint | Secondary Endpoint | Primary Endpoint | Secondary Endpoint | |||||||||
| Variable | HR | CI | p-Value | HR | CI | p-Value | HR | CI | p-Value | HR | CI | p-Value |
| Male sex | - | - | - | - | - | - | 0.81 | 0.48–1.37 | 0.42 | 0.72 | 0.50–1.03 | 0.07 |
| Age, years | 1.03 | 0.99–1.07 | 0.17 | - | - | - | 1.02 | 1.00–1.05 | 0.077 | - | - | - |
| BMI, kg/m2 | - | - | - | - | - | - | 0.94 | 0.89–1.00 | 0.054 | - | - | - |
| LVEF, % | 0.97 | 0.90–1.04 | 0.40 | 0.94 | 0.90–0.97 | <0.001 | 0.96 | 0.93–0.99 | 0.02 | 0.98 | 0.96–1.00 | 0.08 |
| CABG or PCI in the prior medical history | - | - | - | - | - | - | - | - | - | 1.50 | 1.06–2.14 | 0.02 |
| Peripheral artery disease | - | - | - | - | - | - | 1.67 | 0.97–2.88 | 0.06 | 1.35 | 0.93–1.96 | 0.11 |
| CKD | 2.01 | 0.72–5.65 | 0.19 | 1.92 | 1.06–3.49 | 0.03 | 1.48 | 0.90–2.42 | 0.12 | 1.12 | 0.81–1.55 | 0.51 |
| Diabetes | - | - | - | - | - | - | - | - | - | 1.67 | 1.24–2.25 | <0.001 |
| COPD | 1.45 | 0.43–4.94 | 0.55 | - | - | - | 1.38 | 0.83–2.29 | 0.21 | 1.20 | 0.85–1.70 | 0.30 |
| Heart rate, * b.p.m | 1.02 | 1.005–1.04 | 0.011 | 1.00 | 0.99–1.01 | 0.82 | 1.00 | 0.99–1.01 | 0.41 | 1.00 | 1.00–1.01 | 0.21 |
| SBP, * mmHg | - | - | - | 1.00 | 0.99–1.02 | 0.72 | 0.99 | 0.98–0.99 | 0.03 | 0.99 | 0.986–0.999 | 0.04 |
| NYHA class, * class IV or III vs. II or I | 6.23 | 2.02–19.2 | <0.001 | 2.02 | 1.22–3.32 | 0.006 | - | - | - | 1.50 | 1.10–2.06 | 0.01 |
| ACE-I | 0.71 | 0.24–2.13 | 0.54 | 0.76 | 0.43–1.36 | 0.36 | 0.70 | 0.42–1.15 | 0.70 | 0.69 | 0.49–0.97 | 0.03 |
| B-blockers | 0.12 | 0.03–0.4 | <0.001 | 0.42 | 0.16–1.12 | 0.082 | 0.39 | 0.20–0.74 | 0.004 | 0.44 | 0.27–0.73 | <0.001 |
| MRA | - | - | - | 0.91 | 0.49–1.67 | 0.76 | - | - | - | - | - | - |
| Diuretics | - | - | - | 0.71 | 0.27–1.90 | 0.5 | - | - | - | - | - | - |
| Statins | - | - | - | - | - | - | 0.67 | 0.38–1.16 | 0.15 | 0.73 | 0.49–1.08 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tymińska, A.; Ozierański, K.; Balsam, P.; Maciejewski, C.; Wancerz, A.; Brociek, E.; Marchel, M.; Crespo-Leiro, M.G.; Maggioni, A.P.; Drożdż, J.; et al. Ischemic Cardiomyopathy versus Non-Ischemic Dilated Cardiomyopathy in Patients with Reduced Ejection Fraction— Clinical Characteristics and Prognosis Depending on Heart Failure Etiology (Data from European Society of Cardiology Heart Failure Registries). Biology 2022, 11, 341. https://doi.org/10.3390/biology11020341
Tymińska A, Ozierański K, Balsam P, Maciejewski C, Wancerz A, Brociek E, Marchel M, Crespo-Leiro MG, Maggioni AP, Drożdż J, et al. Ischemic Cardiomyopathy versus Non-Ischemic Dilated Cardiomyopathy in Patients with Reduced Ejection Fraction— Clinical Characteristics and Prognosis Depending on Heart Failure Etiology (Data from European Society of Cardiology Heart Failure Registries). Biology. 2022; 11(2):341. https://doi.org/10.3390/biology11020341
Chicago/Turabian StyleTymińska, Agata, Krzysztof Ozierański, Paweł Balsam, Cezary Maciejewski, Anna Wancerz, Emil Brociek, Michał Marchel, Maria G. Crespo-Leiro, Aldo P. Maggioni, Jarosław Drożdż, and et al. 2022. "Ischemic Cardiomyopathy versus Non-Ischemic Dilated Cardiomyopathy in Patients with Reduced Ejection Fraction— Clinical Characteristics and Prognosis Depending on Heart Failure Etiology (Data from European Society of Cardiology Heart Failure Registries)" Biology 11, no. 2: 341. https://doi.org/10.3390/biology11020341
APA StyleTymińska, A., Ozierański, K., Balsam, P., Maciejewski, C., Wancerz, A., Brociek, E., Marchel, M., Crespo-Leiro, M. G., Maggioni, A. P., Drożdż, J., Opolski, G., Grabowski, M., & Kapłon-Cieślicka, A. (2022). Ischemic Cardiomyopathy versus Non-Ischemic Dilated Cardiomyopathy in Patients with Reduced Ejection Fraction— Clinical Characteristics and Prognosis Depending on Heart Failure Etiology (Data from European Society of Cardiology Heart Failure Registries). Biology, 11(2), 341. https://doi.org/10.3390/biology11020341










