Role of Epicardial Adipose Tissue in Cardiovascular Diseases: A Review
Abstract
Simple Summary
Abstract
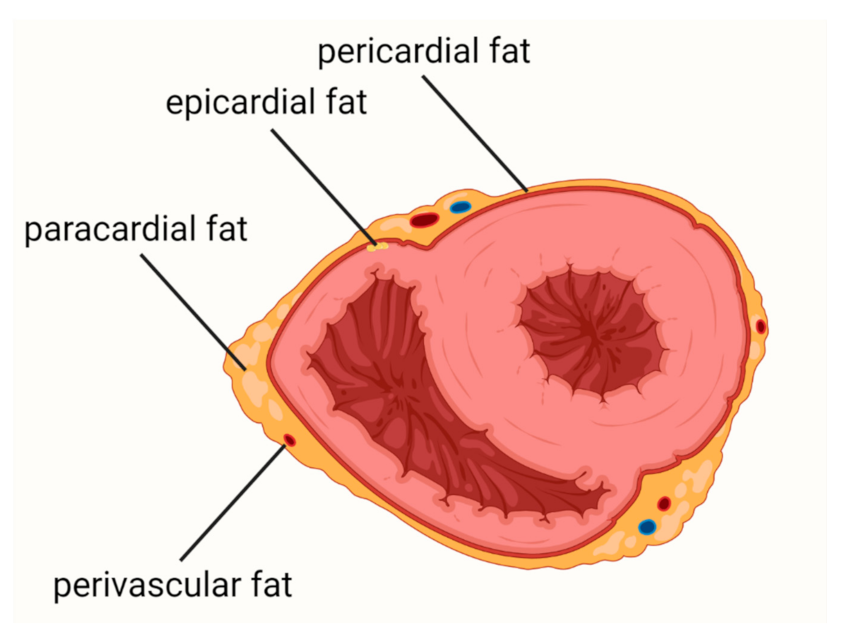
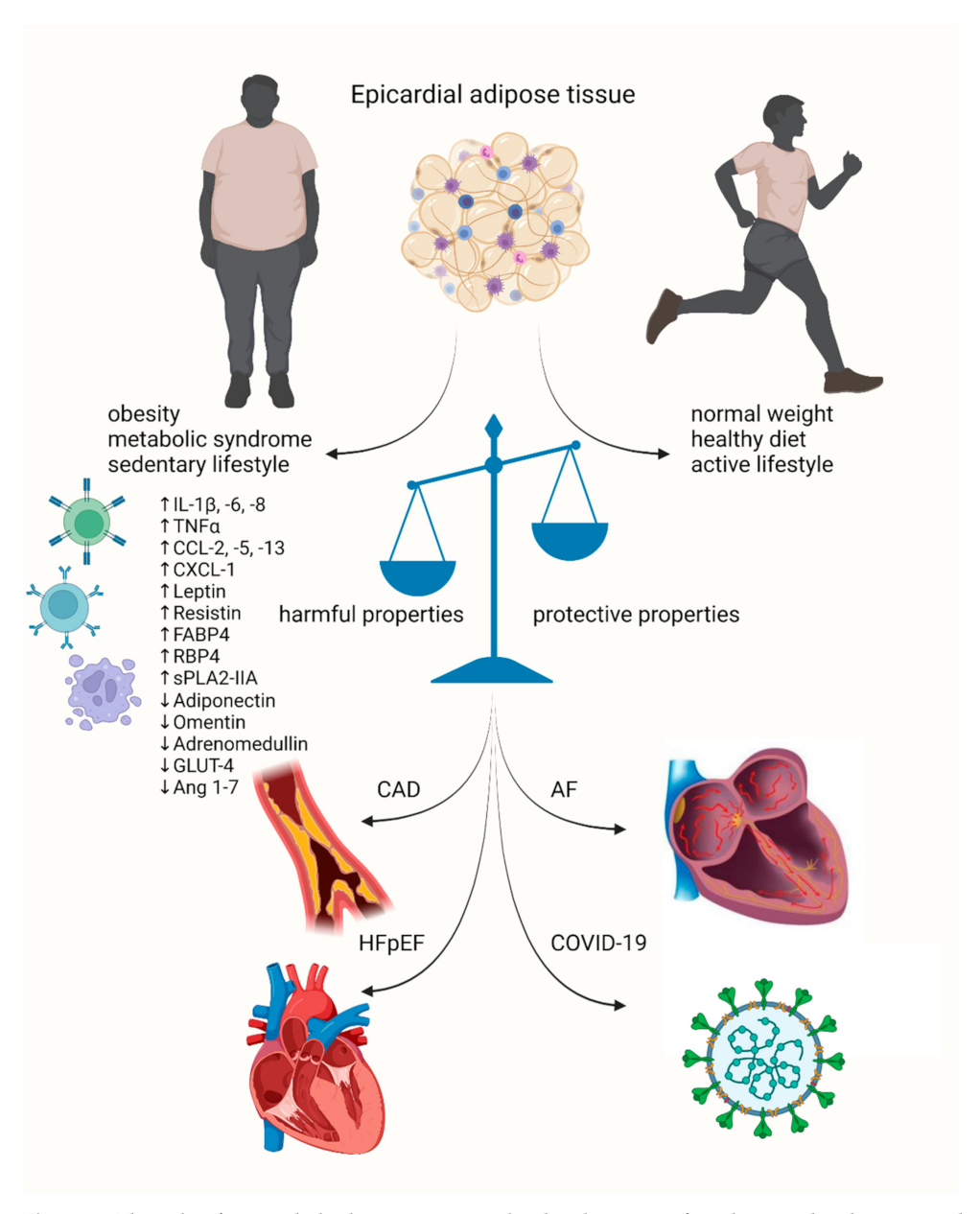
1. Introduction
2. Coronary Artery Disease
3. Heart Failure
4. Atrial Fibrillation
5. COVID-19
6. Therapeutic Options to Affect EAT
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Heron, M. Deaths: Leading Causes for 2013. Natl. Vital Stat. Rep. 2016, 68, 1–77. [Google Scholar]
- Fryar, C.D.; Chen, T.-C.; Li, X. Prevalence of Uncontrolled Risk Factors for Cardiovascular Disease: United States, 1999–2010. NCHS Data Brief. 2012, 103, 1–8. [Google Scholar]
- Sacks, H.S.; Fain, J.N. Human epicardial adipose tissue: A review. Am. Heart J. 2007, 153, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, P.; Pibarot, P.; Larose, É.; Poirier, P.; Marette, A.; Després, J.-P. Visceral obesity and the heart. Int. J. Biochem. Cell Biol. 2008, 40, 821–836. [Google Scholar] [CrossRef]
- Mathieu, P.; Lemieux, I.; Després, J.-P. Obesity, Inflammation, and Cardiovascular Risk. Clin. Pharmacol. Ther. 2010, 87, 407–416. [Google Scholar] [CrossRef]
- Després, J.-P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Rabkin, S.W. Epicardial fat: Properties, function and relationship to obesity. Obes. Rev. 2007, 8, 253–261. [Google Scholar] [CrossRef]
- Iacobellis, G.; Willens, H.J.; Barbaro, G.; Sharma, A.M. Threshold values of high-risk echocardiographic epicardial fat thickness. Obesity 2008, 16, 887–892. [Google Scholar] [CrossRef]
- Mahabadi, A.A.; Berg, M.H.; Lehmann, N.; Kälsch, H.; Bauer, M.; Kara, K.; Dragano, N.; Moebus, S.; Jöckel, K.-H.; Erbel, R.; et al. Association of Epicardial Fat with Cardiovascular Risk Factors and Incident Myocardial Infarction in the General Population. J. Am. Coll. Cardiol. 2013, 61, 1388–1395. [Google Scholar] [CrossRef]
- Mazurek, T.; Opolski, G. Pericoronary Adipose Tissue: A Novel Therapeutic Target in Obesity-Related Coronary Atherosclerosis. J. Am. Coll. Nutr. 2015, 34, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, K.; Zmijewski, P.; Krawczyk, K.; Czajkowska, A.; Kęska, A.; Kapuściński, P.; Mazurek, T. High intensity interval and moderate continuous cycle training in a physical education programme improves health-related fitness in young females. Biol. Sport 2016, 33, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Jermendy, A.L.; Merkely, B.; Maurovich-Horvat, P. Clinical importance of epicardial adipose tissue. Arch. Med Sci. 2017, 4, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zalewski, A.; Liu, Y.; Mazurek, T.; Cowan, S.; Martin, J.L.; Hofmann, S.M.; Vlassara, H.; Shi, Y. Diabetes-Induced Oxidative Stress and Low-Grade Inflammation in Porcine Coronary Arteries. Circulation 2003, 108, 472–478. [Google Scholar] [CrossRef] [PubMed]
- McAninch, E.; Fonseca, T.L.; Poggioli, R.; Panos, A.; Salerno, T.A.; Deng, Y.; Li, Y.; Bianco, A.; Iacobellis, G. Epicardial adipose tissue has a unique transcriptome modified in severe coronary artery disease. Obesity 2015, 23, 1267–1278. [Google Scholar] [CrossRef]
- Iacobellis, G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat. Rev. Endocrinol. 2015, 11, 363–371. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human Epicardial Adipose Tissue Is a Source of Inflammatory Mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef]
- Sacks, H.S.; Fain, J.N.; Holman, B.; Cheema, P.; Chary, A.; Parks, F.; Karas, J.; Optican, R.; Bahouth, S.W.; Garrett, E.; et al. Uncoupling Protein-1 and Related Messenger Ribonucleic Acids in Human Epicardial and Other Adipose Tissues: Epicardial Fat Functioning as Brown Fat. J. Clin. Endocrinol. Metab. 2009, 94, 3611–3615. [Google Scholar] [CrossRef]
- Prati, F.; Arbustini, E.; Labellarte, A.; Sommariva, L.; Pawlowski, T.; Manzoli, A.; Pagano, A.; Motolese, M.; Boccanelli, A. Eccentric atherosclerotic plaques with positive remodelling have a pericardial distribution: A permissive role of epicardial fat? A three-dimensional intravascular ultrasound study of left anterior descending artery lesions. Eur. Heart J. 2003, 24, 329–336. [Google Scholar] [CrossRef]
- Iacobellis, G.; Bianco, A. Epicardial adipose tissue: Emerging physiological, pathophysiological and clinical features. Trends Endocrinol. Metab. 2011, 22, 450–457. [Google Scholar] [CrossRef]
- Iozzo, P. Metabolic toxicity of the heart: Insights from molecular imaging. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, W.-Q.; Zhang, H.; Yang, X.; Fan, Q.; Christopher, T.A.; Lopez, B.L.; Tao, L.; Goldstein, B.J.; Gao, F.; et al. Adiponectin improves endothelial function in hyperlipidemic rats by reducing oxidative/nitrative stress and differential regulation of eNOS/iNOS activity. Am. J. Physiol. Metab. 2007, 293, E1703–E1708. [Google Scholar] [CrossRef]
- Deng, G.; Long, Y.; Yum, Y.R.; Li, M.R. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS pathway. Int. J. Obes. 2010, 34, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Niedziela, M.; Wojciechowska, M.; Zarębiński, M.; Cudnoch-Jędrzejewska, A.; Mazurek, T. Adiponectin promotes ischemic heart preconditioning-PRO and CON. Cytokine 2020, 127, 154981. [Google Scholar] [CrossRef] [PubMed]
- Konwerski, M.; Gromadka, A.; Arendarczyk, A.; Koblowska, M.; Iwanicka-Nowicka, R.; Wilimski, R.; Czub, P.; Filipiak, K.J.; Hendzel, P.; Zielenkiewicz, P.; et al. Atherosclerosis Pathways are Activated in Pericoronary Adipose Tissue of Patients with Coronary Artery Disease. J. Inflamm. Res. 2021, 14, 5419–5431. [Google Scholar] [CrossRef] [PubMed]
- Eiras, S.; Teijeira-Fernández, E.; Shamagian, L.G.; Fernandez, A.L.; Vazquez-Boquete, A.; Gonzalez-Juanatey, J.R. Extension of coronary artery disease is associated with increased IL-6 and decreased adiponectin gene expression in epicardial adipose tissue. Cytokine 2008, 43, 174–180. [Google Scholar] [CrossRef]
- Iacobellis, G.; Pistilli, D.; Gucciardo, M.; Leonetti, F.; Miraldi, F.; Brancaccio, G.; Gallo, P.; Di Gioia, C.R.T. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 2005, 29, 251–255. [Google Scholar] [CrossRef]
- Raman, P.; Khanal, S. Leptin in Atherosclerosis: Focus on Macrophages, Endothelial and Smooth Muscle Cells. Int. J. Mol. Sci. 2021, 22, 5446. [Google Scholar] [CrossRef]
- Iacobellis, G.; Malavazos, A.E.; Corsi, M.M. Epicardial fat: From the biomolecular aspects to the clinical practice. Int. J. Biochem. Cell Biol. 2011, 43, 1651–1654. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef] [PubMed]
- Tavora, F.; Kutys, R.; Li, L.; Ripple, M.; Fowler, D.; Burke, A. Adventitial lymphocytic inflammation in human coronary arteries with intimal atherosclerosis. Cardiovasc. Pathol. 2010, 19, e61–e68. [Google Scholar] [CrossRef]
- Wang, C.P.; Hsu, H.L.; Hung, W.C.; Yu, T.H.; Chen, Y.H.; Chiu, C.A.; Lu, L.F.; Chung, F.M.; Shin, S.J.; Lee, Y.J. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin. Endocrinol. 2009, 70, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Rosito, G.A.; Massaro, J.M.; Hoffmann, U.; Ruberg, F.L.; Mahabadi, A.A.; Vasan, R.S.; O’Donnell, C.J.; Fox, C.S. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: The Framingham Heart Study. Circulation 2008, 117, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, N.; McLean, D.S.; Janik, M.; Arepalli, C.D.; Stillman, A.E.; Raggi, P. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis 2010, 210, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Yamamoto, M.H.; Igawa, W.; Ono, M.; Kido, T.; Ebara, S.; Okabe, T.; Saito, S.; Amemiya, K.; Isomura, N.; et al. Association of Epicardial Adipose Tissue Volume and Total Coronary Plaque Burden in Patients with Coronary Artery Disease. Int. Heart J. 2018, 59, 1219–1226. [Google Scholar] [CrossRef]
- Mazurek, T.; Kochman, J.; Kobylecka, M.; Wilimski, R.; Filipiak, K.J.; Krolicki, L.; Opolski, G. Inflammatory activity of pericoronary adipose tissue may affect plaque composition in patients with acute coronary syndrome without persistent ST-segment elevation: Preliminary results. Kardiol. Pol. 2013, 72, 410–416. [Google Scholar] [CrossRef]
- Mazurek, T.; Kobylecka, M.; Zielenkiewicz, M.; Kurek, A.; Kochman, J.; Filipiak, K.J.; Mazurek, K.; Huczek, Z.; Królicki, L.; Opolski, G. PET/CT evaluation of 18F-FDG uptake in pericoronary adipose tissue in patients with stable coronary artery disease: Independent predictor of atherosclerotic lesions’ formation? J. Nucl. Cardiol. 2016, 24, 1075–1084. [Google Scholar] [CrossRef]
- Özcan, F.; Turak, O.; Canpolat, U.; Kanat, S.; Kadife, I.; Avcı, S.; Işleyen, A.; Cebeci, M.; Tok, D.; Başar, F.; et al. Association of epicardial fat thickness with TIMI risk score in NSTEMI/USAP patients. Herz 2014, 39, 755–760. [Google Scholar] [CrossRef]
- Otsuka, K.; Ishikawa, H.; Yamaura, H.; Shirasawa, K.; Kasayuki, N. Epicardial adipose tissue volume is associated with low-attenuation plaque volume in subjects with or without increased visceral fat: A 3-vessel coronary artery analysis with CT angiography. Eur. Heart J. 2021, 42 (Suppl. 1), ehab724-0208. [Google Scholar] [CrossRef]
- Ma, R.; van Assen, M.; Ties, D.; Pelgrim, G.J.; van Dijk, R.; Sidorenkov, G.; van Ooijen, P.M.A.; van der Harst, P.; Vliegenthart, R. Focal pericoronary adipose tissue attenuation is related to plaque presence, plaque type, and stenosis severity in coronary CTA. Eur. Radiol. 2021, 31, 7251–7261. [Google Scholar] [CrossRef] [PubMed]
- Balcer, B.; Dykun, I.; Schlosser, T.; Forsting, M.; Rassaf, T.; Mahabadi, A.A. Pericoronary fat volume but not attenuation differentiates culprit lesions in patients with myocardial infarction. Atherosclerosis 2018, 276, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Goeller, M.; Achenbach, S.; Cadet, S.; Kwan, A.C.; Commandeur, F.; Slomka, P.J.; Gransar, H.; Albrecht, M.H.; Tamarappoo, B.K.; Berman, D.S.; et al. Pericoronary Adipose Tissue Computed Tomography Attenuation and High-Risk Plaque Characteristics in Acute Coronary Syndrome Compared with Stable Coronary Artery Disease. JAMA Cardiol. 2018, 3, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Goeller, M.; Tamarappoo, B.K.; Kwan, A.C.; Cadet, S.; Commandeur, F.; Razipour, A.; Slomka, P.J.; Gransar, H.; Chen, X.; Otaki, Y.; et al. Relationship between changes in pericoronary adipose tissue attenuation and coronary plaque burden quantified from coronary computed tomography angiography. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 636–643. [Google Scholar] [CrossRef]
- Nogic, J.; Kim, J.; Layland, J.; Chan, J.; Cheng, K.; Wong, D.; Brown, A. TCT-241 Pericoronary Adipose Tissue Is a Predictor of In-Stent Restenosis and Stent Failure in Patients Undergoing Coronary Artery Stent Insertion. J. Am. Coll. Cardiol. 2021, 78 (19 Suppl. S), B98. [Google Scholar] [CrossRef]
- Sade, L.E.; Eroglu, S.; Bozbaş, H.; Özbiçer, S.; Hayran, M.; Haberal, A.; Muderrisoglu, I.H. Relation between epicardial fat thickness and coronary flow reserve in women with chest pain and angiographically normal coronary arteries. Atherosclerosis 2009, 204, 580–585. [Google Scholar] [CrossRef]
- Kanaji, Y.; Sugiyama, T.; Hoshino, M.; Misawa, T.; Nagamine, T.; Yasui, Y.; Nogami, K.; Ueno, H.; Hada, M.; Yamaguchi, M.; et al. The impact of pericoronary adipose tissue attenuation on global coronary flow reserve in patients with stable coronary artery disease. J. Am. Coll. Cardiol. 2021, 77, 1433. [Google Scholar] [CrossRef]
- Pasqualetto, M.C.; Tuttolomondo, D.; Cutruzzolà, A.; Niccoli, G.; Dey, D.; Greco, A.; Martini, C.; Irace, C.; Rigo, F.; Gaibazzi, N. Human coronary inflammation by computed tomography: Relationship with coronary microvascular dysfunction. Int. J. Cardiol. 2021, 336, 8–13. [Google Scholar] [CrossRef]
- Kataoka, T.; Harada, K.; Tanaka, A.; Onishi, T.; Matsunaga, S.; Funakubo, H.; Harada, K.; Nagao, T.; Shinoda, N.; Marui, N.; et al. Relationship between epicardial adipose tissue volume and coronary artery spasm. Int. J. Cardiol. 2021, 324, 8–12. [Google Scholar] [CrossRef]
- Aitken-Buck, H.M.; Moharram, M.; Babakr, A.A.; Reijers, R.; Van Hout, I.; Fomison-Nurse, I.C.; Sugunesegran, R.; Bhagwat, K.; Davis, P.J.; Bunton, R.W.; et al. Relationship between epicardial adipose tissue thickness and epicardial adipocyte size with increasing body mass index. Adipocyte 2019, 8, 412–420. [Google Scholar] [CrossRef]
- Naryzhnaya, N.V.; Koshelskaya, O.A.; Kologrivova, I.V.; Kharitonova, O.A.; Evtushenko, V.V.; Boshchenko, A.A. Hypertrophy and Insulin Resistance of Epicardial Adipose Tissue Adipocytes: Association with the Coronary Artery Disease Severity. Biomedicines 2021, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.; Borlaug, B.A. Heart failure with preserved ejection fraction. Curr. Probl. Cardiol. 2016, 41, 145–188. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Kitzman, D.W.; Borlaug, B.A.; van Heerebeek, L.; Zile, M.R.; Kass, D.A.; Paulus, W.J. Phenotype-specific treatment of heart failure with preserved ejection fraction: A multiorgan roadmap. Circulation 2016, 134, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Kitzman, D.W.; Shah, S.J. The HFpEF obesity phenotype: The elephant in the room. J. Am. Coll. Cardiol. 2016, 68, 200–203. [Google Scholar] [CrossRef]
- Savji, N.; Meijers, W.C.; Bartz, T.M.; Bhambhani, V.; Cushman, M.; Nayor, M.; Kizer, J.R.; Sarma, A.; Blaha, M.J.; Gansevoort, R.T.; et al. The Association of Obesity and Cardiometabolic Traits with Incident HFpEF and HFrEF. JACC Heart Fail. 2018, 6, 701–709. [Google Scholar] [CrossRef]
- Fontes-Carvalho, R.; Fontes-Oliveira, M.; Sampaio, F.; Bettencourt, N.; Teixeira, M.; Rocha Gonçalves, F.; Gama, V.; Leite-Moreira, A. Influence of epicardial and visceral fat on left ventricular diastolic and systolic functions inpatients after myocardial infarction. Am. J. Cardiol. 2014, 114, 1663–1669. [Google Scholar] [CrossRef]
- Liu, J.; Fox, C.S.; Hickson, D.A.; May, W.L.; Ding, J.; Carr, J.J.; Taylor, H.A. Pericardial fat and echocardiographic measures of cardiac abnormalities: The Jackson Heart Study. Diabetes Care 2011, 34, 341–346. [Google Scholar] [CrossRef]
- Cavalcante, J.L.; Tamarappoo, B.K.; Hachamovitch, R.; Kwon, D.H.; Alraies, M.C.; Halliburton, S.; Schoenhagen, P.; Dey, D.; Berman, D.S.; Marwick, T.H. Association of Epicardial Fat, Hypertension, Subclinical Coronary Artery Disease, and Metabolic Syndrome with Left Ventricular Diastolic Dysfunction. Am. J. Cardiol. 2012, 110, 1793–1798. [Google Scholar] [CrossRef]
- Nerlekar, N.; Muthalaly, R.G.; Wong, N.; Thakur, U.; Wong, D.T.L.; Brown, A.J.; Marwick, T.H. Association of Volumetric Epicardial Adipose Tissue Quantification and Cardiac Structure and Function. J. Am. Heart Assoc. 2018, 7, e009975. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.; Pislaru, S.; Melenovsky, V.; Borlaug, B.A. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation 2017, 136, 6–19. [Google Scholar] [CrossRef]
- Peterson, L.R.; Herrero, P.; Schechtman, K.B.; Racette, S.; Waggoner, A.D.; Kisrieva-Ware, Z.; Dence, C.; Klein, S.; Marsala, J.; Meyer, T.; et al. Effect of Obesity and Insulin Resistance on Myocardial Substrate Metabolism and Efficiency in Young Women. Circulation 2004, 109, 2191–2196. [Google Scholar] [CrossRef]
- Iacobellis, G.; Corradi, D.; Sharma, A.M. Epicardial adipose tissue: Anatomic, biomolecular and clinical relationships with the heart. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Koepp, K.E.; Obokata, M.; Reddy, Y.N.; Olson, T.P.; Borlaug, B.A. Hemodynamic and Functional Impact of Epicardial Adipose Tissue in Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2020, 8, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Van Woerden, G.; Gorter, T.M.; Westenbrink, B.D.; Willems, T.P.; Van Veldhuisen, D.J.; Rienstra, M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur. J. Heart Fail. 2018, 20, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.; Lau, D.H.; Sanders, P. Impact of obesity on cardiac metabolism, fibrosis, and function. Trends Cardiovasc. Med. 2015, 25, 119–126. [Google Scholar] [CrossRef]
- Mohammed, S.F.; Hussain, S.; Mirzoyev, S.A.; Edwards, W.D.; Maleszewski, J.J.; Redfield, M.M. Coronary Microvascular Rarefaction and Myocardial Fibrosis in Heart Failure With Preserved Ejection Fraction. Circulation 2015, 131, 550–559. [Google Scholar] [CrossRef]
- Duca, F.; Kammerlander, A.A.; Zotter-Tufaro, C.; Aschauer, S.; Schwaiger, M.L.; Marzluf, B.A.; Bonderman, D.; Mascherbauer, J. Interstitial fibrosis, functional status, and outcomes in heart failure with preserved ejection fraction: Insights from a prospective cardiac magnetic resonance imaging study. Circ. Cardiovasc. Imaging 2016, 9, e005277. [Google Scholar] [CrossRef]
- Packer, M. Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. J. Am. Coll. Cardiol. 2018, 71, 2360–2372. [Google Scholar] [CrossRef]
- Ng, A.C.T.; Strudwick, M.; van der Geest, R.J.; Ng, A.C.C.; Gillinder, L.; Goo, S.Y.; Cowin, G.; Delgado, V.; Wang, W.Y.S.; Bax, J.J. Impact of epicardial adipose tissue, left ventricular myocardial fat content, and interstitial fibrosis on myocardial contractile function. Circ. Cardiovasc. Imaging 2018, 11, e007372. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Antoniades, C. Cardiac Magnetic Resonance Imaging of Epicardial and Intramyocardial Adiposity as an Early Sign of Myocardial Disease. Circ. Cardiovasc. Imaging 2018, 11, e008083. [Google Scholar] [CrossRef]
- Nyman, K.; Granér, M.; Pentikäinen, M.O.; Lundbom, J.; Hakkarainen, A.; Sirén, R.; Nieminen, M.S.; Taskinen, M.-R.; Lundbom, N.; Lauerma, K. Cardiac steatosis and left ventricular function in men with metabolic syndrome. J. Cardiovasc. Magn. Reson. 2013, 15, 103. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Fukuda, S.; Tanaka, A.; Otsuka, K.; Taguchi, H.; Shimada, K. Relationships Between Periventricular Epicardial Adipose Tissue Accumulation, Coronary Microcirculation, and Left Ventricular Diastolic Dysfunction. Can. J. Cardiol. 2017, 33, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, S.W. Is reduction in coronary blood flow themechanism by which epicardial fat produces left ventricular diastolic dysfunction? Can. J. Cardiol. 2017, 33, 1459–1461. [Google Scholar] [CrossRef][Green Version]
- Pugliese, N.; De Biase, N.; Mazzola, M.; Paneni, F.; Del Punta, L.; Gargani, L.; Mengozzi, A.; Virdis, A.; Nesti, L.; Taddei, S.; et al. Prognostic significance of epicardial adipose tissue in heart failure with preserved and reduced ejection fraction. Eur. Heart J. 2021, 42 (Suppl. 1), ehab724-0820. [Google Scholar] [CrossRef]
- Nyawo, T.A.; Dludla, P.V.; Mazibuko-Mbeje, S.E.; Mthembu, S.X.H.; Nyambuya, T.M.; Nkambule, B.B.; Gijsen, H.S.-V.; Strijdom, H.; Pheiffer, C. A systematic review exploring the significance of measuring epicardial fat thickness in correlation to B-type natriuretic peptide levels as prognostic and diagnostic markers in patients with or at risk of heart failure. Heart Fail. Rev. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zulkifly, H.; Lip, G.Y.H.; Lane, D.A. Epidemiology of atrial fibrillation. Int. J. Clin. Pract. 2018, 72, e13070. [Google Scholar] [CrossRef]
- Schneider, M.P.; Hua, T.A.; Böhm, M.; Wachtell, K.; Kjeldsen, S.E.; Schmieder, R.E. Prevention of Atrial Fibrillation by Renin-Angiotensin System Inhibition: A Meta-Analysis. J. Am. Coll. Cardiol. 2010, 55, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Large, S.R.; Hosseinpour, A.R.; Wisbey, C.; Wells, F.C. Spontaneous cardioversion and mitral valve repair: A role for surgical cardioversion (Cox-maze)? Eur. J. Cardiothorac. Surg. 1997, 11, 76–80. [Google Scholar] [CrossRef]
- Hirsh, B.J.; Copeland-Halperin, R.S.; Halperin, J.L. Fibrotic atrial cardiomyo-pathy, atrial fibrillation, and thromboembolism: Mechanistic links and clinical inferences. J. Am. Coll. Cardiol. 2015, 65, 2239–2251. [Google Scholar] [CrossRef]
- Watanabe, T.; Takeishi, Y.; Hirono, O.; Itoh, M.; Matsui, M.; Nakamura, K.; Tamada, Y.; Kubota, I. C-Reactive protein elevation predicts the occurrence of atrial structural remodeling in patients with paroxysmal atrial fibrillation. Heart Vessel. 2005, 20, 45–49. [Google Scholar] [CrossRef]
- Lindhardsen, J.; Ahlehoff, O.; Gislason, G.H.; Madsen, O.R.; Olesen, J.B.; Svendsen, J.H.; Torp-Pedersen, C.; Hansen, P.R. Risk of atrial fibrillation and stroke in rheumatoid arthritis: Danish nationwide cohort study. BMJ 2012, 344, e1257. [Google Scholar] [CrossRef] [PubMed]
- Rhee, T.-M.; Lee, J.H.; Choi, E.-K.; Han, K.-D.; Lee, H.; Park, C.S.; Hwang, D.; Lee, S.-R.; Lim, W.-H.; Kang, S.-H.; et al. Increased Risk of Atrial Fibrillation and Thromboembolism in Patients with Severe Psoriasis: A Nationwide Population-based Study. Sci. Rep. 2017, 7, 9973. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, H.; Okutucu, S.; Sayin, B.Y.; Oto, A. Non-invasive electrocardiographic methods for assessment of atrial conduction heterogeneity in ankylosing spondylitis. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2185–2186. [Google Scholar] [PubMed]
- Efe, T.H.; Cimen, T.; Ertem, A.G.; Coskun, Y.; Bilgin, M.; Sahan, H.F.; Pamukcu, H.E.; Yayla, C.; Sunman, H.; Yuksel, I.; et al. Atrial Electromechanical Properties in Inflammatory Bowel Disease. Echocardiography 2016, 33, 1309–1316. [Google Scholar] [CrossRef]
- Wanahita, N.; Messerli, F.H.; Bangalore, S.; Gami, A.S.; Somers, V.K.; Steinberg, J.S. Atrial fibrillation and obesity—Results of a meta-analysis. Am. Heart J. 2008, 155, 310–315. [Google Scholar] [CrossRef]
- Huxley, R.R.; Alonso, A.; Lopez, F.L.; Filion, K.; Agarwal, S.K.; Loehr, L.; Soliman, E.Z.; Pankow, J.; Selvin, E. Type 2 diabetes, glucose homeostasis and incident atrial fibrillation: The Atherosclerosis Risk in Communities study. Heart 2011, 98, 133–138. [Google Scholar] [CrossRef]
- Graça, B.; Ferreira, M.J.; Donato, P.; Gomes, L.; Castelo-Branco, M.; Caseiro-Alves, F. Left atrial dysfunction in type 2 diabetes mellitus: Insights from cardiac MRI. Eur. Radiol. 2014, 24, 2669–2676. [Google Scholar] [CrossRef]
- Nyman, K.; Graner, M.; Pentikäinen, M.; Lundbom, J.; Hakkarainen, A.; Siren, R.; Nieminen, M.; Taskinen, M.-R.; Lauerma, K.; Lundbom, N. Metabolic syndrome associates with left atrial dysfunction. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 727–734. [Google Scholar] [CrossRef]
- Chechi, K.; Voisine, P.; Mathieu, P.; Laplante, M.; Bonnet, S.; Picard, F.; Joubert, P.; Richard, D. Functional characterization of the Ucp1-associated oxidative phenotype of human epicardial adipose tissue. Sci. Rep. 2017, 7, 15566. [Google Scholar] [CrossRef]
- Pezeshkian, M.; Noori, M.; Najjarpour-Jabbari, H.; Abolfathi, A.; Darabi, M.; Darabi, M.; Shaaker, M.; Shahmohammadi, G. Fatty acid composition of epicardial and subcutaneous human adipose tissue. Metab. Syndr. Relat. Disord. 2009, 7, 125–131. [Google Scholar] [CrossRef]
- Kourliouros, A.; Karastergiou, K.; Nowell, J.; Gukop, P.; Hosseini, M.T.; Valencia, O.; Ali, V.M.; Jahangiri, M. Protective effect of epicardial adiponectin on atrial fibrillation following cardiac surgery. Eur. J. Cardio-Thoracic. Surg. 2011, 39, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Takemori, K.; Mizuguchi, N.; Kimura, M.; Chikugo, T.; Hagiyama, M.; Yoneshige, A.; Mori, T.; Maenishi, O.; Kometani, T.; et al. Heart-bound adiponectin, not serum adiponectin, inversely correlates with cardiac hypertrophy in stroke-prone spontaneously hypertensive rats. Exp. Physiol. 2017, 102, 1435–1447. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Margaritis, M.; Verheule, S.; Recalde, A.; Sanna, F.; Herdman, L.; Psarros, C.; Nasrallah, H.; Coutinho, P.; Akoumianakis, I.; et al. Mutual regulation of epicardial adipose tissue and myocardial redox state by PPARgamma/adiponectin signalling. Circ Res. 2016, 118, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Liu, L.; Yang, Y.; Tamaki, Z.; Wei, J.; Marangoni, R.G.; Bhattacharyya, S.; Summer, R.S.; Ye, B.; Varga, J. The adipokine adiponectin has potent anti-fibrotic effects mediated via adenosine monophosphate-activated protein kinase: Novel target for fibrosis therapy. Arthritis Res. Ther. 2012, 14, R229. [Google Scholar] [CrossRef] [PubMed]
- Vural, B.; Atalar, F.; Ciftci, C.; Demirkan, A.; Susleyici-Duman, B.; Gunay, D.; Akpinar, B.; Sagbas, E.; Ozbek, U.; Buyukdevrim, A.S. Presence of fatty-acid-binding protein 4 expression in human epicardial adipose tissue in metabolic syndrome. Cardiovasc. Pathol. 2008, 17, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, H.S.; Jung, J.W.; Kim, N.S.; Noh, C.I.; Hong, Y.M. Correlation Between Epicardial Fat Thickness by Echocardiography and Other Parameters in Obese Adolescents. Korean Circ. J. 2012, 42, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Karastergiou, K.; Evans, I.; Ogston, N.; Miheisi, N.; Nair, D.; Kaski, J.-C.; Jahangiri, M.; Mohamed-Ali, V. Epicardial Adipokines in Obesity and Coronary Artery Disease Induce Atherogenic Changes in Monocytes and Endothelial Cells. Arter. Thromb. Vasc. Biol. 2010, 30, 1340–1346. [Google Scholar] [CrossRef]
- Bambace, C.; Sepe, A.; Zoico, E.; Telesca, M.; Olioso, D.; Venturi, S.; Rossi, A.; Corzato, F.; Faccioli, S.; Cominacini, L.; et al. Inflammatory profile in subcutaneous and epicardial adipose tissue in men with and without diabetes. Heart Vessel. 2014, 29, 42–48. [Google Scholar] [CrossRef]
- Cheng, K.-H.; Chu, C.-S.; Lee, K.-T.; Lin, T.-H.; Hsieh, C.-C.; Chiu, C.-C.; Voon, W.-C.; Sheu, S.-H.; Lai, W.-T. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int. J. Obes. 2008, 32, 268–274. [Google Scholar] [CrossRef]
- Mazurek, T.; Kiliszek, M.; Kobylecka, M.; Skubisz-Głuchowska, J.; Kochman, J.; Filipiak, K.; Krolicki, L.; Opolski, G. Relation of Proinflammatory Activity of Epicardial Adipose Tissue to the Occurrence of Atrial Fibrillation. Am. J. Cardiol. 2014, 113, 1505–1508. [Google Scholar] [CrossRef]
- Tse, G.; Yan, B.P.; Chan, Y.W.F.; Tian, X.Y.; Huang, Y. Reactive oxygen species, endoplasmic reticulum stress and mitochondrial dysfunction: The link with cardiac arrhythmogenesis. Front. Physiol. 2016, 7, 313. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kattach, H.; Ratnatunga, C.; Pillai, R.; Channon, K.; Casadei, B. Association of Atrial Nicotinamide Adenine Dinucleotide Phosphate Oxidase Activity With the Development of Atrial Fibrillation After Cardiac Surgery. J. Am. Coll. Cardiol. 2008, 51, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Somoza, A.; Teijeira-Fernández, E.; Fernández, Á.L.; González-Juanatey, J.R.; Eiras, S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H202–H209. [Google Scholar] [CrossRef]
- Haemers, P.; Hamdi, H.; Guedj, K.; Suffee, N.; Farahmand, P.; Popovic, N.; Claus, P.; Leprince, P.; Nicoletti, A.; Jalife, J.; et al. Atrial fibrillation is associated with the fibrotic remodelling of adipose tissue in the subepicardium of human and sheep atria. Eur. Heart J. 2017, 38, 53–61. [Google Scholar] [CrossRef]
- Platonov, P.; Mitrofanova, L.B.; Orshanskaya, V.; Ho, S.Y. Structural Abnormalities in Atrial Walls Are Associated with Presence and Persistency of Atrial Fibrillation But Not with Age. J. Am. Coll. Cardiol. 2011, 58, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.; Wang, N.; Meigs, J.B.; Hoffmann, U.; Massaro, J.M.; Fox, C.S.; Magnani, J.W. Pericardial Fat is Associated with Atrial Conduction: The Framingham Heart Study. J. Am. Heart Assoc. 2014, 3, e000477. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H.; Chen, J.; Zhao, L. Epicardial adipose tissue and atrial fibrillation: Possible mechanisms, potential therapies, and future directions. Pacing Clin. Electrophysiol. 2019, 43, 133–145. [Google Scholar] [CrossRef]
- Opolski, M.P.; Staruch, A.D.; Kusmierczyk, M.; Witkowski, A.; Kwiecinska, S.; Kosek, M.; Jastrzebski, J.; Pregowski, J.; Kruk, M.; Rozanski, J.; et al. Computed tomography angiography for prediction of atrial fibrillation after coronary artery bypass grafting: Proof of concept. J. Cardiol. 2015, 65, 285–292. [Google Scholar] [CrossRef]
- Shen, M.J.; Shinohara, T.; Park, H.-W.; Frick, K.; Ice, D.S.; Choi, E.-K.; Han, S.; Maruyama, M.; Sharma, R.; Shen, C.; et al. Continuous Low-Level Vagus Nerve Stimulation Reduces Stellate Ganglion Nerve Activity and Paroxysmal Atrial Tachyarrhythmias in Ambulatory Canines. Circulation 2011, 123, 2204–2212. [Google Scholar] [CrossRef]
- Chen, P.-S.; Chen, L.S.; Fishbein, M.C.; Lin, S.F.; Nattel, S. Role of the autonomic nervous system in atrial fibrillation: Pathophysiology and therapy. Circ Res. 2014, 114, 1500–1515. [Google Scholar] [CrossRef]
- Balcioğlu, A.S.; Çiçek, D.; Akinci, S.; Eldem, H.O.; Bal, U.A.; Okyay, K.; Muderrisoglu, I.H. Arrhythmogenic Evidence for Epicardial Adipose Tissue: Heart Rate Variability and Turbulence are Influenced by Epicardial Fat Thickness. Pacing Clin. Electrophysiol. 2015, 38, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Danik, S.; Neuzil, P.; D’Avila, A.; Malchano, Z.J.; Kralovec, S.; Ruskin, J.N.; Reddy, V. Evaluation of Catheter Ablation of Periatrial Ganglionic Plexi in Patients with Atrial Fibrillation. Am. J. Cardiol. 2008, 102, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.V.; Wang, P.J.; Larson, J.C.; Virnig, B.A.; Cochrane, B.; Curb, J.D.; Klein, L.; Manson, J.E.; Martin, L.W.; Robinson, J.; et al. Effects of postmenopausal hormone therapy on incident atrial fibrillation: TheWomen’s health initiative randomized controlled trials. Circ. Arrhythm. Electrophysiol. 2012, 5, 1108–1116. [Google Scholar] [CrossRef]
- Bernasochi, G.B.; Boon, W.C.; Curl, C.L.; Varma, U.; Pepe, S.; Tare, M.; Parry, L.J.; Dimitriadis, E.; Harrap, S.B.; Nalliah, C.J.; et al. Pericardial adipose and aromatase: A new translational target for aging, obesity and arrhythmogenesis? J. Mol. Cell. Cardiol. 2017, 111, 96–101. [Google Scholar] [CrossRef]
- Ernault, A.C.; Meijborg, V.M.F.; Coronel, R. Modulation of Cardiac Arrhythmogenesis by Epicardial Adipose Tissue: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1730–1745. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.X.; Sullivan, T.; Sun, M.T.; Mahajan, R.; Pathak, R.K.; Middeldorp, M.; Twomey, D.; Ganesan, A.N.; Rangnekar, G.; Roberts-Thomson, K.C.; et al. Obesity and the risk of incident, post-operative, and post-ablation atrial fibrillation: A metaanalysis of 626,603 individuals in 51 studies. JACC Clin. Electrophysiol. 2015, 1, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Thanassoulis, G.; Massaro, J.M.; O’Donnell, C.J.; Hoffmann, U.; Levy, D.; Ellinor, P.T.; Wang, T.J.; Schnabel, R.B.; Vasan, R.S.; Fox, C.S.; et al. Pericardial fat is associated with prevalent atrial fibrillation: The Framingham heart study. Circ. Arrhythm. Electrophysiol. 2010, 3, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Al Chekakie, M.O.; Welles, C.C.; Metoyer, R.; Ibrahim, A.; Shapira, A.R.; Cytron, J.; Santucci, P.; Wilber, D.J.; Akar, J.G. Pericardial fat is independently associated with human atrial fibrillation. J. Am. Coll. Cardiol. 2010, 56, 784–788. [Google Scholar] [CrossRef]
- Batal, O.; Schoenhagen, P.; Shao, M.; Ayyad, A.E.; Van Wagoner, D.R.; Halliburton, S.S.; Tchou, P.J.; Chung, M.K. Left Atrial Epicardial Adiposity and Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2010, 3, 230–236. [Google Scholar] [CrossRef]
- Muhib, S.; Fujino, T.; Sato, N.; Hasebe, N. Epicardial Adipose Tissue Is Associated With Prevalent Atrial Fibrillation in Patients with Hypertrophic Cardiomyopathy. Int. Heart J. 2013, 54, 297–303. [Google Scholar] [CrossRef]
- Wong, C.; Abed, H.S.; Molaee, P.; Nelson, A.; Brooks, A.G.; Sharma, G.; Leong, D.P.; Lau, D.H.; Middeldorp, M.; Roberts-Thomson, K.C.; et al. Pericardial Fat Is Associated with Atrial Fibrillation Severity and Ablation Outcome. J. Am. Coll. Cardiol. 2011, 57, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Tsao, H.-M.; Hu, W.-C.; Wu, M.-H.; Tai, C.-T.; Lin, Y.-J.; Chang, S.-L.; Lo, L.-W.; Hu, Y.-F.; Tuan, T.-C.; Wu, T.-J.; et al. Quantitative Analysis of Quantity and Distribution of Epicardial Adipose Tissue Surrounding the Left Atrium in Patients with Atrial Fibrillation and Effect of Recurrence after Ablation. Am. J. Cardiol. 2011, 107, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.X.; Sun, M.T.; Odutayo, A.; Emdin, C.A.; Mahajan, R.; Lau, D.H.; Pathak, R.; Wong, D.T.; Selvanayagam, J.B.; Sanders, P.; et al. Associations of Epicardial, Abdominal, and Overall Adiposity with Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2016, 9, e004378. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, M.; Bandera, F.; Tassinari, F.; Capasso, L.; Cargnelutti, M.; Pelissero, G.; Malavazos, A.E.; Ricci, C. Is epicardial fat depot associated with atrial fibrillation? A systematic review and meta-analysis. Europace 2017, 19, 747–752. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Feng, X.; Li, S.; Sun, Q.; Zhu, J.; Chen, B.; Xiong, M.; Cao, G. Immune-Inflammatory Parameters in COVID-19 Cases: A Systematic Review and Meta-Analysis. Front. Med. 2020, 7, 301. [Google Scholar] [CrossRef]
- Gąsecka, A.; Filipiak, K.J.; Jaguszewski, M.J. Impaired microcirculation function in COVID-19 and implications for potential therapies. Cardiol. J. 2020, 27, 485–488. [Google Scholar]
- Poggiali, E.; Zaino, D.; Immovilli, P.; Rovero, L.; Losi, G.; Dacrema, A.; Nuccetelli, M.; Vadacca, G.B.; Guidetti, D.; Vercelli, A.; et al. Lactate dehydrogenase and C-reactive protein as predictors of respiratory failure in COVID-19 patients. Clin. Chim. Acta 2020, 509, 135–138. [Google Scholar] [CrossRef]
- Ruetzler, K.; Szarpak, Ł.; Ładny, J.R.; Gąsecka, A.; Gilis-Malinowska, N.; Pruc, M.; Smereka, J.; Nowak, B.; Filipiak, K.J.; Jaguszewski, M.J. D-dimer levels predict COVID-19 severity and mortality. Kardiol. Pol. 2021, 79, 217–218. [Google Scholar] [CrossRef]
- Szarpak, L.; Zaczynski, A.; Kosior, D.; Bialka, S.; Ladny, J.R.; Gilis-Malinowska, N.; Smereka, J.; Kanczuga-Koda, L.; Gasecka, A.; Filipiak, K.J.; et al. Evidence of diagnostic value of ferritin in patients with COVID-19. Cardiol. J. 2020, 27, 886–887. [Google Scholar] [CrossRef]
- Rychter, A.M.; Zawada, A.; Ratajczak, A.E.; Dobrowolska, A.; Krela-Kaźmierczak, I. Should patients with obesity be more afraid of COVID-19? Obes. Rev. 2020, 21, 13083. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.M.; Caplice, N.M. Is Adipose Tissue a Reservoir for Viral Spread, Immune Activation, and Cytokine Amplification in Coronavirus Disease 2019? Obesity 2020, 28, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, A.; Eslami, V.; Baharvand, Z.; Khalili, N.; Saghamanesh, S.; Zarei, E.; Sanei-Taheri, M. Epicardial adipose tissue, inflammatory biomarkers and COVID-19: Is there a possible relationship? Int. Immunopharmacol. 2021, 90, 107174. [Google Scholar] [CrossRef]
- Deng, M.; Qi, Y.; Deng, L.; Wang, H.; Xu, Y.; Li, Z.; Meng, Z.; Tang, J.; Dai, Z. Obesity as a Potential Predictor of Disease Severity in Young COVID-19 Patients: A Retrospective Study. Obesity 2020, 28, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Secchi, F.; Capitanio, G.; Basilico, S.; Schiaffino, S.; Boveri, S.; Sardanelli, F.; Romanelli, M.M.C.; Malavazos, A.E. Epicardial Fat Inflammation in Severe COVID-19. Obesity 2020, 28, 2260–2262. [Google Scholar] [CrossRef] [PubMed]
- Grodecki, K.; Lin, A.; Razipour, A.; Cadet, S.; McElhinney, P.A.; Chan, C.; Pressman, B.D.; Julien, P.; Maurovich-Horvat, P.; Gaibazzi, N.; et al. Epicardial adipose tissue is associated with extent of pneumonia and adverse outcomes in patients with COVID-19. Metabolism 2021, 115, 154436. [Google Scholar] [CrossRef]
- Wei, Z.; Geng, Y.; Huang, J.; Qian, H. Pathogenesis and management of myocardial injury in coronavirus disease 2019. Eur. J. Heart Fail. 2020, 22, 1994–2006. [Google Scholar] [CrossRef]
- Geng, Y.-J.; Wei, Z.-Y.; Qian, H.-Y.; Huang, J.; Lodato, R.; Castriotta, R.J. Pathophysiological characteristics and therapeutic approaches for pulmonary injury and cardiovascular complications of coronavirus disease 2019. Cardiovasc. Pathol. 2020, 47, 107228. [Google Scholar] [CrossRef]
- Gąsecka, A.; Borovac, J.A.; Guerreiro, R.A.; Giustozzi, M.; Parker, W.; Caldeira, D.; Chiva-Blanch, G. Thrombotic Complications in Patients with COVID-19: Pathophysiological Mechanisms, Diagnosis, and Treatment. Cardiovasc. Drugs Ther. 2021, 35, 215–229. [Google Scholar] [CrossRef]
- Szarpak, L.; Filipiak, K.J.; Skwarek, A.; Pruc, M.; Rahnama, M.; Denegri, A.; Jachowicz, M.; Dawidowska, M.; Gasecka, A.; Jaguszewski, M.J.; et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19: A systematic review and meta-analysis. Cardiol. J. 2021. [Google Scholar] [CrossRef]
- Nucera, G.; Chirico, F.; Rafique, Z.; Gilis-Malinowska, N.; Gasecka, A.; Litvinova, N.; Wang, B.; Ilesanmi, O.; Pruc, M.; Jaguszewski, M.J.; et al. Need to update cardiological guidelines to prevent COVID-19 related myocardial infarction and ischemic stroke. Cardiol. J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Gasecka, A.; Pruc, M.; Kukula, K.; Gilis-Malinowska, N.; Filipiak, K.J.; Jaguszewski, M.J.; Szarpak, L. Post-COVID-19 heart syndrome. Cardiol. J. 2021, 28, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Hui, H.; Zhang, Y.; Yang, X.; Wang, X.; He, B.; Li, L.; Li, H.; Tian, J.; Chen, Y. Clinical and radiographic features of cardiac injury in patients with 2019 novel coronavirus pneumonia. MedRχiv 2020. [Google Scholar] [CrossRef]
- Wei, Z.-Y.; Qiao, R.; Chen, J.; Huang, J.; Wang, W.-J.; Yu, H.; Xu, J.; Wu, H.; Wang, C.; Gu, C.-H.; et al. Pre-existing Health Conditions and Epicardial Adipose Tissue Volume: Potential Risk Factors for Myocardial Injury in COVID-19 Patients. Front. Cardiovasc. Med. 2021, 7, 585220. [Google Scholar] [CrossRef] [PubMed]
- Launbo, N.; Zobel, E.H.; Von Scholten, B.J.; Færch, K.; Jørgensen, P.G.; Christensen, R.H. Targeting epicardial adipose tissue with exercise, diet, bariatric surgery or pharmaceutical interventions: A systematic review and meta-analysis. Obes. Rev. 2021, 22, 13136. [Google Scholar] [CrossRef]
- Rabkin, S.W.; Campbell, H. Comparison of reducing epicardial fat by exercise, diet or bariatric surgery weight loss strategies: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 406–415. [Google Scholar] [CrossRef]
- Christensen, R.H.; Wedell-Neergaard, A.S.; Lehrskov, L.L.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Effect of Aerobic and Resistance Exercise on Cardiac Adipose Tissues: Secondary Analyses From a Randomized Clinical Trial. JAMA Cardiol. 2019, 4, 778–787. [Google Scholar] [CrossRef]
- Fernandez-Del-Valle, M.; Gonzales, J.U.; Kloiber, S.; Mitra, S.; Klingensmith, J.; Larumbe-Zabala, E. Effects of resistance training on MRI-derived epicardial fat volume and arterial stiffness in women with obesity: A randomized pilot study. Eur. J. Appl. Physiol. 2018, 118, 1231–1240. [Google Scholar] [CrossRef]
- Kahl, K.; Kerling, A.; Tegtbur, U.; Gützlaff, E.; Herrmann, J.; Borchert, L.; Ates, Z.; Westhoff-Bleck, M.; Hueper, K.; Hartung, D. Effects of additional exercise training on epicardial, intra-abdominal and subcutaneous adipose tissue in major depressive disorder: A randomized pilot study. J. Affect. Disord. 2016, 192, 91–97. [Google Scholar] [CrossRef]
- Fornieles González, G.; Rosety Rodríguez, M.A.; Pery Bohorquez, M.T.; Diaz Ordóñez, A.; Rosety Rodríguez, J.; Pery Bohorquez, M.T.; Brenes Martin, F.; Escribano Ocon, A.; Rosety Rodríguez, M.; Ordóñez Muñoz, F.J.; et al. A home-based treadmill training reduced epicardial and abdominal fat in postmenopausal women with metabolic syndrome. Nutr. Hosp. 2014, 30, 609–613. [Google Scholar] [CrossRef]
- Konwerski, M.; Postuła, M.; Barczuk-Falęcka, M.; Czajkowska, A.; Mróz, A.; Witek, K.; Bakalarski, W.; Gąsecka, A.; Małek, Ł.; Mazurek, T. Epicardial Adipose Tissue and Cardiovascular Risk Assessment in Ultra-Marathon Runners: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 3136. [Google Scholar] [CrossRef]
- Gepner, Y.; Shelef, I.; Schwarzfuchs, D.; Zelicha, H.; Tene, L.; Yaskolka Meir, A.; Tsaban, G.; Cohen, N.; Bril, N.; Rein, M.; et al. Effect of distinct lifestyle interventions on mobilization of fat storage pools CENTRAL magnetic resonance imaging randomized controlled trial. Circulation 2018, 137, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-K.; Tanaka, K.; Matuso, T.; Endo, T.; Tomita, T.; Maeda, S.; Ajisaka, R. Comparison of epicardial, abdominal and regional fat compartments in response to weight loss. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Snel, M.; Jonker, J.T.; Hammer, S.; Kerpershoek, G.; Lamb, H.J.; Meinders, A.E.; Pijl, H.; de Roos, A.; Romijn, J.A.; Smit, J.W.; et al. Long-Term Beneficial Effect of a 16-Week Very Low Calorie Diet on Pericardial Fat in Obese Type 2 Diabetes Mellitus Patients. Obesity 2012, 20, 1572–1576. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Singh, N.; Wharton, S.; Sharma, A.M. Substantial Changes in Epicardial Fat Thickness after Weight Loss in Severely Obese Subjects. Obesity 2008, 16, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Willens, H.J.; Byers, P.; Chirinos, J.A.; Labrador, E.; Hare, J.M.; de Marchena, E. Effects of Weight Loss after Bariatric Surgery on Epicardial Fat Measured Using Echocardiography. Am. J. Cardiol. 2007, 99, 1242–1245. [Google Scholar] [CrossRef]
- Gaborit, B.; Jacquier, A.; Kober, F.; Abdesselam, I.; Cuisset, T.; Boullu-Ciocca, S.; Emungania, O.; Alessi, M.C.; Clément, K.; Bernard, M.; et al. Effects of bariatric surgery on cardiac ectopic fat: Lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J. Am. Coll. Cardiol. 2012, 60, 1381–1389. [Google Scholar] [CrossRef]
- Hannukainen, J.C.; Lautamäki, R.; Pärkkä, J.; Strandberg, M.; Saunavaara, V.; Hurme, S.; Soinio, M.; Dadson, P.; Virtanen, K.A.; Grönroos, T.; et al. Reversibility of myocardial metabolism and remodelling in morbidly obese patients 6 months after bariatric surgery. Diabetes Obes. Metab. 2018, 20, 963–973. [Google Scholar] [CrossRef]
- Graziani, F.; Leone, A.M.; Cialdella, P.; Basile, E.; Pennestrì, F.; Della Bona, R.; Iaconelli, A.; Liuzzo, G.; Biasucci, L.M.; Cardillo, M.T.; et al. Effects of bariatric surgery on cardiac remodeling: Clinical and pathophysiologic implications. Int. J. Cardiol. 2013, 168, 4277–4279. [Google Scholar] [CrossRef]
- Alexopoulos, N.; Melek, B.H.; Arepalli, C.D.; Hartlage, G.R.; Chen, Z.; Kim, S.; Stillman, A.E.; Raggi, P. Effect of intensive versus moderate lipid-lowering therapy on epicardial adipose tissue in hyperlipidemic post-menopausal women: A substudy of the BELLES trial (beyond endorsed lipid lowering with EBT scanning). J. Am. Coll. Cardiol. 2013, 61, 1956–1961. [Google Scholar] [CrossRef]
- Soucek, F.; Covassin, N.; Singh, P.; Ruzek, L.; Kara, T.; Suleiman, M.; Lerman, A.; Koestler, C.; Friedman, P.A.; Lopez-Jimenez, F.; et al. Effects of Atorvastatin (80 mg) Therapy on Quantity of Epicardial Adipose Tissue in Patients Undergoing Pulmonary Vein Isolation for Atrial Fibrillation. Am. J. Cardiol. 2015, 116, 1443–1446. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Petraglia, L.; D’Esposito, V.; Cabaro, S.; Rengo, G.; Caruso, A.; Grimaldi, M.G.; Baldascino, F.; De Bellis, A.; Vitale, D.; et al. Statin therapy modulates thickness and inflammatory profile of human epicardial adipose tissue. Int. J. Cardiol. 2019, 274, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Gadiyaram, V.; Zhang, C.; Chen, Z.; Lopaschuk, G.; Stillman, A.E. Statins Reduce Epicardial Adipose Tissue Attenuation Independent of Lipid Lowering: A Potential Pleiotropic Effect. J. Am. Heart Assoc. 2019, 8, e013104. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.O.; Budoff, M. Effect of statins on atherosclerotic plaque. Trends Cardiovasc. Med. 2019, 29, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Tawakol, A.; Fayad, Z.A.; Mogg, R.; Alon, A.; Klimas, M.T.; Dansky, H.; Subramanian, S.S.; Abdelbaky, A.; Rudd, J.; Farkouh, M.E.; et al. Intensification of Statin Therapy Results in a Rapid Reduction in Atherosclerotic Inflammation: Results of a Multicenter Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography Feasibility Study. J. Am. Coll. Cardiol. 2013, 62, 909–917. [Google Scholar] [CrossRef]
- Yang, Q.; Qi, X.; Dang, Y.; Li, Y.; Song, X.; Hao, X. Effects of atorvastatin on atrial remodeling in a rabbit model of atrial fibrillation produced by rapid atrial pacing. BMC Cardiovasc. Disord. 2016, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, B.; Guo, F.; Li, J.; Han, W.; Tang, Q.; Zhang, Y. Effect of atorvastatin on left atrial function of patients with paroxysmal atrial fibrillation. Genet. Mol. Res. 2013, 12, 3488–3494. [Google Scholar] [CrossRef]
- Fauchier, L.; Pierre, B.; de Labriolle, A.; Grimard, C.; Zannad, N.; Babuty, D. Antiarrhythmic Effect of Statin Therapy and Atrial Fibrillation: A Meta-Analysis of Randomized Controlled Trials. J. Am. Coll. Cardiol. 2008, 51, 828–835. [Google Scholar] [CrossRef]
- Fang, W.; Li, H.-J.; Zhang, H.; Jiang, S. The role of statin therapy in the prevention of atrial fibrillation: A meta-analysis of randomized controlled trials. Br. J. Clin. Pharmacol. 2012, 74, 744–756. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Korantzopoulos, P.; Liu, E.; Li, G. Statin use and development of atrial fibrillation: A systematic review and meta-analysis of randomized clinical trials and observational studies. Int. J. Cardiol. 2008, 126, 160–170. [Google Scholar] [CrossRef]
- Akahori, H.; Tsujino, T.; Naito, Y.; Matsumoto, M.; Sasaki, N.; Iwasaku, T.; Eguchi, A.; Sawada, H.; Hirotani, S.; Masuyama, T. Atorvastatin ameliorates cardiac fibrosis and improves left ventricular diastolic function in hypertensive diastolic heart failure model rats. J. Hypertens. 2014, 32, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Garre, D.; González-Rubio, M.L.; Muñoz-Pacheco, P.; Caro-Vadillo, A.; Aragoncillo, P.; Fernández-Cruz, A. Rosuvastatin added to standard heart failure therapy improves cardiac remodelling in heart failure rats with preserved ejection fraction. Eur. J. Heart Fail. 2010, 12, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.L.; Otto, M.E.; D′avila, L.B.; Netto, F.M.; Armendaris, M.K.; Sposito, A.C. Diastolic function parameters are improved by the addition of simvastatin to enalapril-based treatment in hypertensive individuals. Atherosclerosis 2012, 222, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Okura, H.; Asawa, K.; Kubo, T.; Taguchi, H.; Toda, I.; Yoshiyama, M.; Yoshikawa, J.; Yoshida, K. Impact of Statin Therapy on Systemic Inflammation, Left Ventricular Systolic and Diastolic Function and Prognosis in Low Risk Ischemic Heart Disease Patients without History of Congestive Heart Failure. Intern. Med. 2007, 46, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Preiss, D.; Campbell, R.T.; Murray, H.M.; Ford, I.; Packard, C.J.; Sattar, N.; Rahimi, K.; Colhoun, H.M.; Waters, D.D.; LaRosa, J.C.; et al. The effect of statin therapy on heart failure events: A collaborative meta-analysis of unpublished data from major randomized trials. Eur. Heart J. 2015, 36, 1536–1546. [Google Scholar] [CrossRef]
- Marume, K.; Takashio, S.; Nagai, T.; Tsujita, K.; Saito, Y.; Yoshikawa, T.; Anzai, T. Effect of Statins on Mortality in Heart Failure with Preserved Ejection Fraction without Coronary Artery Disease―Report from the JASPER Study. Circ. J. 2019, 83, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Duan, L.; Clare, R.; Hekimian, A.; Spencer, H.; Chen, W. Comparison of Effects of Statin Use on Mortality in Patients with Heart Failure and Preserved versus Reduced Left Ventricular Ejection Fraction. Am. J. Cardiol. 2018, 122, 405–412. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, X.X.; Xu, Y.L.; Ru, J.; Hui, R.T.; Huang, X.H. Meta-analysis of the effect of statins on mortality in patients with preserved ejection fraction. Am. J. Cardiol. 2014, 113, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, H.; Goto, T.; Wakami, K.; Ohte, N. The effect of statins on mortality in heart failure with preserved ejection fraction: A meta-analysis of propensity score analyses. Int. J. Cardiol. 2016, 214, 301–306. [Google Scholar] [CrossRef]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G.; et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSIHF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1231–1239. [Google Scholar] [CrossRef]
- Rivas Galvez, R.E.; Morales Portano, J.D.; Trujillo Cortes, R.; Gomez Alvarez, E.B.; Sanchez Cubias, S.M.; Zelaya, S.M. Reduction of epicardial adipose tissue thickness with PCSK9 inhibitors. Eur. Heart J. 2020, 41 (Suppl. 2), ehaa946-3008. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Kernan, W.N.; Viscoli, C.M.; Furie, K.L.; Young, L.H.; Inzucchi, S.E.; Gorman, M.; Guarino, P.D.; Lovejoy, A.M.; Peduzzi, P.N.; Conwit, R.; et al. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. New Engl. J. Med. 2016, 374, 1321–1331. [Google Scholar] [CrossRef]
- Mohan, M.; Al-Talabany, S.; McKinnie, A.; Mordi, I.R.; Singh, J.; Gandy, S.J.; Baig, F.; Hussain, M.S.; Bhalraam, U.; Khan, F.; et al. A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: The MET-REMODEL trial. Eur. Heart J. 2019, 40, 3409–3417. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Massey, S.; Story, D.; Li, L. Metformin: An Old Drug with New Applications. Int. J. Mol. Sci. 2018, 19, 2863. [Google Scholar] [CrossRef]
- Jing, Y.; Wu, F.; Li, D.; Yang, L.; Li, Q.; Li, R. Metformin improves obesity-associated inflammation by altering macrophages polarization. Mol. Cell. Endocrinol. 2018, 461, 256–264. [Google Scholar] [CrossRef]
- Salvatore, T.; Pafundi, P.C.; Morgillo, F.; Di Liello, R.; Galiero, R.; Nevola, R.; Marfella, R.; Monaco, L.; Rinaldi, L.; Adinolfi, L.E.; et al. Metformin: An old drug against old age and associated morbidities. Diabetes Res. Clin. Pr. 2020, 160, 108025. [Google Scholar] [CrossRef]
- Morgillo, F.; Fasano, M.; Della Corte, C.M.; Sasso, F.C.; Papaccio, F.; Viscardi, G.; Esposito, G.; Di Liello, R.; Normanno, N.; Capuano, A.; et al. Results of the safety run-in part of the METAL (METformin in Advanced Lung cancer) study: A multicentre, open-label phase I–II study of metformin with erlotinib in second-line therapy of patients with stage IV non-small-cell lung cancer. ESMO Open 2017, 2, e000132. [Google Scholar] [CrossRef]
- Spencer, M.; Yang, L.; Adu, A.; Finlin, B.S.; Zhu, B.; Shipp, L.R.; Rasouli, N.; Peterson, C.A.; Kern, P.A. Pioglitazone Treatment Reduces Adipose Tissue Inflammation through Reduction of Mast Cell and Macrophage Number and by Improving Vascularity. PLoS ONE 2014, 9, e102190. [Google Scholar] [CrossRef]
- Xie, X.; Sinha, S.; Yi, Z.; Langlais, P.R.; Madan, M.; Bowen, B.P.; Willis, W.; Meyer, C. Role of adipocyte mitochondria in inflammation, lipemia and insulin sensitivity in humans: Effects of pioglitazone treatment. Int. J. Obes. 2018, 42, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.R.; Morrison, V.; Levin, D.; Mohan, M.; Forteath, C.; Beall, C.; McNeilly, A.; Balfour, D.J.; Savinko, T.; Wong, A.K.; et al. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ. Res. 2016, 119, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Ziyrek, M.; Kahraman, S.; Ozdemir, E.; Dogan, A. Metformin monotherapy significantly decreases epicardial adipose tissue thickness in newly diagnosed type 2 diabetes patients. Rev Port. Cardiol. 2019, 38, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Tokubuchi, I.; Tajiri, Y.; Iwata, S.; Hara, K.; Wada, N.; Hashinaga, T.; Nakayama, H.; Mifune, H.; Yamada, K. Beneficial effects of metformin on energy metabolism and visceral fat volume through a possible mechanism of fatty acid oxidation in human subjects and rats. PLoS ONE 2017, 12, e0171293. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, Y.; Ling, P.; Feng, X.; Luo, S.; Zheng, X.; Little, P.J.; Xu, S.; Weng, J. Metformin in cardiovascular diabetology: A focused review of its impact on endothelial function. Theranostics 2021, 11, 9376–9396. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Greulich, S.; van der Meer, R.W.; Rijzewijk, L.J.; Lamb, H.J.; de Roos, A.; Smit, J.W.; Romijn, J.A.; Ruige, J.B.; Lammertsma, A.A.; et al. Activin a is associated with impaired myocardial glucose metabolism and left ventricular remodeling in patients with uncomplicated type 2 diabetes. Cardiovasc. Diabetol. 2013, 12, 150. [Google Scholar] [CrossRef]
- Sardu, C.; D’Onofrio, N.; Torella, M.; Portoghese, M.; Loreni, F.; Mureddu, S.; Signoriello, G.; Scisciola, L.; Barbieri, M.; Rizzo, M.R.; et al. Pericoronary fat inflammation and Major Adverse Cardiac Events (MACE) in prediabetic patients with acute myocardial infarction: Effects of metformin. Cardiovasc. Diabetol. 2019, 18, 1–11. [Google Scholar] [CrossRef]
- Varjabedian, L.; Bourji, M.; Pourafkari, L.; Nader, N.D. Cardioprotection by Metformin: Beneficial Effects Beyond Glucose Reduction. Am. J. Cardiovasc. Drugs 2018, 18, 181–193. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Xanthopoulos, A.; Parissis, J.; Butler, J.; Farmakis, D. Pathogenesis of chronic heart failure: Cardiovascular aging, risk factors, comorbidities, and disease modifiers. Hear. Fail. Rev. 2020, 27, 337–344. [Google Scholar] [CrossRef]
- Sag, D.; Carling, D.; Stout, R.D.; Suttles, J. Adenosine 5′-Monophosphate-Activated Protein Kinase Promotes Macrophage Polarization to an Anti-Inflammatory Functional Phenotype. J. Immunol. 2008, 181, 8633–8641. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Park, M.-S.; Choung, J.-S.; Kim, S.-S.; Oh, H.-H.; Choi, C.-S.; Ha, S.-Y.; Kang, Y.; Kim, Y.; Jun, H.-S. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia 2012, 55, 2456–2468. [Google Scholar] [CrossRef]
- Salim, H.M.; Fukuda, D.; Higashikuni, Y.; Tanaka, K.; Hirata, Y.; Yagi, S.; Soeki, T.; Shimabukuro, M.; Sata, M. Teneligliptin, a dipeptidyl peptidase-4 inhibitor, attenuated pro-inflammatory phenotype of perivascular adipose tissue and inhibited atherogenesis in normoglycemic apolipoprotein-E-deficient mice. Vasc. Pharmacol. 2017, 96-98, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Grosso, A.F.; de Oliveira, S.F.; Higuchi Mde, L.; Favarato, D.; Dallan, L.A.; da Luz, P.L. Synergistic antiinflammatory effect: Simvastatin and pioglitazone reduce inflammatory markers of plasma and epicardial adipose tissue of coronary patients with metabolic syndrome. Diabetol. Metab. Syndr. 2014, 6, 47. [Google Scholar] [CrossRef]
- Sacks, H.S.; Fain, J.N.; Cheema, P.; Bahouth, S.W.; Garrett, E.; Wolf, R.Y.; Wolford, D.; Samaha, J. Inflammatory genes in epicardial fat contiguous with coronary atherosclerosis in the metabolic syndrome and type 2 diabetes: Changes associated with pioglitazone. Diabetes Care 2011, 34, 730–733. [Google Scholar] [CrossRef]
- Distel, E.; Penot, G.; Cadoudal, T.; Balguy, I.; Durant, S.; Benelli, C. Early induction of a brown-like phenotype by rosiglitazone in the epicardial adipose tissue of fatty Zucker rats. Biochimie 2012, 94, 1660–1667. [Google Scholar] [CrossRef]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.A.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefèbvre, P.J.; Murray, G.D.; et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Wolski, K.; Nicholls, S.J.; Nissen, S.E. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: A metaanalysis of randomized trials. JAMA 2007, 298, 1180–1188. [Google Scholar] [CrossRef]
- Bouchi, R.; Terashima, M.; Sasahara, Y.; Asakawa, M.; Fukuda, T.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Izumiyama, H.; et al. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: A pilot study. Cardiovasc. Diabetol. 2017, 16, 1–9. [Google Scholar] [CrossRef]
- Fukuda, T.; Bouchi, R.; Terashima, M.; Sasahara, Y.; Asakawa, M.; Takeuchi, T.; Nakano, Y.; Murakami, M.; Minami, I.; Izumiyama, H.; et al. Ipragliflozin Reduces Epicardial Fat Accumulation in Non-Obese Type 2 Diabetic Patients with Visceral Obesity: A Pilot Study. Diabetes Ther. 2017, 8, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Aizawa, Y.; Yuasa, S.; Fujita, S.; Ikeda, Y.; Okabe, M. The Effect of Dapagliflozin Treatment on Epicardial Adipose Tissue Volume and P-Wave Indices: An Ad-hoc Analysis of the Previous Randomized Clinical Trial. J. Atheroscler. Thromb. 2020, 27, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Correale, M.; Lamacchia, O.; Ciccarelli, M.; Dattilo, G.; Tricarico, L.; Brunetti, N.D. Vascular and metabolic effects of SGLT2i and GLP-1 in heart failure patients. Heart Fail. Rev. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Jardine, M.J.; Li, Q.; Neuen, B.L.; Cannon, C.P.; de Zeeuw, D.; Edwards, R.; Levin, A.; Mahaffey, K.W.; Perkovic, V.; et al. Effect of SGLT2 Inhibitors on Stroke and Atrial Fibrillation in Diabetic Kidney Disease: Results from the CREDENCE Trial and Meta-Analysis. Stroke 2021, 52, 1545–1556. [Google Scholar] [CrossRef]
- Bolinder, J.; Ljunggren, Ö.; Kullberg, J.; Johansson, L.; Wilding, J.; Langkilde, A.M.; Sugg, J.; Parikh, S. Effects of Dapagliflozin on Body Weight, Total Fat Mass, and Regional Adipose Tissue Distribution in Patients with Type 2 Diabetes Mellitus with Inadequate Glycemic Control on Metformin. J. Clin. Endocrinol. Metab. 2012, 97, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Gra-Menendez, S. Effects of Dapagliflozin on Epicardial Fat Thickness in Patients with Type 2 Diabetes and Obesity. Obesity 2020, 28, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rodríguez, E.; Agra, R.M.; Fernández, A.L.; Adrio, B.; García-Caballero, T.; Juanatey, J.R.G.; Eiras, S. Effects of dapagliflozin on human epicardial adipose tissue: Modulation of insulin resistance, inflammatory chemokine production, and differentiation ability. Cardiovasc. Res. 2018, 114, 336–346. [Google Scholar] [CrossRef]
- Masson, W.; Lavalle-Cobo, A.; Nogueira, J.P. Effect of SGLT2-Inhibitors on Epicardial Adipose Tissue: A Meta-Analysis. Cells 2021, 10, 2150. [Google Scholar] [CrossRef]
- Iacobellis, G.; Mohseni, M.; Bianco, S.; Banga, P.K. Liraglutide causes large and rapid epicardial fat reduction. Obesity 2017, 25, 311–316. [Google Scholar] [CrossRef]
- Dutour, A.; Abdesselam, I.; Ancel, P.; Kober, F.; Mrad, G.; Darmon, P.; Ronsin, O.; Pradel, V.; Lesavre, N.; Martin, J.C.; et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: A prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes. Metab. 2016, 18, 882–891. [Google Scholar] [CrossRef]
- Pastel, E.; McCulloch, L.J.; Ward, R.; Joshi, S.; Gooding, K.M.; Shore, A.C.; Kos, K. GLP-1 analogue-induced weight loss does not improve obesity-induced AT dysfunction. Clin. Sci. 2017, 131, 343–353. [Google Scholar] [CrossRef]
- Al-Barazanji, K.A.; Arch, J.R.; Buckingham, R.E.; Tadayyon, M. Central exendin-4 infusion reduces body weight without altering plasma leptin in (fa/fa) Zucker rats. Obes. Res. 2000, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Rakipovski, G.; Rolin, B.; Nøhr, J.; Klewe, I.; Frederiksen, K.S.; Augustin, R.; Hecksher-Sørensen, J.; Ingvorsen, C.; Polex-Wolf, J.; Knudsen, L.B. The GLP-1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis in ApoE-/- and LDLr-/- Mice by a Mechanism That Includes Inflammatory Pathways. JACC Basic Transl. Sci. 2018, 3, 844–857. [Google Scholar] [CrossRef]
- Iacobellis, G.; Camarena, V.; Sant, D.W.; Wang, G. Human Epicardial Fat Expresses Glucagon-Like Peptide 1 and 2 Receptors Genes. Horm. Metab. Res. 2017, 49, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ren, J.; Song, J.; Liu, F.; Wu, C.; Wang, X.; Gong, L.; Li, W.; Xiao, F.; Yan, F.; et al. Glucagon-like peptide 1 regulates adipogenesis in 3T3-L1 preadipocytes. Int. J. Mol. Med. 2013, 31, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Beiroa, D.; Imbernon, M.; Gallego, R.; Senra, A.; Herranz, D.; Villarroya, F.; Serrano, M.; Fernø, J.; Salvador, J.; Escalada, J.; et al. GLP-1 Agonism Stimulates Brown Adipose Tissue Thermogenesis and Browning through Hypothalamic AMPK. Diabetes 2014, 63, 3346–3358. [Google Scholar] [CrossRef]
- Lima-Martínez, M.M.; Paoli, M.; Rodney, M.; Balladares, N.; Contreras, M.; D’Marco, L.; Iacobellis, G. Effect of sitagliptin on epicardial fat thickness in subjects with type 2 diabetes and obesity: A pilot study. Endocrine 2015, 51, 448–455. [Google Scholar] [CrossRef]
- Packer, M. Have dipeptidyl peptidase-4 inhibitors ameliorated the vascular complications of type 2 diabetes in large-scale trials? The potential confounding effect of stem-cell chemokines. Cardiovasc. Diabetol. 2018, 17, 9. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, H.; Zhu, H. Blocking CXCR7-mediated adipose tissue macrophages chemotaxis attenuates insulin resistance and inflammation in obesity. Biochem. Biophys. Res. Commun. 2016, 479, 649–655. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Varin, E.M.; Ussher, J.R.; Campbell, J.E.; Bang, K.A.; Abdullah, T.; Baggio, L.L.; Drucker, D.J. Inhibition of Dipeptidyl Peptidase-4 Impairs Ventricular Function and Promotes Cardiac Fibrosis in High Fat–Fed Diabetic Mice. Diabetes 2015, 65, 742–754. [Google Scholar] [CrossRef]
- Aroor, A.; McKarns, S.; Nistala, R.; Demarco, V.; Gardner, M.; Garcia-Touza, M.; Whaley-Connell, A.; Sowers, J.R. DPP-4 Inhibitors as Therapeutic Modulators of Immune Cell Function and Associated Cardiovascular and Renal Insulin Resistance in Obesity and Diabetes. Cardiorenal Med. 2013, 3, 48–56. [Google Scholar] [CrossRef]
- Shah, Z.; Kampfrath, T.; Deiuliis, J.; Zhong, J.; Pineda, C.; Ying, Z.; Xu, X.; Lu, B.; Moffatt-Bruce, S.; Durairaj, R.; et al. Long-Term Dipeptidyl-Peptidase 4 Inhibition Reduces Atherosclerosis and Inflammation via Effects on Monocyte Recruitment and Chemotaxis. Circulation 2011, 124, 2338–2349. [Google Scholar] [CrossRef]
- Tomović, K.; Lazarevic, J.; Kocić, G.; Deljanin-Ilic, M.; Anderluh, M.; Smelcerovic, A. Mechanisms and pathways of anti-inflammatory activity of DPP-4 inhibitors in cardiovascular and renal protection. Med. Res. Rev. 2019, 39, 404–422. [Google Scholar] [CrossRef] [PubMed]
- Sakata, K.; Hayakawa, M.; Yano, Y.; Tamaki, N.; Yokota, N.; Eto, T.; Watanabe, R.; Hirayama, N.; Matsuo, T.; Kuroki, K.; et al. Efficacy of alogliptin, a dipeptidyl peptidase-4 inhibitor, on glucose parameters, the activity of the advanced glycation end product (AGE)—receptor for AGE (RAGE) axis and albuminuria in Japanese type 2 diabetes. Diabetes/Metabolism Res. Rev. 2013, 29, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Nakamura, Y.; Yamadera, S.; Inagaki, M.; Kenmotsu, S.; Saito, H.; Oguchi, T.; Tsuji, M.; Hirokazu, C.; Ohsawa, I.; et al. Linagliptin Inhibits Lipopolysaccharide-Induced Inflammation Concentration-Dependently And -Independently. J. Inflamm. Res. 2019, ume 12, 285–291. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Matsui, T.; Maeda, S.; Higashimoto, Y.; Yamagishi, S.-I. Advanced glycation end products evoke endothelial cell damage by stimulating soluble dipeptidyl peptidase-4 production and its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cardiovasc. Diabetologia 2013, 12, 125. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; MacFadyen, J.G.; Everett, B.M.; Libby, P.; Thuren, T.; Glynn, R.J.; Kastelein, J.; Koenig, W.; Genest, J.; Lorenzatti, A.; et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: A secondary analysis from the CANTOS randomised controlled trial. Lancet 2018, 391, 319–328. [Google Scholar] [CrossRef]
- Cutolo, M.; Sulli, A.; Pizzorni, C.; Seriolo, B.; Straub, R.H. Anti-inflammatory mechanisms of methotrexate in rheumatoid arthritis. Ann. Rheum. Dis. 2001, 60, 729–735. [Google Scholar] [CrossRef]
- Micha, R.; Imamura, F.; von Ballmoos, M.W.; Solomon, D.H.; Hernán, M.; Ridker, P.M.; Mozaffarian, D. Systematic Review and Meta-Analysis of Methotrexate Use and Risk of Cardiovascular Disease. Am. J. Cardiol. 2011, 108, 1362–1370. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef]
- Nidorf, S.M.; Eikelboom, J.; Budgeon, C.A.; Thompson, P.L. Low-Dose Colchicine for Secondary Prevention of Cardiovascular Disease. J. Am. Coll. Cardiol. 2013, 61, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Fiolet, A.T.L.; Mosterd, A.; Eikelboom, J.W.; Schut, A.; Opstal, T.S.J.; The, S.H.K.; Xu, X.-F.; Ireland, M.A.; Lenderink, T.; et al. Colchicine in Patients with Chronic Coronary Disease. N. Engl. J. Med. 2020, 383, 1838–1847. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.-C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
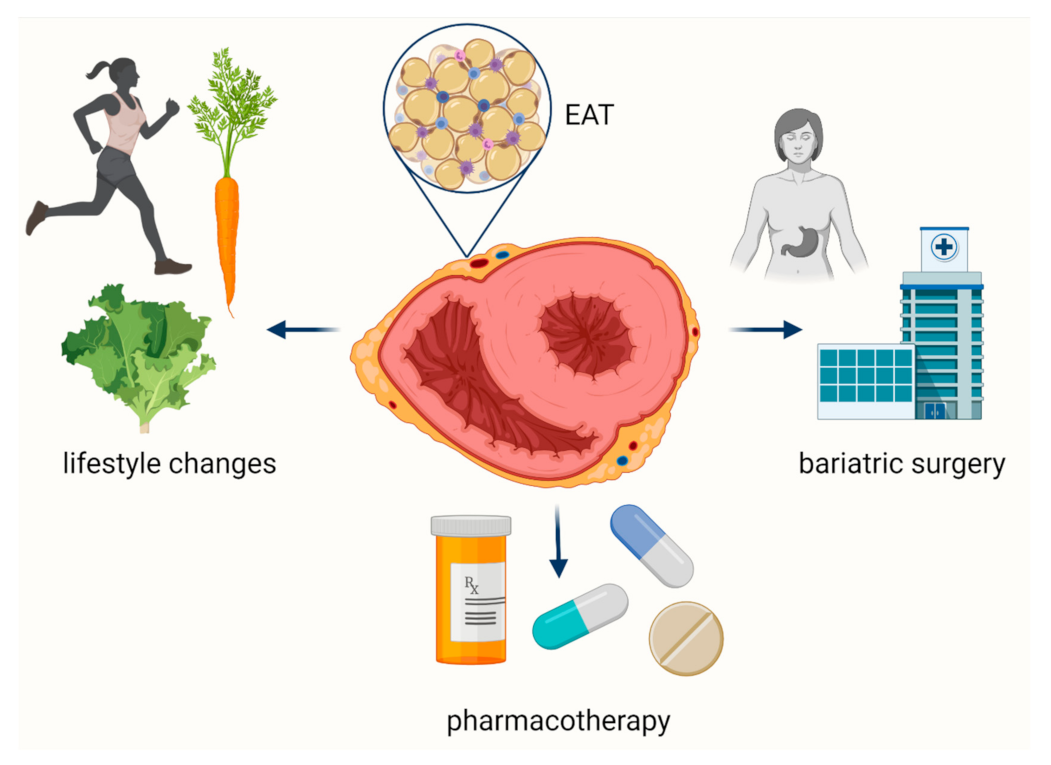
| Pharmacological Therapeutic Options | |
|---|---|
| Group of Drugs | Potential Mechanisms of Action |
| Statins | anti-inflammatory [160,162,163,164,165] modulation of EAT [164] ↓ EAT metabolic activity [163] |
| PCSK-9 inhibitors | unknown |
| Metformin | anti-inflammatory [186,187,196,197,198,200] ↑ fat oxidation and thermogenesis [193,194] ↓ endothelial dysfunction [195] activation of adenosine monophosphate-activated protein kinase [198,200] |
| Thiazolidinediones | anti-inflammatory [190,191,204,205,206] |
| SGLT2 inhibitors | anti-inflammatory [210,217] ↓ endothelial dysfunction [213] stimulation of visceral fat burn [215] ↑ EAT cells sensitivity to insulin [217] |
| GLP-1 agonists | ↑ pre-adipocyte differentiation [225] ↑ thermogenesis [226] ↑ adipocyte browning [226] |
| DPP-4 inhibitors | anti-inflammatory [231,232,233] downregulation of the receptor for advanced glycation end-products [234] ↑ cyclic adenosine monophosphate/protein kinase A signaling and IL-6 production [235] ↓ ROS generation and intercellular adhesion molecule-1 expression [236] |
| Canakinumab | anti-inflammatory [237] |
| Methotrexate | anti-inflammatory [239] |
| Colchicine | anti-inflammatory [242,243] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konwerski, M.; Gąsecka, A.; Opolski, G.; Grabowski, M.; Mazurek, T. Role of Epicardial Adipose Tissue in Cardiovascular Diseases: A Review. Biology 2022, 11, 355. https://doi.org/10.3390/biology11030355
Konwerski M, Gąsecka A, Opolski G, Grabowski M, Mazurek T. Role of Epicardial Adipose Tissue in Cardiovascular Diseases: A Review. Biology. 2022; 11(3):355. https://doi.org/10.3390/biology11030355
Chicago/Turabian StyleKonwerski, Michał, Aleksandra Gąsecka, Grzegorz Opolski, Marcin Grabowski, and Tomasz Mazurek. 2022. "Role of Epicardial Adipose Tissue in Cardiovascular Diseases: A Review" Biology 11, no. 3: 355. https://doi.org/10.3390/biology11030355
APA StyleKonwerski, M., Gąsecka, A., Opolski, G., Grabowski, M., & Mazurek, T. (2022). Role of Epicardial Adipose Tissue in Cardiovascular Diseases: A Review. Biology, 11(3), 355. https://doi.org/10.3390/biology11030355








