Integrating Cytogenetics and Population Genomics: Allopatry and Neo-Sex Chromosomes May Have Shaped the Genetic Divergence in the Erythrinus erythrinus Species Complex (Teleostei, Characiformes)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Sampling
2.2. Chromosome Preparations and Fluorescence In Situ Hybridization (FISH) for rDNA Mapping
2.3. Comparative Genomic Hybridization (CGH)
2.4. Microscopy and Image Processing
2.5. Sequencing and Filtering
2.6. Detection of Markers Putatively under Selection
2.7. Genetic Diversity
2.8. Population Structure Analysis
3. Results
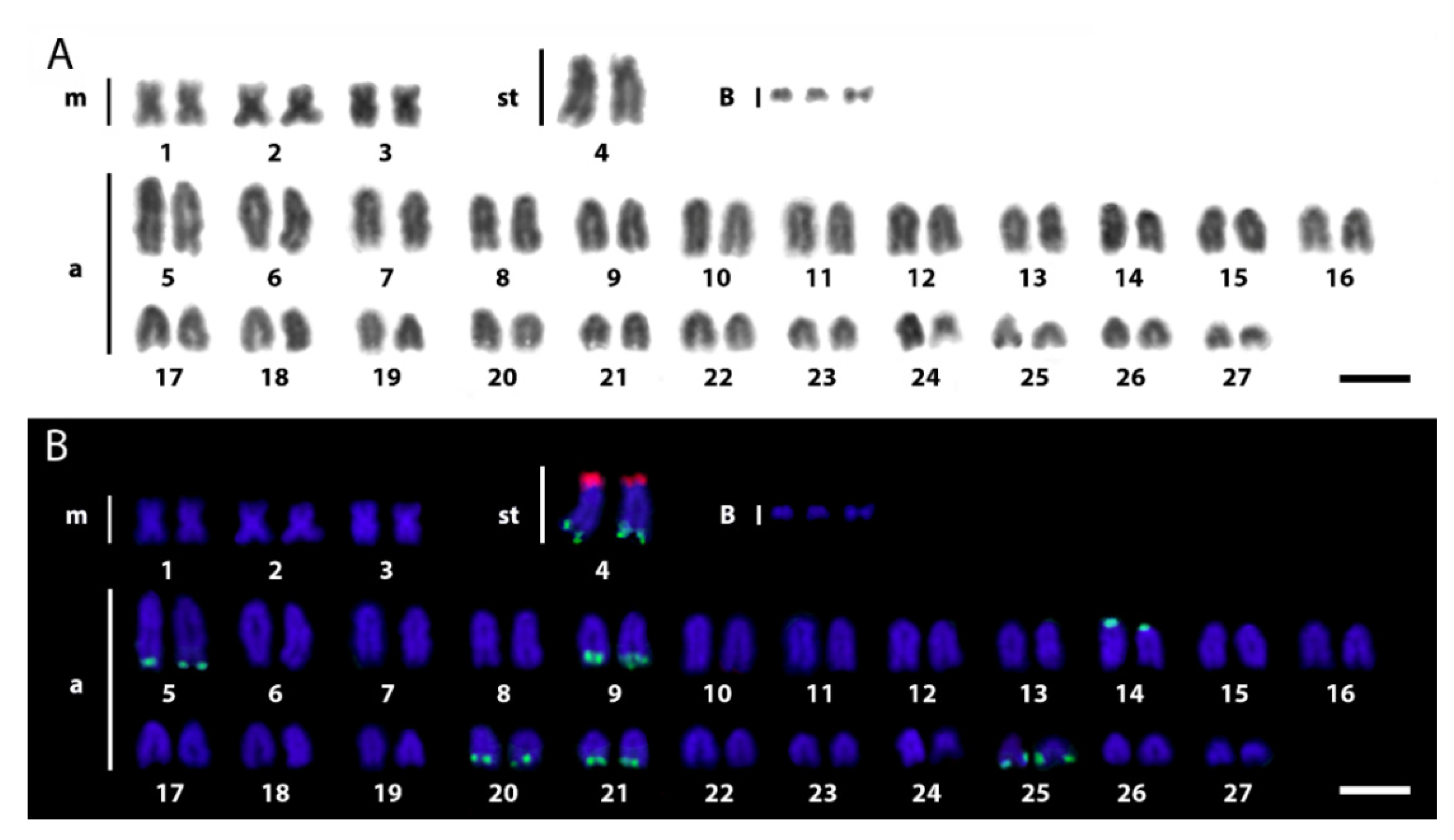
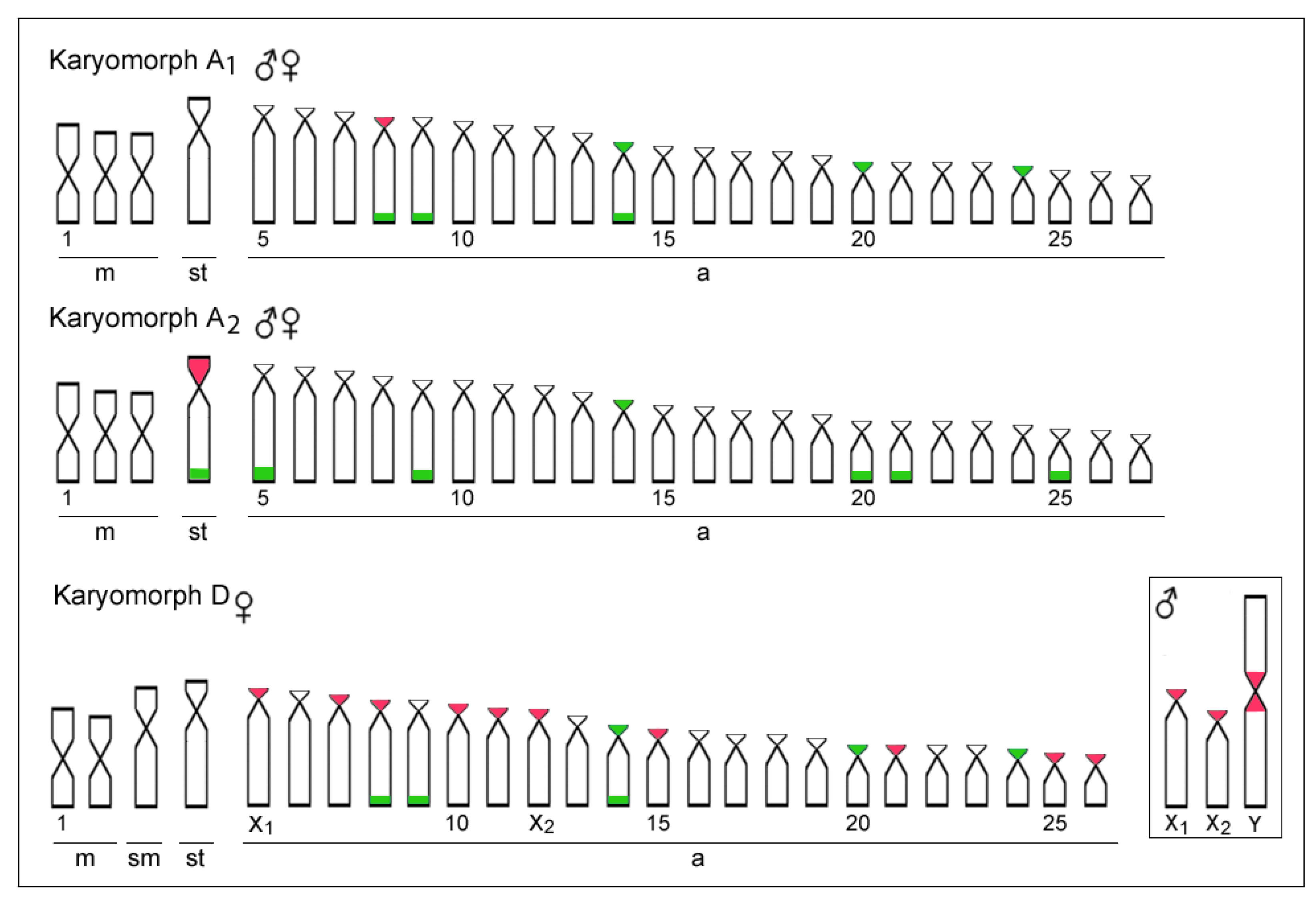
3.1. Karyotypes and rDNA Mapping
3.2. Comparative Genomic Hybridization
3.3. Sequencing, Data Preparation and Detection of Selection Markers
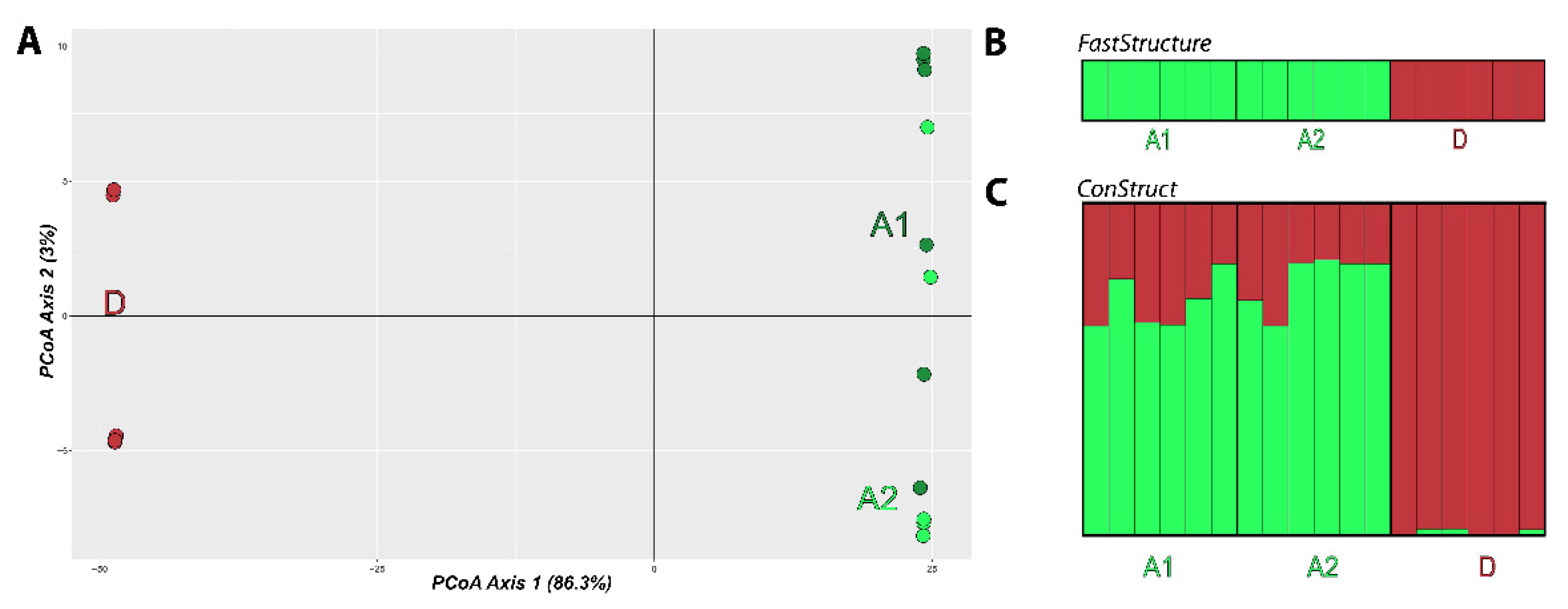
3.4. Population Structure
3.5. Genetic Diversity
4. Discussion
4.1. Chromosomal Diversity among E. erythrinus Populations
4.2. Genetic Diversity and Population Structure
4.3. Multiple Sex Chromosome Systems and the Speciation Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fricke, R.; Eschmeyer, W.N.; van der Laan, R. Eschmeyer’s Catalog of Fishes: Genera, Species, References. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 28 November 2021).
- Oyakawa, O.T. Erythrinidae. In Check List of the Freshwater Fishes of South and Central America; Roberto, E., Sven, R., Kullander, O., Carl, J., Ferraris, J., Eds.; EDIPUCRS: Porto Alegre, Brazil, 2003; pp. 238–241. [Google Scholar]
- Bertollo, L.A.C.; Oliveira, C.; Molina, W.F.; Margarido, V.P.; Fontes, M.S.; Pastori, M.C.; das Falcão, J.N.; Fenocchio, A.S. Chromosome evolution in the erythrinid fish, Erythrinus erythrinus (Teleostei: Characiformes). Heredity 2004, 93, 228–233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cioffi, M.B.; Sánchez, A.; Marchal, J.A.; Kosyakova, N.; Liehr, T.; Trifonov, V.; Bertollo, L.A. Cross-species chromosome painting tracks the independent origin of multiple sex chromosomes in two cofamiliar Erythrinidae fishes. BMC Evol. Biol. 2011, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Oyakawa, O.T.; Mattox, G.M.T. Revision of the Neotropical trahiras of the Hoplias lacerdae species-group (Ostariophysi: Characiformes: Erythrinidae) with descriptions of two new species. Neotrop. Ichthyol. 2009, 7, 117–140. [Google Scholar] [CrossRef]
- Giuliano-Caetano, L. Estudo Citogenético em Hoplerythrinus Unitaeniatus (PISCES, ERYTHRINIDAE) de Diferentes Bacias Hidrográficas Brasileiras. Master’s Thesis, Federal University of São Carlos, São Carlos, Brazil, 1986. [Google Scholar]
- Cioffi, M.B.; Martins, C.; Bertollo, L.A.C. Chromosome spreading of associated transposable elements and ribosomal DNA in the fish Erythrinus erythrinus. Implications for genome change and karyoevolution in fish. BMC Evol. Biol. 2010, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Mayr, E. The evolution of living systems. Proc. Natl. Acad. Sci. USA 1964, 5, 934. [Google Scholar] [CrossRef]
- Haldane, J.B. Sex ratio and unisexual sterility in hybrid animals. J. Genet. 1922, 12, 101–109. [Google Scholar] [CrossRef]
- Bateson, W. Heredity and variation in modern lights. In Darwin and Modern Science; Cambridge University Press: Cambridge, UK, 1909; pp. 85–101. Available online: https://www.scirp.org/(S(i43dyn45teexjx455qlt3d2q))/reference/ReferencesPapers.aspx?ReferenceID=791905 (accessed on 1 January 2022).
- Dobzhansky, T. On the sterility of the interracial hybrids in Drosophila pseudoobscura. Proc. Natl. Acad. Sci. USA 1933, 4, 397. [Google Scholar] [CrossRef]
- Muller, H.J. Isolation mechanisms, evolution and temperature. Biol. Symp. 1942, 6, 71–125. [Google Scholar]
- Coyne, J.A.; Orr, H.A. Speciation; Sinauer Associates: Sunderland, MA, USA, 2004; Volume 37. [Google Scholar]
- Faria, R.; Navarro, A. Chromosomal speciation revisited: Rearranging theory with pieces of evidence. Trends Ecol. Evol. 2010, 25, 660–669. [Google Scholar] [CrossRef]
- Šíchová, J.; Voleníková, A.; Dincă, V.; Nguyen, P.; Vila, R.; Sahara, K.; Marec, F. Dynamic karyotype evolution and unique sex determination systems in Leptidea wood white butterflies. BMC Evol. Biol. 2015, 15, 89. [Google Scholar] [CrossRef]
- Vieira, C.P.; Coelho, P.A.; Vieira, J. Inferences on the evolutionary history of the Drosophila americana polymorphic X/4 fusion from patterns of polymorphism at the X-linked paralytic and elav genes. Genetics 2003, 164, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Presgraves, D.C.; Orr, H.A. Haldane’s rule in taxa lacking a hemizygous X. Science 1998, 282, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.G. Higher levels of sex chromosome heteromorphism are associated with markedly stronger reproductive isolation. Nat. Commun. 2014, 5, 4743. [Google Scholar] [CrossRef] [PubMed]
- Toups, M.A.; Rodrigues, N.; Perrin, N.; Kirkpatrick, M. A reciprocal translocation radically reshapes sex-linked inheritance in the common frog. Mol. Ecol. 2019, 28, 1877–1889. [Google Scholar] [CrossRef]
- Sember, A.; Nguyen, P.; Perez, M.F.; Altmanová, M.; Ráb, P.; Cioffi, M.B. Multiple sex chromosomes in teleost fishes from a cytogenetic perspective: State of the art and future challenges. Philos. Trans. R. Soc. 2021, 376, 20200098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lei, C.; Wu, J.; Zhou, J.; Xiao, M.; Zhu, S.; Xi, Y.; Fu, J.; Sun, Y.; Xu, C.; et al. Meiotic heterogeneity of trivalent structure and interchromosomal effect in blastocysts with Robertsonian translocations. Front. Genet. 2021, 12, 161. [Google Scholar] [CrossRef] [PubMed]
- Ellegren, H. Genome sequencing and population genomics in non-model organisms. Trends Ecol. Evol. 2014, 29, 51–63. [Google Scholar] [CrossRef]
- Martins, N.F.; Bertollo, L.A.C.; Troy, W.P.; Feldberg, E.; de Souza Valentin, F.C.; Cioffi, M.B. Differentiation and evolutionary relationships in Erythrinus erythrinus (Characiformes, Erythrinidae): Comparative chromosome mapping of repetitive se-quences. Rev. Fish Biol. Fish. 2013, 23, 261–269. [Google Scholar] [CrossRef]
- Bertollo, L.A.C.; Cioffi, M.B.; Galetti, P.M.; Moreira-Filho, O. Contributions to the cytogenetics of the Neotropical fish fauna. Comp. Cytogenet. 2017, 11, 665–690. [Google Scholar] [CrossRef]
- Pendas, A.M.; Moran, P.; Freije, J.P.; Garcia-Vazquez, E. Chromosomal mapping and nucleotide sequence of two tandem repeats of Atlantic salmon 5S rDNA. Cytogenet. Genome Res. 1994, 67, 31–36. [Google Scholar] [CrossRef]
- Cioffi, M.B.; Ráb, P.; Ezaz, T.; Bertollo, L.A.C.; Lavoué, S.; de Oliveira, E.A.; Sember, A.; Molina, W.F.; de Souza, F.H.S.; Majtánová, Z.; et al. Deciphering the evolutionary history of arowana fishes (Teleostei, Osteoglossiformes, Osteoglossidae): Insight from comparative cytogenomics. Int. J. Mol. Sci. 2019, 20, 4296. [Google Scholar] [CrossRef]
- Yano, C.F.; Bertollo, L.A.C.; Cioffi, M.B. Fish-FISH: Molecular cytogenetics in fish species. In Fluorescence In Situ Hybridization (FISH); Liehr, T., Ed.; Springer Protocols Handbooks; Springer: Berlin/Heidelberg, Germany, 2017; pp. 429–443. [Google Scholar] [CrossRef]
- Sember, A.; Bertollo, L.A.C.; Ráb, P.; Yano, C.F.; Hatanaka, T.; de Oliveira, E.A.; Cioffi, M.B. Sex chromosome evolution and genomic divergence in the fish Hoplias malabaricus (Characiformes, Erythrinidae). Front. Genet. 2018, 9, 71. [Google Scholar] [CrossRef]
- Toma, G.A.; Moraes, R.L.R.; Sassi, F.M.C.; Bertollo, L.A.C.; De Oliveira, E.A.; Rab, P.; Sember, A.; Liehr, T.; Hatanaka, T.; Viana, P.F.; et al. Cytogenetics of the small-sized fish, Copeina guttata (Characiformes, Lebiasinidae): Novel insights into the karyotype differentiation of the family. PLoS ONE 2019, 14, e0226746. [Google Scholar] [CrossRef] [PubMed]
- Moraes, R.L.R.; Sassi, F.M.C.; Bertollo, L.A.C.; Marinho, M.M.F.; Viana, P.F.; Feldberg, E.; Oliveira, V.C.S.; Deon, G.A.; Al-Rikabi, A.B.H.; Liehr, T.; et al. Tracking the evolutionary trends among small-size fishes of the genus Pyrrhulina (Characiformes, Lebiasinidae): New insights from a molecular cytogenetic perspective. Front. Genet. 2021, 12, 1953. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual Vol. 1, 2, 3; Cold Springs Harb. Lab. Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Zwick, M.S.; Hanson, R.E.; McKnight, T.D.; Islam-Faridi, M.N.; Stelly, D.M.; Wing, R.A.; Price, H.J. A rapid procedure for the isolation of C0t-1 DNA from plants. Genome 2011, 40, 138–142. [Google Scholar] [CrossRef]
- Levan, A.; Fredga, K.; Sandberg, A.A. Nomenclature for centromeric position on chromosomes. Hereditas 1964, 52, 201–220. [Google Scholar] [CrossRef]
- Kilian, A.; Wenzl, P.; Huttner, E.; Carling, J.; Xia, L.; Blois, H.; Caig, V.; Heller-Uszynska, K.; Jaccoud, D.; Hopper, C.; et al. Diversity Arrays Technology: A generic genome profiling technology on open platforms. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2018; Volume 888, pp. 67–89. [Google Scholar] [CrossRef]
- Souza, F.H.S.; Perez, M.F.; Bertollo, L.A.C.; Oliveira, E.A.; Lavoué, S.; Gestich, C.C.; Ráb, P.; Ezaz, T.; Liehr, T.; Viana, P.F.; et al. Interspecific genetic differences and historical demography in south american arowanas (Osteoglossiformes, Osteoglos-sidae, Osteoglossum). Genes 2019, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.M.C.; Perez, M.F.; Oliveira, V.C.S.; Deon, G.A.; Souza, F.H.S.; Ferreira, P.H.N.; de Oliveira, E.A.; Hatanaka, T.; Liehr, T.; Bertollo, L.A.C.; et al. High genetic diversity despite conserved karyotype organization in the giant trahiras from genus Hoplias (Characiformes, erythrinidae). Genes 2021, 12, 252. [Google Scholar] [CrossRef]
- Eaton, D.A.R. PyRAD: Assembly of de novo RADseq loci for phylogenetic analyses. Bioinformatics 2014, 30, 1844–1849. [Google Scholar] [CrossRef]
- Foll, M.; Gaggiotti, O. A genome-scan method to identify selected loci appropriate for both dominant and codominant markers: A Bayesian perspective. Genetics 2008, 180, 977–993. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Stephens, M.; Pritchard, J.K. fastSTRUCTURE: Variational inference of population structure in large SNP data sets. Genetics 2014, 197, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Kopelman, N.M.; Mayzel, J.; Jakobsson, M.; Rosenberg, N.A.; Mayrose, I. Clumpak: A program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 2015, 15, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Bradburd, G.S.; Coop, G.M.; Ralph, P.L. Inferring continuous and discrete population genetic structure across space. Genetics 2018, 210, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.F.; Franco, F.F.; Bombonato, J.R.; Bonatelli, I.A.S.; Khan, G.; Romeiro-Brito, M.; Fegies, A.C.; Ribeiro, P.M.; Silva, G.A.R.; Moraes, E.M. Assessing population structure in the face of isolation by distance: Are we neglecting the problem? Divers. Distrib. 2018, 24, 1883–1889. [Google Scholar] [CrossRef]
- Bradburd, G. How to Run a Construct Analysis. Available online: https://cran.r-project.org/web/packages/conStruct/vignettes/run-conStruct.html (accessed on 12 September 2021).
- Martins, N.F.; Cioffi, M.B.; Troy, W.P.; Martinez, J.F.; Moreira-Filho, O.; Bertollo, L.A.C. Differentiation and evolutionary relationships in Erythrinus erythrinus (Characiformes, Erythrinidae): Occurrence and distribution of B chromosomes. Genet. Mol. Res. 2014, 13, 7094–7101. [Google Scholar] [CrossRef]
- Sochorová, J.; Garcia, S.; Gálvez, F.; Symonová, R.; Kovařík, A. Evolutionary trends in animal ribosomal DNA loci: Intro-duction to a new online database. Chromosoma 2018, 127, 141–150. [Google Scholar] [CrossRef]
- Sochorová, J.; Gálvez, F.; Matyášek, R.; Garcia, S.; Kovařík, A. Analyses of the updated “Animal rDNA Loci Database” with an emphasis on its new features. Int. J. Mol. Sci. 2021, 22, 11403. [Google Scholar] [CrossRef]
- Cioffi, M.B.; Kejnovsk, E.; Marquioni, V.; Poltronieri, J.; Molina, W.F.; Diniz, D.; Bertollo, L.A.C. The key role of repeated DNAs in sex chromosome evolution in two fish species with ZW sex chromosome system. Mol. Cytogenet. 2012, 5, 28. [Google Scholar] [CrossRef]
- Deon, G.A.; Glugoski, L.; Vicari, M.R.; Nogaroto, V.; Sassi, F.M.C.; Cioffi, M.B.; Liehr, T.; Bertollo, L.A.C.; Moreira-Filho, O. Highly rearranged karyotypes and multiple sex chromosome systems in armored catfishes from the genus Harttia (Teleostei, Siluriformes). Genes 2020, 11, 1366. [Google Scholar] [CrossRef] [PubMed]
- Pazza, R.; Kavalco, K.F.; Bertollo, L.A.C. Chromosome polymorphism in Astyanax fasciatus (Teleostei, Characidae). 1. Kary-otype analysis, Ag-NORs and mapping of the 18S and 5S ribosomal genes in sympatric karyotypes and their possible hybrid forms. Cytogenet. Genome Res. 2006, 112, 313–319. [Google Scholar] [CrossRef]
- Ferreira, M.; Garcia, C.; Matoso, D.A.; de Jesus, I.S.; Cioffi, M.B.; Bertollo, L.A.C.; Zuanon, J.; Feldberg, E. The Bunocephalus coracoideus species complex (Siluriformes, Aspredinidae). Signs of a speciation process through chromosomal, genetic and ecological diversity. Front. Genet. 2017, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Gornung, E.; Darwin, C. Twenty years of physical mapping of major ribosomal RNA genes across the teleosts: A review of research. Cytogenet. Genome Res. 2013, 141, 90–102. [Google Scholar] [CrossRef]
- Ruiz-Herrera, A.; Castresana, J.; Robinson, T.J. Is mammalian chromosomal evolution driven by regions of genome fragility? Genome Biol. 2006, 7, R115. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.; Harris, R.A.; Vessere, G.M.; Mootnick, A.R.; Humphray, S.; Rogers, J.; Kim, S.K.; Wall, J.D.; Martin, D.; Jurka, J.; et al. Evolutionary breakpoints in the gibbon suggest association between cytosine methylation and karyotype evolution. PLoS Genet. 2009, 5, e1000538. [Google Scholar] [CrossRef]
- Farré, M.; Bosch, M.; López-Giráldez, F.; Ponsà, M.; Ruiz-Herrera, A. Assessing the role of tandem repeats in shaping the genomic architecture of great apes. PLoS ONE 2011, 6, e0027239. [Google Scholar] [CrossRef]
- Da Silva, A.F.; Feldberg, E.; Moura Carvalho, N.D.; Hernández Rangel, S.M.; Schneider, C.H.; Carvalho-Zilse, G.A.; Fonsêca da Silva, V.; Gross, M.C. Effects of environmental pollution on the rDNAomics of Amazonian fish. Environ. Pollut. 2019, 252, 180–187. [Google Scholar] [CrossRef]
- Leite, P.P.M.; Sassi, F.M.C.; Marinho, M.M.F.; Nirchio, M.; Moraes, R.L.R.; Toma, G.A.; Bertollo, L.A.C.; Cioffi, M.B. Tracking the evolutionary pathways among Brazilian Lebiasina species (Teleostei: Lebiasinidae): A chromosomal and genomic com-parative investigation. Neotrop. Ichthyol. 2021. submitted. [Google Scholar]
- Barby, F.F.; Ráb, P.; Lavoué, S.; Ezaz, T.; Bertollo, L.A.C.; Kilian, A.; Maruyama, S.R.; De Oliveira, E.A.; Artoni, R.F.; Santos, M.H.; et al. From chromosomes to genome: Insights into the evolutionary relationships and biogeography of old world knifefishes (Notopteridae; Osteoglossiformes). Genes 2018, 9, 306. [Google Scholar] [CrossRef]
- Sassi, F.M.C.; Deon, G.A.; Moreira-Filho, O.; Vicari, M.R.; Bertollo, L.A.C.; Liehr, T.; de Oliveira, E.A.; Cioffi, M.B. Multiple sex chromosomes and evolutionary relationships in Amazonian catfishes: The outstanding model of the genus Harttia (Si-luriformes: Loricariidae). Genes 2020, 11, 1179. [Google Scholar] [CrossRef]
- De Oliveira, E.A.; Sember, A.; Bertollo, L.A.C.; Yano, C.F.; Ezaz, T.; Moreira-Filho, O.; Hatanaka, T.; Trifonov, V.; Liehr, T.; Al-Rikabi, A.B.H.; et al. Tracking the evolutionary pathway of sex chromosomes among fishes: Characterizing the unique XX/XY1Y2 system in Hoplias malabaricus (Teleostei, Characiformes). Chromosoma 2017, 127, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Moraes, R.L.; Sember, A.; Bertollo, L.A.; De Oliveira, E.A.; Rab, P.; Hatanaka, T.; Marinho, M.M.; Liehr, T.; Al-Rikabi, A.B.; Feldberg, E.; et al. Comparative cytogenetics and neo-Y formation in small-sized fish species of the genus Pyrrhulina (Characiformes, Lebiasinidae). Front. Genet. 2019, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Yano, C.F.; Sember, A.; Kretschmer, R.; Bertollo, L.A.C.; Ezaz, T.; Hatanaka, T.; Liehr, T.; Ráb, P.; Al-Rikabi, A.; Viana, P.F.; et al. Against the mainstream: Exceptional evolutionary stability of ZW sex chromosomes across the fish families Triportheidae and Gasteropelecidae (Teleostei: Characiformes). Chromosom. Res. 2021, 29, 391–416. [Google Scholar] [CrossRef] [PubMed]
- Beukeboom, L.W.; Perrin, N. The Evolution of Sex Determination; Oxford University Press: Oxford, MA, USA, 2014. [Google Scholar]
- Sherman, K.D.; Paris, J.R.; King, R.A.; Moore, K.A.; Dahlgren, C.P.; Knowles, L.C.; Stump, K.; Tyler, C.R.; Stevens, J.R. RAD-Seq analysis and in situ monitoring of Nassau Grouper reveal fine-scale population structure and origins of aggregating fish. Front. Mar. Sci. 2020, 7, 157. [Google Scholar] [CrossRef]
- Souza, F.H.S.; Perez, M.F.; Charlesworth, D.; Ferreira, P.H.N.; Bertollo, L.A.C.; Ezaz, T.; Cioffi, M.B. Elucidating the complex relationship of Hoplias malabaricus’ (Teleostei; Characiformes) population. A genetic, chromosomal and spatial approach. Evol. Lett. 2021. submitted. [Google Scholar]
- Bertollo, L.A.; Born, G.G.; Dergam, J.A.; Fenocchio, A.S.; Moreira-Filho, O. A biodiversity approach in the neotropical Erythrinidae fish, Hoplias malabaricus. Karyotypic survey, geographic distribution of cytotypes and cytotaxonomic consider-ations. Chromosome Res. 2000, 8, 603–613. [Google Scholar] [CrossRef]
- Kitano, J.; Ross, J.A.; Mori, S.; Kume, M.; Jones, F.C.; Chan, Y.F.; Absher, D.M.; Grimwood, J.; Schmutz, J.; Myers, R.M.; et al. A role for a neo-sex chromosome in stickleback speciation. Nature 2009, 461, 1079–1083. [Google Scholar] [CrossRef]
- Beaudry, F.E.G.; Barrett, S.C.H.; Wright, S.I. Ancestral and neo-sex chromosomes contribute to population divergence in a dioecious plant. Evolution 2020, 74, 256–269. [Google Scholar] [CrossRef]
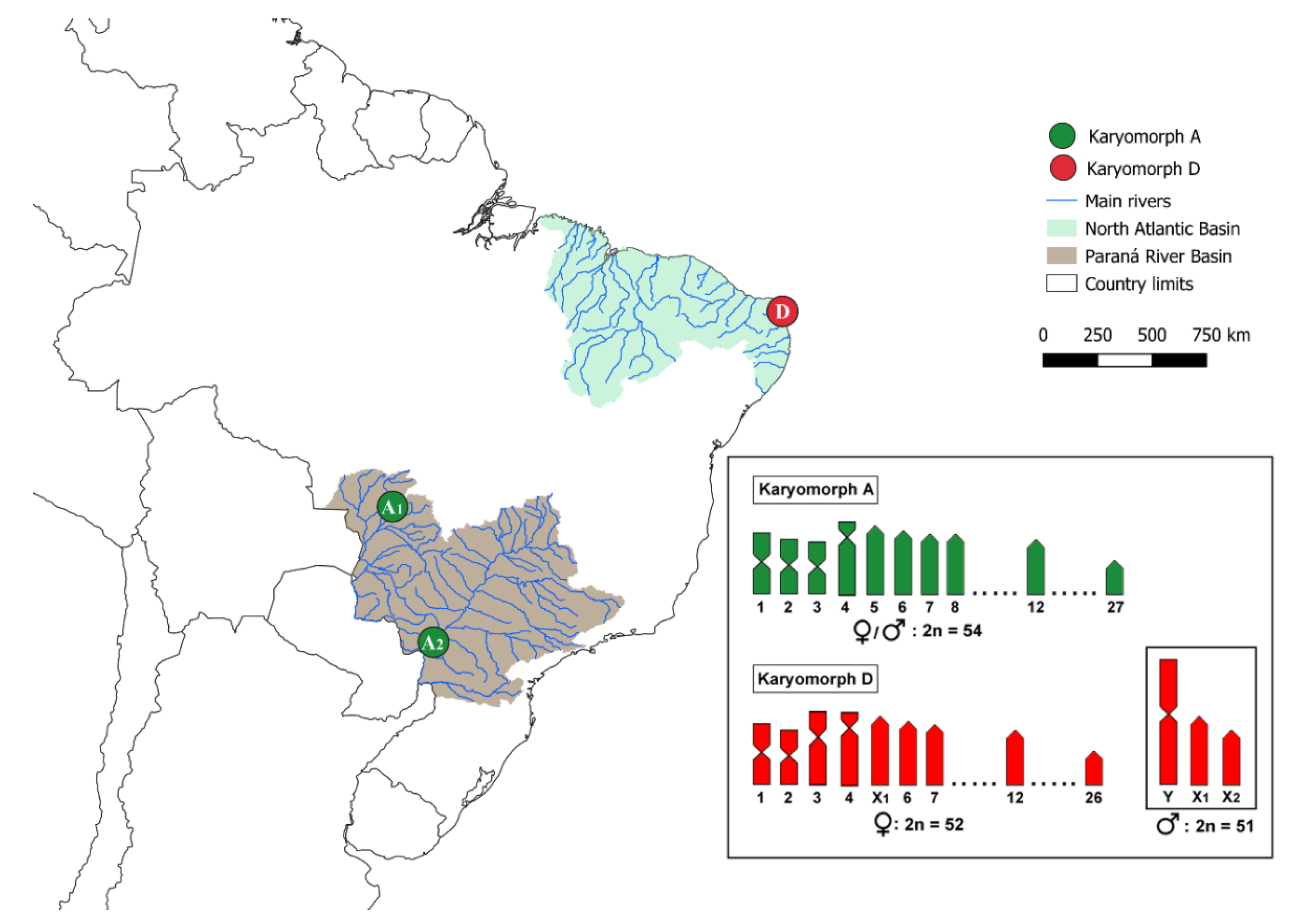

| Karyomorph | 2n | Sex System | Code | Sampling Site | Latitude/Longitude | Cito_N | DArT | Reference |
|---|---|---|---|---|---|---|---|---|
| A | ♀♂ 54 | Homomorphic | A1 | Cuiabá River (MT) | −16.1713/−55.9573 | 14♂ 11♀ | 6 | [23] |
| A | ♀♂ 54 | Homomorphic | A2 | Paraná River (PR) | −23.3774/−53.7805 | 09♂ 07♀ | 5 | Present work |
| D | ♀52/♂51 | ♀X1X1X2X2 ♂X1X2Y | D | Sangue Stream (RN) | −5.7770/−35.2092 | 10♂ 09♀ | 7 | [7] |
| Code | Sample Size | Hd | π | θW | D |
|---|---|---|---|---|---|
| A1 | 6 | 0.05290 | 0.00102 | 0.00125 | −0.48480 |
| A2 | 6 | 0.04306 | 0.00085 | 0.00093 | −0.25292 |
| D1 | 12 | 0.02115 | 0.00044 | 0.00047 | −0.19581 |
| A1 | A2 | D | |
| A1 | 0.00102 | 0.01084 | |
| A2 | 0.00012 | 0.01082 | |
| D | 0.01014 | 0.01022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, F.H.S.d.; Sassi, F.d.M.C.; Ferreira, P.H.N.; Bertollo, L.A.C.; Ezaz, T.; Liehr, T.; Perez, M.F.; Cioffi, M.B. Integrating Cytogenetics and Population Genomics: Allopatry and Neo-Sex Chromosomes May Have Shaped the Genetic Divergence in the Erythrinus erythrinus Species Complex (Teleostei, Characiformes). Biology 2022, 11, 315. https://doi.org/10.3390/biology11020315
Souza FHSd, Sassi FdMC, Ferreira PHN, Bertollo LAC, Ezaz T, Liehr T, Perez MF, Cioffi MB. Integrating Cytogenetics and Population Genomics: Allopatry and Neo-Sex Chromosomes May Have Shaped the Genetic Divergence in the Erythrinus erythrinus Species Complex (Teleostei, Characiformes). Biology. 2022; 11(2):315. https://doi.org/10.3390/biology11020315
Chicago/Turabian StyleSouza, Fernando H. S. de, Francisco de M. C. Sassi, Pedro H. N. Ferreira, Luiz A. C. Bertollo, Tariq Ezaz, Thomas Liehr, Manolo F. Perez, and Marcelo B. Cioffi. 2022. "Integrating Cytogenetics and Population Genomics: Allopatry and Neo-Sex Chromosomes May Have Shaped the Genetic Divergence in the Erythrinus erythrinus Species Complex (Teleostei, Characiformes)" Biology 11, no. 2: 315. https://doi.org/10.3390/biology11020315
APA StyleSouza, F. H. S. d., Sassi, F. d. M. C., Ferreira, P. H. N., Bertollo, L. A. C., Ezaz, T., Liehr, T., Perez, M. F., & Cioffi, M. B. (2022). Integrating Cytogenetics and Population Genomics: Allopatry and Neo-Sex Chromosomes May Have Shaped the Genetic Divergence in the Erythrinus erythrinus Species Complex (Teleostei, Characiformes). Biology, 11(2), 315. https://doi.org/10.3390/biology11020315









