Systemic Administration of the TLR7/8 Agonist Resiquimod (R848) to Mice Is Associated with Transient, In Vivo-Detectable Brain Swelling
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Drugs
2.3. Magnetic Resonance (MR) Scanning Procedures and Data Analysis
2.3.1. MR Spectroscopy
2.3.2. Structural Magnetic Resonance Imaging (MRI)
2.4. Statistics
3. Results
3.1. Sickness Responses
3.2. MRS Metabolite Changes
3.3. Structural MRI Volumetric Changes
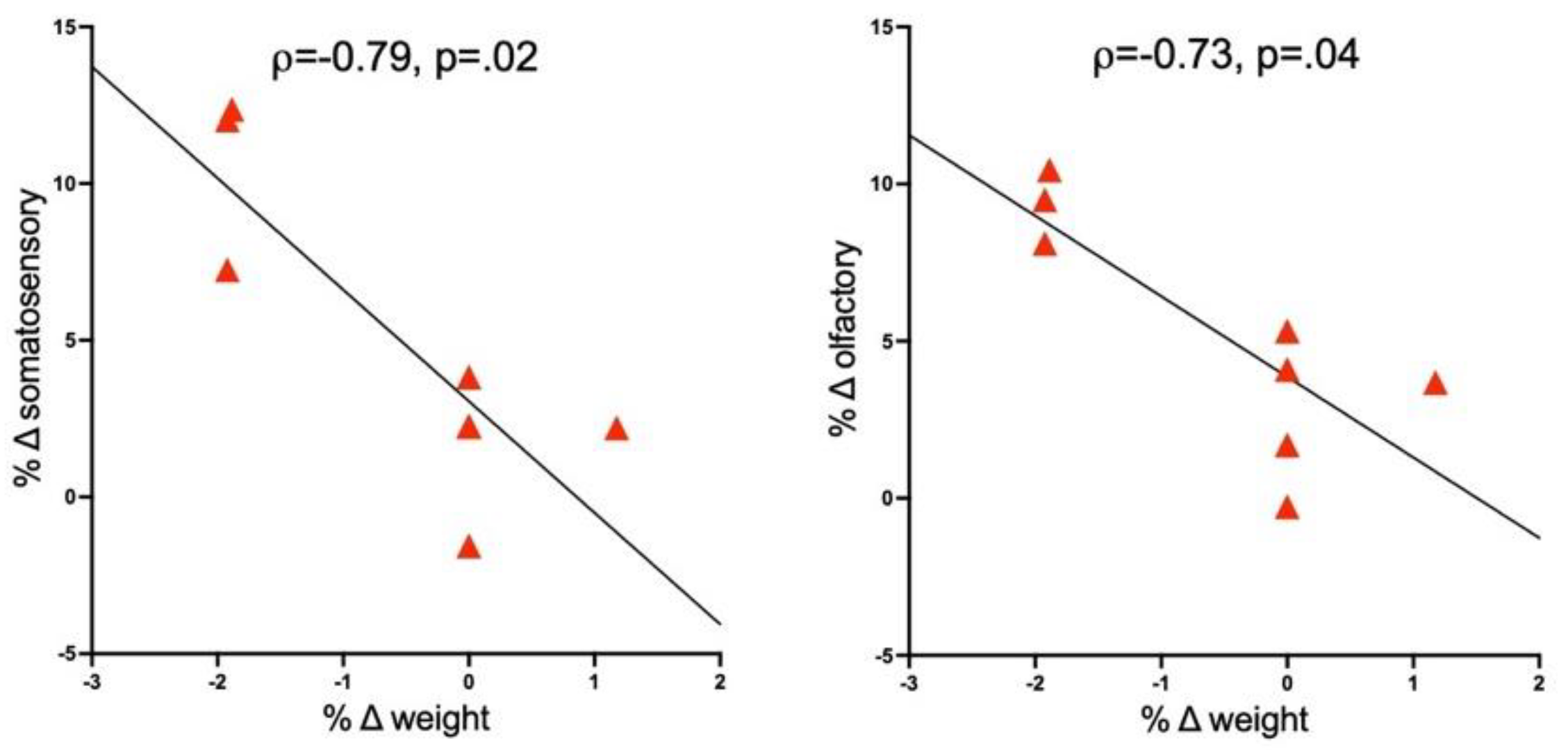
3.4. Correlations between Brain and Response Measures
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Anz, D.; Koelzer, V.H.; Moder, S.; Thaler, R.; Schwerd, T.; Lahl, K.; Sparwasser, T.; Besch, R.; Poeck, H.; Hornung, V.; et al. Immunostimulatory RNA blocks suppression by regulatory T cells. J. Immunol. 2010, 184, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Spinetti, T.; Spagnuolo, L.; Mottas, I.; Secondini, C.; Treinies, M.; Rüegg, C.; Hotz, C.; Bourquin, C. TLR7-based cancer immunotherapy decreases intratumoral myeloid-derived suppressor cells and blocks their immunosuppressive function. Oncoimmunology 2016, 5, e1230578. [Google Scholar] [CrossRef] [Green Version]
- Bourquin, C.; Schmidt, L.; Lanz, A.L.; Storch, B.; Wurzenberger, C.; Anz, D.; Sandholzer, N.; Mocikat, R.; Berger, M.; Poeck, H.; et al. Immunostimulatory RNA oligonucleotides induce an effective antitumoral NK cell response through the TLR7. J. Immunol. 2009, 183, 6078–6086. [Google Scholar] [CrossRef]
- Hotz, C.; Treinies, M.; Mottas, I.; Rötzer, L.C.; Oberson, A.; Spagnuolo, L.; Perdicchio, M.; Spinetti, T.; Herbst, T.; Bourquin, C. Reprogramming of TLR7 signaling enhances antitumor NK and cytotoxic T cell responses. Oncoimmunology 2016, 5, e1232219. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Zhang, Z.; Li, G.; Li, F.; Wang, L.; Zhang, L.; Zurawski, S.M.; Zurawski, G.; Levy, Y.; Su, L. Human innate responses and adjuvant activity of TLR ligands in vivo in mice reconstituted with a human immune system. Vaccine 2017, 35, 6143–6153. [Google Scholar] [CrossRef]
- Ilyinskii, P.O.; Roy, C.J.; O’Neil, C.P.; Browning, E.A.; Pittet, L.A.; Altreuter, D.H.; Alexis, F.; Tonti, E.; Shi, J.; Basto, P.A.; et al. Adjuvant-carrying synthetic vaccine particles augment the immune response to encapsulated antigen and exhibit strong local immune activation without inducing systemic cytokine release. Vaccine 2014, 32, 2882–2895. [Google Scholar] [CrossRef] [Green Version]
- Kato, H.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Uematsu, S.; Matsui, K.; Tsujimura, T.; Takeda, K.; Fujita, T.; Takeuchi, O.; et al. Cell Type-Specific Involvement of RIG-I in Antiviral Response. Immunity 2005, 23, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [Green Version]
- Minoda, Y.; Virshup, I.; Leal Rojas, I.; Haigh, O.; Wong, Y.; Miles, J.J.; Wells, C.A.; Radford, K.J. Human CD141(+) Dendritic Cell and CD1c(+) Dendritic Cell Undergo Concordant Early Genetic Programming after Activation in Humanized Mice In Vivo. Front. Immunol. 2017, 8, 1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butchi, N.B.; Woods, T.; Du, M.; Morgan, T.W.; Peterson, K.E. TLR7 and TLR9 trigger distinct neuroinflammatory responses in the CNS. Am. J. Pathol. 2011, 179, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, S.M.; Krüger, C.; Park, B.; Derkow, K.; Rosenberger, K.; Baumgart, J.; Trimbuch, T.; Eom, G.; Hinz, M.; Kaul, D.; et al. An unconventional role for miRNA: Let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 2012, 15, 827–835. [Google Scholar] [CrossRef]
- Lehmann, S.M.; Rosenberger, K.; Krüger, C.; Habbel, P.; Derkow, K.; Kaul, D.; Rybak, A.; Brandt, C.; Schott, E.; Wulczyn, F.G.; et al. Extracellularly delivered single-stranded viral RNA causes neurodegeneration dependent on TLR7. J. Immunol. 2012, 189, 1448–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaelis, K.A.; Norgard, M.A.; Levasseur, P.R.; Olson, B.; Burfeind, K.G.; Buenafe, A.C.; Zhu, X.; Jeng, S.; McWeeney, S.K.; Marks, D.L. Persistent Toll-like receptor 7 stimulation induces behavioral and molecular innate immune tolerance. Brain Behav. Immun. 2019, 82, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.A.; Race, B.; Williams, K.; Striebel, J.F.; Chesebro, B. Innate immune responses after stimulation with Toll-like receptor agonists in ex vivo microglial cultures and an in vivo model using mice with reduced microglia. J. Neuroinflamm. 2021, 18, 194. [Google Scholar] [CrossRef] [PubMed]
- Hung, I.F.-N.; Zhang, A.J.; To, K.K.-W.; Chan, J.F.-W.; Li, P.; Wong, T.-L.; Zhang, R.; Chan, T.-C.; Chan, B.C.-Y.; Wai, H.H.; et al. Topical imiquimod before intradermal trivalent influenza vaccine for protection against heterologous non-vaccine and antigenically drifted viruses: A single-centre, double-blind, randomised, controlled phase 2b/3 trial. Lancet Infect. Dis. 2016, 16, 209–218. [Google Scholar] [CrossRef]
- Weldon, W.C.; Zarnitsyn, V.G.; Esser, E.S.; Taherbhai, M.T.; Koutsonanos, D.G.; Vassilieva, E.V.; Skountzou, I.; Prausnitz, M.R.; Compans, R.W. Effect of Adjuvants on Responses to Skin Immunization by Microneedles Coated with Influenza Subunit Vaccine. PLoS ONE 2012, 7, e41501. [Google Scholar] [CrossRef]
- Bath-Hextall, F.; Ozolins, M.; Armstrong, S.J.; Colver, G.B.; Perkins, W.; Miller, P.S.; Williams, H.C. Surgical excision versus imiquimod 5% cream for nodular and superficial basal-cell carcinoma (SINS): A multicentre, non-inferiority, randomised controlled trial. Lancet Oncol. 2014, 15, 96–105. [Google Scholar] [CrossRef]
- Schmid, D.; Park, C.G.; Hartl, C.A.; Subedi, N.; Cartwright, A.N.; Puerto, R.B.; Zheng, Y.; Maiarana, J.; Freeman, G.J.; Wucherpfennig, K.W.; et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat. Commun. 2017, 8, 1747. [Google Scholar] [CrossRef]
- Grantham, E.K.; Warden, A.S.; McCarthy, G.S.; DaCosta, A.; Mason, S.; Blednov, Y.; Mayfield, R.D.; Harris, R.A. Role of toll-like receptor 7 (TLR7) in voluntary alcohol consumption. Brain Behav. Immun. 2020, 89, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zou, J.; Barnett, A.; Vetreno, R.P.; Crews, F.T.; Coleman, L.G., Jr. TRAIL Mediates Neuronal Death in AUD: A Link between Neuroinflammation and Neurodegeneration. Int. J. Mol. Sci. 2021, 22, 2547. [Google Scholar] [CrossRef] [PubMed]
- Balan, I.; Aurelian, L.; Schleicher, R.; Boero, G.; O’Buckley, T.; Morrow, A.L. Neurosteroid allopregnanolone (3α,5α-THP) inhibits inflammatory signals induced by activated MyD88-dependent toll-like receptors. Transl. Psychiatry 2021, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Patinote, C.; Karroum, N.B.; Moarbess, G.; Cirnat, N.; Kassab, I.; Bonnet, P.-A.; Deleuze-Masquéfa, C. Agonist and antagonist ligands of toll-like receptors 7 and 8: Ingenious tools for therapeutic purposes. Eur. J. Med. Chem. 2020, 193, 112238. [Google Scholar] [CrossRef] [PubMed]
- van der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice Is Mediated via the IL-23/IL-17 Axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef]
- Thomson, C.A.; McColl, A.; Cavanagh, J.; Graham, G.J. Peripheral inflammation is associated with remote global gene expression changes in the brain. J. Neuroinflamm. 2014, 11, 73. [Google Scholar] [CrossRef] [Green Version]
- McColl, A.; Thomson, C.A.; Nerurkar, L.; Graham, G.J.; Cavanagh, J. TLR7-mediated skin inflammation remotely triggers chemokine expression and leukocyte accumulation in the brain. J. Neuroinflamm. 2016, 13, 102. [Google Scholar] [CrossRef] [Green Version]
- Nerurkar, L.; McColl, A.; Graham, G.; Cavanagh, J. The Systemic Response to Topical Aldara Treatment is Mediated Through Direct TLR7 Stimulation as Imiquimod Enters the Circulation. Sci. Rep. 2017, 7, 16570. [Google Scholar] [CrossRef]
- Kubo, Y.; Yanagawa, Y.; Matsumoto, M.; Hiraide, S.; Togashi, H. Enhanced depressive-like behaviors after Toll-like receptor 7 stimulation in mice. Nihon Shinkei Seishin Yakurigaku Zasshi 2013, 33, 41–47. [Google Scholar]
- Hemmi, H.; Kaisho, T.; Takeuchi, O.; Sato, S.; Sanjo, H.; Hoshino, K.; Horiuchi, T.; Tomizawa, H.; Takeda, K.; Akira, S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 2002, 3, 196–200. [Google Scholar] [CrossRef]
- Jurk, M.; Heil, F.; Vollmer, J.; Schetter, C.; Krieg, A.M.; Wagner, H.; Lipford, G.; Bauer, S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat. Immunol. 2002, 3, 499. [Google Scholar] [CrossRef] [PubMed]
- Dowling, D.J. Recent Advances in the Discovery and Delivery of TLR7/8 Agonists as Vaccine Adjuvants. ImmunoHorizons 2018, 2, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govindaraj, R.G.; Manavalan, B.; Basith, S.; Choi, S. Comparative analysis of species-specific ligand recognition in Toll-like receptor 8 signaling: A hypothesis. PLoS ONE 2011, 6, e25118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damm, J.; Wiegand, F.; Harden, L.; Gerstberger, R.; Rummel, C.; Roth, J. Fever, sickness behavior, and expression of inflammatory genes in the hypothalamus after systemic and localized subcutaneous stimulation of rats with the Toll-like receptor 7 agonist imiquimod. Neuroscience 2012, 201, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Cottam, H.B.; Chan, M.; Jin, G.; Tawatao, R.I.; Crain, B.; Ronacher, L.; Messer, K.; Carson, D.A.; Corr, M. Mast cell-dependent anorexia and hypothermia induced by mucosal activation of Toll-like receptor 7. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R123–R132. [Google Scholar] [CrossRef] [Green Version]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beutler, B. Endotoxin, toll-like receptor 4, and the afferent limb of innate immunity. Curr. Opin. Microbiol. 2000, 3, 23–28. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Fritz, M.; Klawonn, A.M.; Nilsson, A.; Singh, A.K.; Zajdel, J.; Wilhelms, D.B.; Lazarus, M.; Löfberg, A.; Jaarola, M.; Kugelberg, U.; et al. Prostaglandin-dependent modulation of dopaminergic neurotransmission elicits inflammation-induced aversion in mice. J. Clin. Investig. 2016, 126, 695–705. [Google Scholar] [CrossRef] [Green Version]
- Abareshi, A.; Anaeigoudari, A.; Norouzi, F.; Marefati, N.; Beheshti, F.; Saeedjalali, M.; Hosseini, M. The effects of captopril on lipopolysaccharide-induced sickness behaviors in rats. Veter. Res. Forum Int. Q. J. 2019, 10, 199–205. [Google Scholar] [CrossRef]
- Fritz, M.; Klawonn, A.M.; Zhao, Q.; Sullivan, E.V.; Zahr, N.M.; Pfefferbaum, A. Structural and biochemical imaging reveals systemic LPS-induced changes in the rat brain. J. Neuroimmunol. 2020, 348, 577367. [Google Scholar] [CrossRef] [PubMed]
- Sokol, D.K.; Demyer, W.E.; Edwards-Brown, M.; Sanders, S.; Garg, B. From swelling to sclerosis: Acute change in mesial hippocampus after prolonged febrile seizure. Seizure 2003, 12, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Shimosegawa, E.; Hatazawa, J.; Ibaraki, M.; Toyoshima, H.; Suzuki, A. Metabolic penumbra of acute brain infarction: A correlation with infarct growth. Ann. Neurol. 2005, 57, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Baxan, N.; Papanikolaou, A.; Salles-Crawley, I.; Lota, A.; Chowdhury, R.; Dubois, O.; Branca, J.; Hasham, M.G.; Rosenthal, N.; Prasad, S.K.; et al. Characterization of acute TLR-7 agonist-induced hemorrhagic myocarditis in mice by multiparametric quantitative cardiac magnetic resonance imaging. Dis. Model. Mech. 2019, 12, dmm040725. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souyris, M.; Mejía, J.E.; Chaumeil, J.; Guéry, J.C. Female predisposition to TLR7-driven autoimmunity: Gene dosage and the escape from X chromosome inactivation. Semin. Immunopathol. 2019, 41, 153–164. [Google Scholar] [CrossRef]
- Van, L.P.; Bardel, E.; Gregoire, S.; Vanoirbeek, J.; Schneider, E.; Dy, M.; Thieblemont, N. Treatment with the TLR7 agonist R848 induces regulatory T-cell-mediated suppression of established asthma symptoms. Eur. J. Immunol. 2011, 41, 1992–1999. [Google Scholar] [CrossRef]
- Hänel, G.; Angerer, C.; Petry, K.; Lichtenegger, F.S.; Subklewe, M. Blood DCs activated with R848 and poly(I:C) induce antigen-specific immune responses against viral and tumor-associated antigens. Cancer Immunol. Immunother. 2021, 1–14. [Google Scholar] [CrossRef]
- Serrano, R.; Coch, C.; Peters, C.; Hartmann, G.; Wesch, D.; Kabelitz, D. Monocyte-dependent co-stimulation of cytokine induction in human γδ T cells by TLR8 RNA ligands. Sci. Rep. 2021, 11, 15231. [Google Scholar] [CrossRef]
- Provencher, S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993, 30, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.W. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001, 14, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Coupe, P.; Yger, P.; Prima, S.; Hellier, P.; Kervrann, C.; Barillot, C. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Trans. Med. Imaging 2008, 27, 425–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tustison, N.J.; Avants, B.B.; Siqueira, M.; Gee, J.C. Topological well-composedness and glamorous glue: A digital gluing algorithm for topologically constrained front propagation. IEEE Trans. Image Process. A Publ. IEEE Signal Process. Soc. 2011, 20, 1756–1761. [Google Scholar] [CrossRef] [PubMed]
- Tustison, N.J.; Avants, B.B.; Cook, P.A.; Zheng, Y.; Egan, A.; Yushkevich, P.A.; Gee, J.C. N4ITK: Improved N3 bias correction. IEEE Trans. Med. Imaging 2010, 29, 1310–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallast, N.; Diedenhofen, M.; Blaschke, S.; Wieters, F.; Wiedermann, D.; Hoehn, M.; Fink, G.R.; Aswendt, M. Processing Pipeline for Atlas-Based Imaging Data Analysis of Structural and Functional Mouse Brain MRI (AIDAmri). Front. Neuroinform. 2019, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Rohlfing, T. Computational Morphometry Toolkit (CMTK). 2011. Available online: http://nitrc.org/projects/cmtk (accessed on 26 January 2022).
- Karlsson, A.; Jägervall, K.; Utkovic, H.; Karlsson, L.; Rehnström, E.; Fredin, M.F.; Gillberg, P.G.; Jansson, L.; Michaëlsson, E.; Melgar, S. Intra-colonic administration of the TLR7 agonist R-848 induces an acute local and systemic inflammation in mice. Biochem. Biophys. Res. Commun. 2008, 367, 242–248. [Google Scholar] [CrossRef]
- Smith, S.M.; Friedle, S.A.; Watters, J.J. Chronic intermittent hypoxia exerts CNS region-specific effects on rat microglial inflammatory and TLR4 gene expression. PLoS ONE 2013, 8, e81584. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Korgaonkar, A.A.; Swietek, B.; Wang, J.; Elgammal, F.S.; Elkabes, S.; Santhakumar, V. Toll-like receptor 4 enhancement of non-NMDA synaptic currents increases dentate excitability after brain injury. Neurobiol. Dis. 2015, 74, 240–253. [Google Scholar] [CrossRef] [Green Version]
- Kaul, D.; Habbel, P.; Derkow, K.; Krüger, C.; Franzoni, E.; Wulczyn, F.G.; Bereswill, S.; Nitsch, R.; Schott, E.; Veh, R.; et al. Expression of Toll-like receptors in the developing brain. PLoS ONE 2012, 7, e37767. [Google Scholar] [CrossRef] [Green Version]
- Letiembre, M.; Hao, W.; Liu, Y.; Walter, S.; Mihaljevic, I.; Rivest, S.; Hartmann, T.; Fassbender, K. Innate immune receptor expression in normal brain aging. Neuroscience 2007, 146, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Y.; Liang, X.; Xing, X.; Xu, X.; Zhou, C. Toll-Like Receptor 4 Knockdown Attenuates Brain Damage and Neuroinflammation After Traumatic Brain Injury via Inhibiting Neuronal Autophagy and Astrocyte Activation. Cell. Mol. Neurobiol. 2018, 38, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Wu, R.; Gao, F.; Zang, P.; Hu, X.; Gu, C. Effect of cerebral small vessel disease on cognitive function and TLR4 expression in hippocampus. J. Clin. Neurosci. 2019, 67, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Yan, L.R.; Ma, Z.G.; Ji, C. Influences of the TLR4/NF-κB pathway on memory function and inflammatory factors in rats with cerebral small vessel disease. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6264–6271. [Google Scholar] [CrossRef] [PubMed]
- Noll, F.; Behnke, J.; Leiting, S.; Troidl, K.; Alves, G.T.; Müller-Redetzky, H.; Preissner, K.T.; Fischer, S. Self-extracellular RNA acts in synergy with exogenous danger signals to promote inflammation. PLoS ONE 2017, 12, e0190002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahr, N.M.; Mayer, D.; Rohlfing, T.; Hasak, M.; Hsu, O.; Vinco, S.; Orduna, J.; Luong, R.; Sullivan, E.V.; Pfefferbaum, A. Brain Injury and Recovery Following Binge Ethanol: Evidence from In Vivo Magnetic Resonance Spectroscopy. Biol. Psychiatry 2010, 67, 846–854. [Google Scholar] [CrossRef] [Green Version]
- Zahr, N.M.; Mayer, D.; Rohlfing, T.; Orduna, J.; Luong, R.; Sullivan, E.V.; Pfefferbaum, A. A mechanism of rapidly reversible cerebral ventricular enlargement independent of tissue atrophy. Neuropsychopharmacology 2013, 38, 1121–1129. [Google Scholar] [CrossRef] [Green Version]
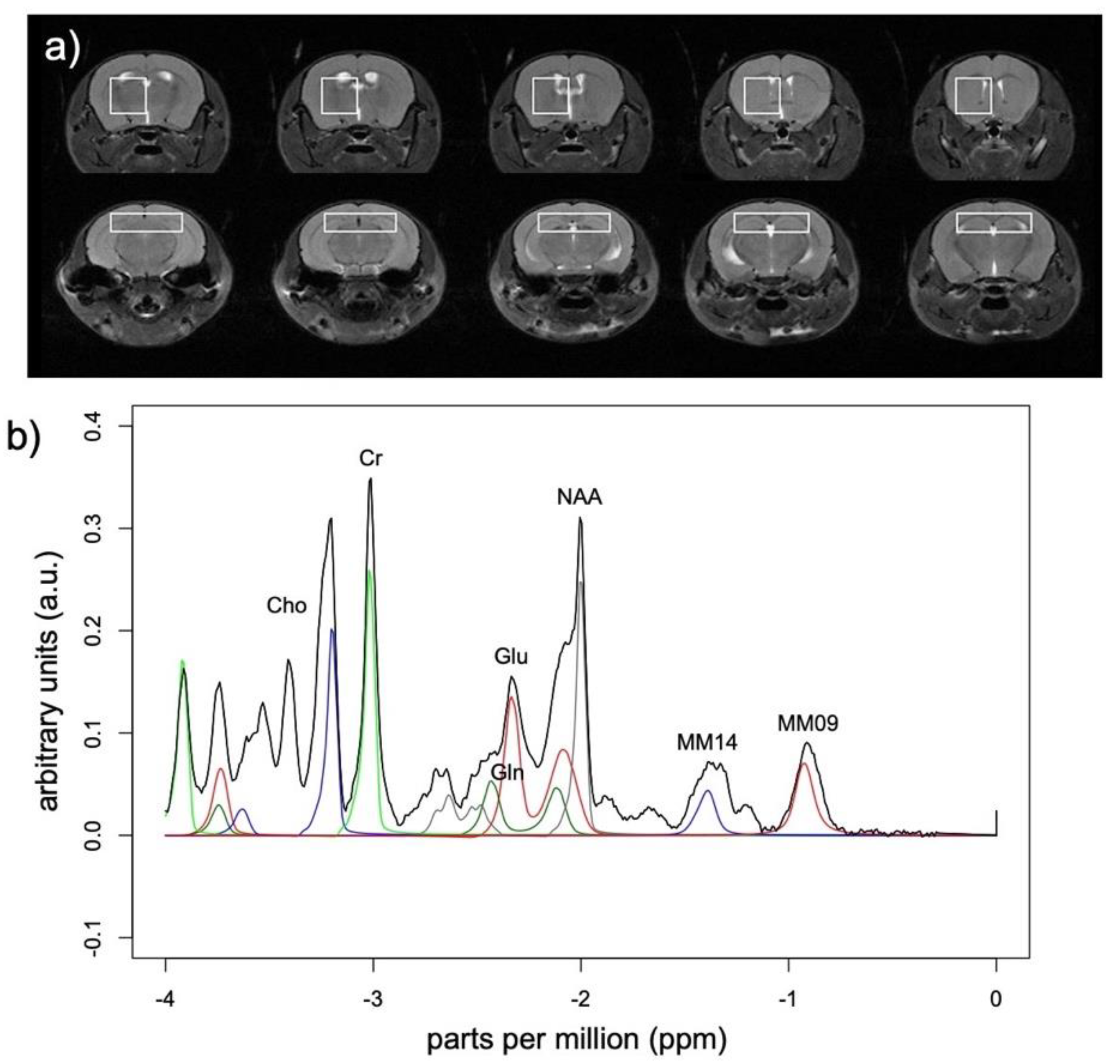
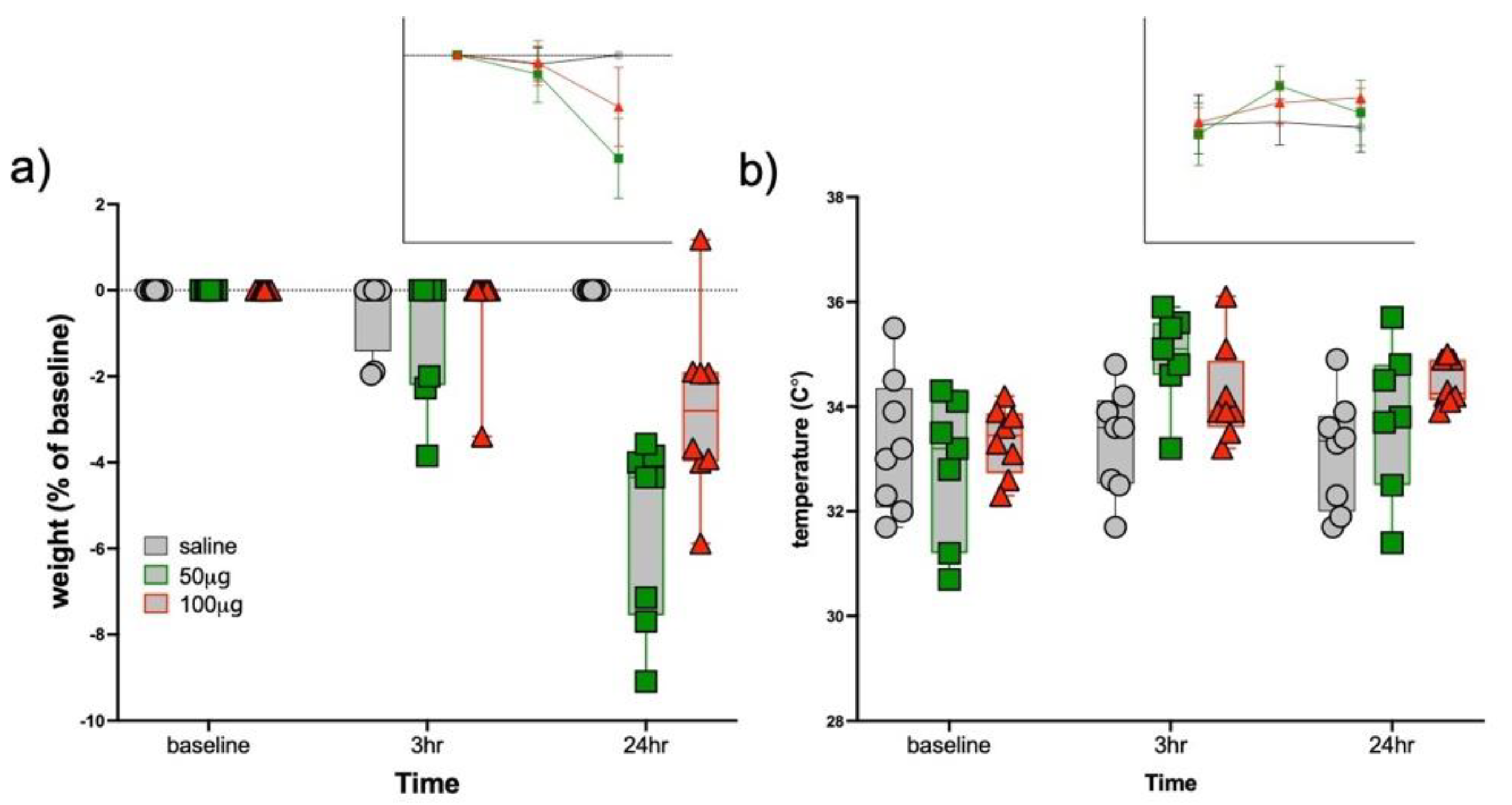
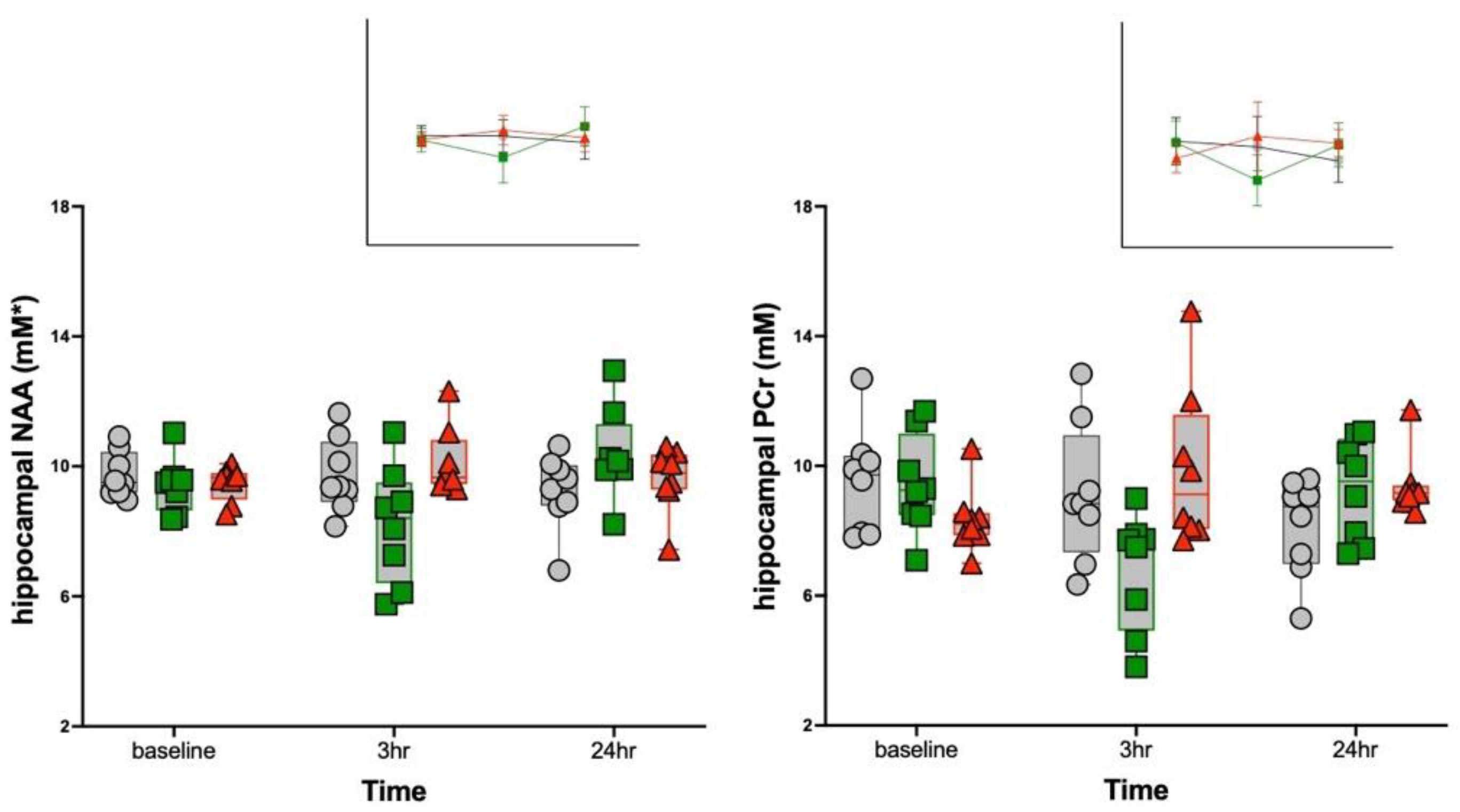
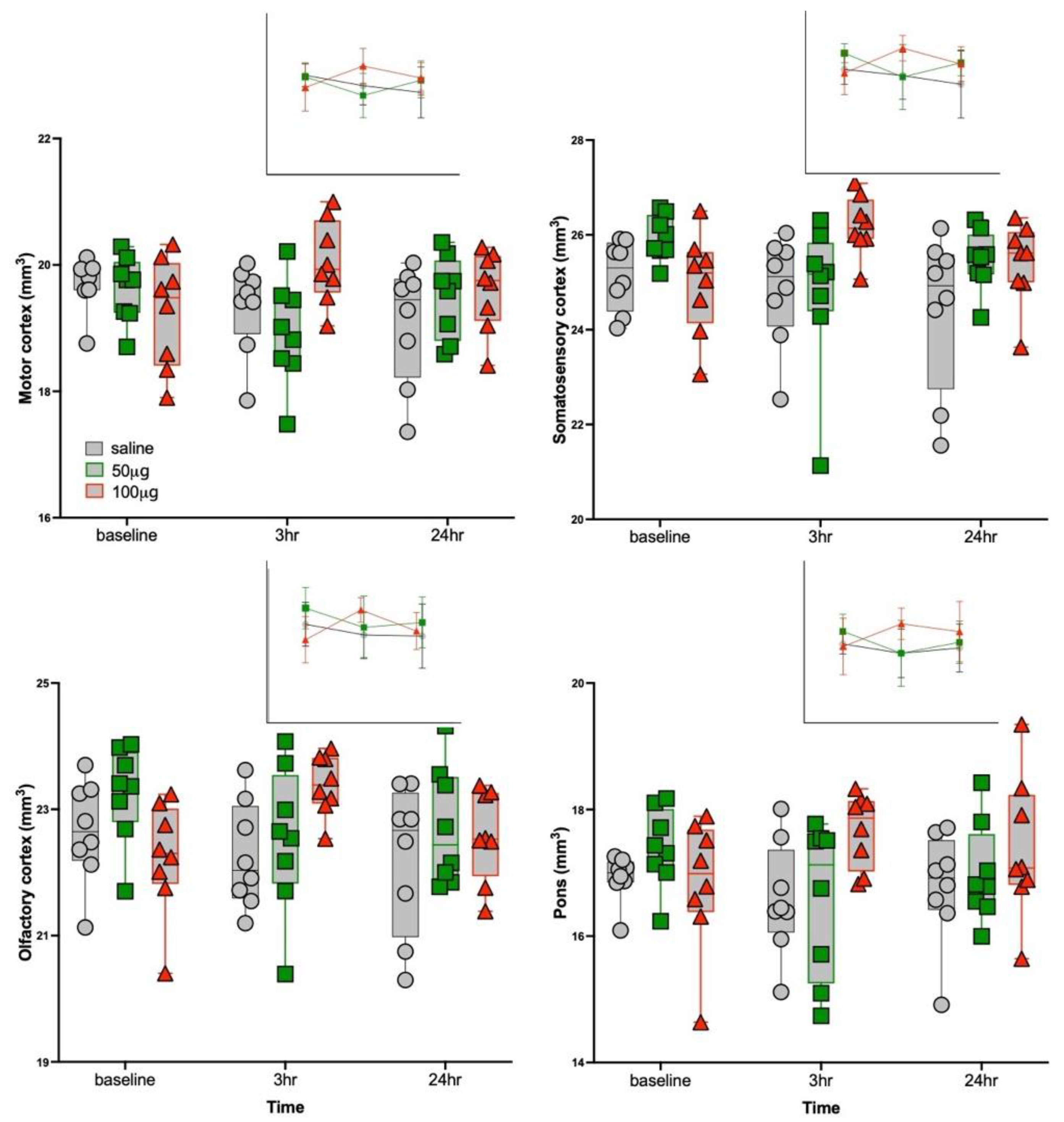
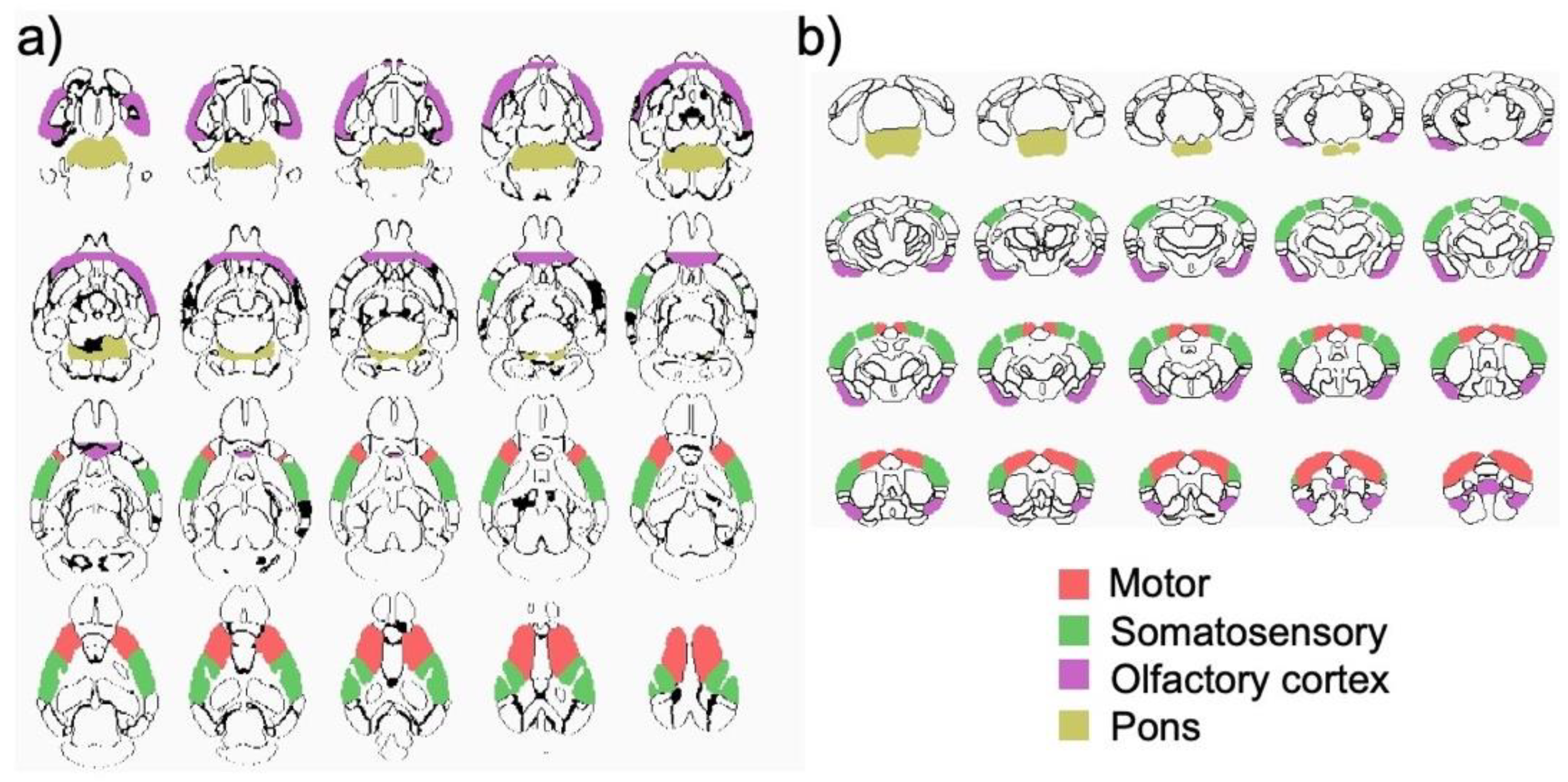
| Hippocampus | Striatum | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metabolite | 3-Group × 3-Time MANOVAs | 3-Group ANOVAs | 3-Group × 3-Time MANOVAs | 3-Group ANOVAs | ||||||
| Baseline | 3 h | 24 h | Baseline | 3 h | 24 h | |||||
| F(4,42) + | p-Value | F(2,24), p | F(2,24), p | F(2,24), p | F(4,42) + | p-Value | F(2,24), p | F(2,24), p | F(2,24), p | |
| Cr + PCr | 1.61 | 0.19 | n.s. | n.s. | n.s. | 2.04 | 0.11 | n.s. | n.s. | n.s. |
| GABA | 1.68 | 0.17 | n.s. | n.s. | n.s. | 0.14 | 0.97 | n.s. | n.s. | n.s. |
| Gln | 0.24 | 0.91 | n.s. | n.s. | n.s. | 3.83 | 0.01 | 2.3, 0.12 | 2.2, 0.13 | 0.5, 0.60 |
| Glu | 0.61 | 0.66 | n.s. | n.s. | n.s. | 1.39 | 0.25 | n.s. | n.s. | n.s. |
| Glu + Gln | 0.15 | 0.96 | n.s. | n.s. | n.s. | 1.77 | 0.15 | n.s. | n.s. | n.s. |
| GPC + PCh | 0.33 | 0.86 | n.s. | n.s. | n.s. | 1.88 | 0.13 | n.s. | n.s. | n.s. |
| GSH | 0.74 | 0.57 | n.s. | n.s. | n.s. | 1.02 | 0.41 | n.s. | n.s. | n.s. |
| Ins | 1.73 | 0.16 | n.s. | n.s. | n.s. | 1.83 | 0.14 | n.s. | n.s. | n.s. |
| MM09 | 0.25 | 0.91 | n.s. | n.s. | n.s. | 0.88 | 0.48 | n.s. | n.s. | n.s. |
| MM09 + Lip09 | 0.45 | 0.77 | n.s. | n.s. | n.s. | 1.05 | 0.39 | n.s. | n.s. | n.s. |
| NAA | 4.67 | 0.003 | 0.5, 0.64 | 4.4, 0.03 * | 1.9, 0.17 | 0.97 | 0.44 | n.s. | n.s. | n.s. |
| NAA + NAAG | 2.06 | 0.10 | n.s. | n.s. | n.s. | 1.03 | 0.41 | n.s. | n.s. | n.s. |
| PCh | 0.80 | 0.53 | n.s. | n.s. | n.s. | 2.60 | 0.05 | 2.3, 0.13 | 1.2, 0.34 | 1.1, 0.37 |
| PCr | 4.96 | 0.002 | 1.7, 0.21 | 4.6, 0.02 + | 2.1, 0.15 | 1.79 | 0.15 | n.s. | n.s. | n.s. |
| Tau | 1.23 | 0.31 | n.s. | n.s. | n.s. | 2.03 | 0.11 | n.s. | n.s. | n.s. |
| Region | Subregion | Size (mm3) * | 3-Group × 3-Time MANOVAs | 3-Group ANOVAs | t-Tests | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 h | 24 h | |||||||
| F(4,42) | p-Value | F(2,24), p | F(2,24), p | F(2,24), p | p-Values | Comparisons | |||
| Isocortex (Cerebral cortex/Cortical plate) | |||||||||
| Fronto-Orbital 1 | 5.2 ± 0.3 | 1.71 | 0.17 | n.s. | n.s. | n.s. | |||
| Motor 2 | 19.5 ± 0.6 | 2.87 | 0.04 | 1.2, 0.32 | 4.7, 0.02 | 1.1, 0.36 | 0.01 | 100μg R848 > 50μg R848 at 3 h | |
| Somatosensory 3 | 25.4 ± 0.9 | 3.16 | 0.02 | 3.4, 0.05 | 3.6, 0.05 | 2.2, 0.14 | 0.02 | 50 μg R848 > 100 μg R848 at baseline | |
| 0.03 | 100μg R848 > 50μg R848 at 3 h | ||||||||
| 0.03 | 100μg R848 > saline at 3 h | ||||||||
| Insula 4 | 9.7 ± 0.5 | 4.43 | 0.005 | 6.0, 0.009 | 1.9, 0.18 | 0.6, 0.56 | 0.003 | 50 μg R848 > 100 μg R848 at baseline | |
| 0.03 | 50 μg R848 > saline at baseline | ||||||||
| Temporal 5 | 6.9 ± 0.4 | 1.51 | 0.22 | n.s. | n.s. | n.s. | |||
| Visual 6 | 21.6 ± 0.9 | 1.05 | 0.39 | n.s. | n.s. | n.s. | |||
| Cingulate 7 | 6.6 ± 0.3 | 2.15 | 0.09 | n.s. | n.s. | n.s. | |||
| Olfactory Areas | |||||||||
| Olfactory bulb | 23.9 ± 1.2 | 1.77 | 0.15 | n.s. | n.s. | n.s. | |||
| Olfactory cortex 8 | 22.7 ± 0.9 | 3.71 | 0.01 | 3.1, 0.07 | 3.7, 0.04 | 0.6, 0.57 | 0.02 | 100μg R848 > saline at 3 h | |
| Hippocampal Formation 9 | 36.8 ± 1.4 | 1.33 | 0.28 | n.s. | n.s. | n.s. | |||
| Cortical Subplate | 6.4 ± 0.3 | 0.80 | 0.53 | n.s. | n.s. | n.s. | |||
| Cerebral Nuclei | |||||||||
| Striatum 10 | 35.1 ± 1.3 | 0.97 | 0.43 | n.s. | n.s. | n.s. | |||
| Pallidum | 8.4 ± 0.4 | 1.28 | 0.29 | n.s. | n.s. | n.s. | |||
| Brainstem | |||||||||
| Thalamus 11 | 16.8 ± 0.6 | 0.73 | 0.57 | n.s. | n.s. | n.s. | |||
| Hypothalamus | 13.9 ± 0.5 | 2.18 | 0.09 | n.s. | n.s. | n.s. | |||
| Midbrain | 35.2 ± 1.2 | 1.70 | 0.17 | n.s. | n.s. | n.s. | |||
| Hindbrain | |||||||||
| Pons | 17.1 ± 0.8 | 3.01 | 0.03 | 1.3, 0.29 | 3.6, 0.04 | 0.9, 0.43 | 0.03 | 100μg R848 > 50μg R848 at 3 h | |
| 0.03 | 100μg R848 > saline at 3 h | ||||||||
| Medulla | 36.5 ± 1.6 | 2.29 | 0.08 | n.s. | n.s. | n.s. | |||
| Cerebellar cortex | 48.5 ± 1.9 | 1.99 | 0.11 | n.s. | n.s. | n.s. | |||
| CSF | 10.7 ± 0.8 | 2.12 | 0.10 | n.s. | n.s. | n.s. | |||
| TOTAL ⨍ | 470.14 ± 15.0 | 2.40 | 0.07 | n.s. | n.s. | n.s. | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahr, N.M.; Zhao, Q.; Goodcase, R.; Pfefferbaum, A. Systemic Administration of the TLR7/8 Agonist Resiquimod (R848) to Mice Is Associated with Transient, In Vivo-Detectable Brain Swelling. Biology 2022, 11, 274. https://doi.org/10.3390/biology11020274
Zahr NM, Zhao Q, Goodcase R, Pfefferbaum A. Systemic Administration of the TLR7/8 Agonist Resiquimod (R848) to Mice Is Associated with Transient, In Vivo-Detectable Brain Swelling. Biology. 2022; 11(2):274. https://doi.org/10.3390/biology11020274
Chicago/Turabian StyleZahr, Natalie May, Qingyu Zhao, Ryan Goodcase, and Adolf Pfefferbaum. 2022. "Systemic Administration of the TLR7/8 Agonist Resiquimod (R848) to Mice Is Associated with Transient, In Vivo-Detectable Brain Swelling" Biology 11, no. 2: 274. https://doi.org/10.3390/biology11020274
APA StyleZahr, N. M., Zhao, Q., Goodcase, R., & Pfefferbaum, A. (2022). Systemic Administration of the TLR7/8 Agonist Resiquimod (R848) to Mice Is Associated with Transient, In Vivo-Detectable Brain Swelling. Biology, 11(2), 274. https://doi.org/10.3390/biology11020274






