The Efficacies of 1-Methylcyclopropene and Chitosan Nanoparticles in Preserving the Postharvest Quality of Damask Rose and Their Underlying Biochemical and Physiological Mechanisms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment
2.2. 1-MCP Treatment
2.3. CSNPs Preparation and Treatment
2.4. Shelf Life and Weight Loss Evaluation
2.5. Relative Water Content (RWC)
2.6. Volatile Oil Content and GC-MS Analysis
2.7. Determination of Total Anthocyanin Content (TAC)
2.8. Determination of Total Carotenoid Content (TCC)
2.9. Determination of Total Phenolics Content (TPC)
2.10. Antioxidant Activity (DPPH Assay)
2.11. Determination of Ethylene Production
2.12. H2O2 Production
2.13. Malondialdehyde Assessment (MDA)
2.14. Membrane Stability Index (MSI)
2.15. Enzyme Activities Determination
2.16. Statistical Analysis
3. Results
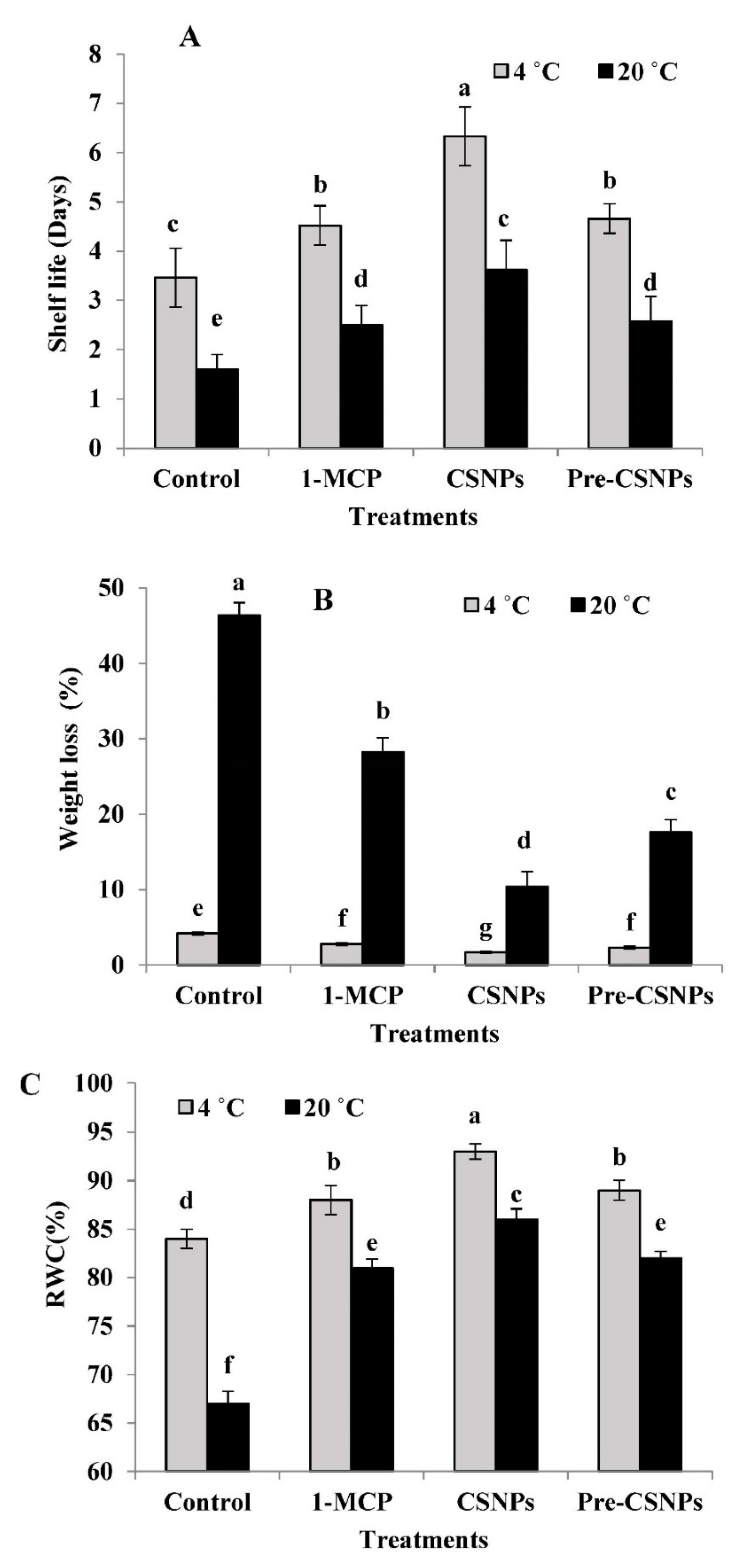
3.1. Shelf Life and Weight Loss
3.2. Relative Water Content (RWC)
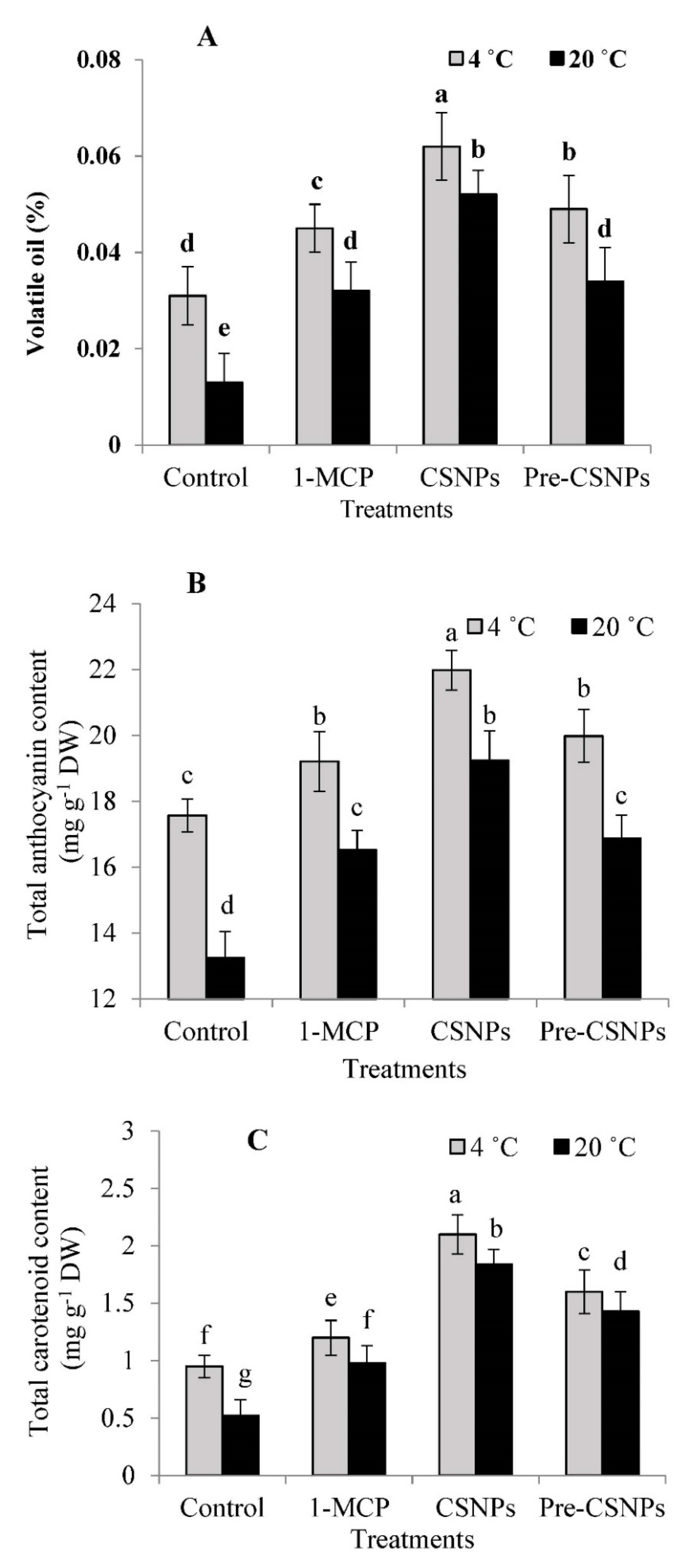
3.3. Volatile Oil Content and GC-MS Results
3.4. Total Anthocyanin Content (TAC)
3.5. Total Carotenoid Content (TCC)
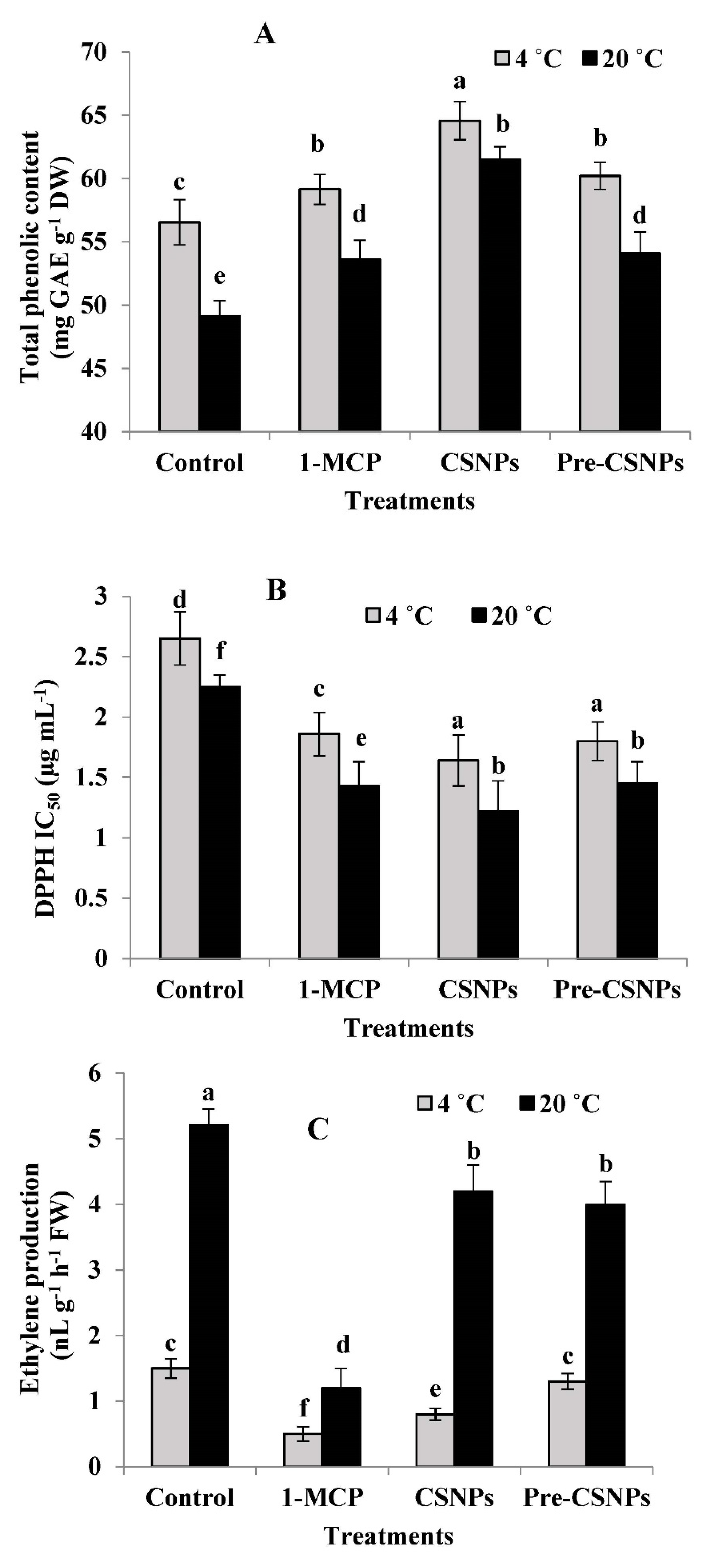
3.6. Total Phenolic Content (TPC)
3.7. Antioxidant Activity
3.8. Ethylene Production
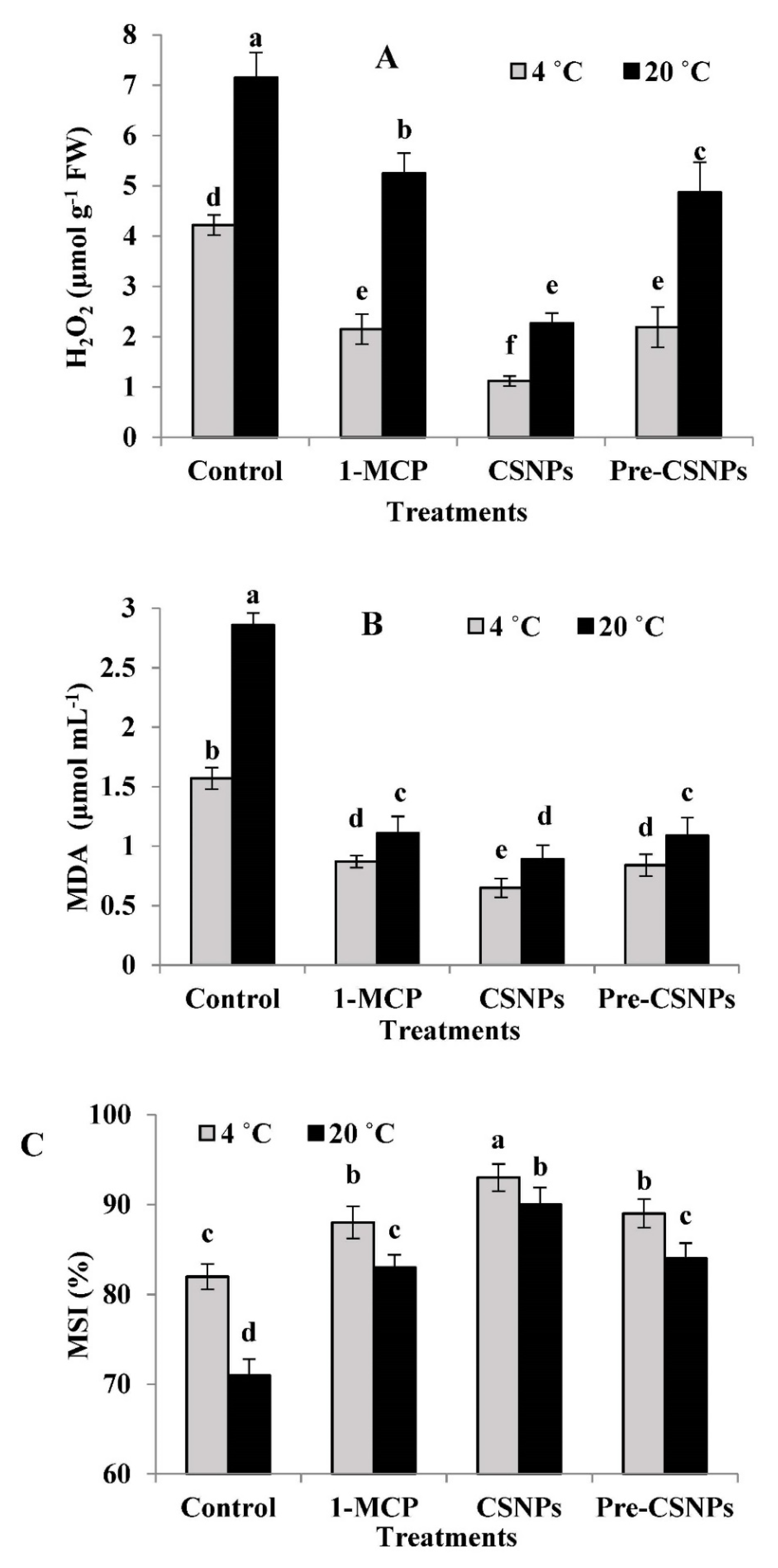
3.9. H2O2 Production
3.10. Malondialdehyde (MDA) Content
3.11. Membrane Stability Index (MSI)
3.12. Enzyme Activities Determination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kashefi, B.; Tabaei-Aghdaei, S.R.; Matinizadeh, M.; Mousavi, A.; Jafari, A. Some physiological and enzymatic characterizations of Damask Rose accessions (‘Rosa damascena’ Mill.). Aust. J. Crop Sci. 2012, 6, 283–290. [Google Scholar]
- Dobreva, A.; Getchovska, K.; Nedeltcheva-Antonova, D. A comparative study of Saudi Arabia and Bulgarian Rose oil chemical profile: The effect of the technology and geographic origin. Flavour Fragr. J. 2020, 35, 584–596. [Google Scholar] [CrossRef]
- Baydar, N.G.; Baydar, H. Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extracts. Ind. Crops Prod. 2013, 41, 375–380. [Google Scholar] [CrossRef]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Esmat, F.; Elshazly, S.; Siddique, K.H.; Hessini, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in Damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef]
- Koksal, N.; Aslancan, H.; Sadighazadi, S.; Kafkas, E. Chemical investigation on Rose damascena Mill. volatiles; effects of storage and drying conditions. Acta Sci. Pol. Hortorum Cultus 2015, 14, 105–114. [Google Scholar]
- Kazaz, S.; Erbas, S.; Baydar, H.; Dilmacunal, T.; Koyuncu, M.A. Cold storage of oil rose (Rosa damascena Mill.) flowers. Sci. Hortic. 2010, 126, 284–290. [Google Scholar] [CrossRef]
- Hassan, F.; Schmidt, G. Post-harvest characteristics of cut carnations as the result of chemical treatments. Acta Agron. Hung. 2004, 52, 125–132. [Google Scholar] [CrossRef]
- Hassan, F.; Ali, E. Effects of salt stress on growth, antioxidant enzyme activity and some other physiological parameters in jojoba [Simmondsia chinensis (Link) Schneider] plant. Aust. J. Crop Sci. 2014, 8, 1615–1624. [Google Scholar]
- Misra, N.; Misra, R.; Mariam, A.; Yusuf, K.; Yusuf, L. Salicylic acid alters antioxidant and phenolics metabolism in Catharanthus roseus grown under salinity stress. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 118–125. [Google Scholar]
- Ali, E.F.; Bazaid, S.A.; Hassan, F. Salinity tolerance of Taif roses by gibberellic acid (GA3). Int. J. Sci. Res. 2014, 3, 184–192. [Google Scholar]
- Hassan, F.; Ali, E. Protective effects of 1-methylcyclopropene and salicylic acid on senescence regulation of gladiolus cut spikes. Sci. Hortic. 2014, 179, 146–152. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Ali, E.F.; Mostafa, N.Y.; Mazrou, R. Shelf-life extension of sweet basil leaves by edible coating with thyme volatile oil encapsulated chitosan nanoparticles. Int. J. Biol. Macromol. 2021, 177, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Sisler, E.C.; Serek, M. Inhibitors of ethylene responses in plants at the receptor level: Recent development. Physiol. Plant. 1997, 100, 577–582. [Google Scholar] [CrossRef]
- Ma, Y.; Ban, Q.; Shi, J.; Dong, T.; Jiang, C.; Wang, Q. 1-Methylcyclopropene (1-MCP), storage time, and shelf life and temperature affect phenolic compounds and antioxidant activity of ‘Jonagold’ apple. Postharvest Biol. Technol. 2019, 150, 71–79. [Google Scholar] [CrossRef]
- Brouwer, B.; Mensink, M.; Hogeveen-van Echtelt, E.; Woltering, E.J. Pre-storage application of 1-methylcyclopropene does not affect the flavour of ‘Conference’ pears ripened after 8 months of commercial-standard controlled atmosphere storage. Postharvest Biol. Technol. 2021, 174, 111448. [Google Scholar] [CrossRef]
- Ozturk, B.; Yildiz, M.; Yildiz, K.; Gun, S. Maintaining the postharvest quality and bioactive compounds of jujube (Ziziphus jujuba Mill. Cv. ‘Li’) fruit by applying 1-methylcyclopropene. Sci. Hortic. 2021, 275, 109671. [Google Scholar] [CrossRef]
- Song, L.; Yi, R.; Luo, H.; Jiang, L.; Gu, S.; Yu, Z. Postharvest 1-methylcyclopropene application delays leaf yellowing of pak choi (Brassica rapa subsp. chinensis) by improving chloroplast antioxidant capacity and maintaining chloroplast structural integrity during storage at 20 °C. Sci. Hortic. 2020, 270, 109466. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, H.; Zhang, L.; Zhou, H.; Li, P. The application of 1-methylcyclopropene preserves the postharvest quality of cabbage by inhibiting ethylene production, delaying chlorophyll breakdown and increasing antioxidant capacity. Sci. Hortic. 2021, 281, 109986. [Google Scholar] [CrossRef]
- Hassan, F.; Mahfouz, S. Effect of 1-methylcyclopropene (1-MCP) on the postharvest senescence of coriander leaves during storage and its relation to antioxidant enzyme activity. Sci. Hortic. 2012, 141, 69–75. [Google Scholar] [CrossRef]
- Patiño, L.S.; Castellanos, D.A.; Herrera, A.O. Influence of 1-MCP and modified atmosphere packaging in the quality and preservation of fresh basil. Postharvest Biol. Technol. 2018, 136, 57–65. [Google Scholar] [CrossRef]
- Azuma, M.; Onozaki, T.; Ichimura, K. Difference of ethylene production and response to ethylene in cut flowers of dahlia (Dahlia variabilis) cultivars. Sci. Hortic. 2020, 273, 109635. [Google Scholar] [CrossRef]
- Hassan, F.; Ali, E.; Mazrou, R. Involvement of ethylene synthetic inhibitors in regulating the senescence of cut carnations through membrane integrity maintenance. J. Hortic. Res. 2020, 28, 39–48. [Google Scholar] [CrossRef]
- Tsai, J.; Wang, T.; Huang, P.; Do, Y. Effects of developmental stages on postharvest performance of White Crane Orchid (Calanthe triplicata) inflorescences. Sci. Hortic. 2021, 281, 109988. [Google Scholar] [CrossRef]
- MacLean, D.D.; Murr, D.P.; DeEll, J.R.; Horvath, C.R. Postharvest variation in apple (Malus × domestica Borkh.) flavonoids following harvest, storage, and 1-MCP treatment. J. Agric. Food Chem. 2006, 54, 870–878. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, K.; Liu, S. Evaluation of 1-methylcyclopropene (1-MCP) and low temperature conditioning (LTC) to control brown of Huangguan pears. Sci. Hortic. 2020, 259, 108738. [Google Scholar] [CrossRef]
- Pichyangkura, R.; Chadchawan, S. Biostimulant activity of chitosan in horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef]
- Choo, K.S.O.; Bollen, M.; Ravensdale, J.T.; Dykes, G.A.; Coorey, R. Effect of chitosan and gum Arabic with natamycin on the aroma profile and bacterial community of Australian grown black P’erigord truffles (Tuber melansoporum) during storage. Food Microbiol. 2021, 97, 103743. [Google Scholar] [CrossRef]
- Khatri, D.; Panigrahi, J.; Prajapati, A.; Bariya, H. Attributes of Aloe vera gel and chitosan treatments on the quality and biochemical traits of post-harvest tomatoes. Sci. Hortic. 2020, 259, 108837. [Google Scholar] [CrossRef]
- Poverenov, E.; Zaitsev, Y.; Arnon, H.; Granit, R.; Alkalai-Tuvia, S.; Perzelan, Y.; Weinberg, T.; Fallik, E. Effects of a composite chitosan Latin edible coating on postharvest quality and storability of red bell peppers. Postharvest Biol. Technol. 2014, 96, 106–109. [Google Scholar] [CrossRef]
- González-Saucedoa, K.A.; Barrera-Nechaa, L.L.; Ventura-Aguilarb, R.I.; Correa-Pachecob, Z.N.; Bautista-Bañosa, S.; Hernández-Lópeza, M. Extension of the postharvest quality of bell pepper by applying nanostructured coatings of chitosan with Byrsonima crassifolia extract (L.). Postharvest Biol. Technol. 2019, 149, 74–82. [Google Scholar] [CrossRef]
- Bañuelos-Hernández, K.P.; García-Nava, J.R.; Leyva-Ovalle, O.R.; Peña-Valdivia, C.B.; Trejo, C.; Ybarra-Moncada, M.C. Chitosan coating effect on vase life of flowering stems of Heliconia bihai (L.) L. cv. Halloween. Postharvest Biol. Technol. 2017, 132, 179–187. [Google Scholar] [CrossRef]
- Hong-Juan, J.; Huan-Qing, L. Chitooligosaccharide prolongs vase life of cut roses by decreasing reactive oxygen species. Korean J. Hortic. Sci. Technol. 2015, 33, 383–389. [Google Scholar]
- Sun, T.; Wu, C.L.; Hao, H.; Dai, Y.; Li, J.R. Preparation and preservation properties of the chitosan coatings modified with the in situ synthesized nano SiOx. Food Hydrocoll. 2016, 54, 130–138. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Saran, R.; Choudhary, K.; Tarafdar, J.C. Biosynthesis and characterization of nanoparticles. J. Adv. Med. Life Sci. 2014, 1, 4. [Google Scholar]
- Mustafa, M.A.; Ali, A.; Manickam, S.; Siddiqui, Y. Ultrasound-Assisted Chitosan- Surfactant Nanostructure Assemblies: Towards Maintaining Postharvest Quality of Tomatoes. Food Bioprocess Technol. 2014, 7, 2102–2111. [Google Scholar] [CrossRef]
- Tokatl, K.; Aslihan, D. Effects of chitosan edible film coatings on the physicochemical and microbiological qualities of sweet cherry (Prunus avium L.). Sci. Hortic. 2020, 259, 108656. [Google Scholar] [CrossRef]
- Amer, A.; Shoala, T. Physiological and phenotypic characters of sweet marjoram in response to pre-harvest application of hydrogen peroxide or chitosan nanoparticles. Sci. Hortic. 2020, 268, 109374. [Google Scholar] [CrossRef]
- Gayed, A.A.N.A.; Shaarawi, S.A.M.A.; Elkhishen, M.A.; Elsherbini, N.R.M. Pre-harvest application of calcium chloride and chitosan on fruit quality and storability of ‘Early Swelling’ peach during cold storage. Ciênc. Agrotecnol. 2017, 41, 220–231. [Google Scholar] [CrossRef] [Green Version]
- Nia, A.E.; Taghipour, S.; Siahmansour, S. Pre-harvest application of chitosan and postharvest Aloe vera gel coating enhances quality of table grape (Vitis vinifera L. cv. ‘Yaghouti’) during postharvest period. Food Chem. 2021, 347, 129012. [Google Scholar]
- Fan, W.; Yan, W.; Xu, Z. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.A.S.; Mazrou, R.; Gaber, A.; Hassan, M. Moringa extract preserved the vase life of cut roses through maintaining water relations and enhancing antioxidant machinery. Postharvest Biol. Technol. 2020, 164, 111156. [Google Scholar] [CrossRef]
- Weatherley, P.E. Studies in the water relations of the cotton plant.1. The field measurements of water deficit in leaves. New Phytol. 1950, 49, 8. [Google Scholar] [CrossRef]
- British Pharmacopea. Determination of Volatile Oil in Drugs; Pharmaceutical Press: London, UK, 1963. [Google Scholar]
- Wrolstad, R.E.; Culbertson, J.D.; Cornwell, C.J.; Mattick, L.R. Detection of adulteration in blackberry juice concentrates and wines. J.-Assoc. Off. Anal. Chem. 1982, 65, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Hnin, K.K.; Zhang, M.; Ju, R.; Wang, B. A novel infrared pulse-spouted freeze drying on the drying kinetics, energy consumption and quality of edible rose flowers. LWT—Food Sci. Technol. 2021, 136, 110318. [Google Scholar] [CrossRef]
- Dóka, O.; Ficzek, G.; Luterotti, S.; Bicanic, D.; Spruijt, R.; Buijnsters, J.G.; Végvári, G. Simple and rapid quantification of total carotenoids in lyophilized apricots (Prunus armeniaca L.) by means of reflectance colorimetry and photoacoustic spectroscopy. Food Technol. Biotechnol. 2013, 51, 453–459. [Google Scholar]
- Kamtekar, S.; Keer, V.; Patil, V. Estimation of phenolic content, flavonoid content, antioxidant and alpha amylase inhibitory activity of marketed polyherbal formulation. J. Appl. Pharm. Sci. 2014, 4, 61–65. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Heiser, I.; Osswald, W.F.; Elstner, E.F. Photodynamic ethane and ethylene formation from linolenic acid catalyzed by cytokinins and copper ions. J. Plant Physiol. 1998, 152, 230–234. [Google Scholar] [CrossRef]
- Patterson, B.D.; Macrae, E.A.; Ferguson, I.B. Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal. Chem. 1984, 134, 487–492. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid reactive-substances assay for estimating lipid peroxidation in plant tissue containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Sairam, R.K.; Deshmukh, P.S.; Shukla, D.S. Tolerance to drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J. Agron. Crop Sci. 1997, 178, 171–177. [Google Scholar] [CrossRef]
- Clairbone, A. Catalase activity. In Handbook of Methods for Oxygen Radical Research; Greenwald, R., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Kumar, V.B.; Mohan, T.C.; Murugan, K. Purification and kinetic characterization of polyphenol oxidase from Barbados cherry (Malpighia glabra L.). Food Chem. 2008, 110, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.F.; Paliyath, G.; Thompson, J.E. Characteristics of a membrane-associated lipoxygenase in tomato fruit. Plant Physiol. 1990, 94, 1225. [Google Scholar] [CrossRef] [Green Version]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics. With special Reference to the Biological Sciences; McGraw-Hill Book Company: New York, NY, USA, 1960; pp. 187–287. [Google Scholar]
- Kerch, G. Chitosan films and coatings prevent losses of fresh fruit nutritional quality: A review. Trends Food Sci. Technol. 2015, 46, 159–166. [Google Scholar] [CrossRef]
- Eshghi, S.; Hashemi, M.; Mohammadi, A.; Badii, F.; Mohammadhoseini, Z.; Ahmadi, K. Effect of nanochitosan-based coating with and without copper loaded on physicochemical and bioactive components of fresh strawberry fruit (Fragaria x ananassa Duchesne) during storage. Food Bioprocess Technol. 2014, 7, 2397–2409. [Google Scholar] [CrossRef]
- Hassan, F.; Ali, E.; Alamer, K. Exogenous application of polyamines alleviates water stress-induced oxidative stress of Rosa damascena Miller var. trigintipetala Dieck. S. Afr. J. Bot. 2018, 116, 96–102. [Google Scholar] [CrossRef]
- Luthria, D.L.; Mukhopadhyay, S.; Krizek, D.T. Content of total phenolics and phenolic acids in tomato (Lycopersicon esculentum Mill.) fruits as influenced by cultivar and solar UV radiation. J. Food Anal. 2006, 19, 771–777. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plants pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Bautista, B.S.; Hernández, L.A.N.; Velázquez del Valle, M.G.; Bosquez, M.E.; Sánchez, D.D. Quitosano: Una alternativa natural para reducir microorganismos postcosecha y mantener la vida de anaquel de productos hotofrutícolas. Rev. Iberoam. Tecnol. Postcosecha 2005, 7, 1–6. [Google Scholar]
- Panigrahi, J.; Patel, M.; Patel, N.; Gheewala, B.; Gantait, S. Changes in antioxidant and biochemical activities in castor oil-coated Capsicum annuum L. during postharvest storage. 3 Biotech 2018, 8, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gull, A.; Bhat, N.; Wani, S.M.; Masoodi, F.A.; Amin, T.; Ganai, S.A. Shelf life extension of apricot fruit by application of nanochitosan emulsion coatings containing pomegranate peel extract. Food Chem. 2021, 349, 129149. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Huang, H. Characterisation and comparison of phenols, flavonoids and isoflavones of soymilk and their correlations with antioxidant activity. Int. J. Food Sci. Technol. 2014, 49, 2290–2298. [Google Scholar] [CrossRef]
- Ranjbar, A.; Ahmadi, N. Effects of 1-MCP and ethylene on antioxidant enzyme activity and postharvest physio-biochemical characteristics of cut carnation flower cv. Fortune. Adv. Hortic. Sci. 2015, 29, 192–198. [Google Scholar]
- Galati, V.; Muniz, A.; Guimarães, J.; Inestroza-Lizardo, C.; Mattiuz, C.; Mattiuz, B. Postharvest conservation of alstroemeria ‘ajax’ using 1-methylcyclopropene. Ciênc. Agrotecnol. 2017, 41, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Kubo, Y. Ethylene, oxygen, carbon dioxide, and temperature in postharvest physiology. In Abiotic Stress Biology in Horticultural Plants; Kanayama, Y., Kochetov, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 14–45. [Google Scholar]
- De Wild, H.P.J.; Otma, E.C.; Peppelenbos, H.W. Carbon dioxide action on ethylene biosynthesis of preclimacteric and climacteric pear fruit. J. Exp. Bot. 2003, 54, 1537–1544. [Google Scholar] [CrossRef] [Green Version]
- Kumarihami, H.M.P.C.; Kim, J.G.; Kim, Y.H.; Lee, M.; Lee, Y.S.; Kwack, Y.B.; Kim, J. Preharvest application of chitosan improves the postharvest life of ‘Garmrok’ kiwifruit through the modulation of genes related to ethylene biosynthesis, cell wall modification and lignin metabolism. Foods 2021, 10, 373. [Google Scholar] [CrossRef]
- He, Y.; Bose, S.K.; Wang, W.; Jia, X.; Lu, H.; Yin, H. Pre-harvest treatment of chitosan oligosaccharides improved strawberry fruit quality. Int. J. Mol. Sci. 2018, 19, 2194. [Google Scholar] [CrossRef] [Green Version]
- Uthaichay, N.; Ketsa, S.; van Doorn, W.G. 1-MCP pretreatment prevents bud and flower abscission in Dendrobium orchids. Postharvest Biol. Technol. 2007, 43, 374–380. [Google Scholar] [CrossRef]
- Serek, M.; Woltering, E.J.; Sisler, E.C.; Frello, S.; Sriskandarajah, S. Controlling ethylene responses in flowers at the receptor level. Biotechnol. Adv. 2006, 24, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.T.; Zhu, X.; Hou, Y.Y.; Wang, X.Y.; Li, X.H. Postharvest nitric oxide treatment delays the senescence of winter jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit during cold storage by regulating reactive oxygen species metabolism. Sci. Hortic. 2020, 261, 109009. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, C.; Cheng, S.C.; Wei, B.D.; Liu, X.Y.; Ji, S.J. Changes in antioxidative metabolism accompanying pitting development in stored blueberry fruit. Postharvest Biol. Technol. 2014, 88, 88–95. [Google Scholar] [CrossRef]
- Bistgani, E.; Zohreh Siadat, S.A.; Bakhshandeh, A.; Ghasemi Pirbalouti, A.; Hashemi, M. Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenensis Celak. Crop. J. 2017, 5, 407–415. [Google Scholar] [CrossRef]
- Fan, X.G.; Shu, C.; Zhao, K.; Wang, X.M.; Cao, J.K.; Jiang, W.B. Regulation of apricot ripening and softening process during shelf life by post-storage treatments of exogenous ethylene and 1-methylcyclopropene. Sci. Hortic. 2018, 232, 63–70. [Google Scholar] [CrossRef]
- Cheng, Y.D.; Liu, L.Q.; Feng, Y.X.; Dong, Y.; Guan, J.F. Effects of 1-MCP on fruit quality and core browning in ‘Yali’ pear during cold storage. Sci. Hortic. 2019, 243, 350–356. [Google Scholar] [CrossRef]
- Rady, M.M.; Boriek, S.H.K.; Abd El-Mageed, T.A.; Seif El-Yazal, M.A.; Ali, E.F.; Hassan, F.A.S.; Abdelkhalik, A. Exogenous Gibberellic Acid or Dilute Bee Honey Boosts Drought Stress Tolerance in Vicia faba by Rebalancing Osmoprotectants, Antioxidants, Nutrients, and Phytohormones. Plants 2021, 10, 748. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Fetouh, M.I. Does moringa leaf ex-tract have preservative effect improving the longevity and postharvest quality of gladiolus cut spikes? Sci. Hortic. 2019, 250, 287–293. [Google Scholar] [CrossRef]
- Ali, E.F.; El-Shehawi, A.M.; Ibrahim, O.H.M.; Abdul-Hafeez, E.Y.; Moussa, M.M.; Hassan, F.A.S. A vital role of chitosan nanoparticles in improvisation the drought stress tolerance in Catharanthus roseus (L.) through biochemical and gene expression modulation. Plant Physiol. Biochem. 2021, 161, 166–175. [Google Scholar] [CrossRef]
- Adiletta, G.; Pasquariello, M.S.; Zampella, L.; Mastrobuoni, F.; Scortichini, M.; Petriccione, M. Chitosan coating: A postharvest treatment to delay oxidative stress in loquat fruits during cold storage. Agronomy 2018, 8, 54. [Google Scholar] [CrossRef] [Green Version]
- Petriccione, M.; Pagano, L.; Forniti, R.; Zampella, L.; Mastrobuoni, F.; Scortichini, M.; Mencarelli, F. Postharvest treatment with chitosan affects the antioxidant metabolism and quality of wine grape during partial dehydration. Postharvest Biol. Technol. 2018, 137, 38–45. [Google Scholar] [CrossRef]
- Cheng, S.; Yu, Y.; Guo, J.; Chen, G.; Guo, M. Effect of 1-methylcyclopropene and chitosan treatment on the storage quality of jujube fruit and its related enzyme activities. Sci. Hortic. 2020, 265, 109281. [Google Scholar] [CrossRef]

| No. | RI | Compound | Control (4 °C) | Control (20 °C) | 1-MCP (4 °C) | 1-MCP (20 °C) | CSNPs (4 °C) | CSNPs (20 °C) | Pre-CSNPs (4 °C) | Pre-CSNPs (20 °C) |
|---|---|---|---|---|---|---|---|---|---|---|
| Relative (%) | ||||||||||
| 1. | 1022 | α-Pinene | 3.47 | 3.45 | 3.52 | 3.51 | 3.56 | 3.54 | 3.49 | 3.46 |
| 2. | 1136 | β-Pinene | 0.54 | 0.55 | 0.59 | 0.58 | 0.61 | 0.63 | 0.58 | 0.56 |
| 3. | 1174 | Myrcene | 1.86 | 1.83 | 1.92 | 1.90 | 1.98 | 1.96 | 1.85 | 1.83 |
| 4. | 1494 | Linalool | 6.98 | 6.92 | 7.11 | 7.08 | 7.22 | 7.19 | 7.14 | 7.12 |
| 5. | 1511 | cis-Rose oxide | 0.65 | 0.62 | 0.69 | 0.66 | 0.75 | 0.73 | 0.68 | 0.69 |
| 6. | 1537 | Phenyl ethyl alcohol | 2.54 | 2.49 | 2.65 | 2.60 | 2.69 | 2.67 | 2.65 | 2.63 |
| 7. | 1541 | trans-Rose oxide | 0.56 | 0.54 | 0.61 | 0.59 | 0.64 | 0.63 | 0.58 | 0.57 |
| 8. | 1552 | Terpinen-4-ol | 1.19 | 1.17 | 1.25 | 1.21 | 1.24 | 1.22 | 1.19 | 1.18 |
| 9. | 1574 | α-Terpineol | 2.47 | 2.45 | 2.57 | 2.54 | 2.59 | 2.58 | 2.56 | 2.52 |
| 10. | 1586 | Nerol | 7.48 | 7.79 | 7.53 | 7.50 | 7.59 | 7.52 | 7.49 | 7.43 |
| 11. | 1657 | Heptadecane | 1.44 | 1.40 | 1.46 | 1.45 | 1.54 | 1.51 | 1.52 | 1.49 |
| 12. | 1688 | Citronellol | 18.86 | 18.75 | 18.91 | 18.88 | 18.97 | 18.90 | 18.89 | 18.81 |
| 13. | 1701 | Geraniol | 15.54 | 15.51 | 15.66 | 15.62 | 16.11 | 15.99 | 15.87 | 15.80 |
| 14. | 1714 | Geranial | 2.83 | 2.79 | 2.96 | 2.85 | 3.08 | 2.99 | 2.97 | 2.92 |
| 15. | 1732 | Eugenol | 1.45 | 1.46 | 1.49 | 1.47 | 1.53 | 1.54 | 1.50 | 1.47 |
| 16. | 1739 | Geranyl acetate | 0.92 | 0.88 | 0.94 | 0.91 | 0.99 | 0.98 | 0.97 | 0.98 |
| 17. | 1747 | Methyl eugenol | 1.29 | 1.25 | 1.33 | 1.31 | 1.32 | 1.33 | 1.36 | 1.34 |
| 18. | 1752 | α-Guaiene | 1.19 | 1.16 | 1.18 | 1.19 | 1.25 | 1.26 | 1.22 | 1.19 |
| 19. | 1794 | Caryophyllene oxide | 0.46 | 0.48 | 0.51 | 0.49 | 0.46 | 0.43 | 0.47 | 0.45 |
| 20. | 1806 | Octadecane | 0.42 | 0.39 | 0.44 | 0.38 | 0.43 | 0.44 | 0.47 | 0.43 |
| 21. | 1812 | Nonadecene | 2.86 | 2.81 | 2.89 | 2.87 | 2.88 | 2.89 | 2.81 | 2.82 |
| 22. | 1819 | Nonadecane | 7.11 | 6.99 | 7.18 | 7.15 | 7.21 | 7.19 | 7.05 | 6.99 |
| 23. | 1984 | Heneicosane | 1.22 | 1.24 | 1.28 | 1.26 | 1.29 | 1.24 | 1.27 | 1.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, E.F.; Issa, A.A.; Al-Yasi, H.M.; Hessini, K.; Hassan, F.A.S. The Efficacies of 1-Methylcyclopropene and Chitosan Nanoparticles in Preserving the Postharvest Quality of Damask Rose and Their Underlying Biochemical and Physiological Mechanisms. Biology 2022, 11, 242. https://doi.org/10.3390/biology11020242
Ali EF, Issa AA, Al-Yasi HM, Hessini K, Hassan FAS. The Efficacies of 1-Methylcyclopropene and Chitosan Nanoparticles in Preserving the Postharvest Quality of Damask Rose and Their Underlying Biochemical and Physiological Mechanisms. Biology. 2022; 11(2):242. https://doi.org/10.3390/biology11020242
Chicago/Turabian StyleAli, Esmat F., Ahmed A. Issa, Hatim M. Al-Yasi, Kamel Hessini, and Fahmy A. S. Hassan. 2022. "The Efficacies of 1-Methylcyclopropene and Chitosan Nanoparticles in Preserving the Postharvest Quality of Damask Rose and Their Underlying Biochemical and Physiological Mechanisms" Biology 11, no. 2: 242. https://doi.org/10.3390/biology11020242
APA StyleAli, E. F., Issa, A. A., Al-Yasi, H. M., Hessini, K., & Hassan, F. A. S. (2022). The Efficacies of 1-Methylcyclopropene and Chitosan Nanoparticles in Preserving the Postharvest Quality of Damask Rose and Their Underlying Biochemical and Physiological Mechanisms. Biology, 11(2), 242. https://doi.org/10.3390/biology11020242







