The Seed and the Metabolism Regulation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Dry Seed: Well-Organized to Resist
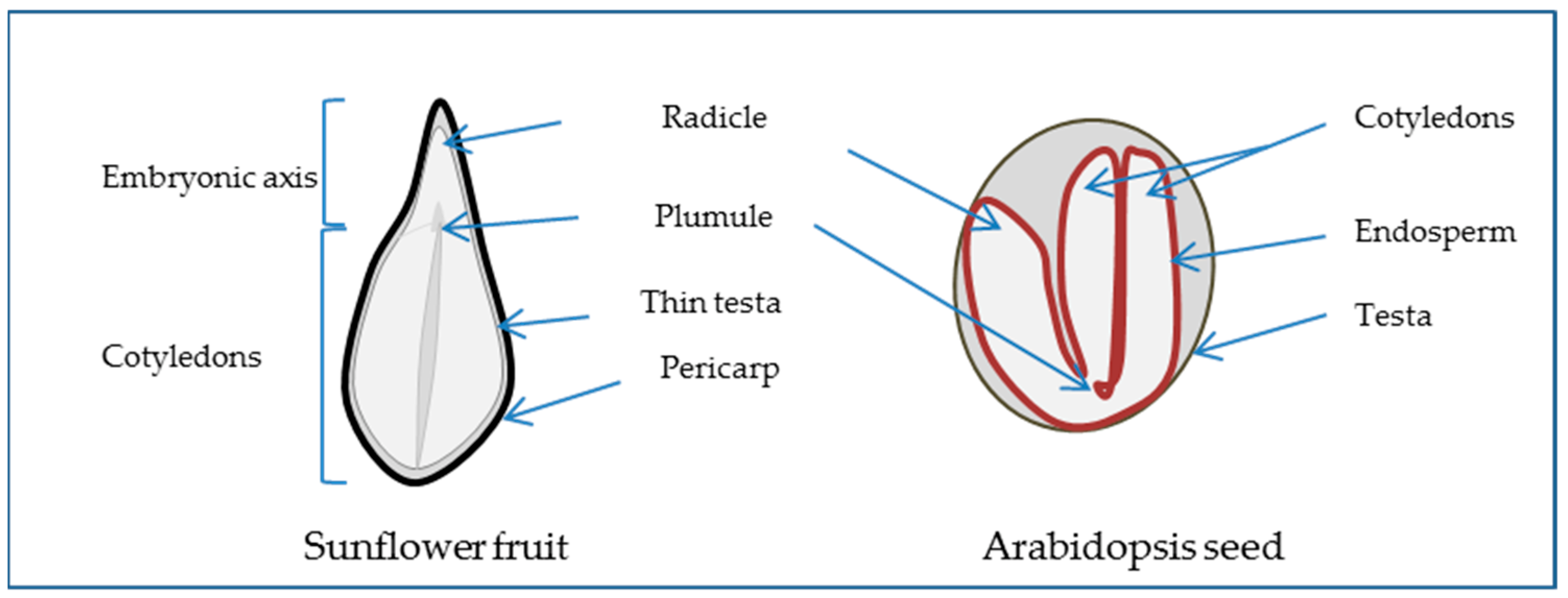
2.1. The Seed, a Special New Individual
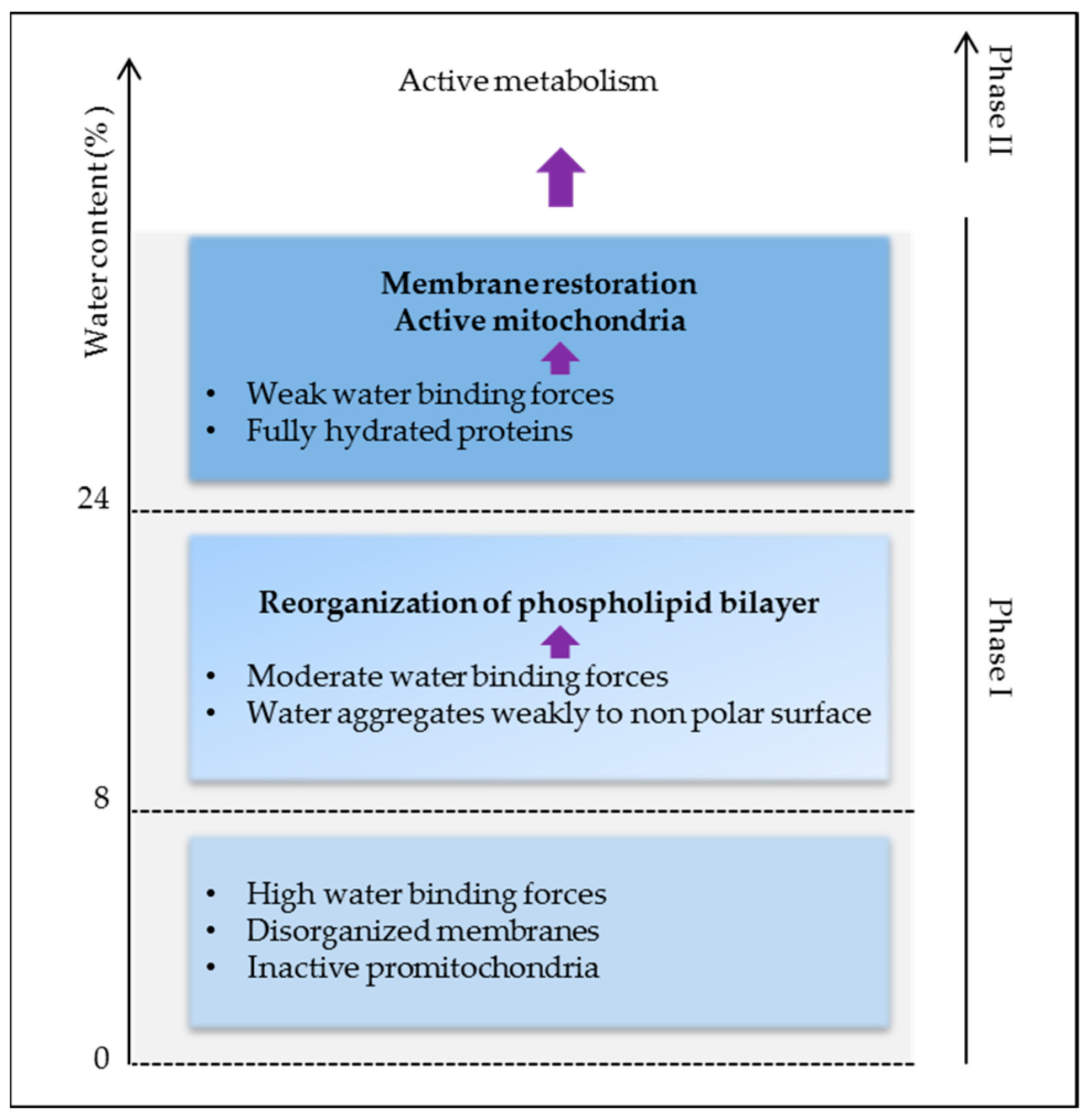
2.2. Water, “Matrix of Life”
2.3. Respiration Resumption
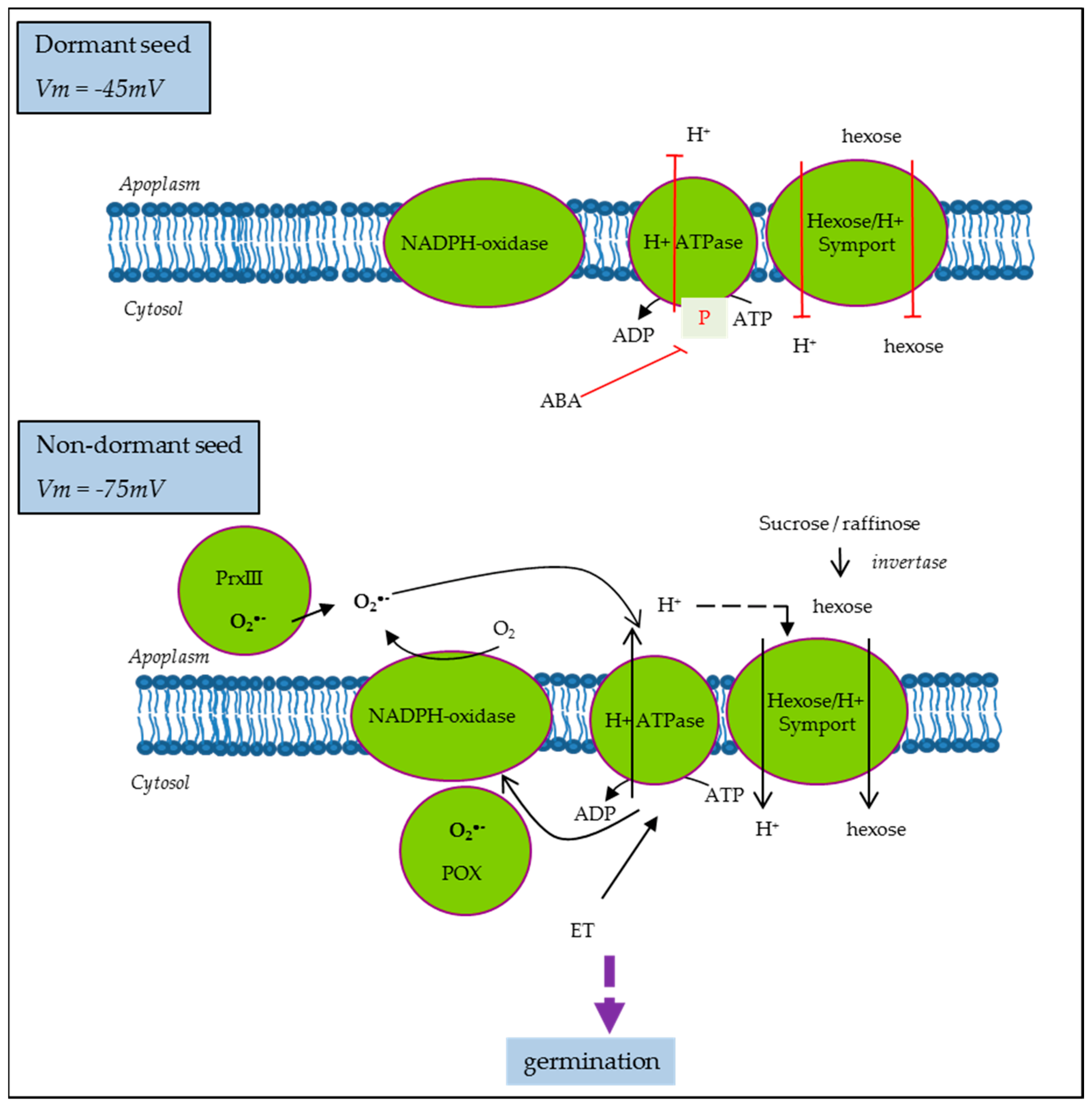
2.4. Plasma Membrane Potential
3. Seed Dormancy: Higher Level of Resistance
3.1. Seed Metabolism and Dormancy
3.2. Internal Determinants of Dormancy
3.3. Environmental Impact on Dormancy
4. Seeds: The Ability to Recover from Ageing
4.1. Seed Ageing
4.2. Seed Priming
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meimoun, P.; Mordret, E.; Langlade, N.B.; Balzergue, S.; Arribat, S.; Bailly, C.; El-Maarouf-Bouteau, H. Is Gene Transcription Involved in Seed Dry After-Ripening? PLoS ONE 2014, 9, e86442. [Google Scholar] [CrossRef]
- Roberts, E.H. Predicting the Storage Life of Seeds. Seed Sci. Technol. 1973, 1, 499–514. [Google Scholar]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. An Intermediate Category of Seed Storage Behaviour? I. Coffee. J. Exp. Bot. 1990, 41, 1167–1174. [Google Scholar] [CrossRef]
- Benner, S.A. Defining Life. Astrobiology 2010, 10, 1021–1030. [Google Scholar] [CrossRef] [Green Version]
- Vitas, M.; Dobovišek, A. In the Beginning Was a Mutualism—On the Origin of Translation. Orig. Life Evol. Biospheres 2018, 48, 223–243. [Google Scholar] [CrossRef]
- Belmonte, M.F.; Kirkbride, R.C.; Stone, S.L.; Pelletier, J.M.; Bui, A.Q.; Yeung, E.C.; Hashimoto, M.; Fei, J.; Harada, C.M.; Munoz, M.D.; et al. Comprehensive Developmental Profiles of Gene Activity in Regions and Subregions of the Arabidopsis Seed. Proc. Natl. Acad. Sci. USA 2013, 110, E435–E444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingram, G.C. Family Life at Close Quarters: Communication and Constraint in Angiosperm Seed Development. Protoplasma 2010, 247, 195–214. [Google Scholar] [CrossRef]
- Ballesteros, D.; Walters, C. Detailed Characterization of Mechanical Properties and Molecular Mobility within Dry Seed Glasses: Relevance to the Physiology of Dry Biological Systems: Molecular Mobility within the Glass of Dry Seed. Plant J. 2011, 68, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Vertucci, C.W.; Leopold, A.C. Bound Water in Soybean Seed and Its Relation to Respiration and Imbibitional Damage. Plant Physiol. 1984, 75, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Paszkiewicz, G.; Gualberto, J.M.; Benamar, A.; Macherel, D.; Logan, D.C. Arabidopsis Seed Mitochondria Are Bioenergetically Active Immediately upon Imbibition and Specialize via Biogenesis in Preparation for Autotrophic Growth. Plant Cell 2017, 29, 109–128. [Google Scholar] [CrossRef]
- Sallon, S.; Solowey, E.; Cohen, Y.; Korchinsky, R.; Egli, M.; Woodhatch, I.; Simchoni, O.; Kislev, M. Germination, Genetics, and Growth of an Ancient Date Seed. Science 2008, 320, 1464. [Google Scholar] [CrossRef]
- Leprince, O.; Pellizzaro, A.; Berriri, S.; Buitink, J. Late Seed Maturation: Drying without Dying. J. Exp. Bot. 2017, 68, 827–841. [Google Scholar] [CrossRef] [Green Version]
- Angelovici, R.; Galili, G.; Fernie, A.R.; Fait, A. Seed Desiccation: A Bridge between Maturation and Germination. Trends Plant Sci. 2010, 15, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Zhen, S.; Zhu, G.; Bian, Y.; Yan, Y. Comparative Metabolome Analysis of Wheat Embryo and Endosperm Reveals the Dynamic Changes of Metabolites during Seed Germination. Plant Physiol. Biochem. 2017, 115, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.A.; Narsai, R.; Carroll, A.; Ivanova, A.; Lohse, M.; Usadel, B.; Millar, A.H.; Whelan, J. Mapping Metabolic and Transcript Temporal Switches during Germination in Rice Highlights Specific Transcription Factors and the Role of RNA Instability in the Germination Process. Plant Physiol. 2009, 149, 961–980. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Ponnaiah, M.; Cueff, G.; Rajjou, L.; Prodhomme, D.; Gibon, Y.; Bailly, C.; Corbineau, F.; Meimoun, P.; El-Maarouf-Bouteau, H. Integrating Proteomics and Enzymatic Profiling to Decipher Seed Metabolism Affected by Temperature in Seed Dormancy and Germination. Plant Sci. Int. J. Exp. Plant Biol. 2018, 269, 118–125. [Google Scholar] [CrossRef]
- Xia, Q.; Saux, M.; Ponnaiah, M.; Gilard, F.; Perreau, F.; Huguet, S.; Balzergue, S.; Langlade, N.; Bailly, C.; Meimoun, P.; et al. One Way to Achieve Germination: Common Molecular Mechanism Induced by Ethylene and After-Ripening in Sunflower Seeds. Int. J. Mol. Sci. 2018, 19, 2464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weitbrecht, K.; Müller, K.; Leubner-Metzger, G. First off the Mark: Early Seed Germination. J. Exp. Bot. 2011, 62, 3289–3309. [Google Scholar] [CrossRef] [Green Version]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination—Still a Mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
- Szent-Györgyi, A. Cell-Associated Water; Academic Press: New York, NY, USA, 1979; pp. 363–413. [Google Scholar]
- Ball, P. Water as an Active Constituent in Cell Biology. Chem. Rev. 2008, 108, 74–108. [Google Scholar] [CrossRef]
- Bellissent-Funel, M.-C.; Hassanali, A.; Havenith, M.; Henchman, R.; Pohl, P.; Sterpone, F.; van der Spoel, D.; Xu, Y.; Garcia, A.E. Water Determines the Structure and Dynamics of Proteins. Chem. Rev. 2016, 116, 7673–7697. [Google Scholar] [CrossRef] [PubMed]
- Vertucci, C.W. Calorimetric Studies of the State of Water in Seed Tissues. Biophys. J. 1990, 58, 1463–1471. [Google Scholar] [CrossRef] [Green Version]
- Buitink, J.; Leprince, O. Intracellular Glasses and Seed Survival in the Dry State. Comptes R. Biol. 2008, 331, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Rupley, J.A.; Gratton, E.; Careri, G. Water and Globular Proteins. Trends Biochem. Sci. 1983, 8, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Richards, F.M.; Richmond, T. Solvents, Interfaces and Protein Structure. In Novartis Foundation Symposia; Porter, R., Fitzsimons, D.W., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 23–45. ISBN 978-0-470-72042-4. [Google Scholar]
- Texter, J. Nucleic Acid-Water Interactions. Prog. Biophys. Mol. Biol. 1979, 33, 83–97. [Google Scholar] [CrossRef]
- Shweta, H.; Sen, S. Dynamics of Water and Ions around DNA: What Is so Special about Them? J. Biosci. 2018, 43, 499–518. [Google Scholar] [CrossRef]
- van Zanten, M.; Koini, M.A.; Geyer, R.; Liu, Y.; Brambilla, V.; Bartels, D.; Koornneef, M.; Fransz, P.; Soppe, W.J.J. Seed Maturation in Arabidopsis Thaliana Is Characterized by Nuclear Size Reduction and Increased Chromatin Condensation. Proc. Natl. Acad. Sci. USA 2011, 108, 20219–20224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borisjuk, L.; Rolletschek, H. The Oxygen Status of the Developing Seed: Tansley Review. New Phytol. 2009, 182, 17–30. [Google Scholar] [CrossRef]
- Logan, D.C.; Millar, A.H.; Sweetlove, L.J.; Hill, S.A.; Leaver, C.J. Mitochondrial Biogenesis during Germination in Maize Embryos. Plant Physiol. 2001, 125, 662–672. [Google Scholar] [CrossRef] [Green Version]
- Howell, K.A.; Millar, A.H.; Whelan, J. Ordered Assembly of Mitochondria During Rice Germination Begins with Promitochondrial Structures Rich in Components of the Protein Import Apparatus. Plant Mol. Biol. 2006, 60, 201–223. [Google Scholar] [CrossRef]
- Nawa, Y.; Asahi, T. Rapid Development of Mitochondria in Pea Cotyledons during the Early Stage of Germination. Plant Physiol. 1971, 48, 671–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morohashi, Y.; Bewley, J.D. Development of Mitochondrial Activities in Pea Cotyledons: Influence of desiccation during and following germination of the axis. Plant Physiol. 1980, 66, 637–640. [Google Scholar] [CrossRef] [Green Version]
- Morohashi, Y.; Bewley, J.D.; Yeung, E.C. Biogenesis of Mitochondria in Imbibed Peanut Cotyledons: II. Development of light and heavy mitochondria. Plant Physiol. 1981, 68, 318–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrenshaft, M.; Brambl, R. Respiration and Mitochondrial Biogenesis in Germinating Embryos of Maize. Plant Physiol. 1990, 93, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Law, S.R.; Narsai, R.; Whelan, J. Mitochondrial Biogenesis in Plants during Seed Germination. Mitochondrion 2014, 19, 214–221. [Google Scholar] [CrossRef]
- Czarna, M.; Kolodziejczak, M.; Janska, H. Mitochondrial Proteome Studies in Seeds during Germination. Proteomes 2016, 4, 19. [Google Scholar] [CrossRef]
- Benamar, A.; Rolletschek, H.; Borisjuk, L.; Avelange-Macherel, M.-H.; Curien, G.; Mostefai, H.A.; Andriantsitohaina, R.; Macherel, D. Nitrite–Nitric Oxide Control of Mitochondrial Respiration at the Frontier of Anoxia. Biochim. Biophys. Acta BBA—Bioenerg. 2008, 1777, 1268–1275. [Google Scholar] [CrossRef]
- Attucci, S.; Carde, J.P.; Raymond, P.; Saint-Gès, V.; Spiteri, A.; Pradet, A. Oxidative Phosphorylation by Mitochondria Extracted from Dry Sunflower Seeds. Plant Physiol. 1991, 95, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Nietzel, T.; Mostertz, J.; Ruberti, C.; Née, G.; Fuchs, P.; Wagner, S.; Moseler, A.; Müller-Schüssele, S.J.; Benamar, A.; Poschet, G.; et al. Redox-Mediated Kick-Start of Mitochondrial Energy Metabolism Drives Resource-Efficient Seed Germination. Proc. Natl. Acad. Sci. USA 2020, 117, 741–751. [Google Scholar] [CrossRef]
- Buchanan, B.B. The Path to Thioredoxin and Redox Regulation Beyond Chloroplasts. Plant Cell Physiol. 2017, 58, 1826–1832. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.W. Phospholipids and plant membrane permeability. New Phytol. 1974, 73, 377–420. [Google Scholar] [CrossRef]
- Yu, X.; Li, A.; Li, W. How Membranes Organize during Seed Germination: Three Patterns of Dynamic Lipid Remodelling Define Chilling Resistance and Affect Plastid Biogenesis: Remodelling of Membrane Lipids during Germination. Plant Cell Environ. 2015, 38, 1391–1403. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xin, X.; Yin, G.; He, J.; Zhou, Y.; Chen, J.; Lu, X. Membrane Phospholipids Remodeling upon Imbibition in Brassica Napus L. Seeds. Biochem. Biophys. Res. Commun. 2019, 515, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Sze, H.; Li, X.; Palmgren, M.G. Energization of Plant Cell Membranes by H+-Pumping ATPases: Regulation and Biosynthesis. Plant Cell 1999, 11, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Haruta, M.; Burch, H.L.; Nelson, R.B.; Barrett-Wilt, G.; Kline, K.G.; Mohsin, S.B.; Young, J.C.; Otegui, M.S.; Sussman, M.R. Molecular Characterization of Mutant Arabidopsis Plants with Reduced Plasma Membrane Proton Pump Activity. J. Biol. Chem. 2010, 285, 17918–17929. [Google Scholar] [CrossRef] [Green Version]
- Falhof, J.; Pedersen, J.T.; Fuglsang, A.T.; Palmgren, M. Plasma Membrane H+-ATPase Regulation in the Center of Plant Physiology. Mol. Plant 2016, 9, 323–337. [Google Scholar] [CrossRef] [Green Version]
- Lang, V.; Pertl-Obermeyer, H.; Safiarian, M.J.; Obermeyer, G. Pump up the Volume—A Central Role for the Plasma Membrane H+ Pump in Pollen Germination and Tube Growth. Protoplasma 2014, 251, 477–488. [Google Scholar] [CrossRef]
- Pedersen, J.T.; Falhof, J.; Ekberg, K.; Buch-Pedersen, M.J.; Palmgren, M. Metal Fluoride Inhibition of a P-Type H+ Pump: Stabilization of the phosphoenzyme intermediate contributes to post-translational pump activation. J. Biol. Chem. 2015, 290, 20396–20406. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, J.T.; Kanashova, T.; Dittmar, G.; Palmgren, M. Isolation of Native Plasma Membrane H+-ATP Ase (Pma1p) in Both the Active and Basal Activation States. FEBS Open Bio 2018, 8, 774–783. [Google Scholar] [CrossRef] [Green Version]
- De Bont, L.; Naim, E.; Arbelet-Bonnin, D.; Xia, Q.; Palm, E.; Meimoun, P.; Mancuso, S.; El-Maarouf-Bouteau, H.; Bouteau, F. Activation of Plasma Membrane H+-ATPases Participates in Dormancy Alleviation in Sunflower Seeds. Plant Sci. 2019, 280, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Leubner-Metzger, G. Beta-1,3-Glucanase Gene Expression in Low-Hydrated Seeds as a Mechanism for Dormancy Release during Tobacco after-Ripening. Plant J. Cell Mol. Biol. 2005, 41, 133–145. [Google Scholar] [CrossRef]
- Bove, J.; Lucas, P.; Godin, B.; Ogé, L.; Jullien, M.; Grappin, P. Gene Expression Analysis by CDNA-AFLP Highlights a Set of New Signaling Networks and Translational Control during Seed Dormancy Breaking in Nicotiana Plumbaginifolia. Plant Mol. Biol. 2005, 57, 593–612. [Google Scholar] [CrossRef] [PubMed]
- Leymarie, J.; Bruneaux, E.; Gibot-Leclerc, S.; Corbineau, F. Identification of Transcripts Potentially Involved in Barley Seed Germination and Dormancy Using CDNA-AFLP. J. Exp. Bot. 2006, 58, 425–437. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Jordan, M.C.; Ayele, B.T. Transcriptional Programs Regulating Seed Dormancy and Its Release by After-Ripening in Common Wheat (Triticum Aestivum L.). Plant Biotechnol. J. 2012, 10, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Gao, F.; Kanno, Y.; Jordan, M.C.; Kamiya, Y.; Seo, M.; Ayele, B.T. Regulation of Wheat Seed Dormancy by After-Ripening Is Mediated by Specific Transcriptional Switches That Induce Changes in Seed Hormone Metabolism and Signaling. PLoS ONE 2013, 8, e56570. [Google Scholar] [CrossRef] [Green Version]
- Dure, L.; Waters, L. Long-lived messenger RNA: Evidence from cotton seed germination. Science 1965, 147, 410–412. [Google Scholar] [CrossRef]
- Sano, N.; Rajjou, L.; North, H.M. Lost in Translation: Physiological Roles of Stored MRNAs in Seed Germination. Plants 2020, 9, 347. [Google Scholar] [CrossRef] [Green Version]
- El-Maarouf-Bouteau, H.; Bailly, C. Oxidative Signaling in Seed Germination and Dormancy. Plant Signal. Behav. 2008, 3, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazin, J.; Langlade, N.; Vincourt, P.; Arribat, S.; Balzergue, S.; El-Maarouf-Bouteau, H.; Bailly, C. Targeted MRNA Oxidation Regulates Sunflower Seed Dormancy Alleviation during Dry After-Ripening. Plant Cell 2011, 23, 2196–2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, F.; Rampitsch, C.; Chitnis, V.R.; Humphreys, G.D.; Jordan, M.C.; Ayele, B.T. Integrated Analysis of Seed Proteome and MRNA Oxidation Reveals Distinct Post-Transcriptional Features Regulating Dormancy in Wheat (Triticum Aestivum L.). Plant Biotechnol. J. 2013, 11, 921–932. [Google Scholar] [CrossRef]
- Katsuya-Gaviria, K.; Caro, E.; Carrillo-Barral, N.; Iglesias-Fernández, R. Reactive Oxygen Species (ROS) and Nucleic Acid Modifications during Seed Dormancy. Plants 2020, 9, 679. [Google Scholar] [CrossRef] [PubMed]
- Buijs, G.; Vogelzang, A.; Nijveen, H.; Bentsink, L. Dormancy Cycling: Translation-related Transcripts Are the Main Difference between Dormant and Non-dormant Seeds in the Field. Plant J. 2020, 102, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Sano, N.; Takebayashi, Y.; To, A.; Mhiri, C.; Rajjou, L.; Nakagami, H.; Kanekatsu, M. Shotgun Proteomic Analysis Highlights the Roles of Long-Lived MRNAs and De Novo Transcribed MRNAs in Rice Seeds upon Imbibition. Plant Cell Physiol. 2019, 60, 2584–2596. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, M.; He, D.; Yang, P. Advances on Post-Translational Modifications Involved in Seed Germination. Front. Plant Sci. 2021, 12, 642979. [Google Scholar] [CrossRef]
- Arc, E.; Galland, M.; Cueff, G.; Godin, B.; Lounifi, I.; Job, D.; Rajjou, L. Reboot the System Thanks to Protein Post-Translational Modifications and Proteome Diversity: How Quiescent Seeds Restart Their Metabolism to Prepare Seedling Establishment. PROTEOMICS 2011, 11, 1606–1618. [Google Scholar] [CrossRef]
- Oracz, K.; El-Maarouf Bouteau, H.; Farrant, J.M.; Cooper, K.; Belghazi, M.; Job, C.; Job, D.; Corbineau, F.; Bailly, C. ROS Production and Protein Oxidation as a Novel Mechanism for Seed Dormancy Alleviation. Plant J. Cell Mol. Biol. 2007, 50, 452–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakabayashi, K.; Okamoto, M.; Koshiba, T.; Kamiya, Y.; Nambara, E. Genome-Wide Profiling of Stored MRNA in Arabidopsis Thaliana Seed Germination: Epigenetic and Genetic Regulation of Transcription in Seed. Plant J. Cell Mol. Biol. 2005, 41, 697–709. [Google Scholar] [CrossRef]
- Nambara, E.; Okamoto, M.; Tatematsu, K.; Yano, R.; Seo, M.; Kamiya, Y. Abscisic Acid and the Control of Seed Dormancy and Germination. Seed Sci. Res. 2010, 20, 55–67. [Google Scholar] [CrossRef]
- Xia, Q.; Ponnaiah, M.; Thanikathansubramanian, K.; Corbineau, F.; Bailly, C.; Nambara, E.; Meimoun, P.; El-Maarouf-Bouteau, H. Re-Localization of Hormone Effectors Is Associated with Dormancy Alleviation by Temperature and after-Ripening in Sunflower Seeds. Sci. Rep. 2019, 9, 4861. [Google Scholar] [CrossRef] [Green Version]
- Shu, K.; Liu, X.; Xie, Q.; He, Z. Two Faces of One Seed: Hormonal Regulation of Dormancy and Germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated Role of ABA in Seed Maturation, Dormancy, and Germination. J. Adv. Res. 2022, 35, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.R.; Gampala, S.S.L.; Rock, C.D. Abscisic Acid Signaling in Seeds and Seedlings. Plant Cell 2002, 14 (Suppl. 1), S15–S45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonogaki, H. The Long-Standing Paradox of Seed Dormancy Unfolded? Trends Plant Sci. 2019, 24, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D.; Black, M. Seeds; Springer: Boston, MA, USA, 1994; pp. 1–33. Available online: https://link.springer.com/chapter/10.1007/978-1-4899-1002-8_1 (accessed on 15 October 2021).
- Planes, M.D.; Niñoles, R.; Rubio, L.; Bissoli, G.; Bueso, E.; García-Sánchez, M.J.; Alejandro, S.; Gonzalez-Guzmán, M.; Hedrich, R.; Rodriguez, P.L.; et al. A Mechanism of Growth Inhibition by Abscisic Acid in Germinating Seeds of Arabidopsis thaliana Based on Inhibition of Plasma Membrane H+-ATPase and Decreased Cytosolic PH, K+, and Anions. J. Exp. Bot. 2015, 66, 813–825. [Google Scholar] [CrossRef] [Green Version]
- Penfield, S. Seed Dormancy and Germination. Curr. Biol. 2017, 27, R874–R878. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Duermeyer, L.; Leoveanu, C.; Nambara, E. The Functions of the Endosperm During Seed Germination. Plant Cell Physiol. 2014, 55, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- de Souza Vidigal, D.; He, H.; Hilhorst, H.W.M.; Willems, L.A.J.; Bentsink, L. Arabidopsis in the Wild—The Effect of Seasons on Seed Performance. Plants 2020, 9, 576. [Google Scholar] [CrossRef]
- Matilla, A.J. Seed Dormancy: Molecular Control of Its Induction and Alleviation. Plants 2020, 9, 1402. [Google Scholar] [CrossRef]
- Roberto Huarte, H.; Luna, V.; Pagano, E.A.; Zavala, J.A.; Benech-Arnold, R.L. Fluctuating Temperatures Terminate Dormancy in Cynara cardunculus Seeds by Turning off ABA Synthesis and Reducing ABA Signalling, but Not Stimulating GA Synthesis or Signalling. Seed Sci. Res. 2014, 24, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Grass, L.; Burris, J.S. Effect of Heat Stress during Seed Development and Maturation on Wheat (Triticum durum) Seed Quality. I. Seed Germination and Seedling Vigor. Can. J. Plant Sci. 1995, 75, 821–829. [Google Scholar] [CrossRef] [Green Version]
- Domergue, J.; Abadie, C.; Limami, A.; Way, D.; Tcherkez, G. Seed Quality and Carbon Primary Metabolism. Plant Cell Environ. 2019, 42, 2776–2788. [Google Scholar] [CrossRef] [Green Version]
- Duermeyer, L.; Khodapanahi, E.; Yan, D.; Krapp, A.; Rothstein, S.J.; Nambara, E. Regulation of Seed Dormancy and Germination by Nitrate. Seed Sci. Res. 2018, 28, 150–157. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. The Control of Seed Dormancy and Germination by Temperature, Light and Nitrate. Bot. Rev. 2020, 86, 39–75. [Google Scholar] [CrossRef]
- Footitt, S.; Huang, Z.; Clay, H.A.; Mead, A.; Finch-Savage, W.E. Temperature, Light and Nitrate Sensing Coordinate Arabidopsis Seed Dormancy Cycling, Resulting in Winter and Summer Annual Phenotypes. Plant J. 2013, 74, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Cadman, C.S.C.; Toorop, P.E.; Lynn, J.R.; Hilhorst, H.W.M. Seed Dormancy Release in Arabidopsis Cvi by Dry After-Ripening, Low Temperature, Nitrate and Light Shows Common Quantitative Patterns of Gene Expression Directed by Environmentally Specific Sensing. Plant J. Cell Mol. Biol. 2007, 51, 60–78. [Google Scholar] [CrossRef]
- Footitt, S.; Douterelo-Soler, I.; Clay, H.; Finch-Savage, W.E. Dormancy Cycling in Arabidopsis Seeds Is Controlled by Seasonally Distinct Hormone-Signaling Pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 20236–20241. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Easwaran, V.; Chau, V.; Okamoto, M.; Ierullo, M.; Kimura, M.; Endo, A.; Yano, R.; Pasha, A.; Gong, Y.; et al. NIN-like Protein 8 Is a Master Regulator of Nitrate-Promoted Seed Germination in Arabidopsis. Nat. Commun. 2016, 7, 13179. [Google Scholar] [CrossRef]
- Delgado-Sánchez, P.; Ortega-Amaro, M.A.; Jiménez-Bremont, J.F.; Flores, J. Are Fungi Important for Breaking Seed Dormancy in Desert Species? Experimental Evidence in Opuntia streptacantha (Cactaceae): Fungi Break Seed Dormancy in Opuntia. Plant Biol. 2011, 13, 154–159. [Google Scholar] [CrossRef]
- Long, R.L.; Gorecki, M.J.; Renton, M.; Scott, J.K.; Colville, L.; Goggin, D.E.; Commander, L.E.; Westcott, D.A.; Cherry, H.; Finch-Savage, W.E. The Ecophysiology of Seed Persistence: A Mechanistic View of the Journey to Germination or Demise: The Ecophysiology of Seed Persistence. Biol. Rev. 2015, 90, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.H. Seed Ageing. By D. A. Priestley. Ithaca and London: Cornell University Press (Comstock Publishing Associates) (1986), pp. 304, $41.25. Exp. Agric. 1987, 23, 227. [Google Scholar] [CrossRef]
- Zinsmeister, J.; Leprince, O.; Buitink, J. Molecular and Environmental Factors Regulating Seed Longevity. Biochem. J. 2020, 477, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Hallam, N.D.; Roberts, B.E.; Osborne, D.J. Embryogenesis and Germination in Rye (Secale Cereale L.): III. Fine Structure and Biochemistry of the Non-Viable Embryo. Planta 1973, 110, 279–290. [Google Scholar] [CrossRef]
- Roberts, B.E.; Payne, P.I.; Osborne, D.J. Protein Synthesis and the Viability of Rye Grains. Loss of Activity of Protein-Synthesizing Systems in Vitro Associated with a Loss of Viability. Biochem. J. 1973, 131, 275–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benamar, A.; Tallon, C.; Macherel, D. Membrane Integrity and Oxidative Properties of Mitochondria Isolated from Imbibing Pea Seeds after Priming or Accelerated Ageing. Seed Sci. Res. 2003, 13, 35–45. [Google Scholar] [CrossRef]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From Intracellular Signaling Networks to Cell Death: The Dual Role of Reactive Oxygen Species in Seed Physiology. Comptes Rendus Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Corbineau, F.; Gay-Mathieu, C.; Vinel, D.; Côme, D. Decrease in Sunflower (Helianthus annuus) Seed Viability Caused by High Temperature as Related to Energy Metabolism, Membrane Damage and Lipid Composition. Physiol. Plant. 2002, 116, 489–496. [Google Scholar] [CrossRef]
- Kibinza, S.; Bazin, J.; Bailly, C.; Farrant, J.M.; Corbineau, F.; El-Maarouf-Bouteau, H. Catalase Is a Key Enzyme in Seed Recovery from Ageing during Priming. Plant Sci. 2011, 181, 309–315. [Google Scholar] [CrossRef]
- Waterworth, W.M.; Bray, C.M.; West, C.E. The Importance of Safeguarding Genome Integrity in Germination and Seed Longevity. J. Exp. Bot. 2015, 66, 3549–3558. [Google Scholar] [CrossRef] [Green Version]
- El-Maarouf-Bouteau, H.; Mazuy, C.; Corbineau, F.; Bailly, C. DNA Alteration and Programmed Cell Death during Ageing of Sunflower Seed. J. Exp. Bot. 2011, 62, 5003–5011. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Osuna, D.; Colville, L.; Lorenzo, O.; Graeber, K.; Küster, H.; Leubner-Metzger, G.; Kranner, I. Transcriptome-Wide Mapping of Pea Seed Ageing Reveals a Pivotal Role for Genes Related to Oxidative Stress and Programmed Cell Death. PLoS ONE 2013, 8, e78471. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, Y.; Xue, H.; Pritchard, H.W.; Wang, X. Reactive Oxygen Species-Provoked Mitochondria-Dependent Cell Death during Ageing of Elm (Ulmus pumila L.) Seeds. Plant J. 2015, 81, 438–452. [Google Scholar] [CrossRef]
- Fleming, M.B.; Richards, C.M.; Walters, C. Decline in RNA Integrity of Dry-Stored Soybean Seeds Correlates with Loss of Germination Potential. J. Exp. Bot. 2017, 68, 2219–2230. [Google Scholar] [CrossRef] [Green Version]
- Fleming, M.B.; Patterson, E.L.; Reeves, P.A.; Richards, C.M.; Gaines, T.A.; Walters, C. Exploring the Fate of MRNA in Aging Seeds: Protection, Destruction, or Slow Decay? J. Exp. Bot. 2018, 69, 4309–4321. [Google Scholar] [CrossRef]
- Kranner, I.; Birtić, S.; Anderson, K.M.; Pritchard, H.W. Glutathione Half-Cell Reduction Potential: A Universal Stress Marker and Modulator of Programmed Cell Death? Free Radic. Biol. Med. 2006, 40, 2155–2165. [Google Scholar] [CrossRef]
- Clerkx, E.J.M.; El-Lithy, M.E.; Vierling, E.; Ruys, G.J.; Blankestijn-De Vries, H.; Groot, S.P.C.; Vreugdenhil, D.; Koornneef, M. Analysis of Natural Allelic Variation of Arabidopsis Seed Germination and Seed Longevity Traits between the Accessions Landsberg Erecta and Shakdara, Using a New Recombinant Inbred Line Population. Plant Physiol. 2004, 135, 432–443. [Google Scholar] [CrossRef] [Green Version]
- Long, R.L.; Kranner, I.; Panetta, F.D.; Birtic, S.; Adkins, S.W.; Steadman, K.J. Wet-Dry Cycling Extends Seed Persistence by Re-Instating Antioxidant Capacity. Plant Soil 2011, 338, 511–519. [Google Scholar] [CrossRef]
- Kibinza, S.; Vinel, D.; Côme, D.; Bailly, C.; Corbineau, F. Sunflower Seed Deterioration as Related to Moisture Content during Ageing, Energy Metabolism and Active Oxygen Species Scavenging. Physiol. Plant. 2006, 128, 496–506. [Google Scholar] [CrossRef]
- Châtelain, E.; Satour, P.; Laugier, E.; Ly Vu, B.; Payet, N.; Rey, P.; Montrichard, F. Evidence for Participation of the Methionine Sulfoxide Reductase Repair System in Plant Seed Longevity. Proc. Natl. Acad. Sci. USA 2013, 110, 3633–3638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogé, L.; Bourdais, G.; Bove, J.; Collet, B.; Godin, B.; Granier, F.; Boutin, J.-P.; Job, D.; Jullien, M.; Grappin, P. Protein Repair L -Isoaspartyl Methyltransferase1 Is Involved in Both Seed Longevity and Germination Vigor in Arabidopsis. Plant Cell 2008, 20, 3022–3037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed Priming: State of the Art and New Perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Lutts, S.; Benincasa, P.; Wojtyla, L.; Kubala, S.; Pace, R.; Lechowska, K.; Quinet, M.; Garnczarska, M. Seed Priming: New Comprehensive Approaches for an Old Empirical Technique. In New Challenges in Seed Biology—Basic and Translational Research Driving Seed Technology; Araujo, S., Balestrazzi, A., Eds.; InTech Open Book Series; InTech: Rijeka, Croatia, 2016; pp. 1–46. ISBN 978-953-51-2658-4. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Maarouf-Bouteau, H. The Seed and the Metabolism Regulation. Biology 2022, 11, 168. https://doi.org/10.3390/biology11020168
El-Maarouf-Bouteau H. The Seed and the Metabolism Regulation. Biology. 2022; 11(2):168. https://doi.org/10.3390/biology11020168
Chicago/Turabian StyleEl-Maarouf-Bouteau, Hayat. 2022. "The Seed and the Metabolism Regulation" Biology 11, no. 2: 168. https://doi.org/10.3390/biology11020168
APA StyleEl-Maarouf-Bouteau, H. (2022). The Seed and the Metabolism Regulation. Biology, 11(2), 168. https://doi.org/10.3390/biology11020168




