Retinoic Acid-Differentiated Neuroblastoma SH-SY5Y Is an Accessible In Vitro Model to Study Native Human Acid-Sensing Ion Channels 1a (ASIC1a)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Ligands
2.2. Cell Culture
2.3. RNA Extraction and cDNA Synthesis
2.4. Quantitative PCR
2.5. Electrophysiology
2.6. Data and Statistical Analysis
3. Results
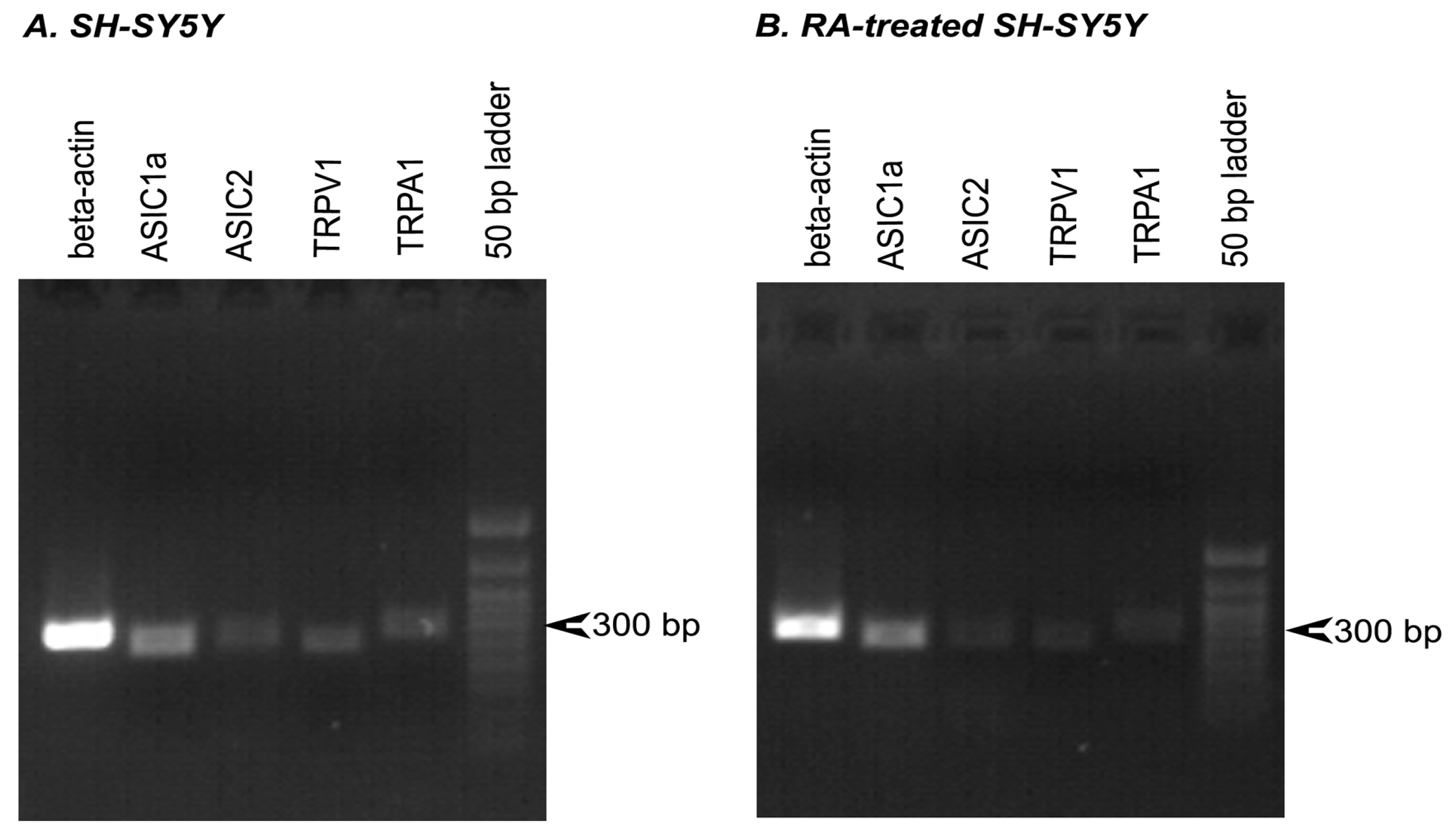
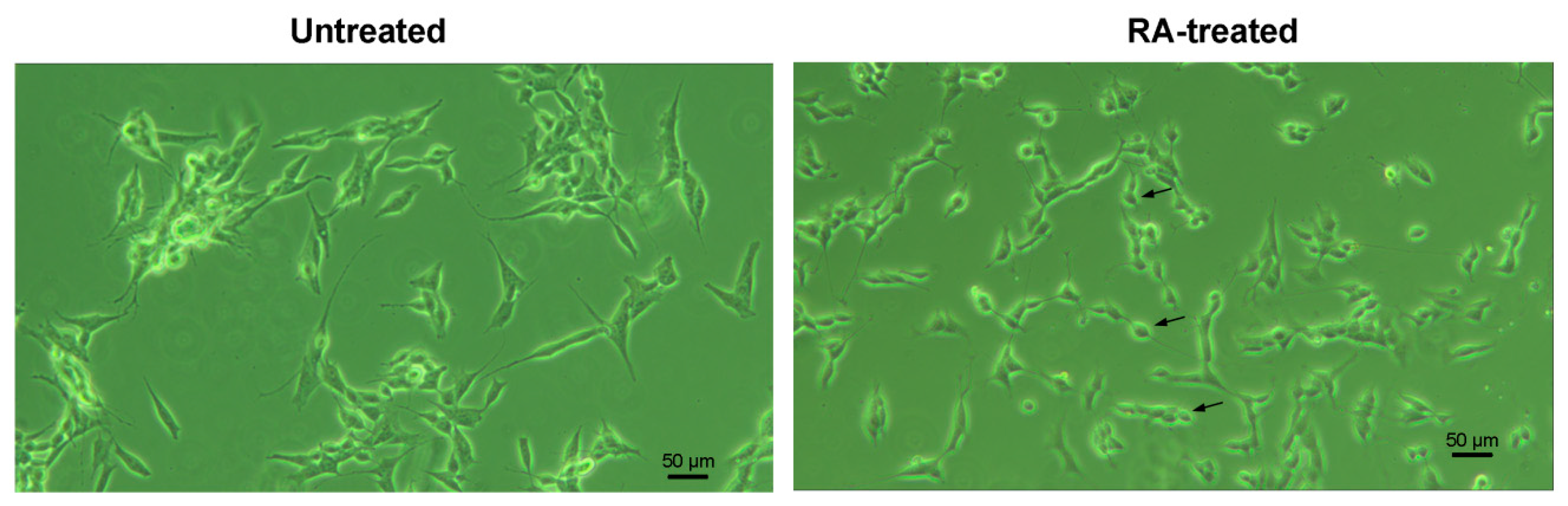
3.1. Quantitative mRNA Difference in Untreated and RA-Treated SH-SY5Y Cells
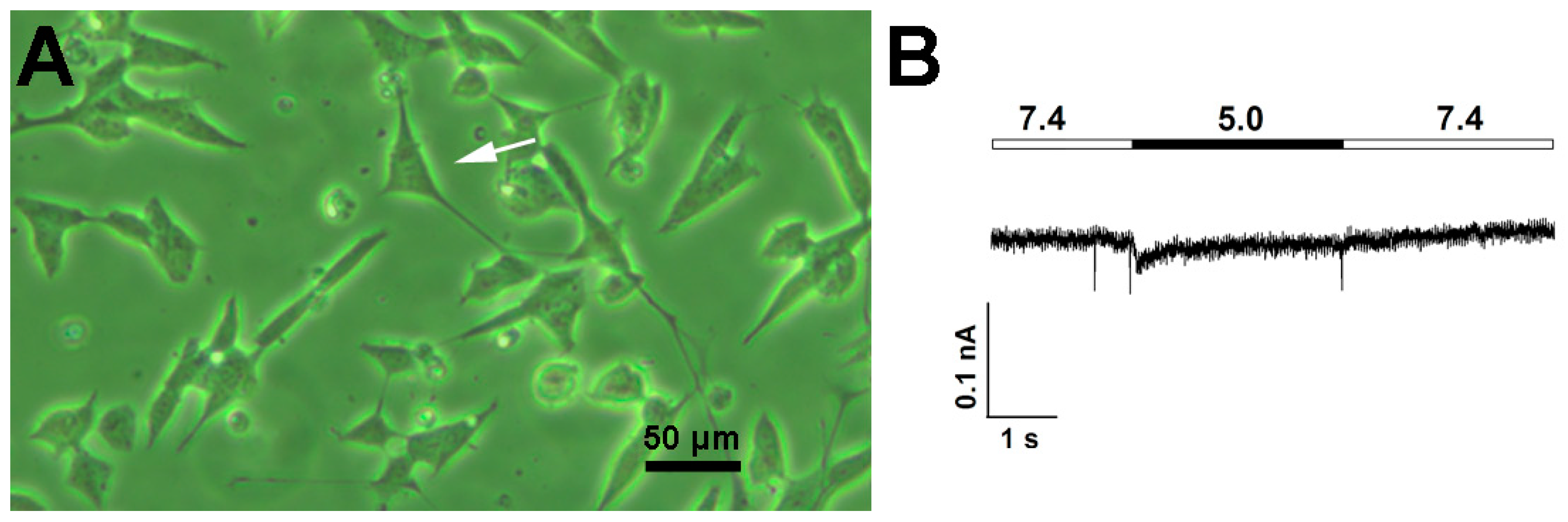
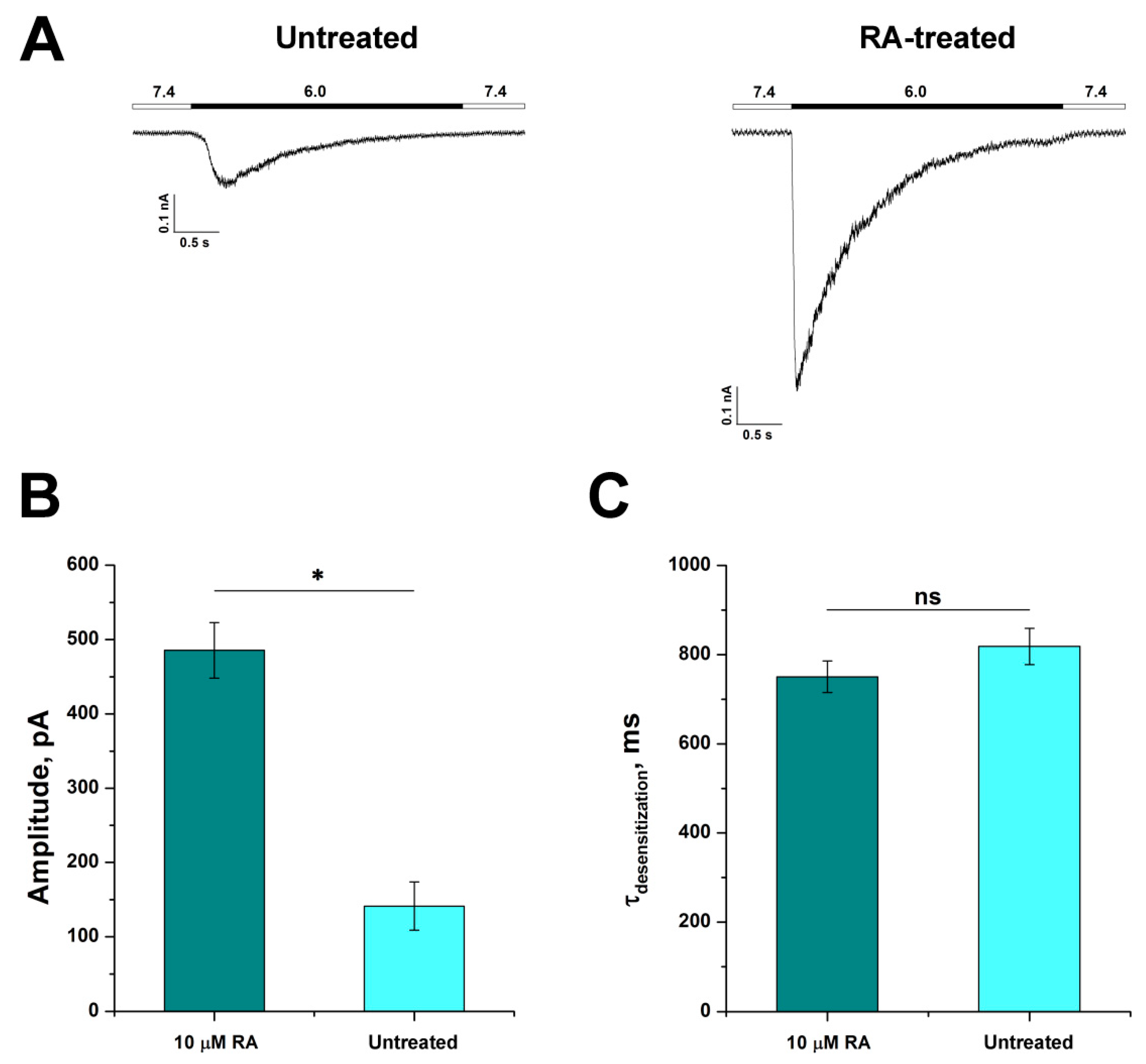
3.2. Comparison of Acid-Induced Currents in Untreated and RA-Treated SH-SY5Y Cells
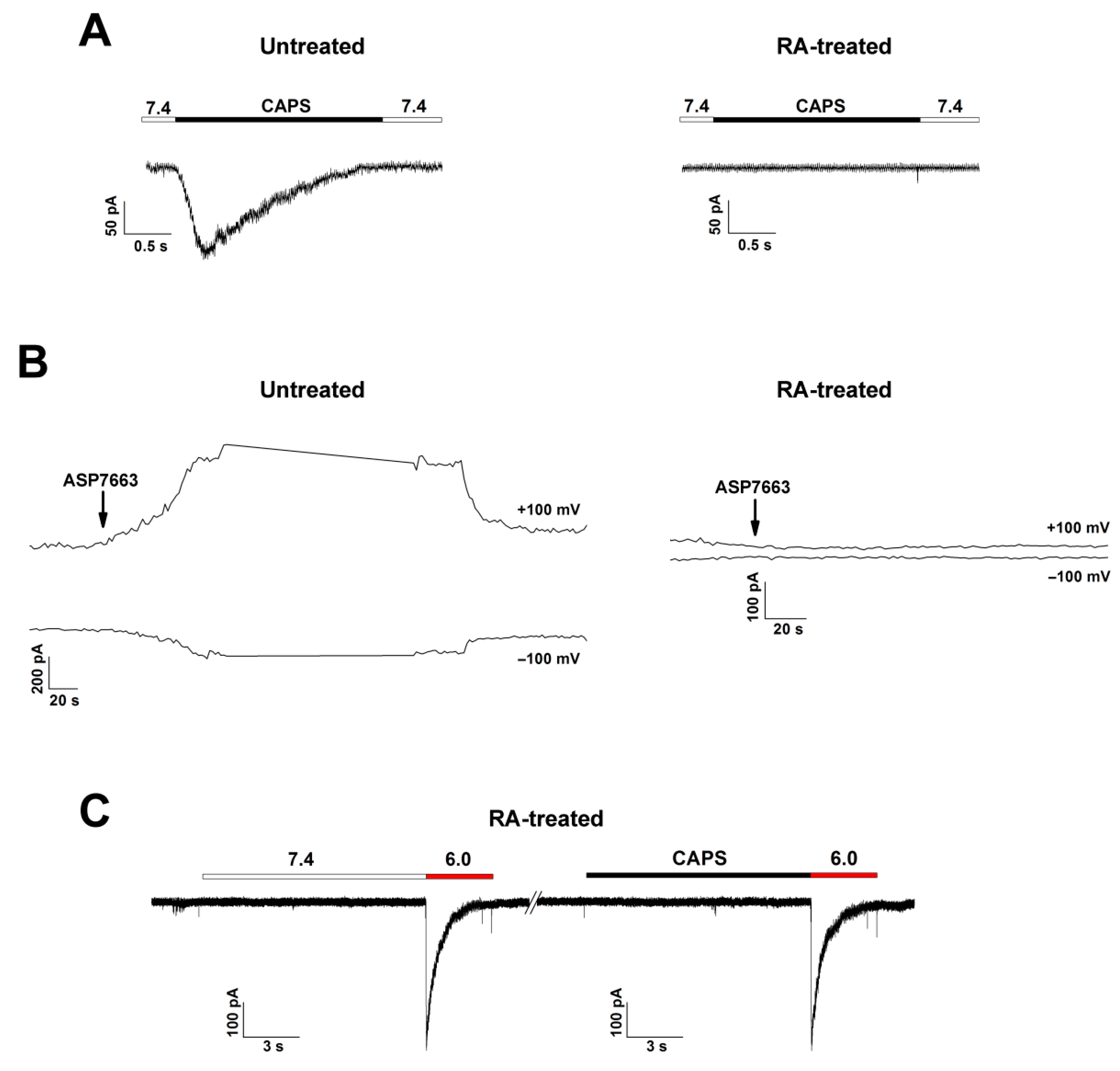
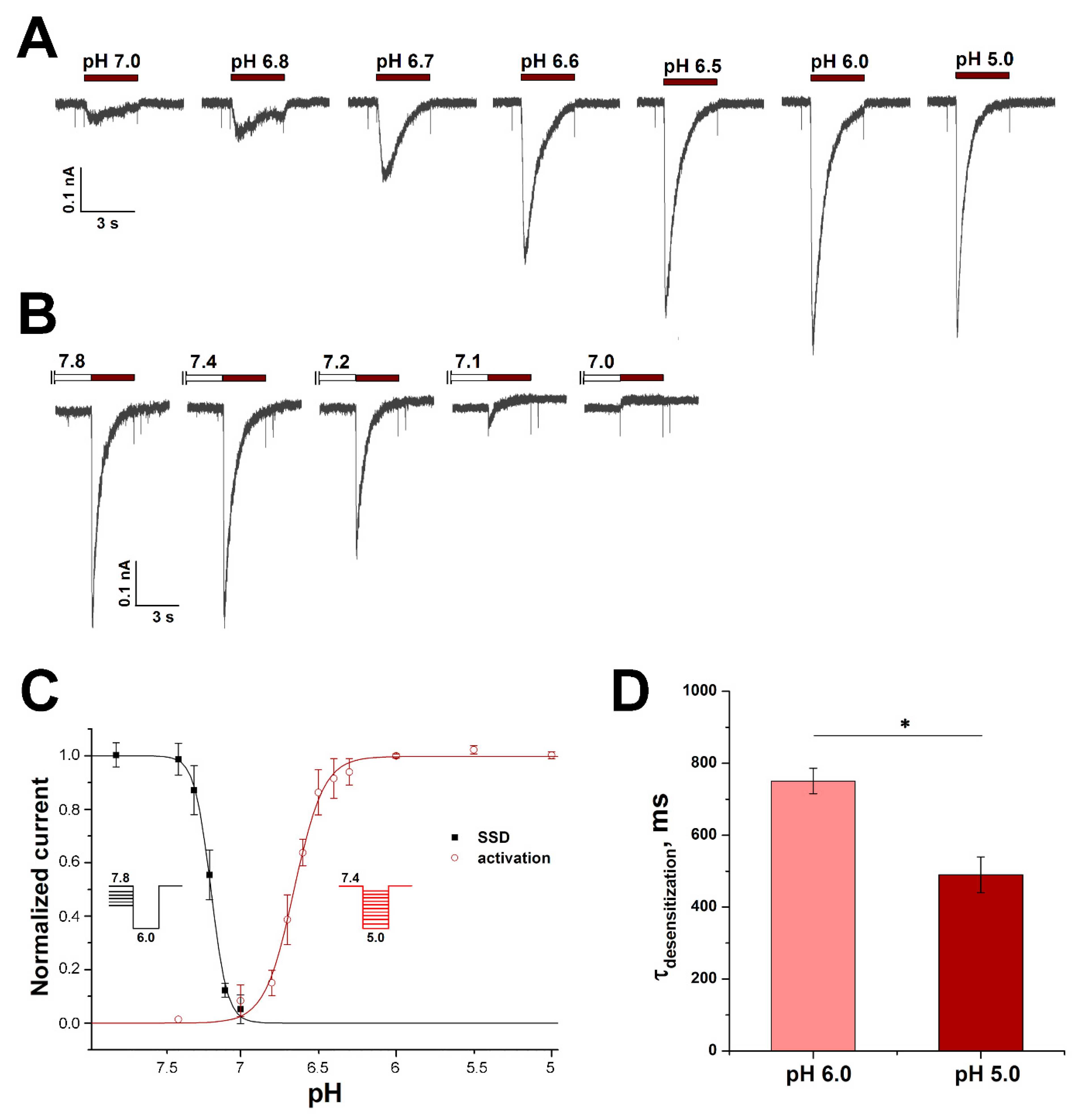
3.3. Characterization of ASIC Currents in RA-Treated SH-SY5Y Cells
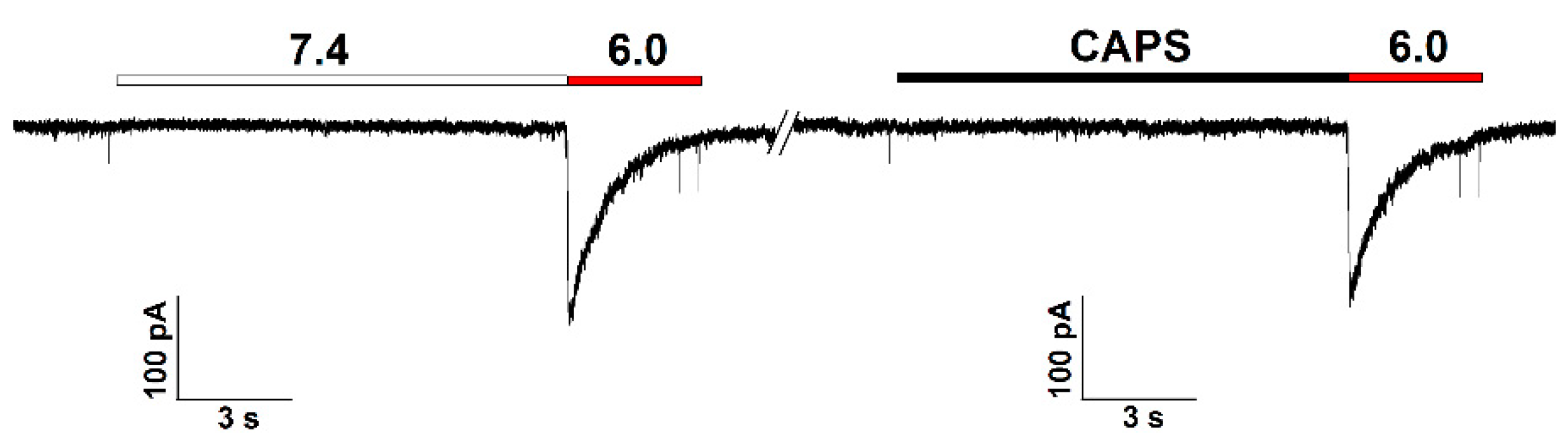
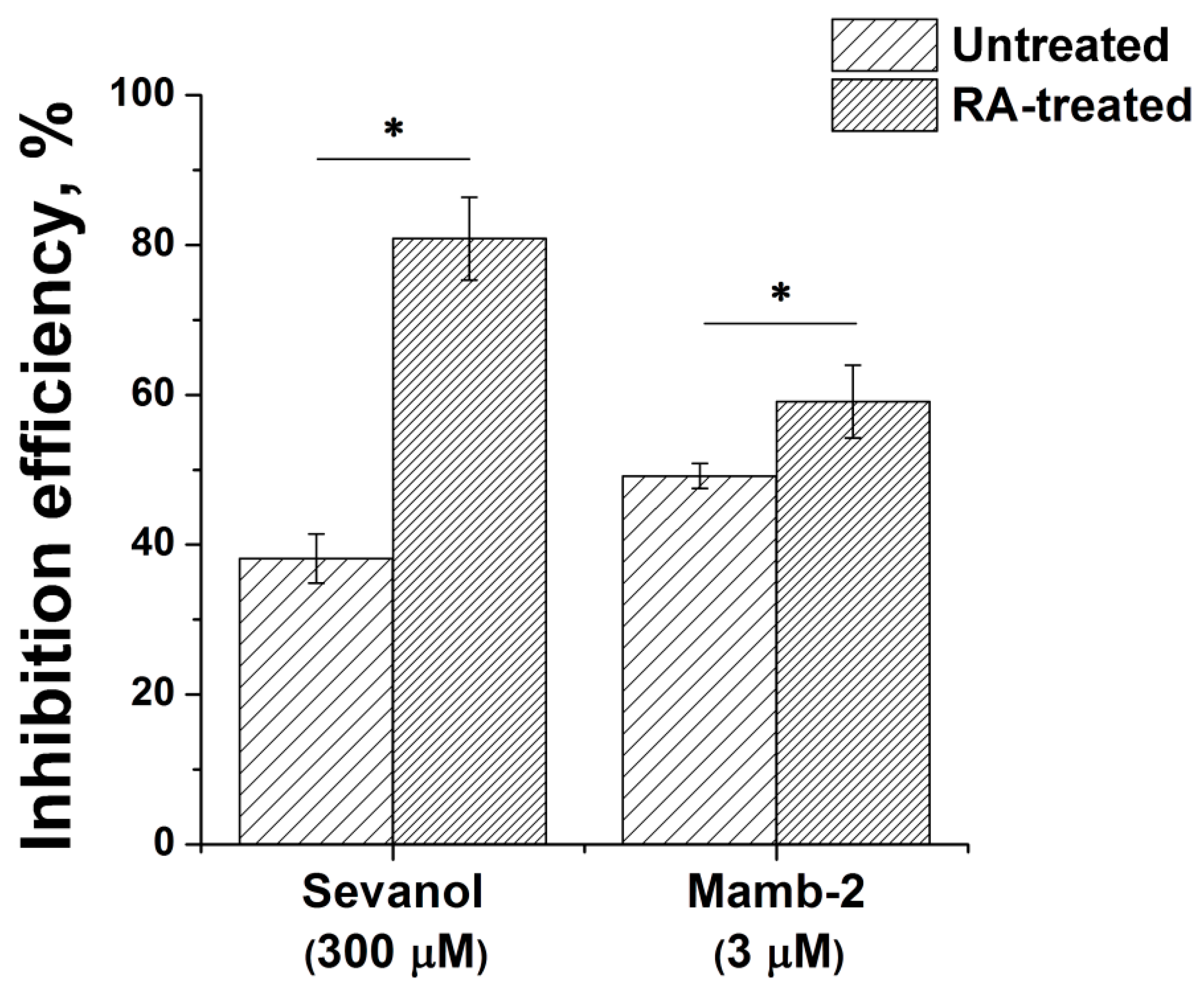
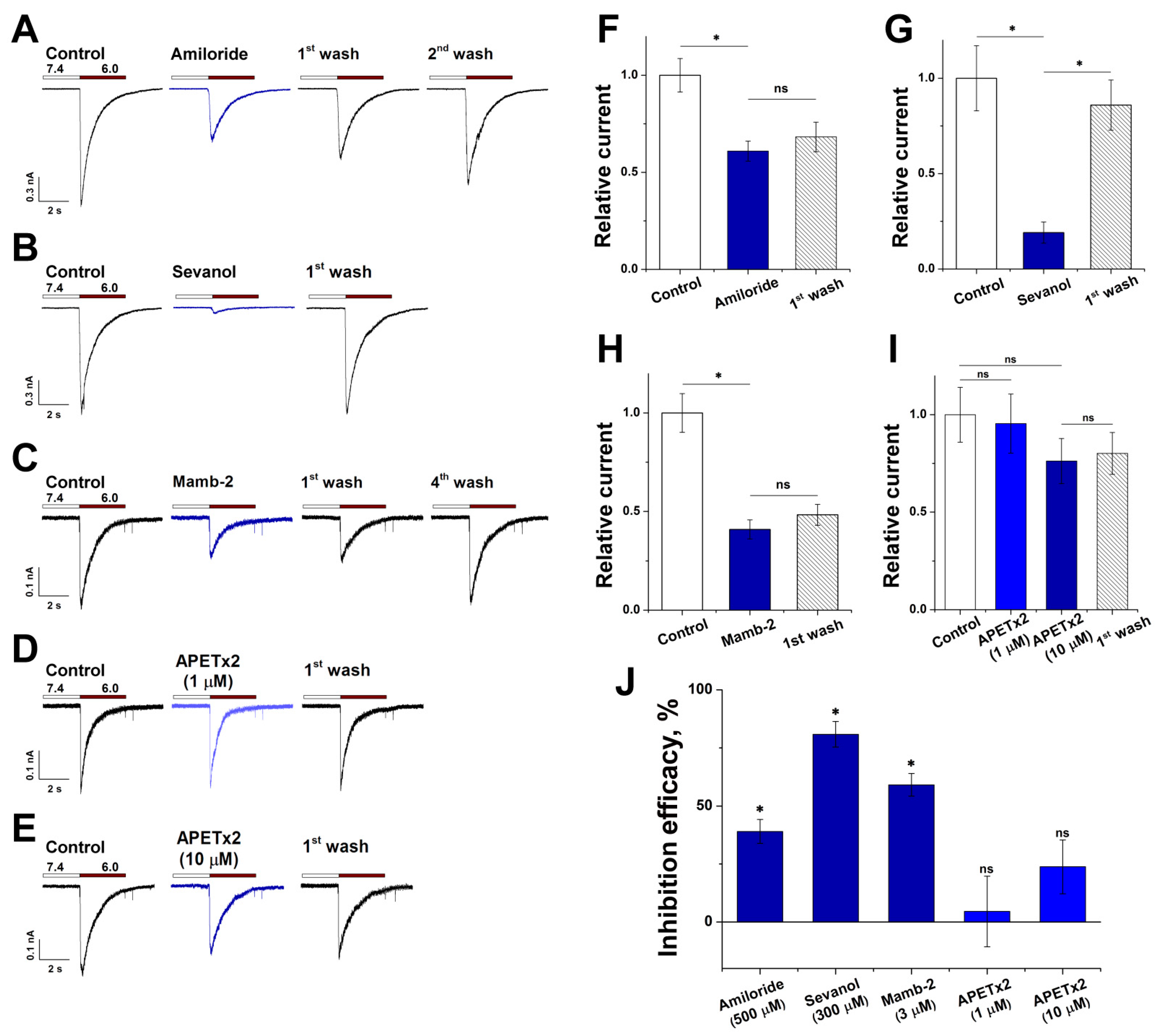
3.4. Pharmacological Characteristics of ASIC1a in RA-Treated SH-SY5Y Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Agholme, L.; Lindström, T.; Kågedal, K.; Marcusson, J.; Hallbeck, M.; Kgedal, K.; Marcusson, J.; Hallbeck, M. An in vitro model for neuroscience: Differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J. Alzheimer’s Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef]
- Liguori, F.; Amadio, S.; Volonté, C. Where and why modeling amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2021, 22, 3977. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Eaton, E.D.; Wills, T.E.; McCann, S.K.; Antonic, A.; Howells, D.W. Human ischaemic cascade studies using SH-SY5Y cells: A systematic review and meta-analysis. Transl. Stroke Res. 2018, 9, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.T.; Lau, W.K.W.; Yu, M.S.; Lai, C.S.W.; Yeung, S.C.; So, K.F.; Chang, R.C.C. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 2009, 30, 127–135. [Google Scholar] [CrossRef]
- Shipley, M.M.; Mangold, C.A.; Kuny, C.V.; Szpara, M.L. Differentiated human SH-SY5Y cells provide a reductionist model of herpes simplex virus 1 neurotropism. J. Virol. 2017, 91, e00958-17. [Google Scholar] [CrossRef]
- Luplertlop, N.; Suwanmanee, S.; Muangkaew, W.; Ampawong, S.; Kitisin, T.; Poovorawan, Y. The impact of zika virus infection on human neuroblastoma (Sh-SY5Y) cell line. J. Vector Borne Dis. 2017, 54, 207–214. [Google Scholar] [CrossRef]
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol. 2013, 1078, 9–21. [Google Scholar] [CrossRef]
- Toselli, M.; Tosetti, P.; Taglietti, V. Functional changes in sodium conductances in the human neuroblastoma cell line SH-SY5Y during in vitro differentiation. J. Neurophysiol. 1996, 76, 3920–3927. [Google Scholar] [CrossRef]
- Liu, M.; Inoue, K.; Leng, T.; Zhou, A.; Guo, S.; Xiong, Z.G. ASIC1 promotes differentiation of neuroblastoma by negatively regulating Notch signaling pathway. Oncotarget 2017, 8, 8283–8293. [Google Scholar] [CrossRef]
- O’Bryant, Z.; Leng, T.; Liu, M.; Inoue, K.; Vann, K.T.; Xiong, Z.G. Acid sensing ion channels (ASICs) in NS20Y cells—Potential role in neuronal differentiation. Mol. Brain 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Neuhof, A.; Tian, Y.; Reska, A.; Falkenburger, B.H.; Gründer, S. Large acid-evoked currents, mediated by ASIC1a, accompany differentiation in human dopaminergic neurons. Front. Cell. Neurosci. 2021, 15, 141. [Google Scholar] [CrossRef]
- Wemmie, J.A.; Taugher, R.J.; Kreple, C.J. Acid-sensing ion channels in pain and disease. Nat. Rev. Neurosci. 2013, 14, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Osmakov, D.I.; Khasanov, T.A.; Andreev, Y.A.; Lyukmanova, E.N.; Kozlov, S.A. Animal, herb, and microbial toxins for structural and pharmacological study of acid-sensing ion channels. Front. Pharmacol. 2020, 11, 1. [Google Scholar]
- Kweon, H.J.; Suh, B.C. Acid-sensing ion channels (ASICs): Therapeutic targets for neurological diseases and their regulation. BMB Rep. 2013, 46, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Vullo, S.; Kellenberger, S. A molecular view of the function and pharmacology of acid-sensing ion channels. Pharmacol. Res. 2020, 154, 104166. [Google Scholar] [CrossRef]
- Bychkov, M.; Shulepko, M.; Osmakov, D.; Andreev, Y.; Sudarikova, A.; Vasileva, V.; Pavlyukov, M.S.; Latyshev, Y.A.; Potapov, A.A.; Kirpichnikov, M.; et al. Mambalgin-2 induces cell cycle arrest and apoptosis in glioma cells via interaction with ASIC1a. Cancers. 2020, 12, 1837. [Google Scholar] [CrossRef]
- Belozerova, O.A.; Osmakov, D.I.; Vladimirov, A.; Koshelev, S.G.; Chugunov, A.O.; Andreev, Y.A.; Palikov, V.A.; Palikova, Y.A.; Shaykhutdinova, E.R.; Gvozd, A.N.; et al. Sevanol and its analogues: Chemical synthesis, biological effects and molecular docking. Pharmaceuticals 2020, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Logashina, Y.A.; Korolkova, Y.V.; Maleeva, E.E.; Osmakov, D.I.; Kozlov, S.A.; Andreev, Y.A. Refolding of disulfide containing peptides in fusion with thioredoxin. Mendeleev Commun. 2020, 30, 214–216. [Google Scholar] [CrossRef]
- Hoagland, E.N.; Sherwood, T.W.; Lee, K.G.; Walker, C.J.; Askwith, C.C. Identification of a calcium permeable human acid-sensing ion channel 1 transcript variant. J. Biol. Chem. 2010, 285, 41852–41862. [Google Scholar] [CrossRef]
- Dwane, S.; Durack, E.; Kiely, P.A. Optimising parameters for the differentiation of SH-SY5Y cells to study cell adhesion and cell migration. BMC Res. Notes 2013, 6, 366. [Google Scholar] [CrossRef] [PubMed]
- Korecka, J.A.; van Kesteren, R.E.; Blaas, E.; Spitzer, S.O.; Kamstra, J.H.; Smit, A.B.; Swaab, D.F.; Verhaagen, J.; Bossers, K. Phenotypic characterization of retinoic acid differentiated SH-SY5Y cells by transcriptional profiling. PLoS ONE 2013, 8, e63862. [Google Scholar] [CrossRef] [PubMed]
- Jahn, K.; Wieltsch, C.; Blumer, N.; Mehlich, M.; Pathak, H.; Khan, A.Q.; Hildebrandt, H.; Frieling, H. A cell culture model for investigation of synapse influenceability: Epigenetics, expression and function of gene targets important for synapse formation and preservation in SH-SY5Y neuroblastoma cells differentiated by retinoic acid. J. Neural Transm. 2017, 124, 1341–1367. [Google Scholar] [CrossRef]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef]
- Kim, S.; Kang, C.; Chan, Y.S.; Sun, W.H.; Young, D.Y.; Won, S.S.; Park, M.Y.; Kim, E.; Kim, M.; Kim, B.M.; et al. TRPV1 recapitulates native capsaicin receptor in sensory neurons in association with Fas-associated factor 1. J. Neurosci. 2006, 26, 2403–2412. [Google Scholar] [CrossRef]
- Kojima, R.; Nozawa, K.; Doihara, H.; Keto, Y.; Kaku, H.; Yokoyama, T.; Itou, H. Effects of novel TRPA1 receptor agonist ASP7663 in models of drug-induced constipation and visceral pain. Eur. J. Pharmacol. 2014, 723, 288–293. [Google Scholar] [CrossRef]
- de la Roche, J.; Eberhardt, M.J.; Klinger, A.B.; Stanslowsky, N.; Wegner, F.; Koppert, W.; Reeh, P.W.; Lampert, A.; Fischer, M.J.M.; Leffler, A. The molecular basis for species-specific activation of human TRPA1 protein by protons involves poorly conserved residues within transmembrane domains 5 and 6. J. Biol. Chem. 2013, 288, 20280–20292. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Chen, J.; Harding, A.M.S.; Price, M.P.; Lu, Y.; Abboud, F.M.; Benson, C.J. ASIC2a and ASIC3 heteromultimerize to Form pH-Sensitive channels in mouse cardiac dorsal root ganglia neurons. Circ. Res. 2009, 105, 279–286. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, Y.-Q.; Li, C.; He, M.; Rusyniak, W.G.; Annamdevula, N.; Ochoa, J.; Leavesley, S.J.; Xu, J.; Rich, T.C.; et al. Human ASIC1a mediates stronger acid-induced responses as compared with mouse ASIC1a. FASEB J. 2018, 32, 3832–3843. [Google Scholar] [CrossRef]
- Vullo, S.; Bonifacio, G.; Roy, S.; Johner, N.; Bernèche, S.; Kellenberger, S. Conformational dynamics and role of the acidic pocket in ASIC pH-dependent gating. Proc. Natl. Acad. Sci. USA 2017, 114, 3768–3773. [Google Scholar] [CrossRef]
- Vaithia, A.; Vullo, S.; Peng, Z.; Alijevic, O.; Kellenberger, S. Accelerated current decay kinetics of a rare human acid-sensing ion channel 1a variant that is used in many studies as wild type. Front. Mol. Neurosci. 2019, 12, 133. [Google Scholar] [CrossRef]
- Waldmann, R.; Champigny, G.; Bassilana, F.; Heurteaux, C.; Lazdunski, M. A proton-gated cation channel involved in acid-sensing. Nature 1997, 386, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Dubinnyi, M.A.; Osmakov, D.I.; Koshelev, S.G.; Kozlov, S.A.; Andreev, Y.A.; Zakaryan, N.A.; Dyachenko, I.A.; Bondarenko, D.A.; Arseniev, A.S.; Grishin, E.V. Lignan from thyme possesses inhibitory effect on ASIC3 channel current. J. Biol. Chem. 2012, 287, 32993–33000. [Google Scholar] [CrossRef]
- Salinas, M.; Besson, T.; Delettre, Q.; Diochot, S.; Boulakirba, S.; Douguet, D.; Lingueglia, E. Binding site and inhibitory mechanism of the mambalgin-2 pain-relieving peptide on acid-sensing ion channel 1a. J. Biol. Chem. 2014, 289, 13363–13373. [Google Scholar] [CrossRef] [PubMed]
- Diochot, S.; Baron, A.; Rash, L.D.; Deval, E.; Escoubas, P.; Scarzello, S.; Salinas, M.; Lazdunski, M. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 2004, 23, 1516–1525. [Google Scholar] [CrossRef]
- Diochot, S.; Baron, A.; Salinas, M.; Douguet, D.; Scarzello, S.; Dabert-Gay, A.S.; Debayle, D.; Friend, V.; Alloui, A.; Lazdunski, M.; et al. Black mamba venom peptides target acid-sensing ion channels to abolish pain. Nature 2012, 490, 552–555. [Google Scholar] [CrossRef]
- Kume, T.; Kawato, Y.; Osakada, F.; Izumi, Y.; Katsuki, H.; Nakagawa, T.; Kaneko, S.; Niidome, T.; Takada-Takatori, Y.; Akaike, A. Dibutyryl cyclic AMP induces differentiation of human neuroblastoma SH-SY5Y cells into a noradrenergic phenotype. Neurosci. Lett. 2008, 443, 199–203. [Google Scholar] [CrossRef]
- Påhlman, S.; Ruusala, A.I.; Abrahamsson, L.; Mattsson, M.E.K.; Esscher, T. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: A comparison with phorbolester-induced differentiation. Cell Differ. 1984, 14, 135–144. [Google Scholar] [CrossRef]
- Encinas, M.; Iglesias, M.; Liu, Y.; Wang, H.; Muhaisen, A.; Ceña, V.; Gallego, C.; Comella, J.X. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J. Neurochem. 2000, 75, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Sarkanen, J.R.; Nykky, J.; Siikanen, J.; Selinummi, J.; Ylikomi, T.; Jalonen, T.O. Cholesterol supports the retinoic acid-induced synaptic vesicle formation in differentiating human SH-SY5Y neuroblastoma cells. J. Neurochem. 2007, 102, 1941–1952. [Google Scholar] [CrossRef]
- Duitama, M.; Vargas-López, V.; Casas, Z.; Albarracin, S.L.; Sutachan, J.-J.; Torres, Y.P. TRP Channels role in pain associated with neurodegenerative diseases. Front. Neurosci. 2020, 14, 782. [Google Scholar] [CrossRef] [PubMed]
- Logashina, Y.A.; Korolkova, Y.V.; Kozlov, S.A.; Andreev, Y.A. TRPA1 channel as a regulator of neurogenic inflammation and pain: Structure, function, role in pathophysiology, and therapeutic potential of ligands. Biochemistry 2019, 84, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.M.; Wemmie, J.A.; Green, S.H.; Welsh, M.J. Acid-sensing ion channel 1a is a postsynaptic proton receptor that affects the density of dendriritic spines. Proc. Natl. Acad. Sci. USA 2006, 103, 16556–16561. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Ye, J.; Zhang, S.; Wang, K.; Su, R. Distribution of acid sensing ion channels in axonal growth cones and presynaptic membrane of cultured hippocampal neurons. Front. Cell. Neurosci. 2020, 14, 205. [Google Scholar] [CrossRef]
- Ortega-Ramírez, A.; Vega, R.; Soto, E. Acid-sensing ion channels as potential therapeutic targets in neurodegeneration and neuroinflammation. Mediat. Inflamm. 2017, 2017, 1–18. [Google Scholar] [CrossRef]
- Taugher, R.J.; Lu, Y.; Fan, R.; Ghobbeh, A.; Kreple, C.J.; Faraci, F.M.; Wemmie, J.A. ASIC1A in neurons is critical for fear-related behaviors. Genes Brain Behav. 2017, 16, 745–755. [Google Scholar] [CrossRef]
- Xiong, Q.-J.; Hu, Z.-L.; Wu, P.-F.; Ni, L.; Deng, Z.-F.; Wu, W.-N.; Chen, J.-G.; Wang, F. Acid-sensing ion channels contribute to the increase in vesicular release from SH-SY5Y cells stimulated by extracellular protons. Am. J. Physiol. Physiol. 2012, 303, C376–C384. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wen, Z.; Guirland, C.; Ming, G.L.; Zheng, J.Q. A CaMKII/calcineurin switch controls the direction of Ca(2+)-dependent growth cone guidance. Neuron 2004, 43, 835–846. [Google Scholar] [CrossRef]
- Xi, F.; Xu, R.; Xu, J.; Ma, J.; Wang, W.; Wang, F.; Ma, Y.; Qi, S.; Zhang, H.; Zhang, H.; et al. Calcium/calmodulin-dependent protein kinase II regulates mammalian axon growth by affecting F-actin length in growth cone. J. Cell. Physiol. 2019, 234, 23053–23065. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Duan, B.; Wang, D.G.; Deng, X.H.; Zhang, G.Y.; Xu, L.; Xu, T.L. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron 2005, 48, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.P.; Saez, N.J.; Cristofori-Armstrong, B.; Anangi, R.; King, G.F.; Smith, M.T.; Rash, L.D. Inhibition of acid-sensing ion channels by diminazene and APETx2 evoke partial and highly variable antihyperalgesia in a rat model of inflammatory pain. Br. J. Pharmacol. 2018, 175, 2204–2218. [Google Scholar] [CrossRef]
- Osmakov, D.I.; Koshelev, S.G.; Andreev, Y.A.; Dubinnyi, M.A.; Kublitski, V.S.; Efremov, R.G.; Sobolevsky, A.I. Kozlov Proton-independent activation of acid-sensing ion channel 3 by an alkaloid, lindoldhamine, from Laurus nobilis. Br. J. Pharmacol. 2018, 175, 924–937. [Google Scholar] [CrossRef]
- Cristofori-Armstrong, B.; Budusan, E.; Rash, L.D. Mambalgin-3 potentiates human acid-sensing ion channel 1b under mild to moderate acidosis: Implications as an analgesic lead. Proc. Natl. Acad. Sci. USA 2021, 118, e2021581118. [Google Scholar] [CrossRef] [PubMed]
- Osmakov, D.I.; Koshelev, S.G.; Andreev, Y.A.; Kozlov, S.A. Endogenous isoquinoline alkaloids agonists of acid-sensing ion channel type 3. Front. Mol. Neurosci. 2017, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Rödelsperger, K.; Woitowitz, H.J. Airborne fibre concentrations and lung burden compared to the tumour response in rats and humans exposed to asbestos. Ann. Occup. Hyg. 1995, 39, 715–725. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinovskii, A.P.; Osmakov, D.I.; Koshelev, S.G.; Lubova, K.I.; Korolkova, Y.V.; Kozlov, S.A.; Andreev, Y.A. Retinoic Acid-Differentiated Neuroblastoma SH-SY5Y Is an Accessible In Vitro Model to Study Native Human Acid-Sensing Ion Channels 1a (ASIC1a). Biology 2022, 11, 167. https://doi.org/10.3390/biology11020167
Kalinovskii AP, Osmakov DI, Koshelev SG, Lubova KI, Korolkova YV, Kozlov SA, Andreev YA. Retinoic Acid-Differentiated Neuroblastoma SH-SY5Y Is an Accessible In Vitro Model to Study Native Human Acid-Sensing Ion Channels 1a (ASIC1a). Biology. 2022; 11(2):167. https://doi.org/10.3390/biology11020167
Chicago/Turabian StyleKalinovskii, Aleksandr P., Dmitry I. Osmakov, Sergey G. Koshelev, Kseniya I. Lubova, Yuliya V. Korolkova, Sergey A. Kozlov, and Yaroslav A. Andreev. 2022. "Retinoic Acid-Differentiated Neuroblastoma SH-SY5Y Is an Accessible In Vitro Model to Study Native Human Acid-Sensing Ion Channels 1a (ASIC1a)" Biology 11, no. 2: 167. https://doi.org/10.3390/biology11020167
APA StyleKalinovskii, A. P., Osmakov, D. I., Koshelev, S. G., Lubova, K. I., Korolkova, Y. V., Kozlov, S. A., & Andreev, Y. A. (2022). Retinoic Acid-Differentiated Neuroblastoma SH-SY5Y Is an Accessible In Vitro Model to Study Native Human Acid-Sensing Ion Channels 1a (ASIC1a). Biology, 11(2), 167. https://doi.org/10.3390/biology11020167





