Toxicity and Starvation Induce Major Trophic Isotope Variation in Daphnia Individuals: A Diet Switch Experiment Using Eight Phytoplankton Species of Differing Nutritional Quality
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Phytoplankton Culturing and Preparation
2.2. Diet Switch Experimental Setup
2.3. Stable Isotope Analysis
2.4. Time-Based Stable Isotope Turnover Rates
2.5. Determination of the Best Model Fit Using AIC
2.6. Discrimination Factor (DF)
2.7. Statistical Analysis
3. Results
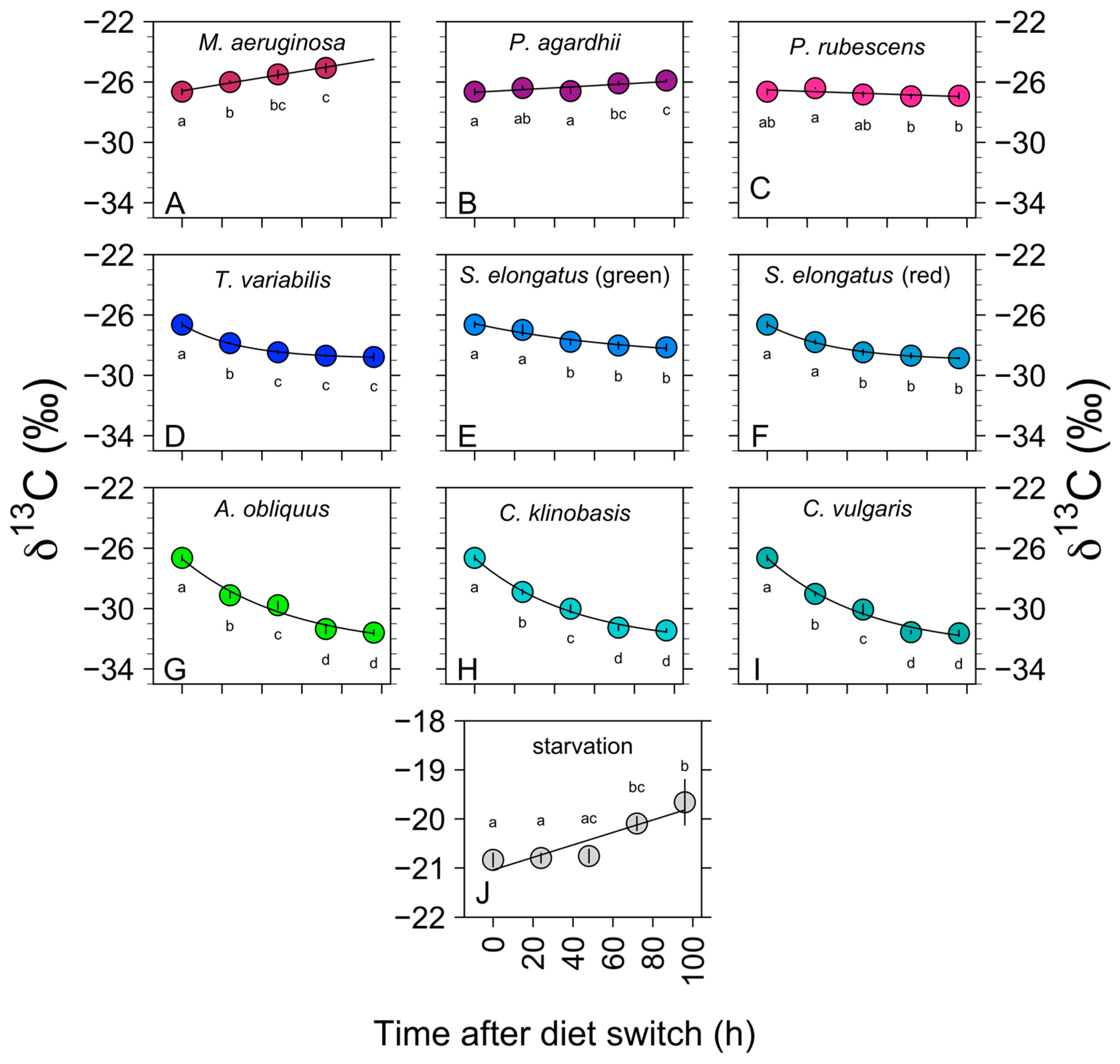
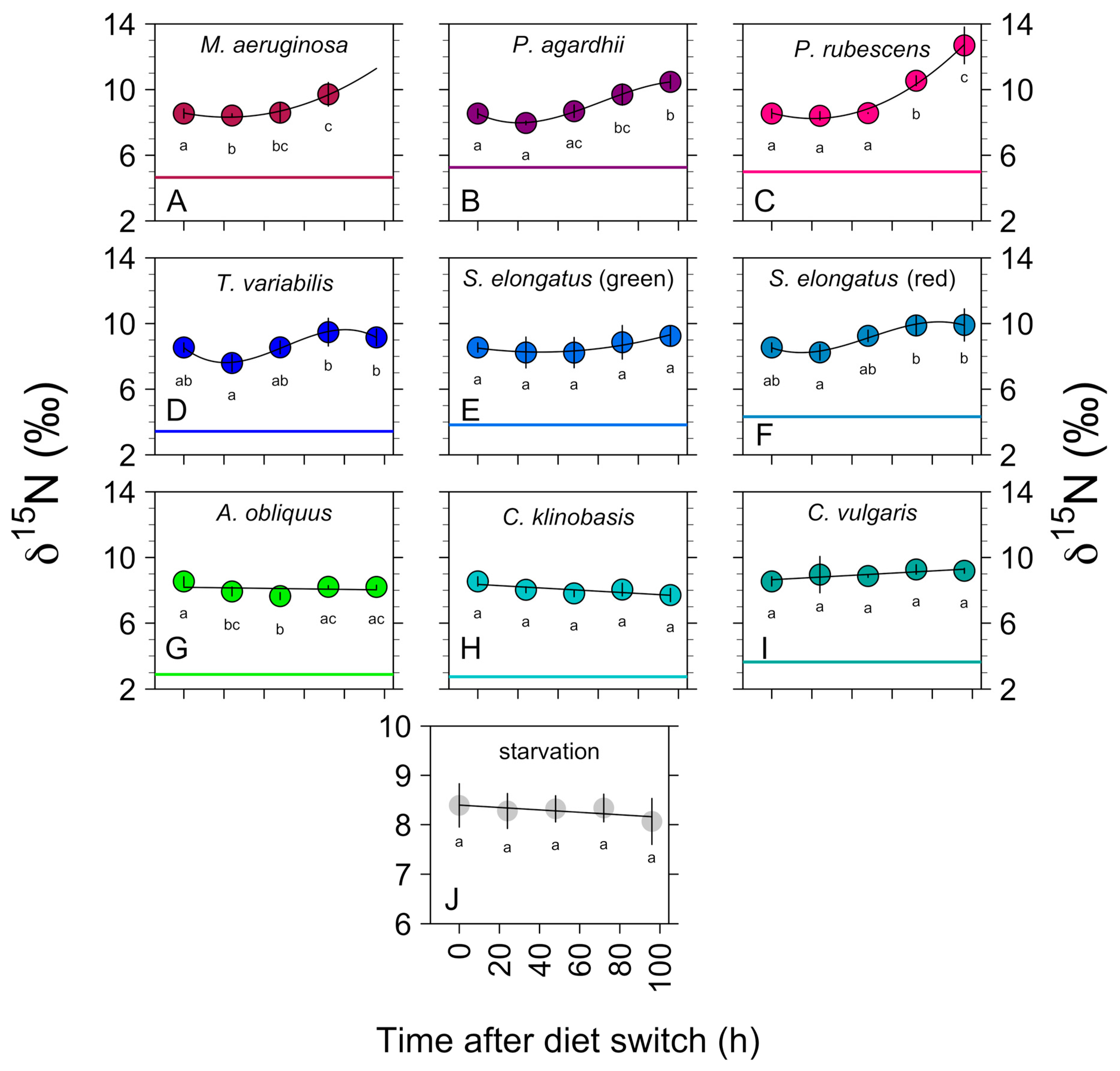
3.1. Isotopic Changes in Daphnia Tissues (δ 13C and δ 15N) after Diet Switch
3.1.1. Chlorophytes: C. klinobasis, C. vulgaris, and A. obliquus
3.1.2. Non-Toxic Cyanobacteria: S. elongatus and T. variabilis
3.1.3. Toxic Filamentous Cyanobacteria: P. rubescens and P. agardhii
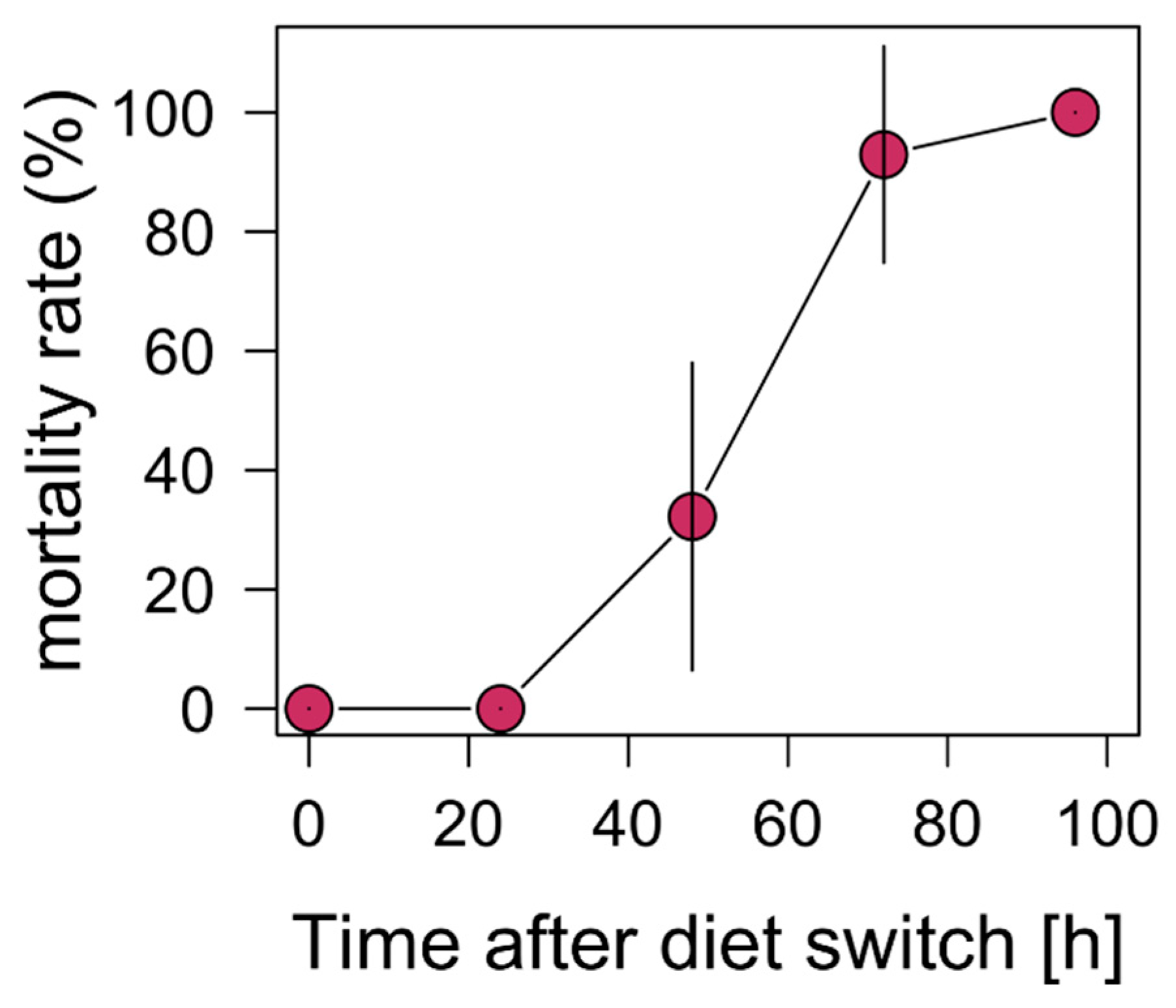
3.1.4. Toxic Unicellular Cyanobacteria and Starvation
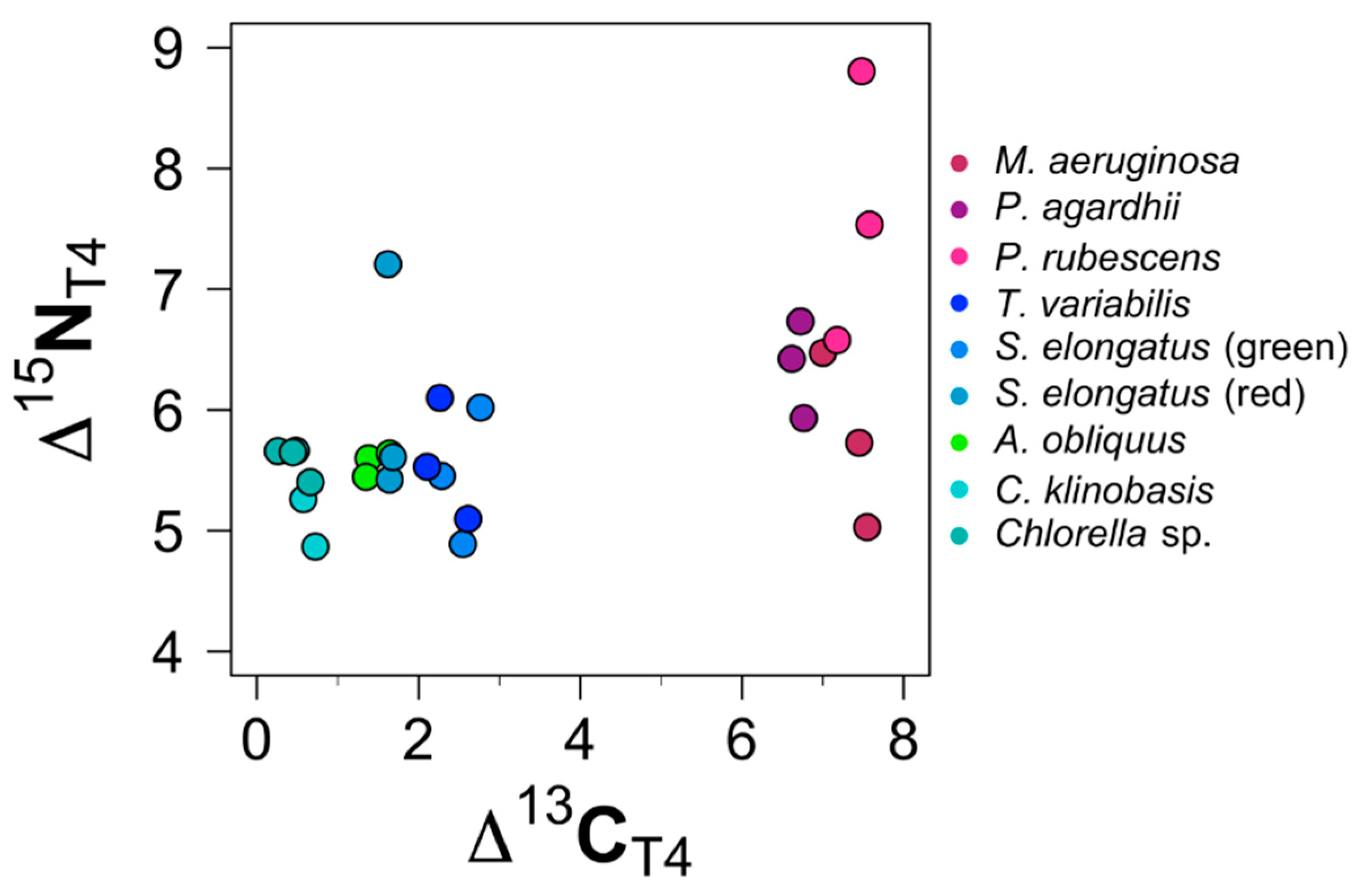
3.2. Trophic Discrimination Factors (TDFs)
3.2.1. TDF: Chlorophytes
3.2.2. TDF: Non-Toxic Cyanobacteria
3.2.3. TDF: Toxic Cyanobacterial
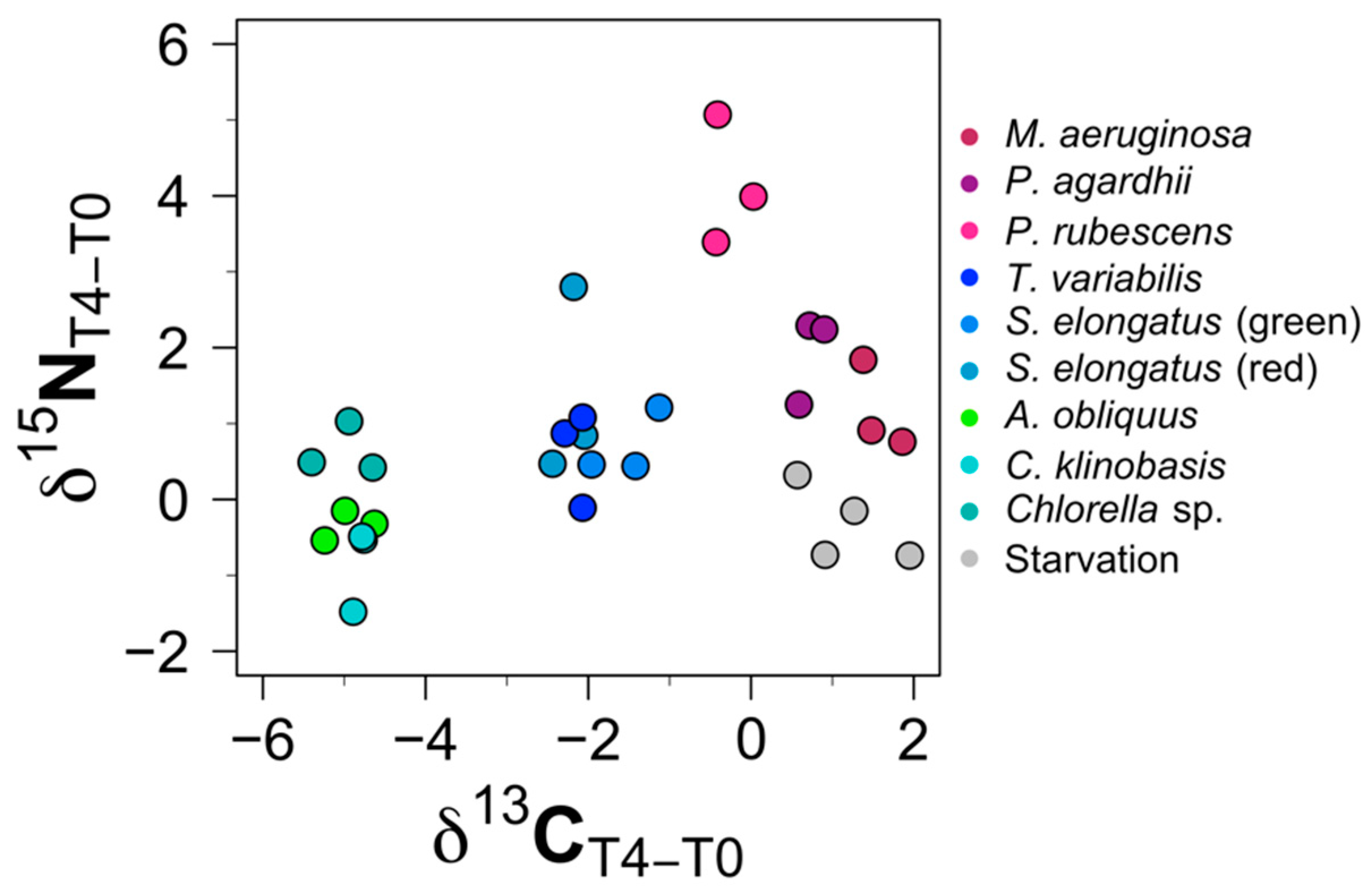
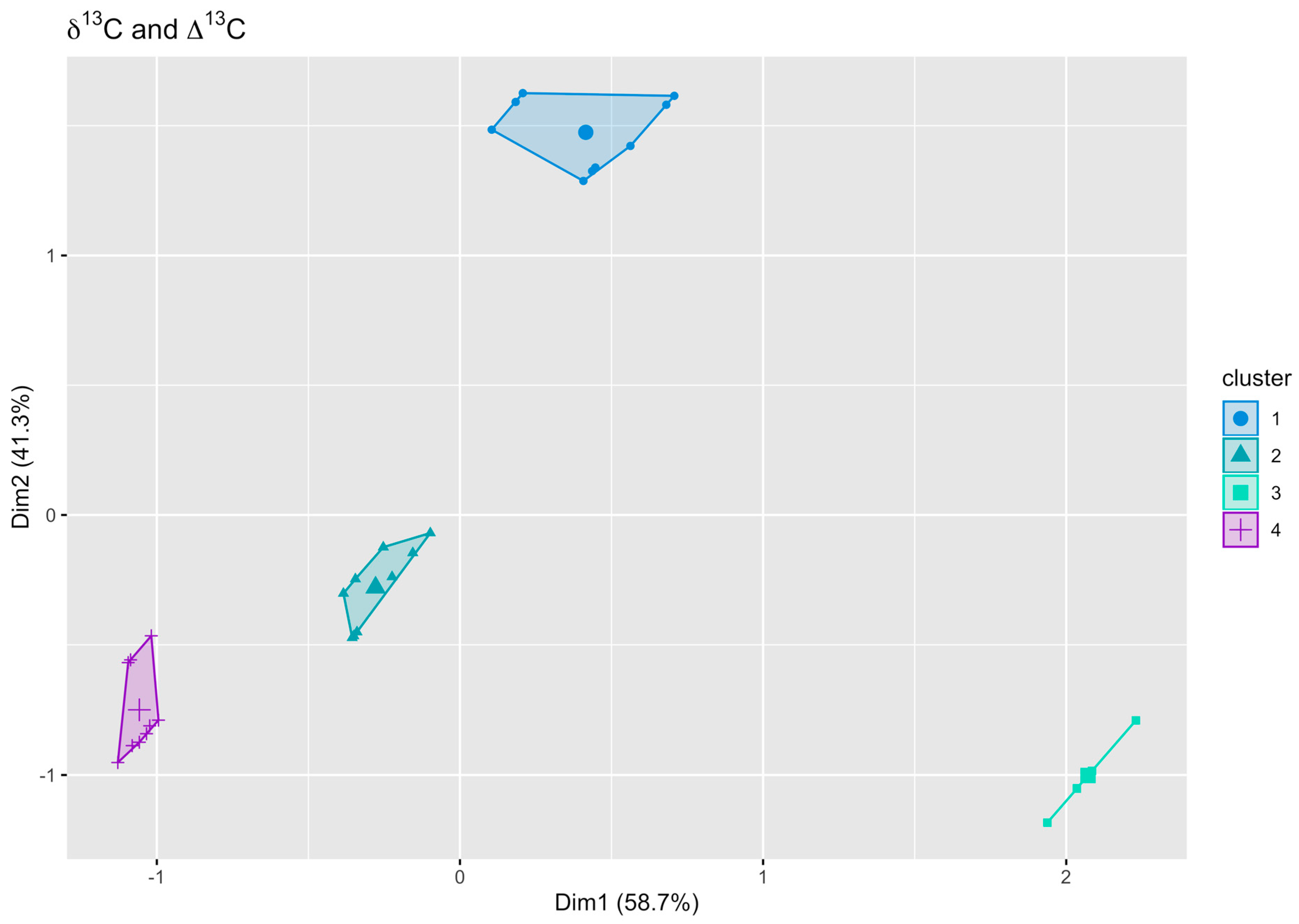
3.3. Cluster Analysis
3.4. Turnover Rates and Decay Models
4. Discussion
4.1. Starvation
4.2. Chlorophytes
4.3. Non-Toxic Cyanobacteria
4.4. Toxic Unicellular Cyanobacteria
4.5. Toxic Filamentous Cyanobacteria
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leibold, M.A.; Chase, J.M.; Shurin, J.B.; Downing, A.L. Species Turnover and the Regulation of Trophic Structure. Annu. Rev. Ecol. Evol. Syst. 1997, 28, 467–494. [Google Scholar] [CrossRef]
- Bell, G. The evolution of trophic structure. Heredity 2007, 99, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Scotti, M.; Bondavalli, C.; Bodini, A.; Allesina, S. Using trophic hierarchy to understand food web structure. Oikos 2009, 118, 1695–1702. [Google Scholar] [CrossRef]
- Pace, M.L.; Cole, J.J.; Carpenter, S.R.; Kitchell, J.F. Trophic cascades revealed in diverse ecosystems. Trends Ecol. Evol. 1999, 14, 483–488. [Google Scholar] [CrossRef]
- Eddy, T.D.; Bernhardt, J.R.; Blanchard, J.L.; Cheung, W.W.; Colléter, M.; Du Pontavice, H.; Fulton, E.A.; Gascuel, D.; Kearney, K.A.; Petrik, C.M. Energy flow through marine ecosystems: Confronting transfer efficiency. Trends Ecol. Evol. 2021, 36, 76–86. [Google Scholar] [CrossRef]
- Nielsen, J.M.; Clare, E.L.; Hayden, B.; Brett, M.T.; Kratina, P. Diet tracing in ecology: Method comparison and selection. Methods Ecol. Evol. 2018, 9, 278–291. [Google Scholar] [CrossRef]
- Webb, B.W.; Hannah, D.M.; Moore, R.D.; Brown, L.E.; Nobilis, F. Recent advances in stream and river temperature research. Hydrol. Process. 2008, 22, 902–918. [Google Scholar] [CrossRef]
- IPCC. Climate change 2021: The physical science basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; p. 2391. [Google Scholar]
- IPCC. Summary for Policymakers. In Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Shukla, P.R., Skea, J., Slade, R., Al Khourdajie, A., van Diemen, R., McCollum, D., Pathak, M., Some, S., Vyas, P., Fradera, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Nazari-Sharabian, M.; Ahmad, S.; Karakouzian, M. Climate change and eutrophication: A short review. Eng. Technol. Appl. Sci. Res. 2018, 8, 3668. [Google Scholar] [CrossRef]
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. Anthropogenic nitrogen loads to freshwater: A high-resolution global study. In Just Enough Nitrogen; Springer: Berlin/Heidelberg, Germany, 2020; pp. 303–317. [Google Scholar]
- Anderson, N.J.; Heathcote, A.; Engstrom, D.; contributors, G.d. Anthropogenic alteration of nutrient supply increases the global freshwater carbon sink. Sci. Adv. 2020, 6, eaaw2145. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Nakahara, H. The formation and degradation of cyanobacterium Aphanizomenon flos-aquae blooms: The importance of pH, water temperature, and day length. Limnology 2005, 6, 1–6. [Google Scholar] [CrossRef]
- Paerl, H.W.; Hall, N.S.; Calandrino, E.S. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 2011, 409, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tang, H.; Zhang, X.; Zhu, X.; Huang, Y.; Yang, Z. High temperature and pH favor Microcystis aeruginosa to outcompete Scenedesmus obliquus. Environ. Sci. Pollut. Res. 2018, 25, 4794–4802. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.A. Is the future blue-green? A review of the current model predictions of how climate change could affect pelagic freshwater cyanobacteria. Water Res. 2012, 46, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Burford, M.; Carey, C.; Hamilton, D.; Huisman, J.; Paerl, H.; Wood, S.; Wulff, A. Perspective: Advancing the research agenda for improving understanding of cyanobacteria in a future of global change. Harmful Algae 2020, 91, 101601. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.A. The seasonal sensitivity of cyanobacteria and other phytoplankton to changes in flushing rate and water temperature. Glob. Chang. Biol. 2010, 16, 864–876. [Google Scholar] [CrossRef]
- Joehnk, K.D.; Huisman, J.; Sharples, J.; Sommeijer, B.; Visser, P.M.; Stroom, J.M. Summer heatwaves promote blooms of harmful cyanobacteria. Glob. Chang. Biol. 2008, 14, 495–512. [Google Scholar] [CrossRef]
- Visser, P.M.; Verspagen, J.M.H.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.P.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- You, J.; Mallery, K.; Hong, J.; Hondzo, M. Temperature effects on growth and buoyancy of Microcystis aeruginosa. J. Plankton Res. 2018, 40, 16–28. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, S.; Briand, J.-F.; Leboulanger, C.; Avois-Jacquet, C.; Oberhaus, L.; Tassin, B.; Vinçon-Leite, B.; Paolini, G.; Druart, J.-C.; Anneville, O. The proliferation of the toxic cyanobacterium Planktothrix rubescens following restoration of the largest natural French lake (Lac du Bourget). Harmful Algae 2005, 4, 651–672. [Google Scholar] [CrossRef]
- Dokulil, M.T.; Teubner, K. Deep living Planktothrix rubescens modulated by environmental constraints and climate forcing. In Phytoplankton Responses to Human Impacts at Different Scales; Springer: Berlin/Heidelberg, Germany, 2012; pp. 29–46. [Google Scholar]
- Legnani, E.; Copetti, D.; Oggioni, A.; Tartari, G.; Palumbo, M.T.; Morabito, G. Planktothrix rubescens’ seasonal dynamics and vertical distribution in Lake Pusiano (North Italy). J. Limnol. 2005, 64, 61–73. [Google Scholar] [CrossRef]
- Li, X.; Dreher, T.W.; Li, R. An overview of diversity, occurrence, genetics and toxin production of bloom-forming Dolichospermum (Anabaena) species. Harmful Algae 2016, 54, 54–68. [Google Scholar] [CrossRef]
- Madigan, D.J.; Baumann, Z.; Carlisle, A.B.; Hoen, D.K.; Popp, B.N.; Dewar, H.; Snodgrass, O.E.; Block, B.A.; Fisher, N.S. Reconstructing transoceanic migration patterns of Pacific bluefin tuna using a chemical tracer toolbox. Ecology 2014, 95, 1674–1683. [Google Scholar] [CrossRef]
- Miner, B.E.; De Meester, L.; Pfrender, M.E.; Lampert, W.; Hairston, N.G., Jr. Linking genes to communities and ecosystems: Daphnia as an ecogenomic model. Proc. R. Soc. B 2012, 279, 1873–1882. [Google Scholar] [CrossRef]
- Jüttner, F.; Leonhardt, J.; MöHren, S. Environmental factors affecting the formation of mesityloxide, dimethylallylic alcohol and other volatile compounds excreted by Anabaena cylindrica. Microbiology 1983, 129, 407–412. [Google Scholar] [CrossRef]
- Guillard, R.R.; Lorenzen, C.J. Yellow-green algae with chlorophyllide C 1, 2. J. Phycol. 1972, 8, 10–14. [Google Scholar] [CrossRef]
- Tonk, L.; Welker, M.; Huisman, J.; Visser, P.M. Production of cyanopeptolins, anabaenopeptins, and microcystins by the harmful cyanobacteria Anabaena 90 and Microcystis PCC 7806. Harmful Algae 2009, 8, 219–224. [Google Scholar] [CrossRef]
- Martin, C.; Oberer, L.; Ino, T.; König, W.A.; Busch, M.; Weckesser, J. Cyanopeptolins, new depsipeptides from the cyanobacterium Microcystis sp. PCC 7806. J. Antibiot. 1993, 46, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Bister, B.; Keller, S.; Baumann, H.I.; Nicholson, G.; Weist, S.; Jung, G.; Süssmuth, R.D.; Jüttner, F. Cyanopeptolin 963A, a chymotrypsin inhibitor of Microcystis PCC 7806. J. Nat. Prod. 2004, 67, 1755–1757. [Google Scholar] [CrossRef]
- Portmann, C.; Blom, J.F.; Gademann, K.; Jüttner, F. Aerucyclamides A and B: Isolation and synthesis of toxic ribosomal heterocyclic peptides from the cyanobacterium Microcystis aeruginosa PCC 7806. J. Nat. Prod. 2008, 71, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Portmann, C.; Blom, J.F.; Kaiser, M.; Brun, R.; Jüttner, F.; Gademann, K. Isolation of aerucyclamides C and D and structure revision of microcyclamide 7806A: Heterocyclic ribosomal peptides from Microcystis aeruginosa PCC 7806 and their antiparasite evaluation. J. Nat. Prod. 2008, 71, 1891–1896. [Google Scholar] [CrossRef]
- Martin-Creuzburg, D.; von Elert, E.; Hoffmann, K.H. Nutritional constraints at the cyanobacteria—Daphnia magna interface: The role of sterols. Limnol. Oceanogr. 2008, 53, 456–468. [Google Scholar] [CrossRef]
- Bec, A.; Martin-Creuzburg, D.; von Elert, E. Trophic upgrading of autotrophic picoplankton by the heterotrophic nanoflagellate Paraphysomonas sp. Limnol. Oceanogr. 2006, 51, 1699–1707. [Google Scholar] [CrossRef]
- Entfellner, E.; Frei, M.; Christiansen, G.; Deng, L.; Blom, J.; Kurmayer, R. Evolution of anabaenopeptin peptide structural variability in the cyanobacterium Planktothrix. Front. Microbiol. 2017, 8, 219. [Google Scholar] [CrossRef]
- Kosol, S.; Schmidt, J.; Kurmayer, R. Variation in peptide net production and growth among strains of the toxic cyanobacterium Planktothrix spp. Eur. J. Phycol. 2009, 44, 49–62. [Google Scholar] [CrossRef]
- Kurmayer, R.; Blom, J.F.; Deng, L.; Pernthaler, J. Integrating phylogeny, geographic niche partitioning and secondary metabolite synthesis in bloom-forming Planktothrix. ISME J. 2015, 9, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Thiel, T.; Pratte, B.S.; Zhong, J.; Goodwin, L.; Copeland, A.; Lucas, S.; Han, C.; Pitluck, S.; Land, M.L.; Kyrpides, N.C. Complete genome sequence of Anabaena variabilis ATCC 29413. Stand. Genom. Sci. 2014, 9, 562–573. [Google Scholar] [CrossRef]
- Basen, T.; Martin-Creuzburg, D.; Rothhaupt, K.-O. Role of essential lipids in determining food quality for the invasive freshwater clam Corbicula fluminea. J. N. Am. Benthol. Soc. 2011, 30, 653–664. [Google Scholar] [CrossRef]
- Basen, T.; Gergs, R.; Rothhaupt, K.-O.; Martin-Creuzburg, D. Phytoplankton food quality effects on gammarids: Benthic–pelagic coupling mediated by an invasive freshwater clam. Can. J. Fish. Aquat. 2013, 70, 198–207. [Google Scholar] [CrossRef]
- Martin-Creuzburg, D.; von Elert, E. Good food versus bad food: The role of sterols and polyunsaturated fatty acids in determining growth and reproduction of Daphnia magna. Aquat. Ecol. 2009, 43, 943–950. [Google Scholar] [CrossRef]
- Martin-Creuzburg, D.; Merkel, P. Sterols of freshwater microalgae: Potential implications for zooplankton nutrition. J. Plankton Res. 2016, 38, 865–877. [Google Scholar] [CrossRef]
- Elert, E.V.; Martin-Creuzburg, D.; Le Coz, J.R. Absence of sterols constrains carbon transfer between cyanobacteria and a freshwater herbivore (Daphnia galeata). Proc. R. Soc. B 2003, 270, 1209–1214. [Google Scholar] [CrossRef]
- Schlotz, N.; Roulin, A.; Ebert, D.; Martin-Creuzburg, D. Combined effects of dietary polyunsaturated fatty acids and parasite exposure on eicosanoid-related gene expression in an invertebrate model. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 201, 115–123. [Google Scholar] [CrossRef]
- Piepho, M.; Martin-Creuzburg, D.; Wacker, A. Simultaneous effects of light intensity and phosphorus supply on the sterol content of phytoplankton. PLoS ONE 2010, 5, e15828. [Google Scholar] [CrossRef]
- Amini Khoeyi, Z.; Seyfabadi, J.; Ramezanpour, Z. Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquac. Int. 2012, 20, 41–49. [Google Scholar] [CrossRef]
- Hobson, K.A.; Clark, R.G. Assessing avian diets using stable isotopes I: Turnover of 13C in tissues. Condor 1992, 94, 181–188. [Google Scholar] [CrossRef]
- Tibshirani, R.; Walther, G.; Hastie, T. Estimating the number of clusters in a data set via the gap statistic. J. R. Stat. Soc. B Stat. Methodol. 2001, 63, 411–423. [Google Scholar] [CrossRef]
- Webb, S.; Hedges, R.; Simpson, S. Diet quality influences the C-13 and N-15 of locusts and their biochemical components. J. Exp. Biol. 1998, 201, 2903–2911. [Google Scholar] [CrossRef]
- Oelbermann, K.; Scheu, S. Stable isotope enrichment (δ 15N and δ 13C) in a generalist predator (Pardosa lugubris, Araneae: Lycosidae): Effects of prey quality. Oecologia 2002, 130, 337–344. [Google Scholar] [CrossRef]
- Doi, H.; Akamatsu, F.; González, A.L. Starvation effects on nitrogen and carbon stable isotopes of animals: An insight from meta-analysis of fasting experiments. R. Soc. Open Sci. 2017, 4, 170633. [Google Scholar] [CrossRef]
- Adams, T.S.; Sterner, R.W. The effect of dietary nitrogen content on trophic level 15N enrichment. Limnol. Oceanogr. 2000, 45, 601–607. [Google Scholar] [CrossRef]
- Hobson, K.A.; Alisauskas, R.T.; Clark, R.G. Stable-nitrogen isotope enrichment in avian tissues due to fasting and nutritional stress: Implications for isotopic analyses of diet. Condor 1993, 95, 388–394. [Google Scholar] [CrossRef]
- Doi, H.; Kikuchi, E.; Takagi, S.; Shikano, S. Changes in carbon and nitrogen stable isotopes of chironomid larvae during growth, starvation and metamorphosis. Rapid Commun. Mass Spectrom. 2007, 21, 997–1002. [Google Scholar] [CrossRef]
- Gorokhova, E.; Hansson, S. An experimental study on variations in stable carbon and nitrogen isotope fractionation during growth of Mysis mixta and Neomysis integer. Can. J. Fish. Aquat. 1999, 56, 2203–2210. [Google Scholar] [CrossRef]
- Minagawa, M.; Wada, E. Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ 15N and animal age. Geochim. Cosmochim. Acta 1984, 48, 1135–1140. [Google Scholar] [CrossRef]
- Petkov, G.; Garcia, G. Which are fatty acids of the green alga Chlorella? Biochem. Syst. Ecol. 2007, 35, 281–285. [Google Scholar] [CrossRef]
- Rosa, A.P.C.d.; Moraes, L.; Morais, E.G.d.; Costa, J.A.V. Fatty acid biosynthesis from Chlorella in autotrophic and mixotrophic cultivation. Braz. Arch. Biol. Technol. 2020, 63, e20180534. [Google Scholar] [CrossRef]
- Raya, I.; Anshar, A.M.; Mayasari, E.; Dwiyana, Z.; Asdar, M. Chorella vulgaris and Spirulina platensis: Concentration of protein, Docosahexaenoic Acid Chorella (DHA), Eicosapentaenoic Acid (EPA) and variation concentration of maltodextrin via microencapsulation method. Int. J. Appl. Chem. 2016, 12, 539–548. [Google Scholar]
- Tokuşoglu, Ö.; Üunal, M. Biomass nutrient profiles of three microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J. Food Sci. 2003, 68, 1144–1148. [Google Scholar] [CrossRef]
- Rismani, S.; Shariati, M. Changes of the total lipid and omega-3 fatty acid contents in two microalgae Dunaliella salina and Chlorella vulgaris under salt stress. Braz. Arch. Biol. Technol. 2017, 60, e17160555. [Google Scholar] [CrossRef]
- Martin-Creuzburg, D.; Sperfeld, E.; Wacker, A. Colimitation of a freshwater herbivore by sterols and polyunsaturated fatty acids. Proc. R. Soc. B 2009, 276, 1805–1814. [Google Scholar] [CrossRef]
- Ahlgren, G.; Gustafsson, I.B.; Boberg, M. Fatty acid content and chemical composition of freshwater microalgae. J. Phycol. 1992, 28, 37–50. [Google Scholar] [CrossRef]
- Volkman, J. Sterols in microorganisms. Appl. Microbiol. Biotechnol. 2003, 60, 495–506. [Google Scholar] [CrossRef]
- Carmichael, W.W. The toxins of cyanobacteria. Sci. Am. 1994, 270, 78–86. [Google Scholar] [CrossRef]
- Boag, B.; Neilson, R.; Scrimgeour, C.M. The effect of starvation on the planarian Arthurdendyus triangulatus (Tricladida: Terricola) as measured by stable isotopes. Biol. Fertil. Soils 2006, 43, 267–270. [Google Scholar] [CrossRef]
- Gaye-Siessegger, J.; Focken, U.; Muetzel, S.; Abel, H.; Becker, K. Feeding level and individual metabolic rate affect δ 13C and δ 15N values in carp: Implications for food web studies. Oecologia 2004, 138, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Gaye-Siessegger, J.; Focken, U.; Abel, H.; Becker, K. Starvation and low feeding levels result in an enrichment of 13C in lipids and 15N in protein of Nile tilapia Oreochromis niloticus L. J. Fish Biol. 2007, 71, 90–100. [Google Scholar] [CrossRef]
- Ambrose, S.H.; DeNiro, M.J. The isotopic ecology of East African mammals. Oecologia 1986, 69, 395–406. [Google Scholar] [CrossRef]
- Müller-Navarra, D.C.; Brett, M.T.; Liston, A.M.; Goldman, C.R. A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 2000, 403, 74–77. [Google Scholar] [CrossRef]
- Masclaux, H.; Richoux, N.B. Effects of temperature and food quality on isotopic turnover and discrimination in a cladoceran. Aquat. Ecol. 2017, 51, 33–44. [Google Scholar] [CrossRef]
- Ek, C.; Karlson, A.M.; Hansson, S.; Garbaras, A.; Gorokhova, E. Stable isotope composition in Daphnia is modulated by growth, temperature, and toxic exposure: Implications for trophic magnification factor assessment. Environ. Sci. Technol. 2015, 49, 6934–6942. [Google Scholar] [CrossRef]
- Mohan, J.A.; Smith, S.D.; Connelly, T.L.; Attwood, E.T.; McClelland, J.W.; Herzka, S.Z.; Walther, B.D. Tissue-specific isotope turnover and discrimination factors are affected by diet quality and lipid content in an omnivorous consumer. J. Exp. Mar. Biol. Ecol. 2016, 479, 35–45. [Google Scholar] [CrossRef]
- Kurmayer, R.; Jüttner, F. Strategies for the co-existence of zooplankton with the toxic cyanobacterium Planktothrix rubescens in Lake Zurich. J. Plankton Res. 1999, 21, 659–683. [Google Scholar] [CrossRef]
- Brzeziński, T.; Czub, M.; Nawała, J.; Gordon, D.; Dziedzic, D.; Dawidziuk, B.; Popiel, S.; Maszczyk, P. The effects of chemical warfare agent Clark I on the life histories and stable isotopes composition of Daphnia magna. Environ. Pollut. 2020, 266, 115142. [Google Scholar] [CrossRef]
- Shaw-Allen, P.L.; Romanek, C.S.; Bryan, A.L.; Brant, H.; Jagoe, C.H. Shifts in Relative Tissue δ 15N Values in Snowy Egret Nestlings with Dietary Mercury Exposure: A Marker for Increased Protein Degradation. Environ. Sci. Technol. 2005, 39, 4226–4233. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.J.; Barber, I.; Calow, P. Clonal Variation in General Responses of Daphnia magna Straus to Toxic Stress. I. Chronic Life-History Effects. Funct. Ecol. 1990, 4, 399–407. [Google Scholar] [CrossRef]
- Arzate-Cárdenas, M.A.; Martínez-Jerónimo, F. Energy reserve modification in different age groups of Daphnia schoedleri (Anomopoda: Daphniidae) exposed to hexavalent chromium. Environ. Toxicol. Pharmacol. 2012, 34, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Staaden, S.; Milcu, A.; Rohlfs, M.; Scheu, S. Fungal toxins affect the fitness and stable isotope fractionation of Collembola. Soil Biol. Biochem. 2010, 42, 1766–1773. [Google Scholar] [CrossRef]
- Pawlik-Skowrońska, B.; Bownik, A. Cyanobacterial anabaenopeptin-B, microcystins and their mixture cause toxic effects on the behavior of the freshwater crustacean Daphnia magna (Cladocera). Toxicon 2021, 198, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pawlik-Skowrońska, B.; Bownik, A. Synergistic toxicity of some cyanobacterial oligopeptides to physiological activities of Daphnia magna (Crustacea). Toxicon 2022, 206, 74–84. [Google Scholar] [CrossRef]
- Padisák, J.; Barbosa, F.; Koschel, R.; Krienitz, L. Deep layer cyanoprokaryota maxima in temperate and tropical lakes. Arch. Hydrobiol. Spec. Issues Adv. Limnol. 2003, 58, 175–199. [Google Scholar]
- Kirk, K.L.; Gilbert, J.J. Variation in herbivore response to chemical defenses: Zooplankton foraging on toxic cyanobacteria. Ecology 1992, 73, 2208–2217. [Google Scholar] [CrossRef]
- Ger, K.A.; Hansson, L.A.; Lürling, M. Understanding cyanobacteria-zooplankton interactions in a more eutrophic world. Freshw. Biol. 2014, 59, 1783–1798. [Google Scholar] [CrossRef]
- Gliwicz, Z.M. Why do cladocerans fail to control algal blooms? In Biomanipulation Tool for Water Management; Springer: Dordrecht, The Netherlands, 1990; pp. 83–97. [Google Scholar]
- Porter, K.G.; McDonough, R. The energetic cost of response to blue-green algal filaments by cladocerans 1. Limnol. Oceanogr. 1984, 29, 365–369. [Google Scholar] [CrossRef]
- Brendelberger, H. Filter mesh size of cladocerans predicts retention efficiency for bacteria. Limnol. Oceanogr. 1991, 36, 884–894. [Google Scholar] [CrossRef]
- Holm, N.P.; Ganf, G.G.; Shapiro, J. Feeding and assimilation rates of Daphnia pulex fed Aphanizomenon flos-aquae. Limnol. Oceanogr. 1983, 28, 677–687. [Google Scholar] [CrossRef]
- Schwarzenberger, A.; Kurmayer, R.; Martin-Creuzburg, D. Toward Disentangling the Multiple Nutritional Constraints Imposed by Planktothrix: The Significance of Harmful Secondary Metabolites and Sterol Limitation. Front. Microbiol. 2020, 11, 586120. [Google Scholar] [CrossRef] [PubMed]
| Phytoplankton | Origin | Toxic (+) Yes (−) No | PUFAs (+) Yes (−) No | Potentially Relevant PUFAs | Sterols (+) Yes (−) No | Potentially Relevant Phytosterols |
|---|---|---|---|---|---|---|
| Microcystis aeruginosa | PCC 7806 | (+) [37,38,39,40,41] | <C 18 (+) [42] >C 18 (−) [42,43] | ALA [42,43] SDA [42] | (−) [42] | (−) [42] |
| Planktothrix rubescens No 91/1 | MON (isolated Mondsee 2001, Kurmayer) | (+) [41,44,45,46] | no info. | no info. | no info. | no info. |
| Planktothrix agardhii No 829 | MON (isolated Russland 2008, Kurmayer) | (+) [41,44] | no info. | no info. | no info. | no info. |
| Trichormus variabilis P9 | ATCC 29413 | (−) [47] | <C 18 (+) [42,48] >C 18 (−) [42,49] | ALA [42,48,49] SDA [42,49] | (−) [42] | (−) [42] |
| Synechococcus elongatus Bo 8801 (green) | KON 76 (isolated Lake Constance) | no info. | (−) [42,48,49] * | (−) [42,48,49] * | (−) [42,50] * | (−) [42,50] * |
| Synechococcus elongatus Bo 8809 (red) | KON 77 (isolated Lake Constance) | no info. | (−) [42,48,49] * | (−) [42,48,49] * | (−) [42,50] * | (−) [42,50] * |
| Acutodesmus obliquus | SAG 276-3a | no info. | <C 18 (+) [42,48] >C 18 (−) [49] | ALA [48] SDA [48] | (+) [51,52] | chondrillasterol [51] fungisterol [51] 22-dihydrochondrillasterol [51] |
| Chlamydomonas klinobasis | KON 56 (isolated lake constance) | no info. | >C 18 (−) [53] | ALA [53] | (+) [51,54] | ergosterol [51] 7-dehydroporiferasterol [51] |
| Chlorella vulgaris | KON 65 (isolated lake constance) | no info. | <C 18 (+) [55] >C 18 (+) [55] * | ALA [55] * EPA [55] * DHA [55] * | (+) [51] | ergosterol [51] * fungisterol [51] * |
| Diet | δ 13C | δ 15N | C/N Ratio |
|---|---|---|---|
| A. obliquus (Cyano-Medium) | −25.59 ± 0.09 | 3.10 ± 0.01 | 2.86 ± 0.16 |
| M. aeruginosa | −32.28 ± 0.18 | 4.65 ± 0.85 | 3.43 ± 1.22 |
| P. agardhii | −32.80 ± 0.17 | 5.26 ± 1.35 | 3.10 ± 0.65 |
| P. rubescens | −34.35 ± 0.15 | 4.99 ± 0.36 | 2.74 ± 0.20 |
| T. variabilis | −31.44 ± 0.22 | 3.43 ± 0.17 | 2.50 ± 0.06 |
| S. elongatus (green) | −30.53 ± 0.16 | 3.83 ± 0.12 | 2.75 ± 0.21 |
| S. elongatus (red) | −30.40 ± 0.08 | 4.32 ± 0.35 | 2.56 ± 0.21 |
| A. obliquus | −32.99 ± 0.18 | 2.80 ± 0.23 | 2.19 ± 0.09 |
| C. klinobasis | −32.05 ± 0.10 | 2.75 ± 0.48 | 2.63 ± 0.15 |
| C. vulgaris | −32.08 ± 0.04 | 3.63 ± 0.12 | 2.89 ± 0.21 |
| Diet | t | df | Significance Level | Statistical Test | Significance Level |
|---|---|---|---|---|---|
| M. aeruginosa | 48.336 | 2 | *** | Paired t-test | *** |
| P. agardhii | −50.803 | 2 | *** | ||
| P. rubescens | −40.07 | 2 | *** | ||
| S. elongatus (green) | −10.877 | 2 | ** | Paired Wilcoxon test | ** |
| S. elongatus (red) | −20.385 | 2 | ** | ||
| T. variabilis | −11.746 | 2 | ** | ||
| A. obliquus | −13.183 | 2 | ** | Paired Wilcoxon test | ** |
| C. klinobasis | −8.8552 | 2 | * | ||
| C. vulgaris | −3.6104 | 2 | n.s. |
| Time (h) | Diet | δ0 | δeq | logλ | λ | AIC | ∆AIC | Half-Life δ 13C | |
|---|---|---|---|---|---|---|---|---|---|
| 96 | T. variabilis | −26.64 ± 0.03 | −28.89 ± 0.03 | −3.39 ± 0.05 | 0.034 | −18.28 (E) | 27.23 | 20.46 | 27.63 |
| 96 | S. elongatus (green) | −26.58 ± 0.18 | −28.92 ± 1.20 | −4.39 ± 0.87 | 0.013 | 0.95 (E) | 5.84 | 55.69 | |
| 96 | S. elongatus (red) | −26.63 ± 0.05 | −29.01 ± 0.07 | −3.54 ± 0.08 | 0.029 | −13.18 (E) | 21.24 | 23.78 | |
| 96 | A. obliquus | −26.70 ± 0.41 | −32.64 ± 1.26 | −3.99 ± 0.45 | 0.019 | 8.78 (E) | 4.6 | 37.28 | 35.52 |
| 96 | C. klinobasis | −26.65 ± 0.22 | −32.38 ± 0.60 | −3.91 ± 0.23 | 0.020 | 2.67 (E) | 10.52 | 34.48 | |
| 96 | C. vulgaris | −26.65 ± 0.33 | −32.68 ± 0.91 | −3.92 ± 0.34 | 0.020 | 6.60 (E) | 7.34 | 35.02 | |
| 72 | M. aeruginosa | −6.05 (L) | - | ||||||
| 96 | P. agardhii | 11.05 (L) | |||||||
| 96 | P. rubescens | 17.74 (L) | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helmer, M.; Helmer, D.; Martin-Creuzburg, D.; Rothhaupt, K.-O.; Yohannes, E. Toxicity and Starvation Induce Major Trophic Isotope Variation in Daphnia Individuals: A Diet Switch Experiment Using Eight Phytoplankton Species of Differing Nutritional Quality. Biology 2022, 11, 1816. https://doi.org/10.3390/biology11121816
Helmer M, Helmer D, Martin-Creuzburg D, Rothhaupt K-O, Yohannes E. Toxicity and Starvation Induce Major Trophic Isotope Variation in Daphnia Individuals: A Diet Switch Experiment Using Eight Phytoplankton Species of Differing Nutritional Quality. Biology. 2022; 11(12):1816. https://doi.org/10.3390/biology11121816
Chicago/Turabian StyleHelmer, Michelle, Desiree Helmer, Dominik Martin-Creuzburg, Karl-Otto Rothhaupt, and Elizabeth Yohannes. 2022. "Toxicity and Starvation Induce Major Trophic Isotope Variation in Daphnia Individuals: A Diet Switch Experiment Using Eight Phytoplankton Species of Differing Nutritional Quality" Biology 11, no. 12: 1816. https://doi.org/10.3390/biology11121816
APA StyleHelmer, M., Helmer, D., Martin-Creuzburg, D., Rothhaupt, K.-O., & Yohannes, E. (2022). Toxicity and Starvation Induce Major Trophic Isotope Variation in Daphnia Individuals: A Diet Switch Experiment Using Eight Phytoplankton Species of Differing Nutritional Quality. Biology, 11(12), 1816. https://doi.org/10.3390/biology11121816






