Temperature and Humidity Regulate Sporulation of Corynespora cassiicola That Is Associated with Pathogenicity in Cucumber (Cucumis sativus L.)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strain and Culture Media
2.2. Seedling Cultivation
2.3. Inoculum Preparation
2.4. Temperature and Moisture Conditions
2.5. Sporulation Dynamics of C. cassiicola In Vitro and In Vivo
2.6. Effects of Temperature and Moisture on Sporulation of C. cassiicola
2.7. Effects of Spore Size in the Virulence of C. cassiicola on Cucumber
2.8. Data Analysis
3. Results
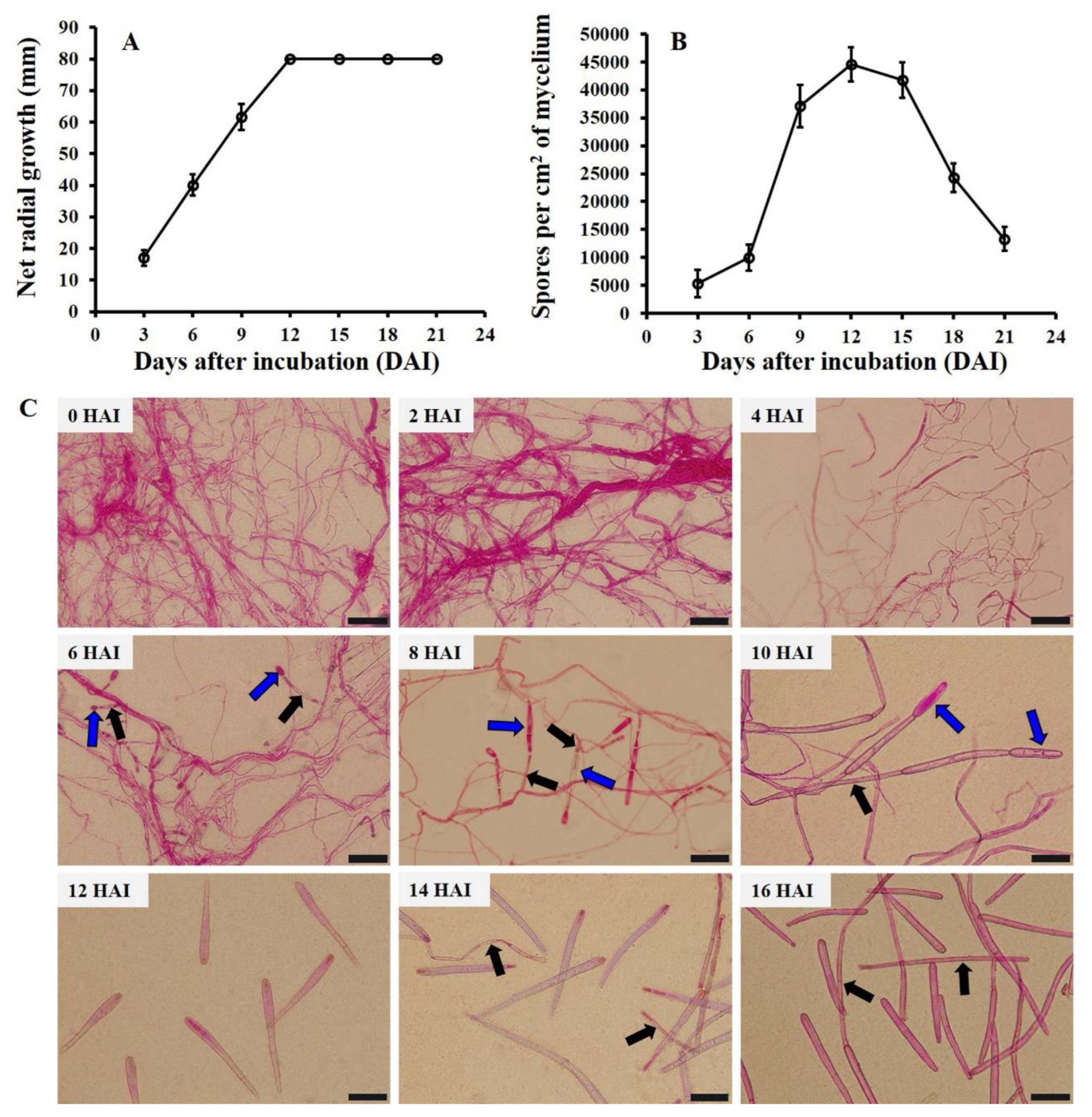
3.1. Sporulation Dynamics of C. cassiicola In Vitro
3.2. Sporulation Dynamics of C. cassiicola In Vivo
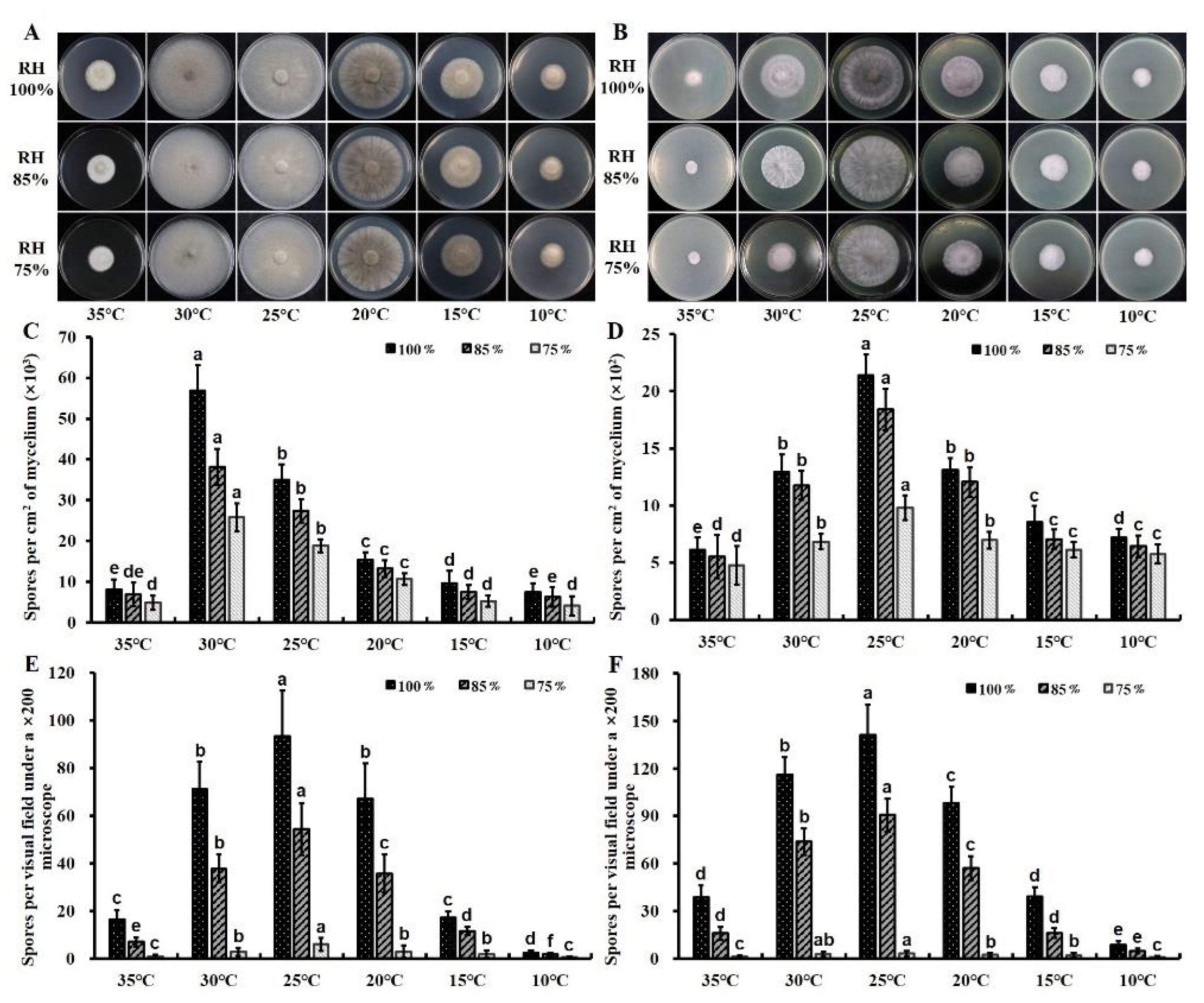
3.3. Effects of Temperature and Moisture on Spore Production
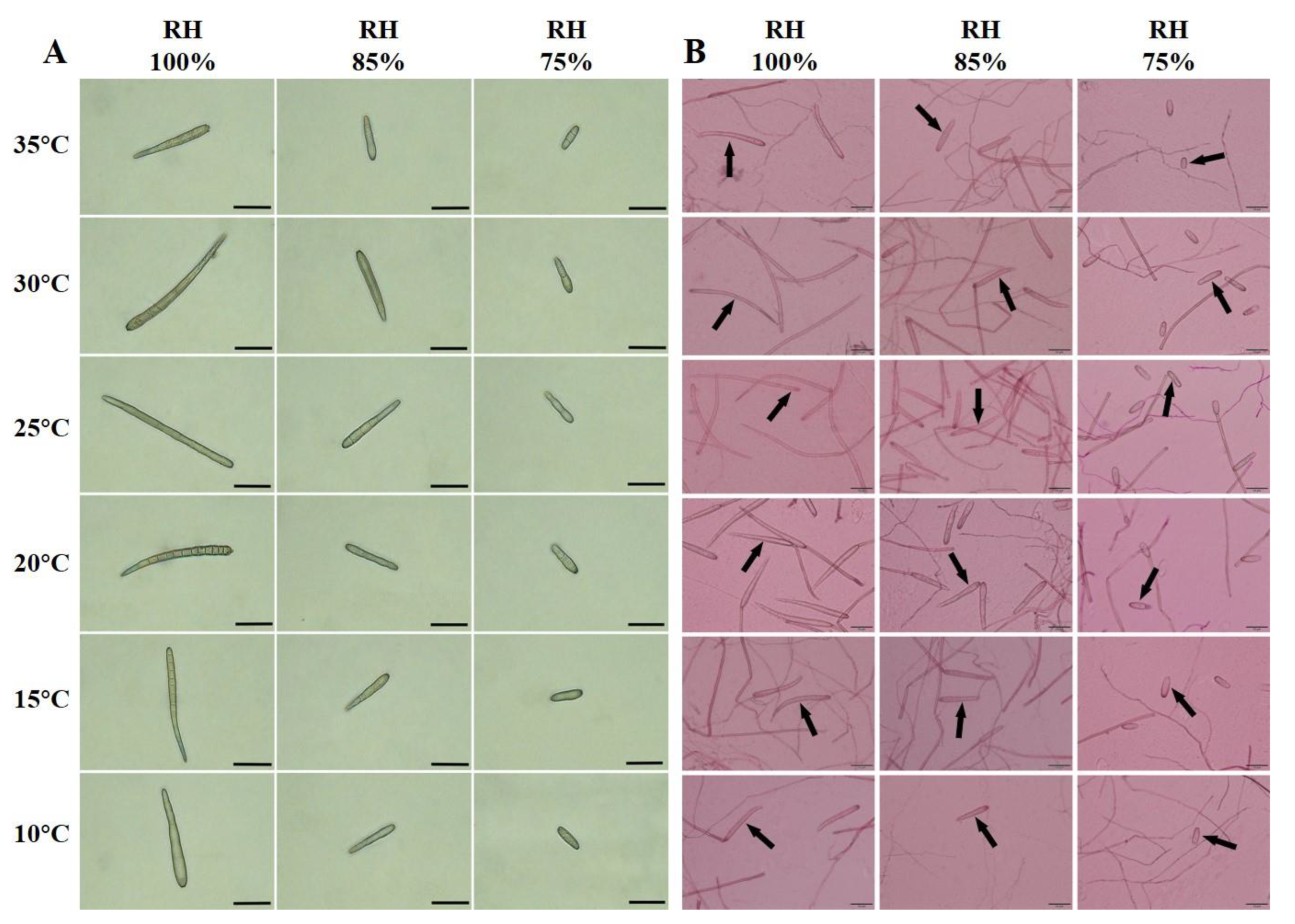
3.4. Effects of Temperature and Moisture on Spore Size
3.5. The Role of Spore Size in the Virulence of C. cassiicola on Cucumber
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dixon, L.J.; Schlub, R.L.; Pernezny, K.; Datnoff, L.E. Host specialization and phylogenetic diversity of Corynespora cassiicola. Phytopathology 2009, 99, 1015–1027. [Google Scholar] [CrossRef] [Green Version]
- Looi, H.K.; Toh, Y.F.; Yew, S.M.; Na, S.L.; Tan, Y.C.; Chong, P.S.; Khoo, J.S.; Yee, W.Y.; Ng, K.P.; Kuan, C.S. Genomic insight into pathogenicity of dematiaceous fungus Corynespora cassiicola. PeerJ 2017, 5, e2841. [Google Scholar] [CrossRef] [Green Version]
- Berkeley, M.J.; Curtis, M.A. Fungi Cubenses (Hymenomycetes). J. Linn. Soc. 1869, 10, 280–392. [Google Scholar] [CrossRef]
- Güssow, U.T. Über eine neue Krankheit an Gurken in England (Corynespora mazei Güssow gen. et sp. nov.). Z. Pflanzenk. Pflanzen. 1906, 16, 10–13. [Google Scholar]
- Wei, C.T. Notes on Corynespora. Mycol. Pap. 1950, 34, 1–10. [Google Scholar]
- Ishii, H.; Yano, K.; Date, H.; Furuta, A.; Sagehashi, Y.; Yamaguchi, T.; Sugiyama, T.; Nishimura, K.; Hasama, W. Molecular characterization and diagnosis of QoI resistance in cucumber and eggplant fungal pathogens. Phytopathology 2007, 97, 1458–1466. [Google Scholar] [CrossRef] [Green Version]
- Castro, M.A.S. Leaf blight caused by Corynespora: A new disease on cucumber (Cucumis sativus) in the Valley of Culiacan, Sinaloa, Mexico, and its chemical control. Plant Dis. Rep. 1979, 63, 599–601. [Google Scholar]
- Kwon, M.K.; Kang, B.R.; Cho, B.H.; Kim, Y.C. Occurrence of target leaf spot disease caused by Corynespora cassicola on cucumber in Korea. Plant Pathol. 2003, 52, 424. [Google Scholar] [CrossRef]
- Miyamoto, T.; Ishii, H.; Stammler, G.; Koch, A.; Ogawara, T.; Tomita, Y.; Fountaine, J.M.; Ushio, S.; Seko, T.; Kobori, S. Distribution and molecular characterization of Corynespora cassiicola isolates resistant to boscalid. Plant Pathol. 2010, 59, 873–881. [Google Scholar] [CrossRef]
- Li, B.J.; Gao, W.; Shi, Y.X.; Xie, X.W. Progress in researches on Corynespora leaf spot. Acta Phytophy. Sin. 2012, 39, 171–176. [Google Scholar]
- Yang, S.J.; Gu, X.F.; Zhang, S.P.; Miao, H.; Li, B.J. Research progress on cucumber target leaf spot (Corynespora cassiicola). China Veg. 2012, 1, 1–9. [Google Scholar]
- Liu, D.; Xin, M.; Zhou, X.Y.; Wang, C.H.; Zhang, Y.J.; Qin, Z.W. Expression and functional analysis of the transcription factor−encoding Gene CsERF004 in cucumber during Pseudoperonospora cubensis and Corynespora cassiicola infection. BMC Plant Biol. 2017, 17, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.Y.; Yu, G.C.; Zhao, J.Y.; Cui, N.; Yu, Y.; Fan, H.Y. Functional identification of Corynespora cassiicola−responsive miRNAs and their targets in cucumber. Front. Plant Sci. 2019, 10, 668. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zhang, D.; Cui, N.; Yu, Y.; Yu, G.C.; Fan, H.Y. Transcriptome and miRNA analyses of the response to Corynespora cassiicola in cucumber. Sci. Rep. 2018, 8, 7798. [Google Scholar] [CrossRef] [PubMed]
- Fitt, B.D.L.; McCartney, H.A.; Walklate, P.J. The role of rain in dispersal of pathogen inoculum. Annu. Rev. Phytopathol. 1989, 27, 241–270. [Google Scholar] [CrossRef]
- Kennedy, R.; Wakeham, A.J.; Byrne, K.G.; Meyer, U.M.; Dewey, F.M. A new method to monitor airborne inoculum of the fungal plant pathogens Mycosphaerella brassicicola and Botrytis cinerea. Appl. Environ. Microbiol. 2000, 66, 2996–3003. [Google Scholar] [CrossRef] [Green Version]
- Stepanov, K.M. Dissemination of infective diseases of plants by air currents. Phytopathology 1935, 8, 1–68. [Google Scholar]
- Nelson, R.R.; Tung, G. Influence of some climatic factors on sporulation by an isolate of race T of Helminthosporium maydis on a susceptible malesterile corn hybrid. Plant Dis. Rep. 1973, 57, 304–307. [Google Scholar]
- Brown, J.K.M.; Hovmøller, M.S. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 2002, 297, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Hovmøller, M.S.; Yahyaoui, A.H.; Milus, E.A.; Justesen, A.F. Rapid global spread of two aggressive strains of a wheat rust fungus. Mol. Ecol. 2008, 17, 3818–3826. [Google Scholar] [CrossRef]
- Kim, S.; Park, H.; Gruszewski, H.A.; Schmale, D.G.; Jung, S. Vortex−induced dispersal of a plant pathogen by raindrop impact. Proc. Natl. Acad. Sci. USA 2019, 116, 4917–4922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spotts, R.A.; Cervantes, L.A. Disease incidence—Inoculum dose relationships for Botrytis cinerea and Penicillium expansum and decay of pear fruit using dry, airborne conidia. Plant Dis. 2001, 85, 755–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, D.S. Violent spore release in some fungi imperfecti. Ann. Bot. 1963, 27, 39–47. [Google Scholar] [CrossRef]
- Shukla, P.; Yadav, R.N.; Kusum, D. Studies on perpetuation of Corynespora blight of sesame. Indian J. Plant Pathol. 1987, 5, 10–13. [Google Scholar]
- Lee, C.H.; Park, Y.K.; Jeong, I.M.; Kwun, K.C.; Jeong, T.W. Relationship between disease occurrence and dispersal of airborne conidia of Corynespora cassiicola on sesame in field. Research Reports of the Rural Development Administration. Crop Prot. 1990, 32, 13–18. [Google Scholar]
- Patiño-Medina, J.A.; Reyes-Mares, N.Y.; Valle-Maldonado, M.I.; Jacome-Galarza, I.E.; Perez-Arques, C.; Nuñez-Anita, R.E.; Campos-Garcia, J.; Anaya-Martinez, V.; Ortiz-Alvarado, R.; Ramirez-Diaz, M.I.; et al. Heterotrimeric G−alpha subunits Gpa11 and Gpa12 define a transduction pathway that control spore size and virulence in Mucor circinelloides. PLoS ONE 2019, 14, e0226682. [Google Scholar] [CrossRef] [Green Version]
- Velagapudi, R.; Hsueh, Y.P.; Geunes-Boyer, S.; Wright, J.R.; Heitman, J. Spores as infectious propagules of Cryptococcus neoformans. Infect Immun. 2009, 77, 4345–4355. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Zhu, X.G.; Ying, S.H.; Feng, M.G. Effect of vacuolar ATPase subunit H (VmaH) on cellular pH, asexual cycle, stress tolerance and virulence in Beauveria bassiana. Fungal Genet. Biol. 2017, 98, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Arthurs, S.; Thomas, M.B. Effects of temperature and relative humidity on sporulation of Metarhizium anisopliae var. acridum in Mycosed Cadavers of Schistocerca gregaria. J. Invertebr. Pathol. 2001, 78, 59–65. [Google Scholar] [CrossRef]
- Lawrence, E.G.; Zehr, E.I. Environmental effects on the development and dissemination of Cladosporium carpophilum on peach. Phytopathology 1982, 72, 773–776. [Google Scholar] [CrossRef]
- Lalancette, N.; Foster, K.A.; Robison, D.M. Quantitative models for describing temperature and moisture effects on sporulation of Phomopsis amygdali on peach. Phytopathology 2003, 93, 1165–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalancette, N.; McFarland, K.A.; Burnett, A.L. Modeling sporulation of Fusicladium carpophilum on nectarine twig lesions: Relative humidity and temperature effects. Phytopathology 2012, 102, 421–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.L.; Lian, S.; Feng, S.L.; Dong, X.L.; Wang, C.X.; Li, B.H.; Liang, W.X. Effects of temperature and moisture on sporulation and infection by Pseudoperonospora cubensis. Plant Dis. 2017, 101, 562–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becktell, M.C.; Daughtrey, M.L.; Fry, W.E. Temperature and leaf wetness requirements for pathogen establishment, incubation period, and sporulation of Phytophthora infestans on Petunia hybrida. Plant Dis. 2005, 89, 975–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciliberti, N.; Fermaud, M.; Roudet, J.; Languasco, L.; Rossi, V. Environmental effects on the production of Botrytis cinerea conidia on different media, grape bunch trash, and mature berries. Aust. J. Grape Wine Res. 2016, 22, 262–270. [Google Scholar] [CrossRef]
- Jat, M.K.; Ahir, R.R. Effect of temperature, relative humidity and pH on mycelial growth and sporulation of Fusarium solani causing root rot of Indian Aloe (Aloe barbadensis MILL.). J. Plant Sci. Res. 2013, 29, 181–183. [Google Scholar]
- Li, X.; Li, B.H.; Lian, S.; Dong, X.L.; Wang, C.X.; Liang, W.X. Effects of temperature, moisture and nutrition on conidial germination, survival, colonization and sporulation of Trichothecium roseum. Eur. J. Plant Pathol. 2019, 153, 557–570. [Google Scholar] [CrossRef]
- Ward, S.V.; Manners, J.G. Environmental effects on the quantity and viability of conidia produced by Erysiphe graminis. Trans. Brit. Mycol. Soc. 1974, 62, 119–128. [Google Scholar] [CrossRef]
- Reuveni, R.; Rotem, J. Effect of humidity on epidemiological patterns of the powdery mildew (Sphaerotheca fuliginea) on squash. Phytoparasitica 1974, 2, 25–33. [Google Scholar] [CrossRef]
- Guan, G.J.; Liang, G.L.; Li, J.X.; Zhang, Z.Y.; Zhang, C.H.; Li, X.L. Effects of temperature on growth, sporification, and germination of flue−cured tobacco leaf spot causing Corynespora cassiicola in Guizhou province. Acta Tob. Sin. 2006, 12, 27–31. [Google Scholar]
- Sharma, N.; Bowen, K. Enhancing sporulation of Corynespora cassiicola to use for conducting research on epidemiology of the target spot of cotton. Phytopathology 2016, 106, 13. [Google Scholar]
- Liang, P.; Ning, P.; Feng, J.; Huang, Y.H.; Wang, L.R.; Chen, L.H. Identification and biological characteristics of Corynespora cassiicola causing leaf spot on Sanchezia nobilis. Acta Phytopathol. Sin. 2018, 48, 758–765. [Google Scholar]
- Rotem, J.; Cohen, Y.; Bashi, E. Host and environmental influences on sporulation in vivo. Ann. Rev. Phytopathol. 1978, 16, 83–101. [Google Scholar] [CrossRef]
- Uppala, S.S.; Zhou, X.G.; Liu, B.; Wu, M. Plant−based culture media for improved growth and sporulation of Cercospora janseana. Plant Dis. 2019, 103, 504–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winston, P.W.; Bates, D.H. Saturated solutions for the control of humidity in biological research. Ecology 1960, 41, 232–237. [Google Scholar] [CrossRef]
- Lang, A. Osmotic coefficients and water potentials of sodium chloride solutions from 0 to 40 °C. Aust. J. Chem. 1967, 20, 2017–2023. [Google Scholar] [CrossRef]
- Chee, K.H. Studies on sporulation, pathogenicity and epidemiology of Corynespora cassiicola on Hevea rubber. J. Nat. Rubber Res. 1988, 3, 21–29. [Google Scholar]
- Melo, M.M.; Reis, E.M. Effect of substrates, light and filter paper on Corynespora cassiicola sporulation. Summa Phytopathol. 2010, 36, 251–253. [Google Scholar] [CrossRef] [Green Version]
- Onesirosan, P.; Arny, D.; Durbin, R.D. Increasing sporulation of Corynespora cassiicola. Mycopathologia 1975, 55, 121–123. [Google Scholar] [CrossRef]
- Kang, S.W.; Kwon, J.H.; Chung, B.K.; Cho, J.K.; Lee, Y.S.; Kim, H.K. Identification and etiological of new disease, Corynespora leaf spot of cucumber caused by Corynespora melonis (Cook) Lindaw under greenhouse cultivation in Korea. RDA J. Agric. Sci. 1993, 35, 332–336. [Google Scholar]
- Shi, T.; Cai, J.M.; Li, C.P.; Lin, C.H.; Liu, X.B.; Huang, G.X. Optimization of sporulation conditions for Corynespora cassiicola infecting rubber tree in lab experiments. Chin. J. Trop. Crops. 2010, 31, 98–105. [Google Scholar]
- Waggoner, P.E.; Horsfall, J.G.; Lukens, R.J. Epimay, a simulator of southern corn leaf blight. Bull. Conn. Agric. Exp. Stn. 1972, 729, 84. [Google Scholar]
- Clement, A.; Olatunde, M.; Patrick, O.; Joyce, O. Effect of drying temperature on nutritional content of Moringa oleifera leave. World J. Food Sci. Techol. 2017, 1, 93–96. [Google Scholar]
- Giorni, P.; Leggieri, M.C.; Magan, N.; Battilani, P. Comparison of temperature and moisture requirements for sporulation of Aspergillus flavus sclerotia on natural and artificial substrates. Fungal Biol. 2012, 116, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Hollier, C. Effect of amended media, temperature, and light on the growth and development of Cercospora janseana. In Proceedings of the America Phytopathological Society Annual Meeting, Austin, TX, USA, 10–14 August 2013. [Google Scholar]
- Gilles, T.; Phelps, K.; Clarkson, J.P.; Kennedy, R. Development of MILIONCAST, an improved model for predicting downy mildew sporulation on onions. Plant Dis. 2004, 88, 695–702. [Google Scholar] [CrossRef] [Green Version]
- Trujillo, E.E. The effects of humidity and temperature on Phytophthora blight of taro. Phytopathology 1965, 55, 183–188. [Google Scholar]
- King, W.T.; Madden, L.V.; Ellis, M.A.; Wilson, L.L. Effects of temperature on sporulation and latent period of Colletotrichum spp. infecting strawberry fruit. Plant Dis. 1997, 81, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Rotem, J.; Clare, B.G.; Carter, M.V. Effects of temperature, leaf wetness, leaf bacteria and leaf and bacterial diffusates on production and lysis of Rhynchosporium secalis spores. Physiol. Plant Pathol. 1976, 8, 297–305. [Google Scholar] [CrossRef]
- Mathur, R.S.; Barnett, H.L.; Lilly, V.G. Sporulation of Colletotrichum lindemuthianum in culture. Phytopathology 1950, 40, 104–114. [Google Scholar]
- Zhao, H.C.; Wang, Q.; Liu, C.; Shang, Y.L.; Wen, F.P.; Wang, F.; Liu, W.X.; Xiao, W.; Li, W. A role for respiration in regulating meiosis initiation in Saccharomyces cerevisiae. Genetics 2018, 208, 1181–1194. [Google Scholar] [CrossRef]
- Halbwachs, H.; Heilmann-Clausen, J.; Bässler, C. Mean spore size and shape in ectomycorrhizal and saprotrophic assemblages show strong responses under resource constraints. Fungal Ecol. 2017, 26, 59–64. [Google Scholar] [CrossRef]
- Song, X.D.; Walla, J.A.; Xu, G.J.; Liu, G.R.; Chen, J.Y. Comparative studies on the morphology of Sphaeropsis sapinea spores and mycelia. Forest Pest Dis. 2004, 23, 5–9. [Google Scholar]
- Li, C.H.; Cervantes, M.; Springer, D.J.; Boekhout, T.; Ruiz-Vazquez, R.M.; Torres-Martinez, S.R.; Heitmsn, J.; Lee, S.C. Sporangiospore size dimorphism is linked to virulence of Mucor circinelloides. PLoS Pathog. 2011, 7, e1002086. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Kim, K.R.; Park, E.W. Effects of interrupted wetness periods on conidial germination, germ tube elongation and infection periods of Botryosphaeria dothidea causing apple white rot. Plant Pathol. J. 2016, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Shi, Y.; Wang, Y.; Xie, X.; Li, L.; Fan, T.; Guo, L.; Chai, A.; Li, B. Temperature and Humidity Regulate Sporulation of Corynespora cassiicola That Is Associated with Pathogenicity in Cucumber (Cucumis sativus L.). Biology 2022, 11, 1675. https://doi.org/10.3390/biology11111675
Zhao Q, Shi Y, Wang Y, Xie X, Li L, Fan T, Guo L, Chai A, Li B. Temperature and Humidity Regulate Sporulation of Corynespora cassiicola That Is Associated with Pathogenicity in Cucumber (Cucumis sativus L.). Biology. 2022; 11(11):1675. https://doi.org/10.3390/biology11111675
Chicago/Turabian StyleZhao, Qian, Yanxia Shi, Yikai Wang, Xuewen Xie, Lei Li, Tengfei Fan, Liyun Guo, Ali Chai, and Baoju Li. 2022. "Temperature and Humidity Regulate Sporulation of Corynespora cassiicola That Is Associated with Pathogenicity in Cucumber (Cucumis sativus L.)" Biology 11, no. 11: 1675. https://doi.org/10.3390/biology11111675
APA StyleZhao, Q., Shi, Y., Wang, Y., Xie, X., Li, L., Fan, T., Guo, L., Chai, A., & Li, B. (2022). Temperature and Humidity Regulate Sporulation of Corynespora cassiicola That Is Associated with Pathogenicity in Cucumber (Cucumis sativus L.). Biology, 11(11), 1675. https://doi.org/10.3390/biology11111675






