Antibiotics from Insect-Associated Actinobacteria
Abstract
Simple Summary
Abstract
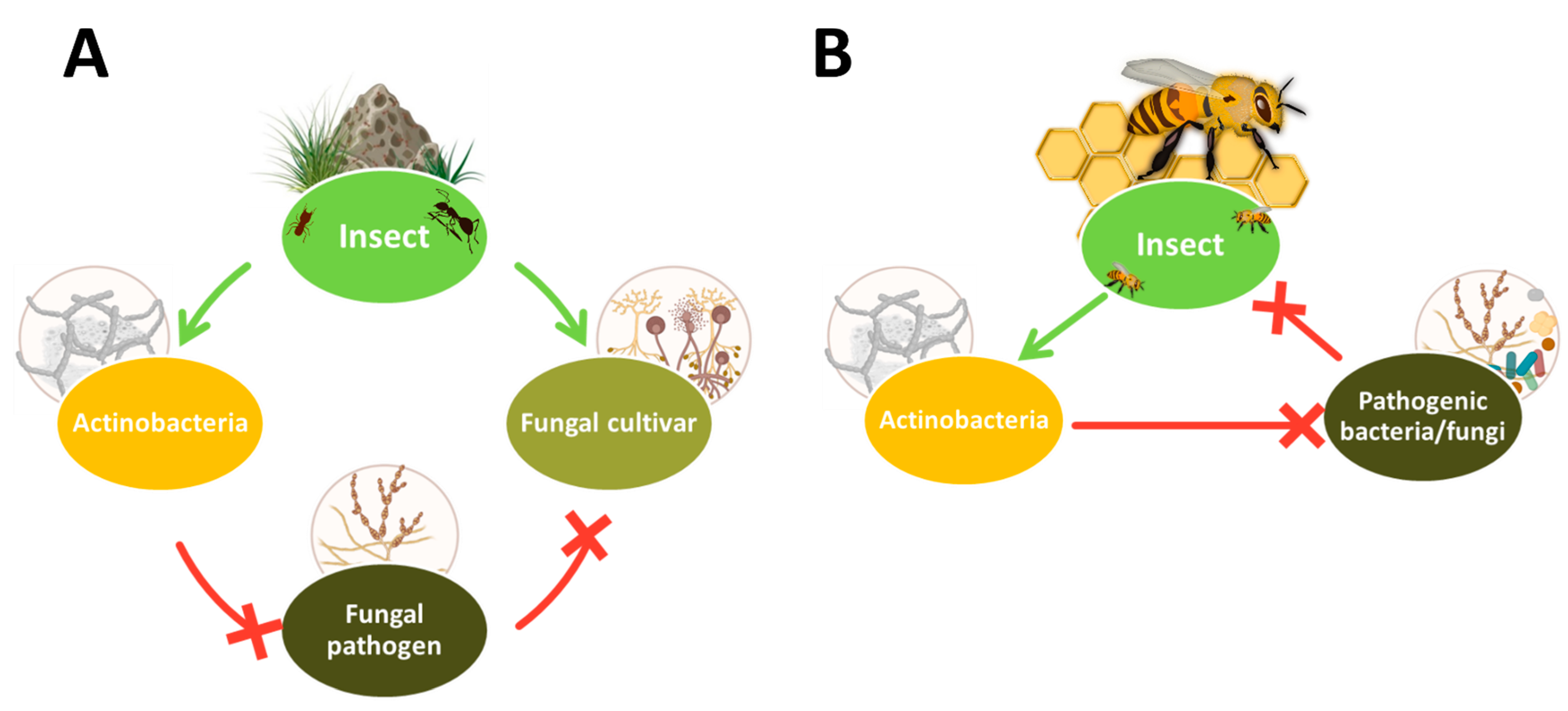
1. Introduction
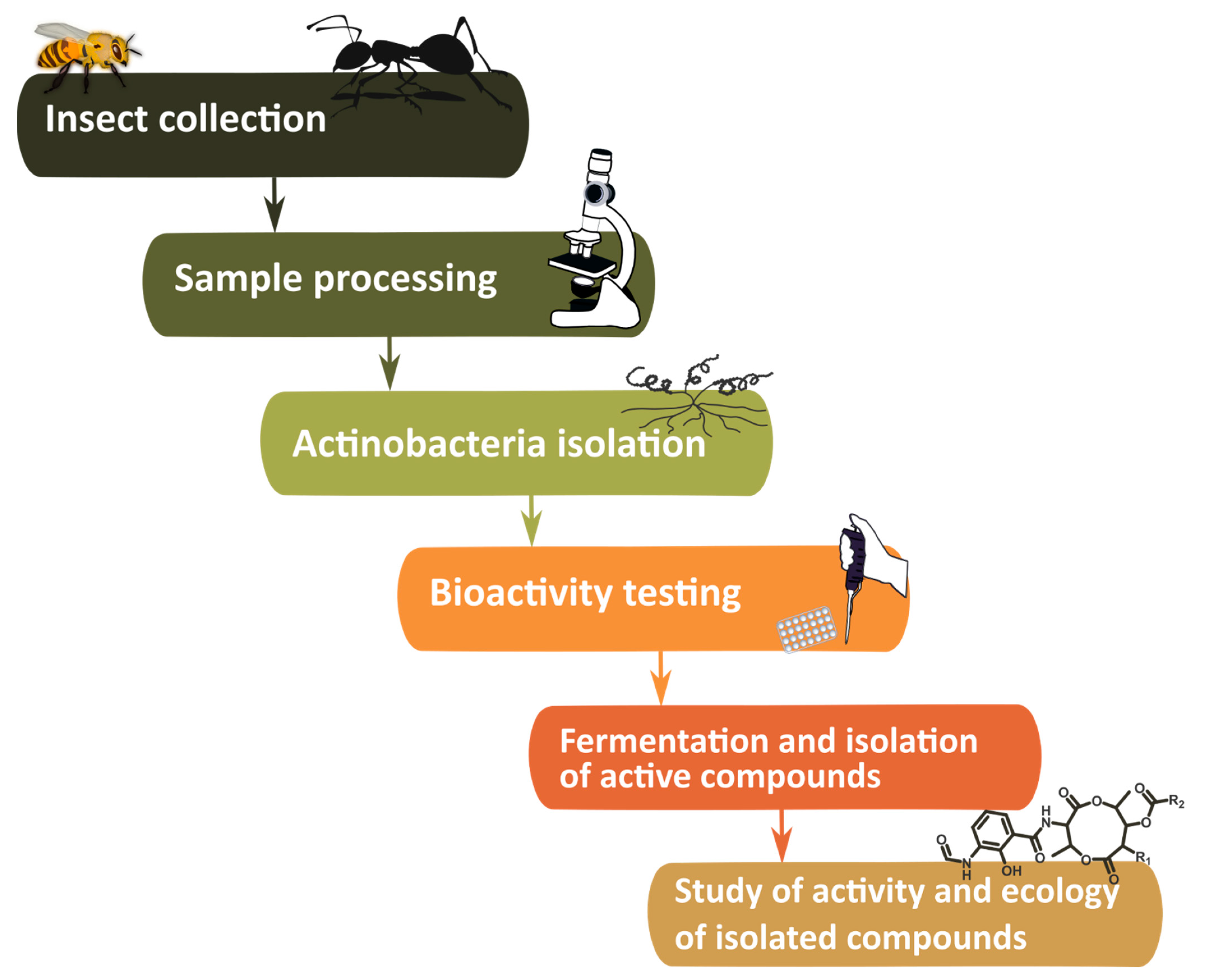
2. Methodology
- The actinobacteria (Phylum: Actinomycetota) have been isolated directly from insects (Class: Insecta) (whole animals, cuticle, gut or other internal/external organs) or from freshly sampled secreted substances. Ant nest fragments and other environmental samples were excluded;
- The antibiotic compounds (i.e., having some antagonistic or enzyme-inhibition activity) produced by actinomycetes have been described or, at least, antagonistic action was confirmed by bioassay;
- Only regular articles, reviews and book chapters were included. Patents, conference materials and preprints were excluded.
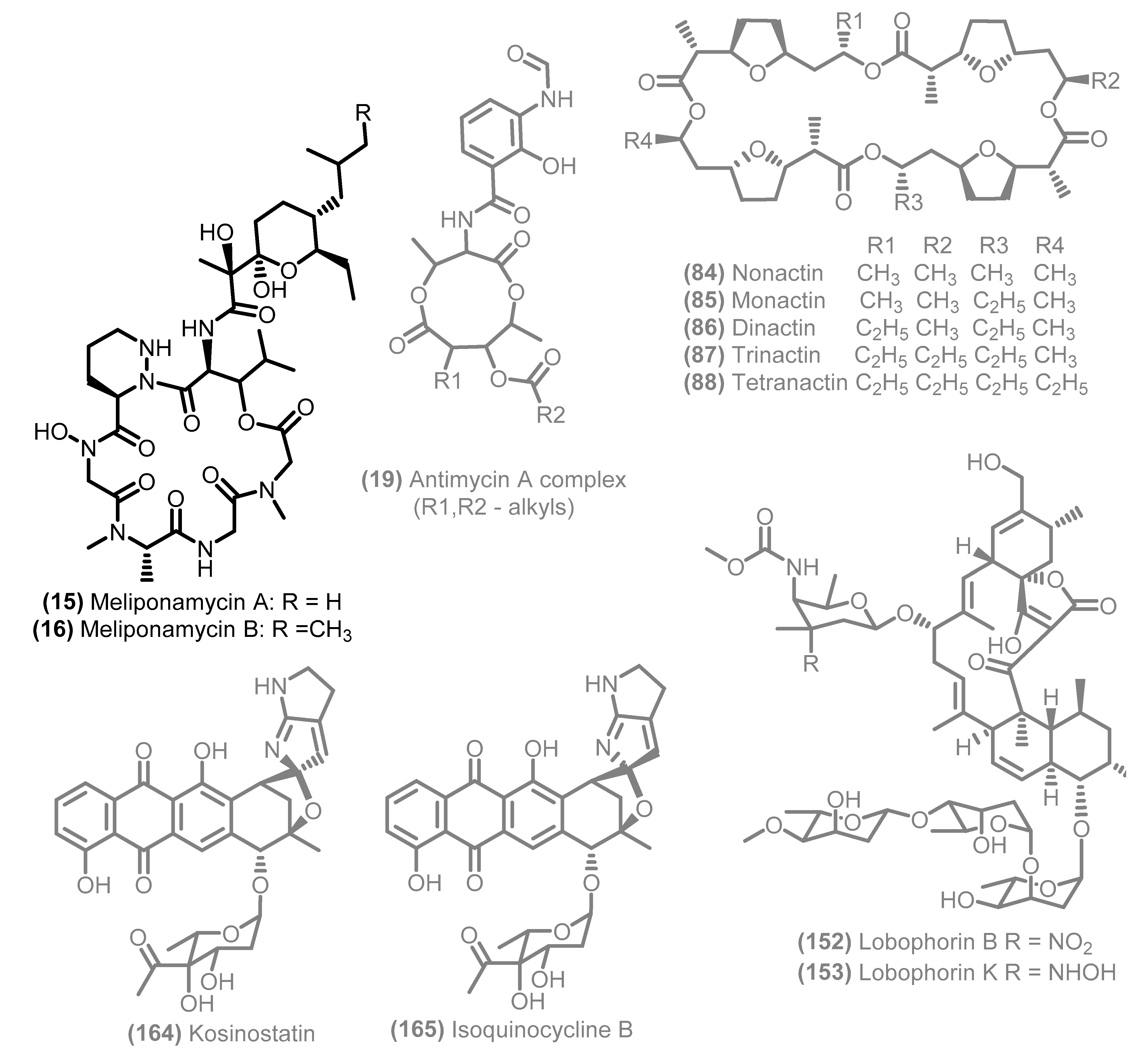
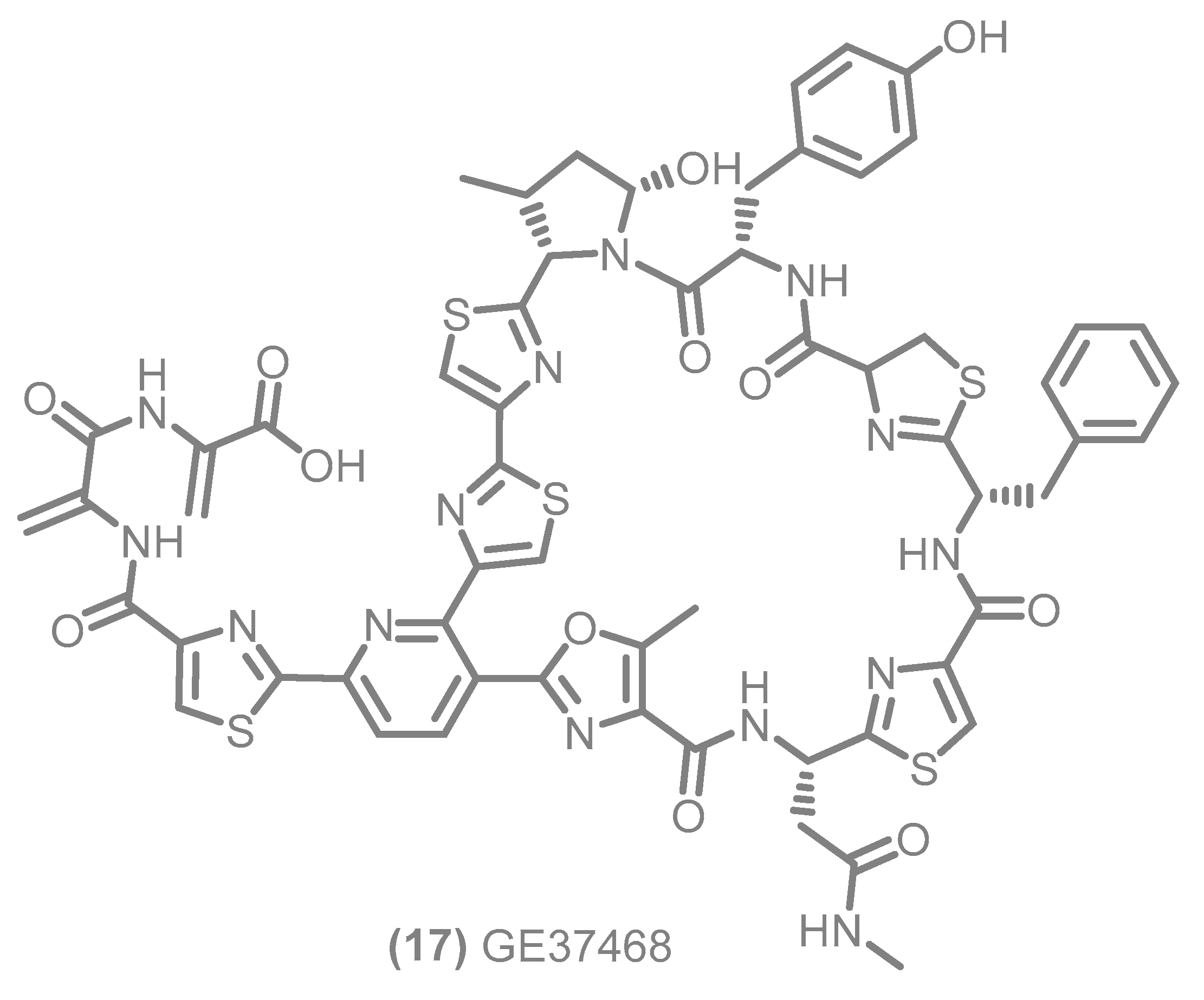
3. General Remarks: Data Pre-Processing
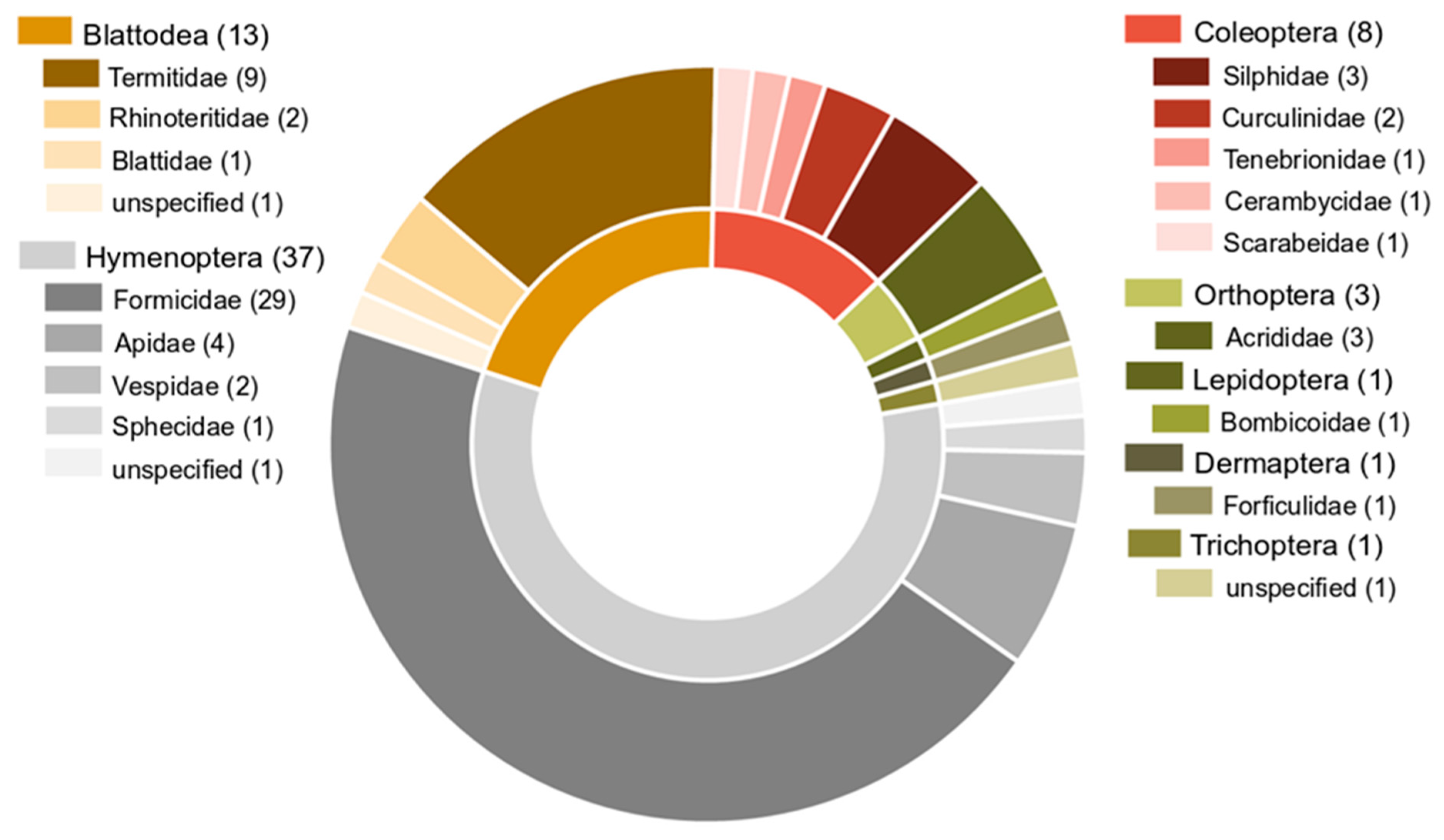
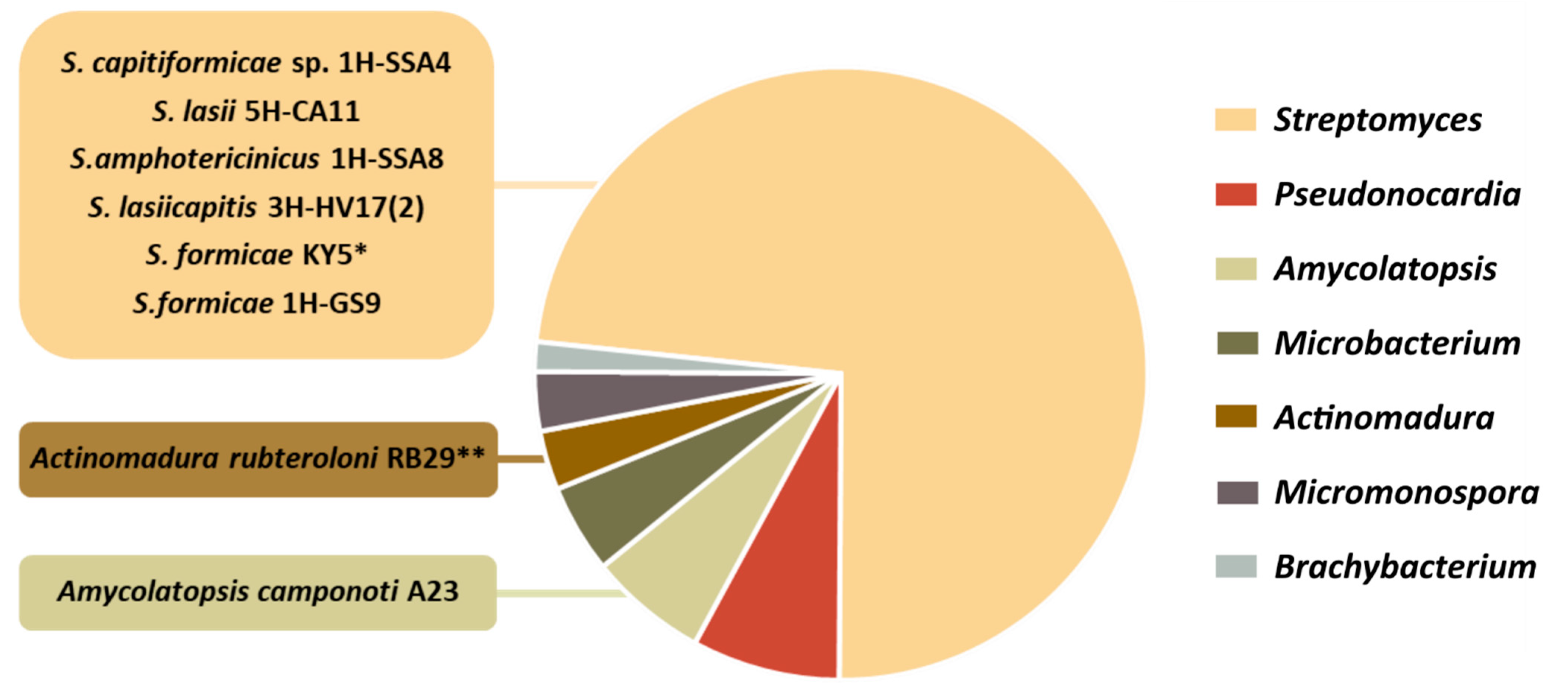
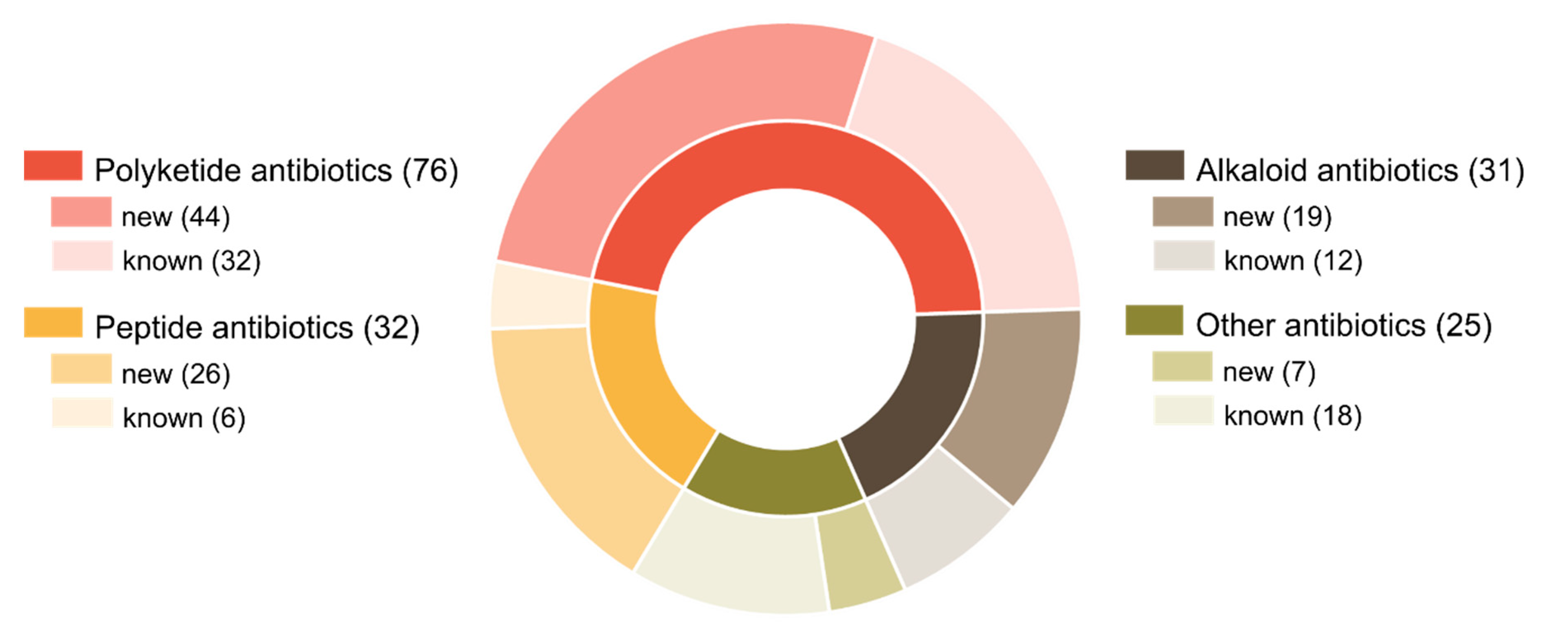
4. Data Analysis: Main Trends
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bar-On, Y.M.; Phillips, R.; Milo, R. The Biomass Distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [PubMed]
- Eggleton, P. The State of the World’s Insects. Annu. Rev. Environ. Resour. 2020, 45, 61–82. [Google Scholar] [CrossRef]
- Forister, M.L.; Jahner, J.P.; Casner, K.L.; Wilson, J.S.; Shapiro, A.M. The Race Is Not to the Swift: Long-Term Data Reveal Pervasive Declines in California’s Low-Elevation Butterfly Fauna. Ecology 2011, 92, 2222–2235. [Google Scholar] [CrossRef]
- Valtonen, A.; Hirka, A.; Szőcs, L.; Ayres, M.P.; Roininen, H.; Csóka, G. Long-Term Species Loss and Homogenization of Moth Communities in Central Europe. J. Anim. Ecol. 2017, 86, 730–738. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 Percent Decline over 27 Years in Total Flying Insect Biomass in Protected Areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide Decline of the Entomofauna: A Review of Its Drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- van Klink, R.; Bowler, D.E.; Gongalsky, K.B.; Swengel, A.B.; Gentile, A.; Chase, J.M. Meta-Analysis Reveals Declines in Terrestrial but Increases in Freshwater Insect Abundances. Science 2020, 368, 417–420. [Google Scholar] [CrossRef]
- Kaur, R.; Shropshire, J.D.; Cross, K.L.; Leigh, B.; Mansueto, A.J.; Stewart, V.; Bordenstein, S.R.; Bordenstein, S.R. Living in the Endosymbiotic World of Wolbachia: A Centennial Review. Cell Host Microbe 2021, 29, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.O. Chemical Communication in the Social Insects: Insect Societies Are Organized Principally by Complex Systems of Chemical Signals. Science 1965, 149, 1064–1071. [Google Scholar] [CrossRef]
- Hoffmann, K.H.; Dettner, K.; Tomaschko, K. Chemical Signals in Insects and Other Arthropods: From Molecular Structure to Physiological Functions. Physiol. Biochem. Zool. 2006, 79, 344–356. [Google Scholar] [CrossRef]
- de Bruyne, M.; Baker, T.C. Odor Detection in Insects: Volatile Codes. J. Chem. Ecol. 2008, 34, 882–897. [Google Scholar] [CrossRef]
- Klowden, M.J.; Palli, S.R. Communication Systems. In Physiological Systems in Insects; Elsevier: Amsterdam, The Netherlands, 2022; pp. 607–653. ISBN 978-0-12-820359-0. [Google Scholar]
- Genilloud, O. Actinomycetes: Still a Source of Novel Antibiotics. Nat. Prod. Rep. 2017, 34, 1203–1232. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, J.; Poorinmohammad, N.; Wink, J. The Role of Actinobacteria in Biotechnology. In Biology and Biotechnology of Actinobacteria; Wink, J., Mohammadipanah, F., Hamedi, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 269–328. ISBN 978-3-319-60338-4. [Google Scholar]
- Ossai, J.; Khatabi, B.; Nybo, S.E.; Kharel, M.K. Renewed Interests in the Discovery of Bioactive Actinomycete Metabolites Driven by Emerging Technologies. J. Appl. Microbiol. 2022, 132, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.K.; Daniel-Ivad, M.; Jeedigunta, S.P.; Li, J.; Iliadi, K.G.; Boulianne, G.L.; Hurd, T.R.; Smibert, C.A.; Nodwell, J.R. Chemical Entrapment and Killing of Insects by Bacteria. Nat. Commun. 2020, 11, 4608. [Google Scholar] [CrossRef]
- Huang, H.; Ren, L.; Li, H.; Schmidt, A.; Gershenzon, J.; Lu, Y.; Cheng, D. The Nesting Preference of an Invasive Ant Is Associated with the Cues Produced by Actinobacteria in Soil. PLoS Pathog. 2020, 16, e1008800. [Google Scholar] [CrossRef]
- Van Moll, L.; De Smet, J.; Cos, P.; Van Campenhout, L. Microbial Symbionts of Insects as a Source of New Antimicrobials: A Review. Crit. Rev. Microbiol. 2021, 47, 562–579. [Google Scholar] [CrossRef]
- Kaltenpoth, M. Actinobacteria as Mutualists: General Healthcare for Insects? Trends Microbiol. 2009, 17, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.B. Insects: True Pioneers in Anti-Infective Therapy and What We Can Learn from Them. Angew. Chem. Int. Ed. 2009, 48, 6394–6396. [Google Scholar] [CrossRef]
- Seipke, R.F.; Kaltenpoth, M.; Hutchings, M.I. Streptomyces as Symbionts: An Emerging and Widespread Theme? FEMS Microbiol. Rev. 2012, 36, 862–876. [Google Scholar] [CrossRef]
- Klassen, J.L. Microbial Secondary Metabolites and Their Impacts on Insect Symbioses. Curr. Opin. Insect Sci. 2014, 4, 15–22. [Google Scholar] [CrossRef]
- Kaltenpoth, M.; Engl, T. Defensive Microbial Symbionts in Hymenoptera. Funct. Ecol. 2014, 28, 315–327. [Google Scholar] [CrossRef]
- Kurtböke, D.İ.; French, J.R.J.; Hayes, R.A.; Quinn, R.J. Eco-Taxonomic Insights into Actinomycete Symbionts of Termites for Discovery of Novel Bioactive Compounds. In Biotechnological Applications of Biodiversity; Mukherjee, J., Ed.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 147, pp. 111–135. ISBN 978-3-662-45096-3. [Google Scholar]
- Berasategui, A.; Shukla, S.; Salem, H.; Kaltenpoth, M. Potential Applications of Insect Symbionts in Biotechnology. Appl. Microbiol. Biotechnol. 2016, 100, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Van Arnam, E.B.; Ruzzini, A.C.; Sit, C.S.; Horn, H.; Pinto-Tomás, A.A.; Currie, C.R.; Clardy, J. Selvamicin, an Atypical Antifungal Polyene from Two Alternative Genomic Contexts. Proc. Natl. Acad. Sci. USA 2016, 113, 12940–12945. [Google Scholar] [CrossRef] [PubMed]
- Worsley, S.F.; Innocent, T.M.; Heine, D.; Murrell, J.C.; Yu, D.W.; Wilkinson, B.; Hutchings, M.I.; Boomsma, J.J.; Holmes, N.A. Symbiotic Partnerships and Their Chemical Interactions in the Leafcutter Ants (Hymenoptera: Formicidae). Myrmecol. News 2018, 27, 59–74. [Google Scholar] [CrossRef]
- Beemelmanns, C.; Guo, H.; Rischer, M.; Poulsen, M. Natural Products from Microbes Associated with Insects. Beilstein J. Org. Chem. 2016, 12, 314–327. [Google Scholar] [CrossRef]
- Batey, S.F.D.; Greco, C.; Hutchings, M.I.; Wilkinson, B. Chemical Warfare between Fungus-Growing Ants and Their Pathogens. Curr. Opin. Chem. Biol. 2020, 59, 172–181. [Google Scholar] [CrossRef]
- Mudalungu, C.M.; Tanga, C.M.; Kelemu, S.; Torto, B. An Overview of Antimicrobial Compounds from African Edible Insects and Their Associated Microbiota. Antibiotics 2021, 10, 621. [Google Scholar] [CrossRef]
- Menegatti, C.; Fukuda, T.T.H.; Pupo, M.T. Chemical Ecology in Insect-Microbe Interactions in the Neotropics. Planta Med. 2021, 87, 38–48. [Google Scholar] [CrossRef]
- Schmidt, S.; Kildgaard, S.; Guo, H.; Beemelmanns, C.; Poulsen, M. The Chemical Ecology of the Fungus-Farming Termite Symbiosis. Nat. Prod. Rep. 2022, 39, 231–248. [Google Scholar] [CrossRef]
- Zakalyukina, Y.V.; Osterman, I.A.; Wolf, J.; Neumann-Schaal, M.; Nouioui, I.; Biryukov, M.V. Amycolatopsis camponoti sp. nov., New Tetracenomycin-Producing Actinomycete Isolated from Carpenter Ant Camponotus vagus. Antonie Leeuwenhoek 2022, 115, 533–544. [Google Scholar] [CrossRef]
- An, J.S.; Lim, H.-J.; Lee, J.Y.; Jang, Y.-J.; Nam, S.-J.; Lee, S.K.; Oh, D.-C. Hamuramicin C, a Cytotoxic Bicyclic Macrolide Isolated from a Wasp Gut Bacterium. J. Nat. Prod. 2022, 85, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Y.; Yu, J.; Xu, Y.; Chen, S. Observation of the Antimicrobial Activities of Two Actinomycetes in the Harvester Ant Messor orientalis. Insects 2022, 13, 691. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, B.; Zhang, S.; Guo, Z.; Li, J. Isolation and Identification of Symbiotic Actinomycetes from Termites in Hainan and Their Activity Against Tropical Plants Pathogenic Fungi. Chin. J. Trop. Crops 2022, 43, 1240–1247. [Google Scholar] [CrossRef]
- Lee, S.R.; Schalk, F.; Schwitalla, J.W.; Guo, H.; Yu, J.S.; Song, M.; Jung, W.H.; de Beer, Z.W.; Beemelmanns, C.; Kim, K.H. GNPS-Guided Discovery of Madurastatin Siderophores from the Termite-Associated Actinomadura sp. RB99**. Chem. Eur. J. 2022, 28, e202200612. [Google Scholar] [CrossRef] [PubMed]
- Promnuan, Y.; Promsai, S.; Pathom-aree, W.; Meelai, S. Apis andreniformis Associated Actinomycetes Show Antimicrobial Activity against Black Rot Pathogen (Xanthomonas campestris pv. Campestris). PeerJ 2021, 9, e12097. [Google Scholar] [CrossRef]
- Dhodary, B.; Spiteller, D. Ammonia Production by Streptomyces Symbionts of Acromyrmex Leaf-Cutting Ants Strongly Inhibits the Fungal Pathogen Escovopsis. Microorganisms 2021, 9, 1622. [Google Scholar] [CrossRef]
- Shin, Y.-H.; Ban, Y.H.; Shin, J.; Park, I.W.; Yoon, S.; Ko, K.; Shin, J.; Nam, S.-J.; Winter, J.M.; Kim, Y.; et al. Azetidine-Bearing Non-Ribosomal Peptides, Bonnevillamides D and E, Isolated from a Carrion Beetle-Associated Actinomycete. J. Org. Chem. 2021, 86, 11149–11159. [Google Scholar] [CrossRef]
- Shin, Y.-H.; Ban, Y.H.; Kim, T.H.; Bae, E.S.; Shin, J.; Lee, S.K.; Jang, J.; Yoon, Y.J.; Oh, D.-C. Structures and Biosynthetic Pathway of Coprisamides C and D, 2-Alkenylcinnamic Acid-Containing Peptides from the Gut Bacterium of the Carrion Beetle Silpha perforata. J. Nat. Prod. 2021, 84, 239–246. [Google Scholar] [CrossRef]
- Li, J.; Sang, M.; Jiang, Y.; Wei, J.; Shen, Y.; Huang, Q.; Li, Y.; Ni, J. Polyene-Producing Streptomyces Spp. From the Fungus-Growing Termite Macrotermes barneyi Exhibit High Inhibitory Activity Against the Antagonistic Fungus Xylaria. Front. Microbiol. 2021, 12, 649962. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, J.; Wu, W.C.; Chen, B.T.; Zhang, S.Q.; Wang, R.; Huang, J.; Guo, Z.K. Discovery of an Unprecedented Benz[a]Anthraquinone-Type Heterodimer from a Rare Actinomycete Amycolatopsis sp. HCa1. Fitoterapia 2021, 155, 105039. [Google Scholar] [CrossRef]
- Ortega, H.E.; Lourenzon, V.B.; Chevrette, M.G.; Ferreira, L.L.G.; Alvarenga, R.F.R.; Melo, W.G.P.; Venâncio, T.; Currie, C.R.; Andricopulo, A.D.; Bugni, T.S.; et al. Antileishmanial Macrolides from Ant-Associated Streptomyces sp. ISID311. Bioorg. Med. Chem. 2021, 32, 116016. [Google Scholar] [CrossRef] [PubMed]
- Matarrita-Carranza, B.; Murillo-Cruz, C.; Avendaño, R.; Ríos, M.I.; Chavarría, M.; Gómez-Calvo, M.L.; Tamayo-Castillo, G.; Araya, J.J.; Pinto-Tomás, A.A. Streptomyces sp. M54: An Actinobacteria Associated with a Neotropical Social Wasp with High Potential for Antibiotic Production. Antonie Leeuwenhoek 2021, 114, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-F.; Wu, J.; Li, S.; Li, Q.; Jin, L.-P.; Yin, C.-P.; Zhang, Y.-L. Antibacterial Potential of Termite-Associated Streptomyces spp. ACS Omega 2021, 6, 4329–4334. [Google Scholar] [CrossRef]
- Fukuda, T.T.H.; Helfrich, E.J.N.; Mevers, E.; Melo, W.G.P.; Van Arnam, E.B.; Andes, D.R.; Currie, C.R.; Pupo, M.T.; Clardy, J. Specialized Metabolites Reveal Evolutionary History and Geographic Dispersion of a Multilateral Symbiosis. ACS Cent. Sci. 2021, 7, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Mevers, E.; Pishchany, G.; Whaley, S.G.; Rock, C.O.; Andes, D.R.; Currie, C.R.; Pupo, M.T.; Clardy, J. Chemical Exchanges between Multilateral Symbionts. Org. Lett. 2021, 23, 1648–1652. [Google Scholar] [CrossRef]
- Du, Y.E.; Byun, W.S.; Lee, S.B.; Hwang, S.; Shin, Y.-H.; Shin, B.; Jang, Y.-J.; Hong, S.; Shin, J.; Lee, S.K.; et al. Formicins, N-Acetylcysteamine-Bearing Indenone Thioesters from a Wood Ant-Associated Bacterium. Org. Lett. 2020, 22, 5337–5341. [Google Scholar] [CrossRef]
- Santamaría, R.I.; Martínez-Carrasco, A.; Sánchez de la Nieta, R.; Torres-Vila, L.M.; Bonal, R.; Martín, J.; Tormo, R.; Reyes, F.; Genilloud, O.; Díaz, M. Characterization of Actinomycetes Strains Isolated from the Intestinal Tract and Feces of the Larvae of the Longhorn Beetle Cerambyx welensii. Microorganisms 2020, 8, 2013. [Google Scholar] [CrossRef]
- Baranova, A.A.; Chistov, A.A.; Tyurin, A.P.; Prokhorenko, I.A.; Korshun, V.A.; Biryukov, M.V.; Alferova, V.A.; Zakalyukina, Y.V. Chemical Ecology of Streptomyces albidoflavus Strain A10 Associated with Carpenter Ant Camponotus vagus. Microorganisms 2020, 8, 1948. [Google Scholar] [CrossRef]
- Hwang, S.; Le, L.T.H.L.; Jo, S.-I.; Shin, J.; Lee, M.J.; Oh, D.-C. Pentaminomycins C–E: Cyclic Pentapeptides as Autophagy Inducers from a Mealworm Beetle Gut Bacterium. Microorganisms 2020, 8, 1390. [Google Scholar] [CrossRef]
- Hwang, S.; Shin, D.; Kim, T.H.; An, J.S.; Jo, S.-I.; Jang, J.; Hong, S.; Shin, J.; Oh, D.-C. Structural Revision of Lydiamycin A by Reinvestigation of the Stereochemistry. Org. Lett. 2020, 22, 3855–3859. [Google Scholar] [CrossRef]
- Chang, P.T.; Rao, K.; Longo, L.O.; Lawton, E.S.; Scherer, G.; Van Arnam, E.B. Thiopeptide Defense by an Ant’s Bacterial Symbiont. J. Nat. Prod. 2020, 83, 725–729. [Google Scholar] [CrossRef] [PubMed]
- An, J.S.; Lee, J.Y.; Kim, E.; Ahn, H.; Jang, Y.-J.; Shin, B.; Hwang, S.; Shin, J.; Yoon, Y.J.; Lee, S.K.; et al. Formicolides A and B, Antioxidative and Antiangiogenic 20-Membered Macrolides from a Wood Ant Gut Bacterium. J. Nat. Prod. 2020, 83, 2776–2784. [Google Scholar] [CrossRef] [PubMed]
- Menegatti, C.; Lourenzon, V.B.; Rodríguez-Hernández, D.; da Paixão Melo, W.G.; Ferreira, L.L.G.; Andricopulo, A.D.; do Nascimento, F.S.; Pupo, M.T. Meliponamycins: Antimicrobials from Stingless Bee-Associated Streptomyces sp. J. Nat. Prod. 2020, 83, 610–616. [Google Scholar] [CrossRef]
- Zhang, L.; Song, T.; Wu, J.; Zhang, S.; Yin, C.; Huang, F.; Hang, Y.; Abbas, N.; Liu, X.; Zhang, Y. Antibacterial and Cytotoxic Metabolites of Termite-Associated Streptomyces sp. BYF63. J. Antibiot. 2020, 73, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, Z.; Zhao, J.; Zhuang, X.; Cao, P.; Guo, X.; Liu, C.; Xiang, W. Community Composition, Antifungal Activity and Chemical Analyses of Ant-Derived Actinobacteria. Front. Microbiol. 2020, 11, 201. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ou, P.; Liu, L.; Jin, X. Anti-MRSA Activity of Actinomycin X2 and Collismycin A Produced by Streptomyces globisporus WA5-2-37 From the Intestinal Tract of American Cockroach (Periplaneta americana). Front. Microbiol. 2020, 11, 555. [Google Scholar] [CrossRef]
- Grubbs, K.J.; Surup, F.; Biedermann, P.H.W.; McDonald, B.R.; Klassen, J.L.; Carlson, C.M.; Clardy, J.; Currie, C.R. Cycloheximide-Producing Streptomyces Associated With Xyleborinus saxesenii and Xyleborus affinis Fungus-Farming Ambrosia Beetles. Front. Microbiol. 2020, 11, 562140. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, A.; Xie, W.; Wang, M.; Zheng, D.; Feng, M. Hexokinase II Inhibitory Effect of Secondary Metabolites Derived from a Streptomyces sp. Associated with Mud Dauber Wasp. Chem. Biodivers. 2020, 17, e2000140. [Google Scholar] [CrossRef]
- Vikeli, E.; Widdick, D.A.; Batey, S.F.D.; Heine, D.; Holmes, N.A.; Bibb, M.J.; Martins, D.J.; Pierce, N.E.; Hutchings, M.I.; Wilkinson, B. In Situ Activation and Heterologous Production of a Cryptic Lantibiotic from an African Plant Ant-Derived Saccharopolyspora Species. Appl. Environ. Microbiol. 2020, 86, e01876-19. [Google Scholar] [CrossRef]
- Zakalyukina, Y.V.; Birykov, M.V.; Lukianov, D.A.; Shiriaev, D.I.; Komarova, E.S.; Skvortsov, D.A.; Kostyukevich, Y.; Tashlitsky, V.N.; Polshakov, V.I.; Nikolaev, E.; et al. Nybomycin-Producing Streptomyces Isolated from Carpenter Ant Camponotus vagus. Biochimie 2019, 160, 93–99. [Google Scholar] [CrossRef]
- Ortega, H.; Batista, J.; Melo, W.; de Paula, G.; Pupo, M. Structure and Absolute Configuration of Secondary Metabolites from Two Strains of Streptomyces chartreusis Associated with Attine Ants. J. Braz. Chem. Soc. 2019, 30, 2672–2680. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, D.; Melo, W.G.P.; Menegatti, C.; Lourenzon, V.B.; do Nascimento, F.S.; Pupo, M.T. Actinobacteria Associated with Stingless Bees Biosynthesize Bioactive Polyketides against Bacterial Pathogens. New J. Chem. 2019, 43, 10109–10117. [Google Scholar] [CrossRef]
- Ortega, H.E.; Ferreira, L.L.G.; Melo, W.G.P.; Oliveira, A.L.L.; Ramos Alvarenga, R.F.; Lopes, N.P.; Bugni, T.S.; Andricopulo, A.D.; Pupo, M.T. Antifungal Compounds from Streptomyces Associated with Attine Ants Also Inhibit Leishmania donovani. PLoS Negl. Trop. Dis. 2019, 13, e0007643. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.-H.; Beom, J.Y.; Chung, B.; Shin, Y.; Byun, W.S.; Moon, K.; Bae, M.; Lee, S.K.; Oh, K.-B.; Shin, J.; et al. Bombyxamycins A and B, Cytotoxic Macrocyclic Lactams from an Intestinal Bacterium of the Silkworm Bombyx mori. Org. Lett. 2019, 21, 1804–1808. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-Y.; Lee, S.R.; Hwang, J.Y.; Benndorf, R.; Beemelmanns, C.; Chung, S.J.; Kim, K.H. Fridamycin A, a Microbial Natural Product, Stimulates Glucose Uptake without Inducing Adipogenesis. Nutrients 2019, 11, 765. [Google Scholar] [CrossRef]
- Chevrette, M.G.; Carlson, C.M.; Ortega, H.E.; Thomas, C.; Ananiev, G.E.; Barns, K.J.; Book, A.J.; Cagnazzo, J.; Carlos, C.; Flanigan, W.; et al. The Antimicrobial Potential of Streptomyces from Insect Microbiomes. Nat. Commun. 2019, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Cambronero-Heinrichs, J.C.; Matarrita-Carranza, B.; Murillo-Cruz, C.; Araya-Valverde, E.; Chavarría, M.; Pinto-Tomás, A.A. Phylogenetic Analyses of Antibiotic-Producing Streptomyces sp. Isolates Obtained from the Stingless-Bee Tetragonisca angustula (Apidae: Meliponini). Microbiology 2019, 165, 292–301. [Google Scholar] [CrossRef]
- Martinez, A.F.C.; de Almeida, L.G.; Moraes, L.A.B.; Cônsoli, F.L. Microbial Diversity and Chemical Multiplicity of Culturable, Taxonomically Similar Bacterial Symbionts of the Leaf-Cutting Ant Acromyrmex coronatus. Microb. Ecol. 2019, 77, 1067–1081. [Google Scholar] [CrossRef]
- Song, Y.-J.; Zheng, H.-B.; Peng, A.-H.; Ma, J.-H.; Lu, D.-D.; Li, X.; Zhang, H.-Y.; Xie, W.-D. Strepantibins A–C: Hexokinase II Inhibitors from a Mud Dauber Wasp Associated Streptomyces sp. J. Nat. Prod. 2019, 82, 1114–1119. [Google Scholar] [CrossRef]
- Hong, S.-H.; Ban, Y.H.; Byun, W.S.; Kim, D.; Jang, Y.-J.; An, J.S.; Shin, B.; Lee, S.K.; Shin, J.; Yoon, Y.J.; et al. Camporidines A and B: Antimetastatic and Anti-Inflammatory Polyketide Alkaloids from a Gut Bacterium of Camponotus kiusiuensis. J. Nat. Prod. 2019, 82, 903–910. [Google Scholar] [CrossRef]
- Han, H.; Guo, Z.-K.; Zhang, B.; Zhang, M.; Shi, J.; Li, W.; Jiao, R.-H.; Tan, R.-X.; Ge, H.-M. Bioactive Phenazines from an Earwig-Associated Streptomyces sp. Chin. J. Nat. Med. 2019, 17, 475–480. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, D.; Yu, J.S.; Benndorf, R.; Lee, S.; Lee, D.-S.; Huh, J.; de Beer, Z.W.; Kim, Y.H.; Beemelmanns, C.; et al. Natalenamides A–C, Cyclic Tripeptides from the Termite-Associated Actinomadura sp. RB99. Molecules 2018, 23, 3003. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Piao, C.; Yu, Y.; Cao, P.; Li, C.; Yang, F.; Li, M.; Xiang, W.; Liu, C. Streptomyces capitiformicae sp. nov., a Novel Actinomycete Producing Angucyclinone Antibiotics Isolated from the Head of Camponotus japonicus Mayr. Int. J. Syst. Evol. Microbiol. 2018, 68, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kang, K.; Lee, H.-J.; Kim, K. Chemical Characterization of a Renoprotective Metabolite from Termite-Associated Streptomyces sp. RB1 against Cisplatin-Induced Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 174. [Google Scholar] [CrossRef]
- Guo, Z.K.; Wang, R.; Chen, F.X.; Liu, T.M. Streptoxamine, an Unprecedented Benzoisoindole-Deferoxamine Hybrid from the Locust-Derived Streptomyces sp. HKHCa2. Fitoterapia 2018, 127, 25–28. [Google Scholar] [CrossRef]
- Liu, C.; Han, C.; Jiang, S.; Zhao, X.; Tian, Y.; Yan, K.; Wang, X.; Xiang, W. Streptomyces lasii sp. nov., a Novel Actinomycete with Antifungal Activity Isolated from the Head of an Ant (Lasius flavus). Curr. Microbiol. 2018, 75, 353–358. [Google Scholar] [CrossRef]
- Guo, H.; Benndorf, R.; König, S.; Leichnitz, D.; Weigel, C.; Peschel, G.; Berthel, P.; Kaiser, M.; Steinbeck, C.; Werz, O.; et al. Expanding the Rubterolone Family: Intrinsic Reactivity and Directed Diversification of PKS-Derived Pyrans. Chem. Eur. J. 2018, 24, 11319–11324. [Google Scholar] [CrossRef]
- Benndorf, R.; Guo, H.; Sommerwerk, E.; Weigel, C.; Garcia-Altares, M.; Martin, K.; Hu, H.; Küfner, M.; de Beer, Z.; Poulsen, M.; et al. Natural Products from Actinobacteria Associated with Fungus-Growing Termites. Antibiotics 2018, 7, 83. [Google Scholar] [CrossRef]
- Guo, H.; Benndorf, R.; Leichnitz, D.; Klassen, J.L.; Vollmers, J.; Görls, H.; Steinacker, M.; Weigel, C.; Dahse, H.-M.; Kaster, A.-K.; et al. Isolation, Biosynthesis and Chemical Modifications of Rubterolones A-F: Rare Tropolone Alkaloids from Actinomadura sp. 5-2. Chem. Eur. J. 2017, 23, 9338–9345. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, C.L.; Xiao, Y.S.; Zhang, B.; Deng, X.Z.; Yang, L.; Shi, J.; Wang, Y.S.; Li, W.; Jiao, R.H.; et al. Aurachin SS, a New Antibiotic from Streptomyces sp. NA04227. J. Antibiot. 2017, 70, 853–855. [Google Scholar] [CrossRef]
- Cao, T.; Mu, S.; Lu, C.; Zhao, S.; Li, D.; Yan, K.; Xiang, W.; Liu, C. Streptomyces amphotericinicus sp. nov., an Amphotericin-Producing Actinomycete Isolated from the Head of an Ant (Camponotus japonicus Mayr). Int. J. Syst. Evol. Microbiol. 2017, 67, 4967–4973. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, S.; Li, Y.; Jiang, S.; Zhao, Y.; Li, J.; Yan, K.; Wang, X.; Xiang, W.; Liu, C. Streptomyces lasiicapitis sp. nov., an Actinomycete That Produces Kanchanamycin, Isolated from the Head of an Ant (Lasius fuliginosus L.). Int. J. Syst. Evol. Microbiol. 2017, 67, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.E.; Batista, J.M.; Melo, W.G.P.; Clardy, J.; Pupo, M.T. Absolute Configurations of Griseorhodins A and C. Tetrahedron Lett. 2017, 58, 4721–4723. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Munnoch, J.T.; Devine, R.; Holmes, N.A.; Seipke, R.F.; Wilkinson, K.A.; Wilkinson, B.; Hutchings, M.I. Formicamycins, Antibacterial Polyketides Produced by Streptomyces formicae Isolated from African Tetraponera Plant-Ants. Chem. Sci. 2017, 8, 3218–3227. [Google Scholar] [CrossRef] [PubMed]
- Mevers, E.; Chouvenc, T.; Su, N.-Y.; Clardy, J. Chemical Interaction among Termite-Associated Microbes. J. Chem. Ecol. 2017, 43, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.-H.; Bae, S.; Sim, J.; Hur, J.; Jo, S.-I.; Shin, J.; Suh, Y.-G.; Oh, K.-B.; Oh, D.-C. Nicrophorusamides A and B, Antibacterial Chlorinated Cyclic Peptides from a Gut Bacterium of the Carrion Beetle Nicrophorus concolor. J. Nat. Prod. 2017, 80, 2962–2968. [Google Scholar] [CrossRef]
- Beemelmanns, C.; Ramadhar, T.R.; Kim, K.H.; Klassen, J.L.; Cao, S.; Wyche, T.P.; Hou, Y.; Poulsen, M.; Bugni, T.S.; Currie, C.R.; et al. Macrotermycins A–D, Glycosylated Macrolactams from a Termite-Associated Amycolatopsis sp. M39. Org. Lett. 2017, 19, 1000–1003. [Google Scholar] [CrossRef]
- Wyche, T.P.; Ruzzini, A.C.; Beemelmanns, C.; Kim, K.H.; Klassen, J.L.; Cao, S.; Poulsen, M.; Bugni, T.S.; Currie, C.R.; Clardy, J. Linear Peptides Are the Major Products of a Biosynthetic Pathway That Encodes for Cyclic Depsipeptides. Org. Lett. 2017, 19, 1772–1775. [Google Scholar] [CrossRef]
- Ma, S.Y.; Xiao, Y.S.; Zhang, B.; Shao, F.L.; Guo, Z.K.; Zhang, J.J.; Jiao, R.H.; Sun, Y.; Xu, Q.; Tan, R.X.; et al. Amycolamycins A and B, Two Enediyne-Derived Compounds from a Locust-Associated Actinomycete. Org. Lett. 2017, 19, 6208–6211. [Google Scholar] [CrossRef]
- Xiao, Y.S.; Zhang, B.; Zhang, M.; Guo, Z.K.; Deng, X.Z.; Shi, J.; Li, W.; Jiao, R.H.; Tan, R.X.; Ge, H.M. Rifamorpholines A–E, Potential Antibiotics from Locust-Associated Actinobacteria Amycolatopsis sp. Hca4. Org. Biomol. Chem. 2017, 15, 3909–3916. [Google Scholar] [CrossRef]
- Human, Z.R.; Slippers, B.; De Beer, Z.W.; Wingfield, M.J.; Venter, S.N. Antifungal Actinomycetes Associated with the Pine Bark Beetle, Orthotomicus erosus, in South Africa. S. Afr. J. Sci. 2017, 113, 1–7. [Google Scholar] [CrossRef]
- Arango, R.A.; Carlson, C.M.; Currie, C.R.; McDonald, B.R.; Book, A.J.; Green, F.; Lebow, N.K.; Raffa, K.F. Antimicrobial Activity of Actinobacteria Isolated From the Guts of Subterranean Termites. Environ. Entomol. 2016, 45, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Axenov-Gribanov, D.; Rebets, Y.; Tokovenko, B.; Voytsekhovskaya, I.; Timofeyev, M.; Luzhetskyy, A. The Isolation and Characterization of Actinobacteria from Dominant Benthic Macroinvertebrates Endemic to Lake Baikal. Folia Microbiol. 2016, 61, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Holmes, N.A.; Innocent, T.M.; Heine, D.; Bassam, M.A.; Worsley, S.F.; Trottmann, F.; Patrick, E.H.; Yu, D.W.; Murrell, J.C.; Schiøtt, M.; et al. Genome Analysis of Two Pseudonocardia Phylotypes Associated with Acromyrmex Leafcutter Ants Reveals Their Biosynthetic Potential. Front. Microbiol. 2016, 7, 2073. [Google Scholar] [CrossRef]
- Liu, S.; Xu, M.; Zhang, H.; Qi, H.; Zhang, J.; Liu, C.; Wang, J.; Xiang, W.; Wang, X. New Cytotoxic Spectinabilin Derivative from Ant-Associated Streptomyces sp. 1H-GS5. J. Antibiot. 2016, 69, 128–131. [Google Scholar] [CrossRef]
- Liu, C.-X.; Liu, S.-H.; Zhao, J.-W.; Zhang, J.; Wang, X.-J.; Li, J.-S.; Wang, J.-D.; Xiang, W.-S. A New Spectinabilin Derivative with Cytotoxic Activity from Ant-Derived Streptomyces sp. 1H-GS5. J. As. Nat. Prod. Res. 2017, 19, 924–929. [Google Scholar] [CrossRef]
- Kang, H.R.; Lee, D.; Benndorf, R.; Jung, W.H.; Beemelmanns, C.; Kang, K.S.; Kim, K.H. Termisoflavones A–C, Isoflavonoid Glycosides from Termite-Associated Streptomyces sp. RB1. J. Nat. Prod. 2016, 79, 3072–3078. [Google Scholar] [CrossRef]
- Um, S.; Bach, D.-H.; Shin, B.; Ahn, C.-H.; Kim, S.-H.; Bang, H.-S.; Oh, K.-B.; Lee, S.K.; Shin, J.; Oh, D.-C. Naphthoquinone–Oxindole Alkaloids, Coprisidins A and B, from a Gut-Associated Bacterium in the Dung Beetle, Copris tripartitus. Org. Lett. 2016, 18, 5792–5795. [Google Scholar] [CrossRef]
- Bai, L.; Liu, C.; Guo, L.; Piao, C.; Li, Z.; Li, J.; Jia, F.; Wang, X.; Xiang, W. Streptomyces formicae Sp. Nov., a Novel Actinomycete Isolated from the Head of Camponotus japonicus Mayr. Antonie Leeuwenhoek 2016, 109, 253–261. [Google Scholar] [CrossRef]
- The genus Escovopsis was recently separated into 3 distinct genera, for details see: Montoya, Q.V.; Martiarena, M.J.S.; Bizarria, R.; Gerardo, N.M.; Rodrigues, A. Fungi Inhabiting Attine Ant Colonies: Reassessment of the Genus Escovopsis and Description of Luteomyces and Sympodiorosea Gens. Nov. IMA Fungus 2021, 12, 23. [CrossRef]
- Alferova, V.A.; Shuvalov, M.V.; Korshun, V.A.; Tyurin, A.P. Naphthoquinone-Derived Polyol Macrolides from Natural Sources. Russ. Chem. Bull. 2019, 68, 955–966. [Google Scholar] [CrossRef]
- Berdy, B.; Spoering, A.L.; Ling, L.L.; Epstein, S.S. In Situ Cultivation of Previously Uncultivable Microorganisms Using the Ichip. Nat. Protoc. 2017, 12, 2232–2242. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranova, A.A.; Zakalyukina, Y.V.; Ovcharenko, A.A.; Korshun, V.A.; Tyurin, A.P. Antibiotics from Insect-Associated Actinobacteria. Biology 2022, 11, 1676. https://doi.org/10.3390/biology11111676
Baranova AA, Zakalyukina YV, Ovcharenko AA, Korshun VA, Tyurin AP. Antibiotics from Insect-Associated Actinobacteria. Biology. 2022; 11(11):1676. https://doi.org/10.3390/biology11111676
Chicago/Turabian StyleBaranova, Anna A., Yuliya V. Zakalyukina, Anna A. Ovcharenko, Vladimir A. Korshun, and Anton P. Tyurin. 2022. "Antibiotics from Insect-Associated Actinobacteria" Biology 11, no. 11: 1676. https://doi.org/10.3390/biology11111676
APA StyleBaranova, A. A., Zakalyukina, Y. V., Ovcharenko, A. A., Korshun, V. A., & Tyurin, A. P. (2022). Antibiotics from Insect-Associated Actinobacteria. Biology, 11(11), 1676. https://doi.org/10.3390/biology11111676