Phase Response Curve to Light under Ambulatory Conditions: A Pilot Study for Potential Application to Daylight Saving Time Transitions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
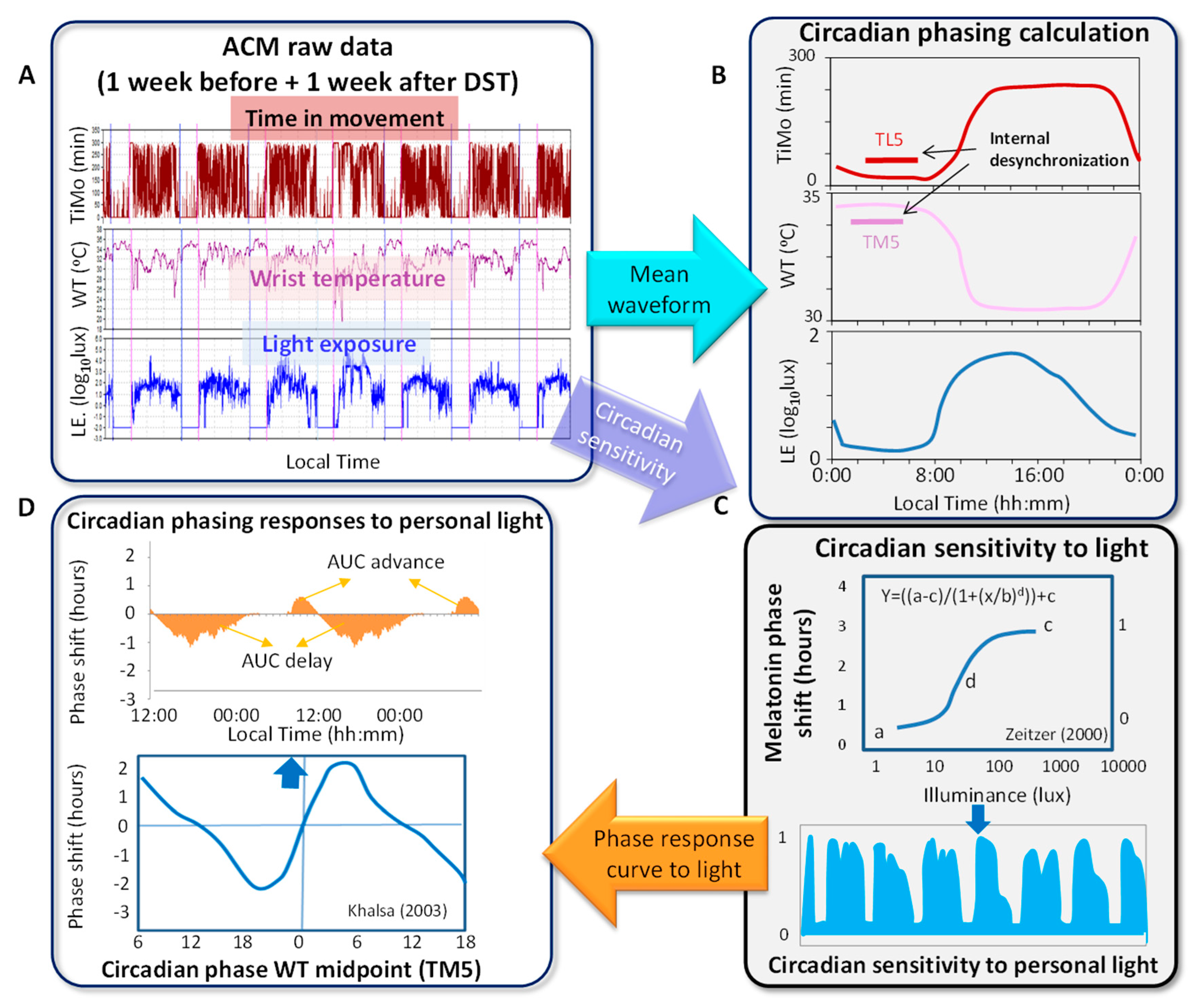
2.2. Ambulatory Circadian Monitoring
2.3. Circadian and Sleep Parameters
2.4. Phase Response Curve
2.5. Chronotype
2.6. Internal Desynchronization
2.7. Statistical Analysis
3. Results
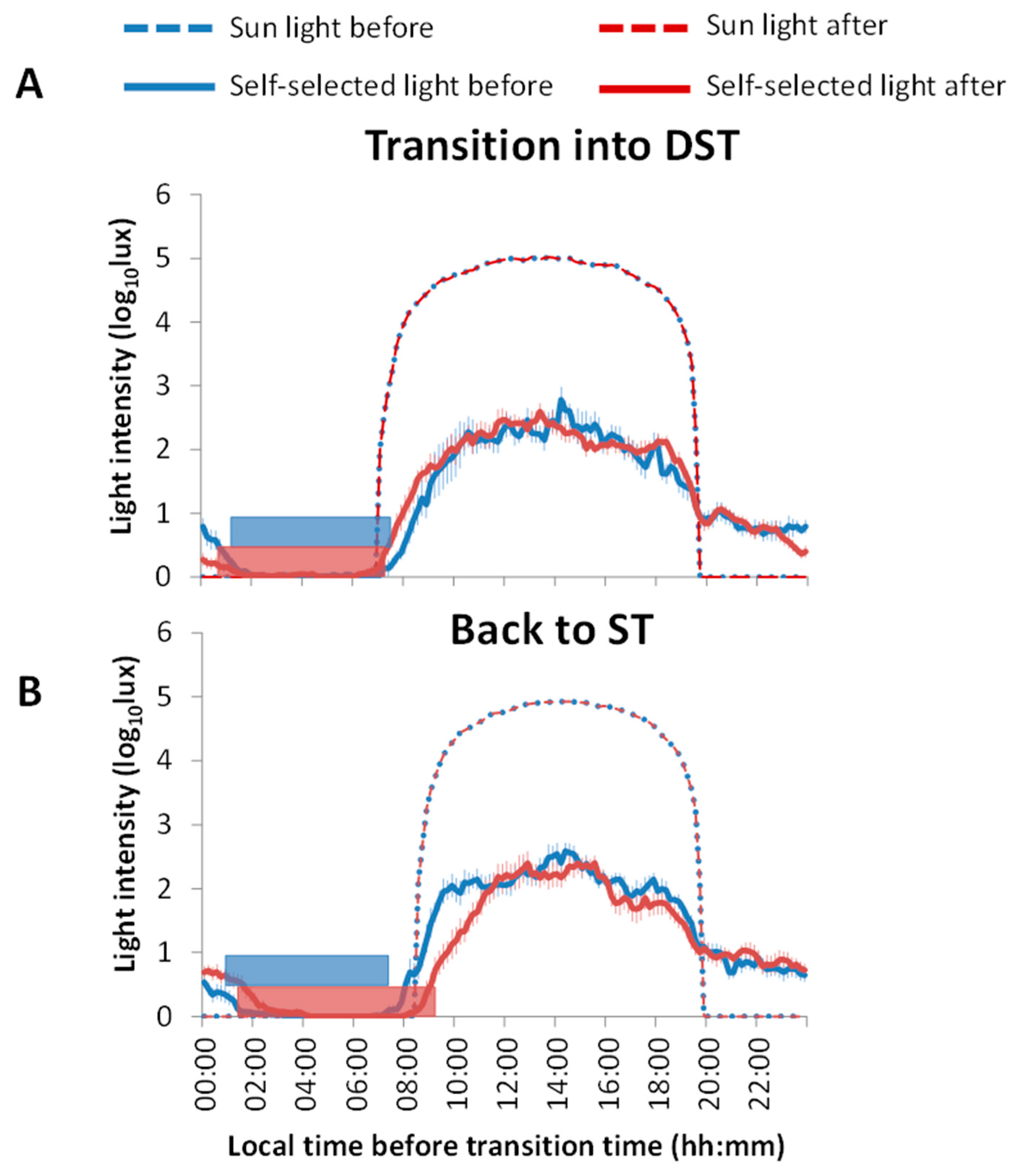
3.1. Light Exposure and Sleep Patterns
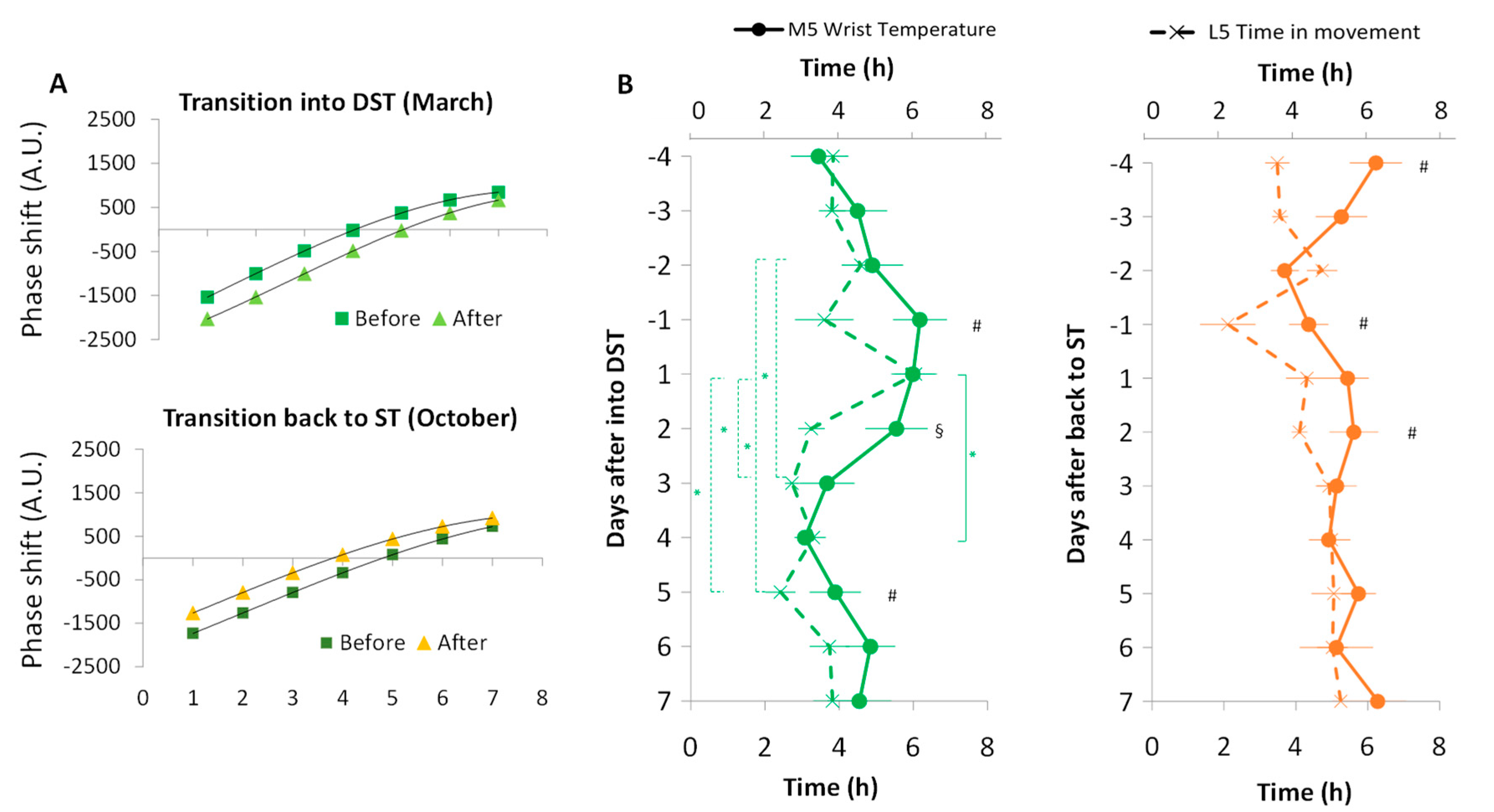
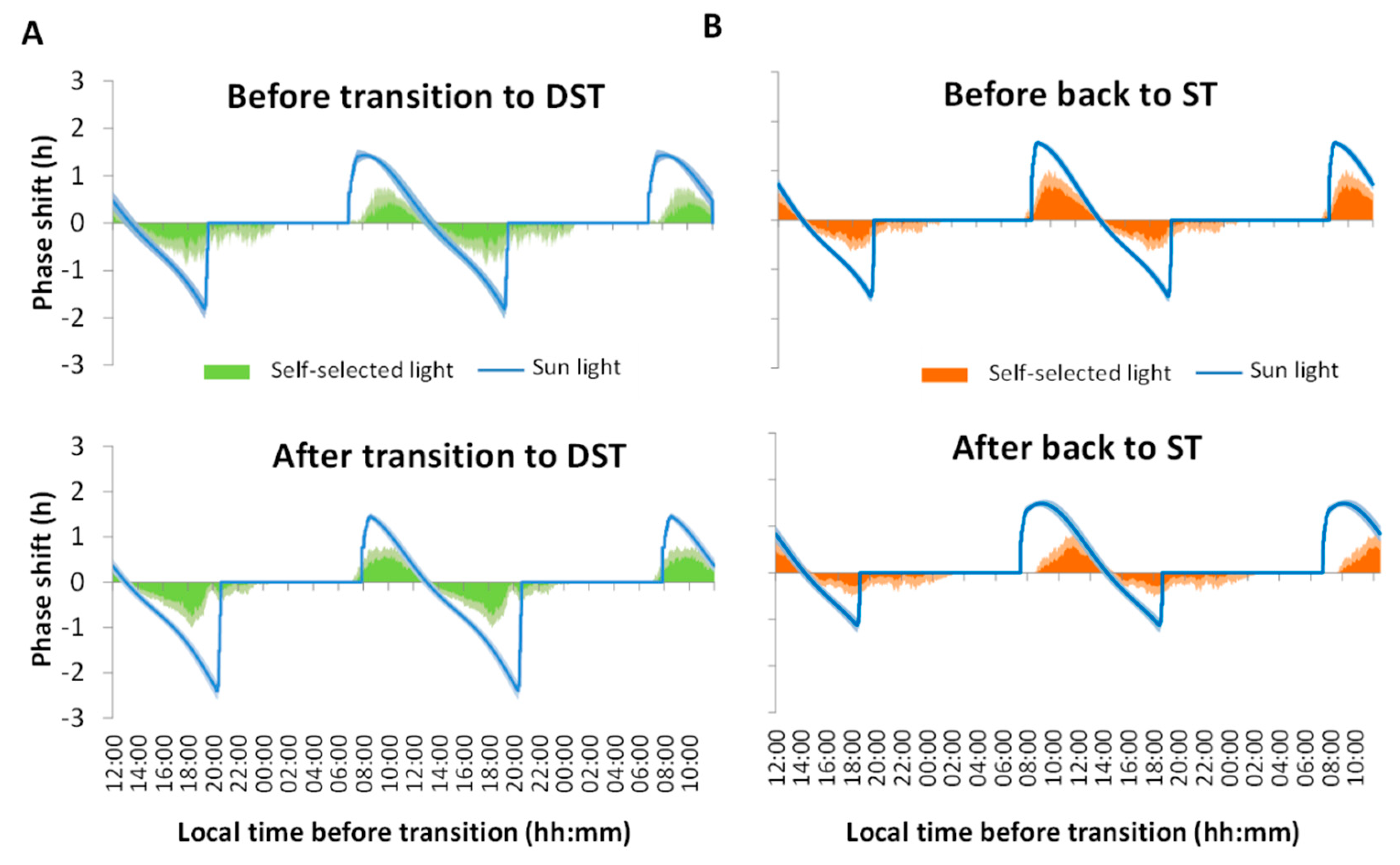
3.2. Phase Shifts
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kantermann, T.; Juda, M.; Merrow, M.; Roenneberg, T. The Human Circadian Clock’s Seasonal Adjustment Is Disrupted by Daylight Saving Time. Curr. Biol. 2007, 17, 1996–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aries, M.B.C.; Newsham, G.R. Effect of daylight saving time on lighting energy use: A literature review. Energy Policy 2008, 36, 1858–1866. [Google Scholar] [CrossRef] [Green Version]
- Thorsen, S.; Bikos, K.; Brastad, I.; Buckle, A.; Gundersen, M.; Jones, G.; Kher, A.; Rehberger, G. Time and Date-Daylight Saving Time. Available online: https://www.timeanddate.com/time/dst/ (accessed on 25 February 2022).
- Roenneberg, T.; Winnebeck, E.C.; Klerman, E.B. Daylight saving time and artificial time zones—A battle between biological and social times. Front. Physiol. 2019, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gaski, J.F.; Sagarin, J. Detrimental effects of daylight-saving time on SAT scores. J. Neurosci. Psychol. Econ. 2011, 4, 44–53. [Google Scholar] [CrossRef]
- Kotchen, M.J.; Grant, L.E. Does Daylight Saving Time Save Energy? Evidence from a Natural Experiment in Indiana. Natl. Bur. Econ. Res. 2008, 93, 14429. [Google Scholar] [CrossRef]
- Choi, S.; Pellen, A.; Masson, V. How does daylight saving time affect electricity demand? An answer using aggregate data from a natural experiment in Western Australia. Energy Econ. 2017, 66, 247–260. [Google Scholar] [CrossRef]
- Karasu, S. The effect of daylight saving time options on electricity consumption of Turkey. Energy 2010, 35, 3773–3782. [Google Scholar] [CrossRef]
- Alencar, J.C.N.; Leocadio-Miguel, M.A.; Duarte, L.L.; Louzada, F.; Fontenele Araujo, J.; Pedrazzoli, M. Self-reported discomfort associated with Daylight Saving Time in Brazilian tropical and subtropical zones. Ann. Hum. Biol. 2017, 44, 628–635. [Google Scholar] [CrossRef]
- Bellia, L.; Acosta, I.; Campano, M.Á.; Fragliasso, F. Impact of daylight saving time on lighting energy consumption and on the biological clock for occupants in office buildings. Sol. Energy 2020, 211, 1347–1364. [Google Scholar] [CrossRef]
- Küfeoğlu, S.; Üçler, Ş.; Eskicioğlu, F.; Öztürk, E.B.; Chen, H. Daylight Saving Time policy and energy consumption. Energy Rep. 2021, 7, 5013–5025. [Google Scholar] [CrossRef]
- Hill, S.I.; Desobry, F.; Garnsey, E.W.; Chong, Y.F. The impact on energy consumption of daylight saving clock changes. Energy Policy 2010, 38, 4955–4965. [Google Scholar] [CrossRef]
- Verdejo, H.; Becker, C.; Echiburu, D.; Escudero, W.; Fucks, E.; Jose Reveco, M. Impact of daylight saving time on the Chilean residential consumption. Energy Policy 2016, 88, 456–464. [Google Scholar] [CrossRef]
- Kudela, P.; Havranek, T.; Herman, D.; Irsova, Z. Does daylight saving time save electricity? Evidence from Slovakia. Energy Policy 2020, 137, 111146. [Google Scholar] [CrossRef] [Green Version]
- Awad Momani, M.; Yatim, B.; Ali, M.A.M. The impact of the daylight saving time on electricity consumption—A case study from Jordan. Energy Policy 2009, 37, 2042–2051. [Google Scholar] [CrossRef]
- Mirza, F.M.; Bergland, O. The impact of daylight saving time on electricity consumption: Evidence from southern Norway and Sweden. Energy Policy 2011, 39, 3558–3571. [Google Scholar] [CrossRef]
- Harrison, Y. The impact of daylight saving time on sleep and related behaviours. Sleep Med. Rev. 2013, 17, 285–292. [Google Scholar] [CrossRef]
- Tarquini, R.; Carbone, A.; Martinez, M.; Mazzoccoli, G. Daylight saving time and circadian rhythms in the neuro-endocrine-immune system: Impact on cardiovascular health. Intern. Emerg. Med. 2019, 14, 17–19. [Google Scholar] [CrossRef]
- Manfredini, R.; Fabbian, F.; Cappadona, R.; Modesti, P.A. Daylight saving time, circadian rhythms, and cardiovascular health. Intern. Emerg. Med. 2018, 13, 641–646. [Google Scholar] [CrossRef] [Green Version]
- Manfredini, R.; Fabbian, F.; Cappadona, R.; De Giorgi, A.; Bravi, F.; Carradori, T.; Flacco, M.E.; Manzoli, L. Daylight Saving Time and acute myocardial infarction: A meta-analysis. J. Clin. Med. 2019, 8, 404. [Google Scholar] [CrossRef] [Green Version]
- Sipilä, J.O.T.; Ruuskanen, J.O.; Rautava, P.; Kytö, V. Changes in ischemic stroke occurrence following daylight saving time transitions. Sleep Med. 2016, 27–28, 20–24. [Google Scholar] [CrossRef]
- Varughese, J.; Allen, R.P. Fatal accidents following changes in daylight savings time: The American experience. Sleep Med. 2001, 2, 31–36. [Google Scholar] [CrossRef]
- Coren, S. Daylight savings time and traffic accidents. N. Engl. J. Med. 1996, 334, 924–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, S.A.; Preusser, D.F.; Lund, A.K.; Zador, P.L.; Ulmer, R.G. Daylight Saving Time and Motor Vehicle Crashes: The Reduction in Pedestrian and Vehicle Occupant Fatalities. Am. J. Public Health 1995, 85, 92–95. [Google Scholar] [CrossRef] [Green Version]
- Lambe, M.; Cummings, P. The shift to and from daylight savings time and motor vehicle crashes. Accid. Anal. Prev. 2000, 32, 609–611. [Google Scholar] [CrossRef]
- Huang, A.; Levinson, D. The effects of daylight saving time on vehicle crashes in Minnesota. J. Saf. Res. 2010, 41, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Prats-Uribe, A.; Tobías, A.; Prieto-Alhambra, D. Excess Risk of Fatal Road Traffic Accidents on the Day of Daylight Saving Time Change. Epidemiology 2018, 29, E44–E45. [Google Scholar] [CrossRef]
- Lahti, T.A.; Haukka, J.; Lönnqvist, J.; Partonen, T. Daylight saving time transitions and hospital treatments due to accidents or manic episodes. BMC Public Health 2008, 8, 74. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, C.M.; Blake, F.; Fossey, E.; Adams, B. Daylight saving time in psychiatric illness. J. Affect. Disord. 1990, 19, 177–181. [Google Scholar] [CrossRef]
- Berk, M.; Dodd, S.; Hallam, K.; Berk, L.; Gleeson, J.; Henry, M. Small shifts in diurnal rhythms are associated with an increase in suicide: The effect of daylight saving. Sleep Biol. Rhythm. 2008, 6, 22–25. [Google Scholar] [CrossRef]
- Monk, T.H.; Folkard, S. Adjusting to the changes to and from Daylight Saving Time. Nature 1976, 261, 688–689. [Google Scholar] [CrossRef]
- Harrison, Y. Individual response to the end of Daylight Saving Time is largely dependent on habitual sleep duration. Biol. Rhythm Res. 2012, 44, 391–401. [Google Scholar] [CrossRef]
- Monk, T.H.; Aplin, L.C. Spring and Autumn daylight saving time changes: Studies of adjustment in sleep timings, mood, and efficiency. Ergonomics 1980, 23, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Lahti, T.A.; Leppämäki, S.; Lönnqvist, J.; Partonen, T. Transition to daylight saving time reduces sleep duration plus sleep efficiency of the deprived sleep. Neurosci. Lett. 2006, 406, 174–177. [Google Scholar] [CrossRef]
- Lahti, T.A.; Leppämäki, S.; Ojanen, S.M.; Haukka, J.; Tuulio-Henriksson, A.; Lönnqvist, J.; Partonen, T. Transition into daylight saving time influences the fragmentation of the rest-activity cycle. J. Circadian Rhythm. 2006, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahti, T.A.; Leppämäki, S.; Lönnqvist, J.; Partonen, T. Transitions into and out of daylight saving time compromise sleep and the rest-activity cycles. BMC Physiol. 2008, 8, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelson, W. Sleep Time: Media Hype vs. Diary Data. Soc. Indic. Res. 2010, 101, 275–280. [Google Scholar] [CrossRef]
- Allebrandt, K.V.; Teder-Laving, M.; Kantermann, T.; Peters, A.; Campbell, H.; Rudan, I.; Wilson, J.F.; Metspalu, A.; Roenneberg, T. Chronotype and sleep duration: The influence of season of assessment. Chronobiol. Int. 2014, 31, 731–740. [Google Scholar] [CrossRef]
- Schneider, A.M.; Randler, C. Daytime sleepiness during transition into daylight saving time in adolescents: Are owls higher at risk? Sleep Med. 2009, 10, 1047–1050. [Google Scholar] [CrossRef]
- Toth Quintilham, M.C.; Adamowicz, T.; Pereira, É.F.; Pedrazzoli, M.; Louzada, F.M. Does the transition into daylight saving time really cause partial sleep deprivation? Ann. Hum. Biol. 2014, 41, 554–560. [Google Scholar] [CrossRef]
- Johnsen, M.T.; Wynn, R.; Allebrandt, K.; Bratlid, T. Lack of major seasonal variations in self reported sleep-wake rhythms and chronotypes among middle aged and older people at 69 degrees North: The Tromsø Study. Sleep Med. 2013, 14, 140–148. [Google Scholar] [CrossRef]
- Shochat, T.; Santhi, N.; Herer, P.; Flavell, S.A.; Skeldon, A.C.; Dijk, D.J. Sleep timing in late autumn and late spring associates with light exposure rather than Sun time in college students. Front. Neurosci. 2019, 13, 882. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.M.; Wagner, D.T. Changing to Daylight Saving Time cuts into sleep and increases workplace injuries. J. Appl. Psychol. 2009, 94, 1305–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, J.C.; Leong, R.L.F.; Loh, K.K.; Dijk, D.J.; Chee, M.W.L. Young adults’ sleep duration on work days: Differences between East and West. Front. Neurol. 2014, 5, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonetti, L.; Erbacci, A.; Fabbri, M.; Martoni, M.; Natale, V. Effects of transitions into and out of daylight saving time on the quality of the sleep/wake cycle: An actigraphic study in healthy university students. Chronobiol. Int. 2013, 30, 1218–1222. [Google Scholar] [CrossRef]
- Minors, D.S.; Waterhourse, J.M.; Wirz-Justice, A. A human phase-response curve to light. Neurosci. Lett. 1991, 133, 36–40. [Google Scholar] [CrossRef]
- De Coursey, P.J. Daily Light Sensitivity Rhythm in a Rodent. Science 1960, 131, 33–35. [Google Scholar] [CrossRef]
- Meira e Cruz, M.; Miyazawa, M.; Manfredini, R.; Cardinali, D.; Madrid, J.A.; Reiter, R.; Araujo, J.F.; Agostinho, R.; Acuña-Castroviejo, D. Impact of Daylight Saving Time on circadian timing system: An expert statement. Eur. J. Intern. Med. 2019, 60, 1–3. [Google Scholar] [CrossRef]
- Blattner, M.S.; Mahoney, M.M. Photic phase-response curve in 2 strains of mice with impaired responsiveness to estrogens. J. Biol. Rhythm. 2013, 28, 291–300. [Google Scholar] [CrossRef]
- Revell, V.L.; Molina, T.A.; Eastman, C.I. Human phase response curve to intermittent blue light using a commercially available device. J. Physiol. 2012, 590, 4859–4868. [Google Scholar] [CrossRef]
- Khalsa, S.B.S.; Jewett, M.E.; Cajochen, C.; Czeisler, C.A. A phase response curve to single bright light pulses in human subjects. J. Physiol. 2003, 549, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Madrid-Navarro, C.J.; Escamilla-Sevilla, F.; Mínguez-Castellanos, A.; Campos, M.; Ruiz-Abellán, F.; Madrid, J.A.; Rol, M.A. Multidimensional circadian monitoring by wearable biosensors in Parkinson’s disease. Front. Neurol. 2018, 9, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz-Tudela, E.; Martinez-Nicolas, A.; Campos, M.; Rol, M.Á.; Madrid, J.A. A new integrated variable based on thermometry, actimetry and body position (TAP) to evaluate circadian system status in humans. PLoS Comput. Biol. 2010, 6, e1000996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonmati-Carrion, M.A.; Middleton, B.; Revell, V.L.; Skene, D.J.; Rol, M.A.; Madrid, J.A. Validation of an innovative method, based on tilt sensing, for the assessment of activity and body position. Chronobiol. Int. 2015, 32, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Bonmati-Carrion, M.A.; Middleton, B.; Revell, V.; Skene, D.J.; Rol, M.A.; Madrid, J.A. Circadian phase assessment by ambulatory monitoring in humans: Correlation with dim light melatonin onset. Chronobiol. Int. 2014, 31, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Bonmati-Carrion, M.-A.; Revell, V.L.; Cook, T.; Welch, T.; Rol, M.Á.; Skene, D.J.; Madrid, J.A. Living without temporal cues: A case study. Front. Physiol. 2020, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Solar Forecasting & Solar Irradiance Data. Available online: https://solcast.com/ (accessed on 18 May 2022).
- Zeitzer, J.M.; Dijk, D.J.; Kronauer, R.E.; Brown, E.N.; Czeisler, C.A. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J. Physiol. 2000, 526, 695. [Google Scholar] [CrossRef]
- Sarabia, J.A.; Rol, M.Á.; Mendiola, P.; Madrid, J.A. Circadian rhythm of wrist temperature in normal-living subjects A candidate of new index of the circadian system. Physiol. Behav. 2008, 95, 570–580. [Google Scholar] [CrossRef]
- Krauchi, K.; Deboer, T. The interrelationship between sleep regulation and thermoregulation. Front. Biosci.-Landmark Ed. 2010, 15, 604–625. [Google Scholar] [CrossRef] [Green Version]
- Witting, W.; Kwa, I.H.; Eikelenboom, P.; Mirmiran, M.; Swaab, D.F. Alteration in the circadian rest-activity rhythm in aging and alzheimer’s disease. Biol. Psychiatry 1990, 27, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Roenneberg, T.; Wirz-Justice, A.; Merrow, M. Life between clocks: Daily temporal patterns of human chronotypes. J. Biol. Rhythm. 2003, 18, 80–90. [Google Scholar] [CrossRef]
- Bonmati-Carrion, M.A.; Hild, K.; Isherwood, C.; Sweeney, S.J.; Revell, V.L.; Skene, D.J.; Rol, M.A.; Madrid, J.A. Relationship between human pupillary light reflex and circadian system status. PLoS ONE 2016, 11, e0162476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, K.E.E.; Wegmann, H.M.M.; Hunt, B.I.I. Desynchronization of body temperature and performance circadian rhythm as a result of outgoing and homegoing transmeridian flights. Aerosp. Med. 1972, 43, 119–132. [Google Scholar] [PubMed]
- Czeisler, C.A.; Duffy, J.F.; Shanahan, T.L.; Brown, E.N.; Mitchell, J.F.; Rimmer, D.W.; Ronda, J.M.; Silva, E.J.; Allan, J.S.; Emens, J.S.; et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 1999, 284, 2177–2181. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.N.; Stone, B.M. Adaptation of sleep to British Summer Time [proceedings]. J. Physiol. 1978, 275, 22P–23P. [Google Scholar]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended amount of sleep for a healthy adult: A joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015, 38, 843–844. [Google Scholar] [CrossRef] [PubMed]
- Sládek, M.; Kudrnáčová Röschová, M.; Adámková, V.; Hamplová, D.; Sumová, A. Chronotype assessment via a large scale socio-demographic survey favours yearlong Standard time over Daylight Saving Time in central Europe. Sci. Rep. 2020, 10, 1419. [Google Scholar] [CrossRef] [Green Version]
- Poteser, M.; Moshammer, H. Daylight saving time transitions: Impact on total mortality. Int. J. Environ. Res. Public Health 2020, 17, 1611. [Google Scholar] [CrossRef] [Green Version]
- McDougal, D.H.; Gamlin, P.D. The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vis. Res. 2010, 50, 72–87. [Google Scholar] [CrossRef] [Green Version]
- Danilenko, K.V.; Kobelev, E.; Semenova, E.A.; Aftanas, L.I. Summer-winter difference in 24-h melatonin rhythms in subjects on a 5-workdays schedule in Siberia without daylight saving time transitions. Physiol. Behav. 2019, 212, 112686. [Google Scholar] [CrossRef]
- Bonmatí-Carrión, M.-Á.; Casado-Ramírez, E.; Moreno-Casbas, M.T.; Campos, M.; Consortium, M.; Madrid, J.A.; Rol, M.-A. Living at the wrong time: Effects of unmatching official time in Portugal and Western Spain. Biology 2022, 11, 1130. [Google Scholar] [CrossRef]
- Valdez, P.; Ramírez, C.; García, A. Adjustment of the sleep-wake cycle to small (1–2 h) changes in schedule. Biol. Rhythm Res. 2003, 34, 145–155. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arguelles-Prieto, R.; Madrid, J.A.; Rol, M.A.; Bonmatí-Carrión, M.Á. Phase Response Curve to Light under Ambulatory Conditions: A Pilot Study for Potential Application to Daylight Saving Time Transitions. Biology 2022, 11, 1584. https://doi.org/10.3390/biology11111584
Arguelles-Prieto R, Madrid JA, Rol MA, Bonmatí-Carrión MÁ. Phase Response Curve to Light under Ambulatory Conditions: A Pilot Study for Potential Application to Daylight Saving Time Transitions. Biology. 2022; 11(11):1584. https://doi.org/10.3390/biology11111584
Chicago/Turabian StyleArguelles-Prieto, Raquel, Juan Antonio Madrid, Maria Angeles Rol, and María Ángeles Bonmatí-Carrión. 2022. "Phase Response Curve to Light under Ambulatory Conditions: A Pilot Study for Potential Application to Daylight Saving Time Transitions" Biology 11, no. 11: 1584. https://doi.org/10.3390/biology11111584
APA StyleArguelles-Prieto, R., Madrid, J. A., Rol, M. A., & Bonmatí-Carrión, M. Á. (2022). Phase Response Curve to Light under Ambulatory Conditions: A Pilot Study for Potential Application to Daylight Saving Time Transitions. Biology, 11(11), 1584. https://doi.org/10.3390/biology11111584







