Negative Magnetic Sorting Preserves the Functionality of Ex Vivo Cultivated Non-Adherent Human Monocytes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Monocytes
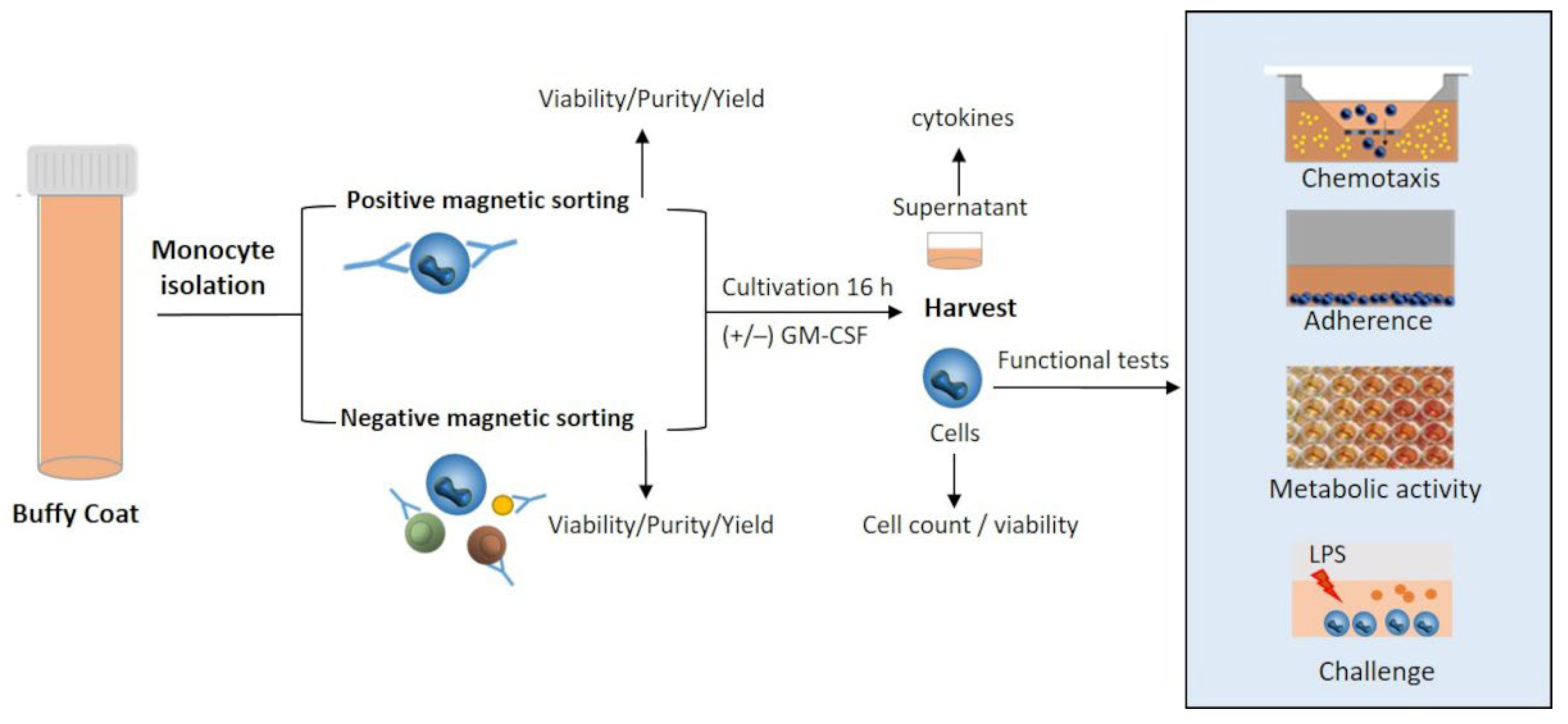
2.2. Experimental Design
2.3. Monocyte Isolation by Positive and Negative Selection
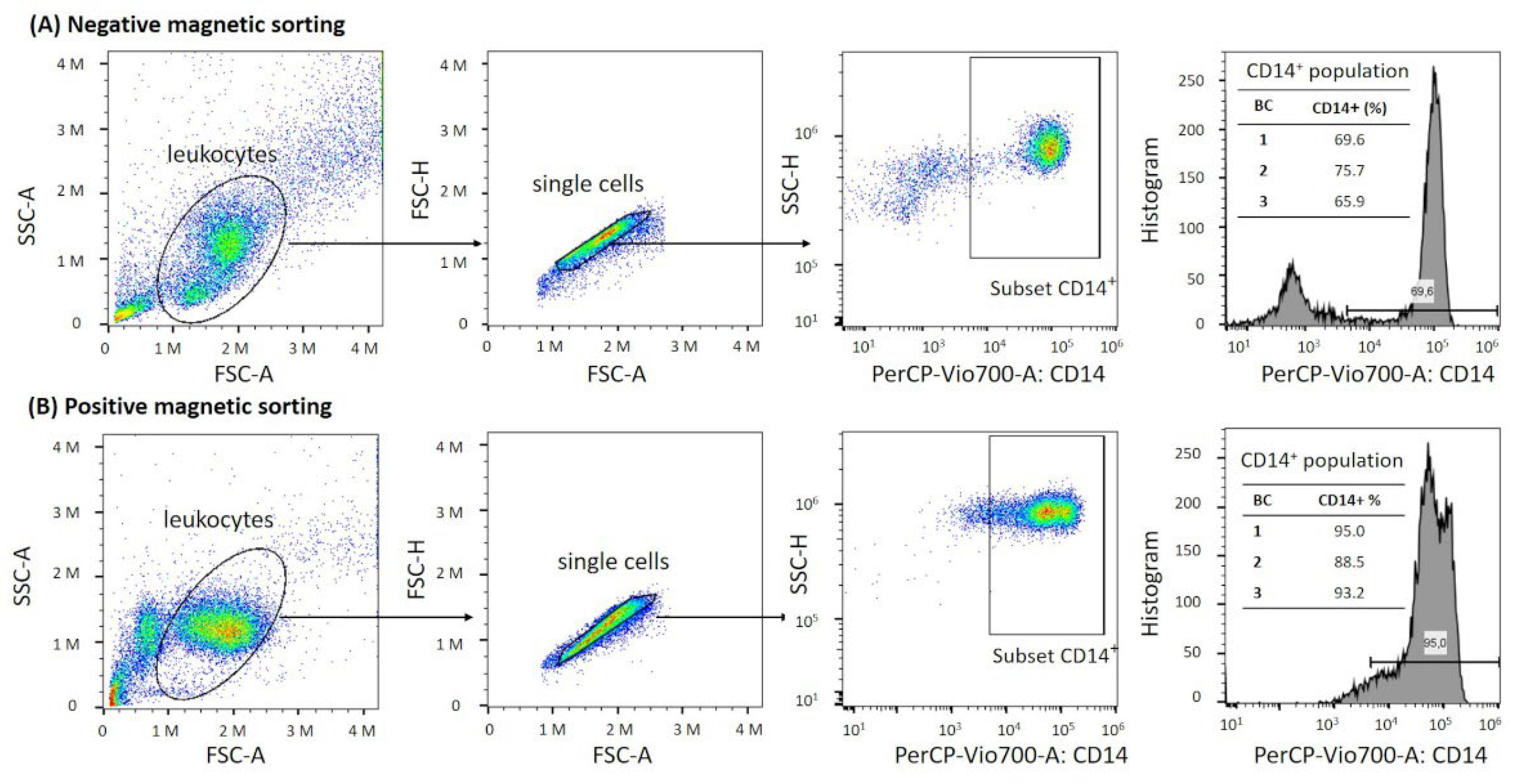
2.4. Flow Cytometry Analysis
2.5. Migratory Capacity, Adherence, Metabolic Activity
2.6. Response to Lipopolysaccharides
2.7. Quantification of Cytokines
2.8. Statistical Analyses
3. Results
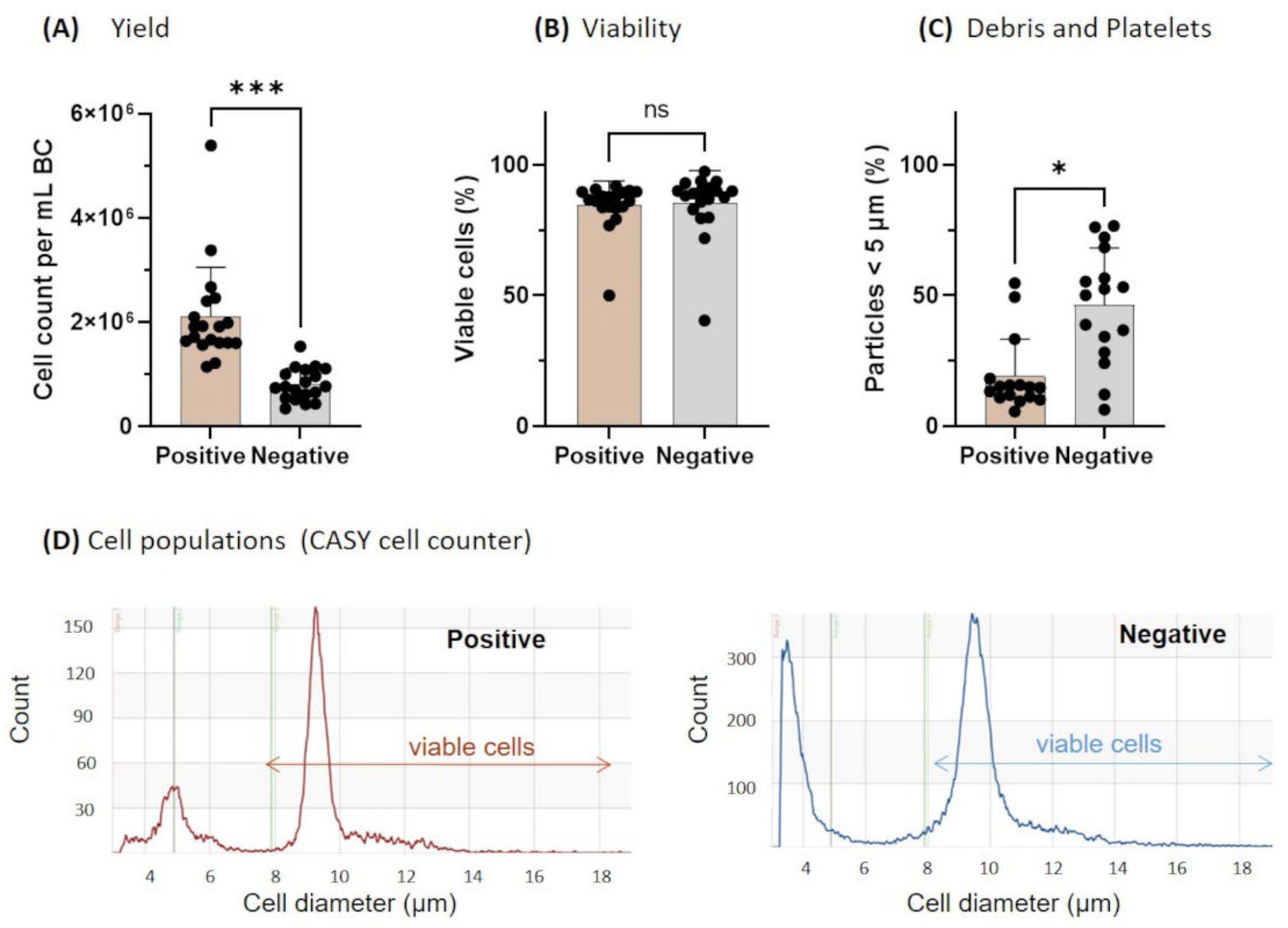
3.1. Yield, Viability, and Purity Directly after Monocyte Isolation
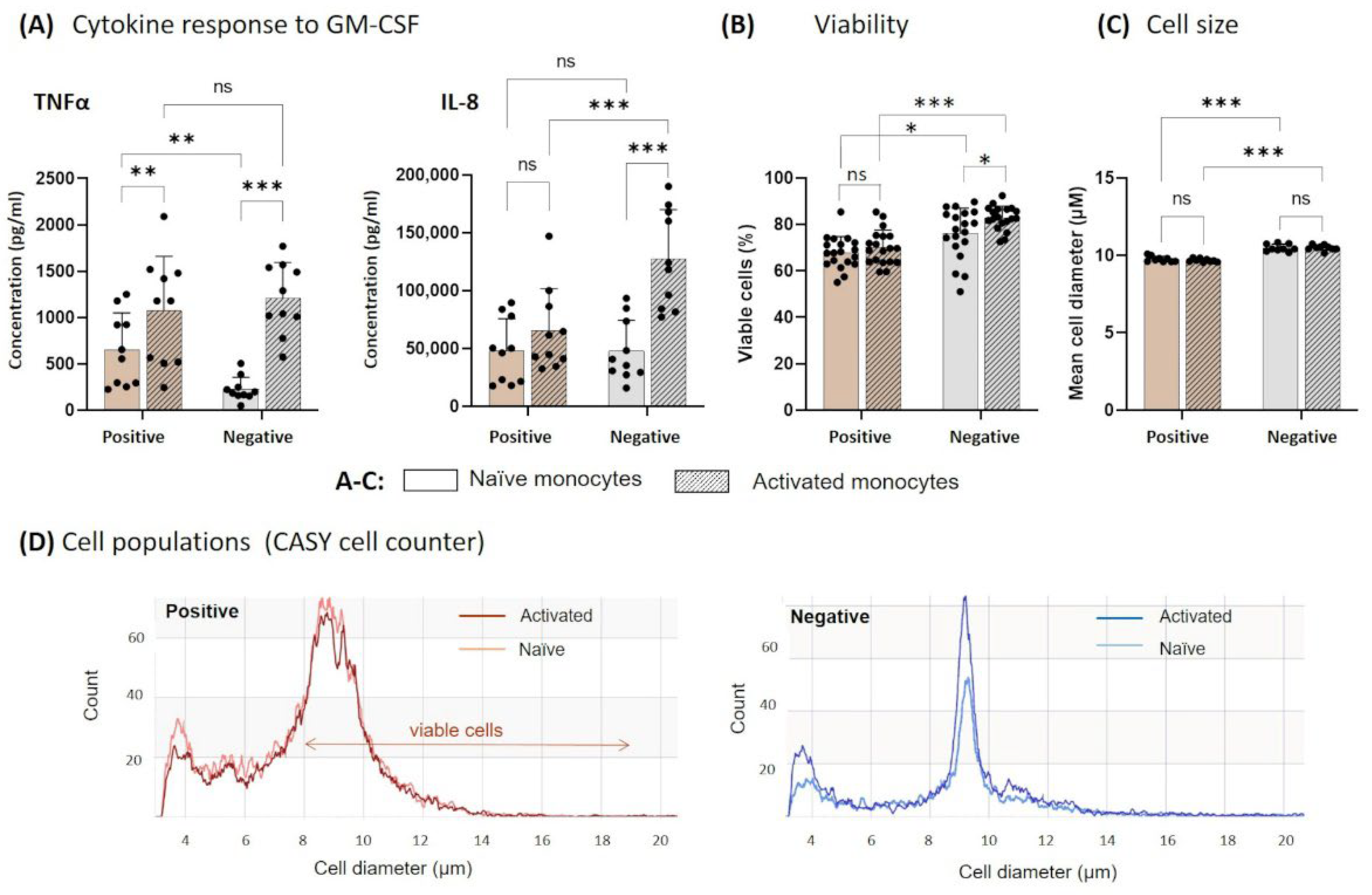
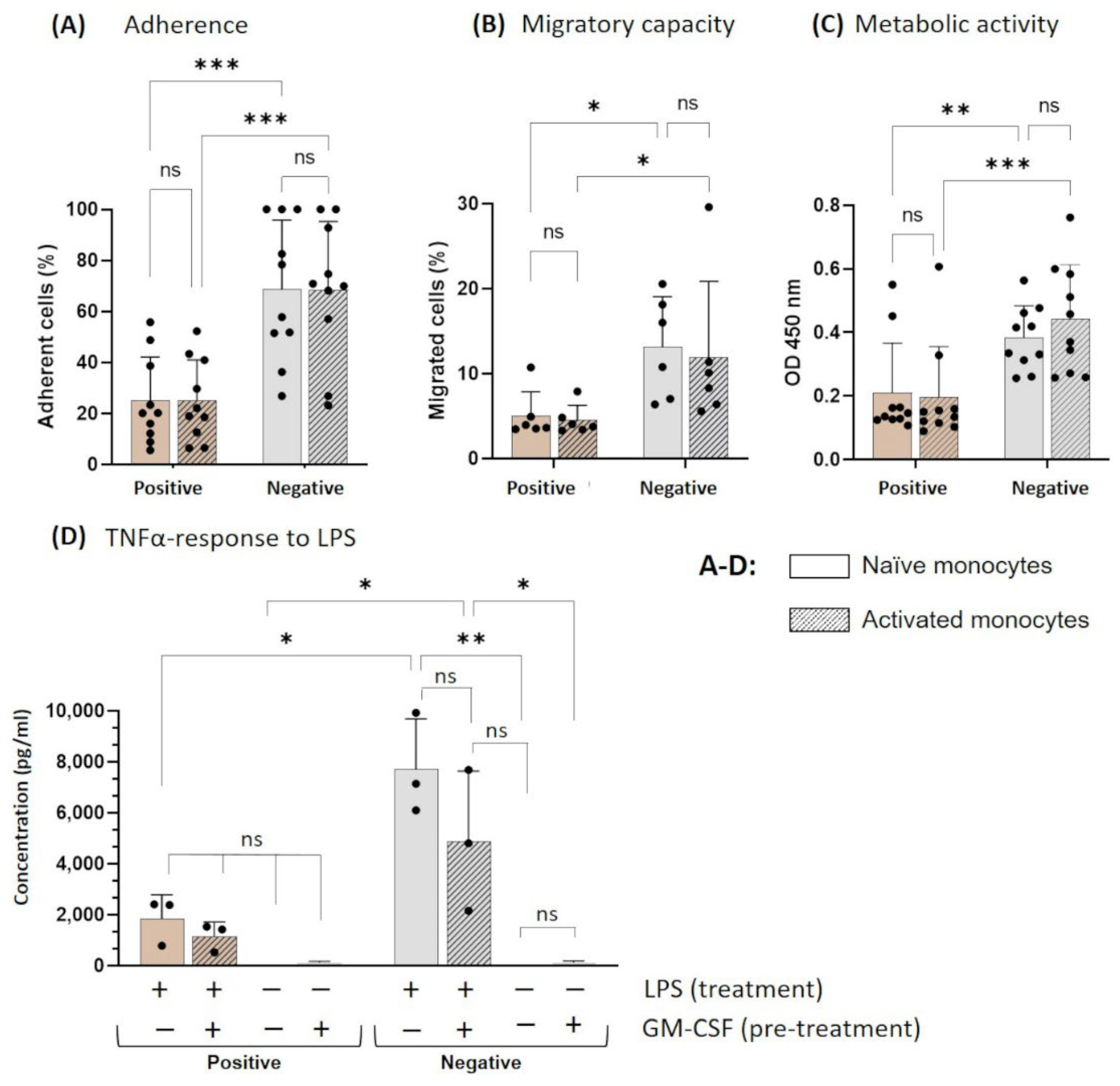
3.2. Functionality of Naïve and Activated Monocytes after Cultivation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jakubzick, C.V.; Randolph, G.J.; Henson, P.M. Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol. 2017, 17, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Coillard, A.; Segura, E. In vivo differentiation of human monocytes. Front. Immunol. 2019, 10, 1907. [Google Scholar] [CrossRef] [PubMed]
- Herold, J.; Tillmanns, H.; Xing, Z.; Strasser, R.H.; Braun-Dullaeus, R.C. Isolation and transduction of monocytes: Promising vehicles for therapeutic arteriogenesis. Langenbeck’s Arch. Surg. 2006, 391, 72–82. [Google Scholar] [CrossRef]
- De Palma, M.; Mazzieri, R.; Politi, L.S.; Pucci, F.; Zonari, E.; Sitia, G.; Mazzoleni, S.; Moi, D.; Venneri, M.A.; Indraccolo, S.; et al. Tumor-targeted interferon-α delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell 2008, 14, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Lebson, L.; Nash, K.; Kamath, S.; Herber, D.; Carty, N.; Lee, D.C.; Li, Q.; Szekeres, K.; Jinwal, U.; Koren, J.; et al. Trafficking CD11b-positive blood cells deliver therapeutic genes to the brain of amyloid-depositing transgenic mice. J. Neurosci. 2010, 30, 9651–9658. [Google Scholar] [CrossRef] [PubMed]
- Däbritz, J.; Weinhage, T.; Varga, G.; Wirth, T.; Walscheid, K.; Brockhausen, A.; Schwarzmaier, D.; Bruckner, M.; Ross, M.; Bettenworth, D.; et al. Reprogramming of monocytes by GM-CSF contributes to regulatory immune functions during intestinal inflammation. J. Immunol. 2015, 194, 2424–2438. [Google Scholar] [CrossRef]
- Weinhage, T.; Däbritz, J.; Brockhausen, A.; Wirth, T.; Brückner, M.; Belz, M.; Foell, D.; Varga, G. Granulocyte macrophage colony-stimulating factor-activated CD39+/CD73+ murine monocytes modulate intestinal inflammation via induction of regulatory T cells. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 433–449. [Google Scholar] [CrossRef]
- Green, D.S.; Nunes, A.T.; Tosh, K.W.; David-Ocampo, V.; Fellowes, V.S.; Ren, J.; Jin, J.; Frodigh, S.-E.; Pham, C.; Procter, J. Production of a cellular product consisting of monocytes stimulated with Sylatron® (Peginterferon alfa-2b) and Actimmune® (Interferon gamma-1b) for human use. J. Transl. Med. 2019, 17, 82. [Google Scholar] [CrossRef]
- Smith, C.; Økern, G.; Rehan, S.; Beagley, L.; Lee, S.K.; Aarvak, T.; Schjetne, K.W.; Khanna, R. Ex vivo expansion of human T cells for adoptive immunotherapy using the novel Xeno-free CTS immune cell serum replacement. Clin. Transl. Immunol. 2015, 4, e31. [Google Scholar] [CrossRef]
- Ghaffari, S.; Torabi-Rahvar, M.; Aghayan, S.; Jabbarpour, Z.; Moradzadeh, K.; Omidkhoda, A.; Ahmadbeigi, N. Optimizing interleukin-2 concentration, seeding density and bead-to-cell ratio of T-cell expansion for adoptive immunotherapy. BMC Immunol. 2021, 22, 43. [Google Scholar] [CrossRef]
- Pakala, R.; Benedict, C.R. Endothelial cells regulate the proliferation of monocytes in vitro. Atherosclerosis 1999, 147, 25–32. [Google Scholar] [CrossRef]
- Jenkins, S.J.; Knipper, J.A.; Zaiss, D.M. Local proliferation of monocytes. J. Leukoc. Biol. 2020, 107, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.; Breit, S.N. Variables in the isolation and culture of human monocytes that are of particular relevance to studies of HIV. J. Leukoc. Biol. 1994, 56, 236. [Google Scholar] [PubMed]
- de Almeida, M.C.; Silva, A.C.; Barral, A.; Barral Netto, M. A simple method for human peripheral blood monocyte isolation. Mem. Inst. Oswaldo Cruz 2000, 95, 221–223. [Google Scholar] [CrossRef]
- Fluks, A. Three-step isolation of human blood monocytes using discontinuous density gradients of Percoll. J. Immunol. Methods 1981, 41, 225–233. [Google Scholar] [CrossRef]
- Bøyum, A. Isolation of Human Blood Monocytes with Nycodenz, a New Non-Ionic lodinated Gradient Medium. Scand. J. Immunol. 1983, 17, 429–436. [Google Scholar] [CrossRef]
- Klinder, A.; Markhoff, J.; Jonitz-Heincke, A.; Sterna, P.; Salamon, A.; Bader, R. Comparison of different cell culture plates for the enrichment of non-adherent human mononuclear cells. Exp. Ther. Med. 2019, 17, 2004–2012. [Google Scholar] [CrossRef]
- Nielsen, M.C.; Andersen, M.N.; Møller, H.J. Monocyte isolation techniques significantly impact the phenotype of both isolated monocytes and derived macrophages in vitro. Immunology 2020, 159, 63–74. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.Y. Anti-CD14 antibody reduces LPS responsiveness via TLR4 internalization in human monocytes. Mol. Immunol. 2014, 57, 210–215. [Google Scholar] [CrossRef]
- Elkord, E.; Williams, P.E.; Kynaston, H.; Rowbottom, A.W. Human monocyte isolation methods influence cytokine production from in vitro generated dendritic cells. Immunology 2005, 114, 204–212. [Google Scholar] [CrossRef]
- Delirezh, N.; Shojaeefar, E.; Parvin, P.; Asadi, B. Comparison the effects of two monocyte isolation methods, plastic adherence and magnetic activated cell sorting methods, on phagocytic activity of generated dendritic cells. Cell J. 2013, 15, 218. [Google Scholar] [PubMed]
- Wirthgen, E.; Hornschuh, M.; Wrobel, I.M.; Manteuffel, C.; Däbritz, J. Mimicking of Blood Flow Results in a Distinct Functional Phenotype in Human Non-Adherent Classical Monocytes. Biology 2021, 10, 748. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.L.; Rozek, M.M.; Suenram, C.A.; Schwartz, C.J. Activation of human blood monocytes by adherence to tissue culture plastic surfaces. Exp. Mol. Pathol. 1987, 46, 266–278. [Google Scholar] [CrossRef]
- Tsubota, Y.; Frey, J.M.; Raines, E.W. Novel ex vivo culture method for human monocytes uses shear flow to prevent total loss of transendothelial diapedesis function. J. Leukoc. Biol. 2014, 95, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 2008, 8, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A. GM-CSF in inflammation. J. Exp. Med. 2020, 217, e20190945. [Google Scholar] [CrossRef] [PubMed]
- de Waal Malefyt, R.; Abrams, J.; Bennett, B.; Figdor, C.G.; De Vries, J.E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991, 174, 1209–1220. [Google Scholar] [CrossRef]
- van der Bruggen, T.; Nijenhuis, S.; van Raaij, E.; Verhoef, J.; Sweder van Asbeck, B. Lipopolysaccharide-induced tumor necrosis factor alpha production by human monocytes involves the raf-1/MEK1-MEK2/ERK1-ERK2 pathway. Infect. Immun. 1999, 67, 3824–3829. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, Aka Least-Squares Means; R Core Team: Vienna, Austria, 2018. [Google Scholar]
- Kramer, C.Y. Extension of multiple range tests to group means with unequal numbers of replications. Biometrics 1956, 12, 307–310. [Google Scholar] [CrossRef]
- Palmer, C.S.; Crowe, S.M. How does monocyte metabolism impact inflammation and aging during chronic HIV infection? AIDS Res. Hum. Retrovir. 2014, 30, 335–336. [Google Scholar] [CrossRef]
- Suzuki, H.; Hisamatsu, T.; Chiba, S.; Mori, K.; Kitazume, M.T.; Shimamura, K.; Nakamoto, N.; Matsuoka, K.; Ebinuma, H.; Naganuma, M.; et al. Glycolytic pathway affects differentiation of human monocytes to regulatory macrophages. Immunol. Lett. 2016, 176, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Groh, L.; Keating, S.T.; Joosten, L.A.; Netea, M.G.; Riksen, N.P. Monocyte and macrophage immunometabolism in atherosclerosis. Semin. Immunopathol. 2018, 40, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; von Ungern-Sternberg, S.N.; Seizer, P.; Schlegel, F.; Büttcher, M.; Sindhu, N.; Müller, S.; Mack, A.; Gawaz, M. Platelet-derived CXCL12 regulates monocyte function, survival, differentiation into macrophages and foam cells through differential involvement of CXCR4–CXCR7. Cell Death Dis. 2015, 6, e1989. [Google Scholar] [CrossRef] [PubMed]
- Scheuerer, B.; Ernst, M.; Dürrbaum-Landmann, I.; Fleischer, J.; Grage-Griebenow, E.; Brandt, E.; Flad, H.-D.; Petersen, F. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood J. Am. Soc. Hematol. 2000, 95, 1158–1166. [Google Scholar] [CrossRef]
- Arroyo-Espliguero, R.; Avanzas, P.; Jeffery, S.; Kaski, J.C. CD14 and toll-like receptor 4: A link between infection and acute coronary events? Heart 2004, 90, 983–988. [Google Scholar] [CrossRef]
- Bhattacharjee, J.; Das, B.; Mishra, A.; Sahay, P.; Upadhyay, P. Monocytes isolated by positive and negative magnetic sorting techniques show different molecular characteristics and immunophenotypic behaviour. F1000Res 2017, 6, 2045. [Google Scholar] [CrossRef]
- Mengozzi, M.; Fantuzzi, G.; Sironi, M.; Bianchi, M.; Fratelli, M.; Peri, G.; Bernasconi, S.; Ghezzi, P. Early down-regulation of TNF production by LPS tolerance in human monocytes: Comparison with IL-1 beta, IL-6, and IL-8. Lymphokine Cytokine Res. 1993, 12, 231–236. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hornschuh, M.; Haas, V.; Winkel, P.P.; Gökyildirim, M.Y.; Mullins, C.S.; Wrobel, I.M.; Manteuffel, C.; Wirthgen, E. Negative Magnetic Sorting Preserves the Functionality of Ex Vivo Cultivated Non-Adherent Human Monocytes. Biology 2022, 11, 1583. https://doi.org/10.3390/biology11111583
Hornschuh M, Haas V, Winkel PP, Gökyildirim MY, Mullins CS, Wrobel IM, Manteuffel C, Wirthgen E. Negative Magnetic Sorting Preserves the Functionality of Ex Vivo Cultivated Non-Adherent Human Monocytes. Biology. 2022; 11(11):1583. https://doi.org/10.3390/biology11111583
Chicago/Turabian StyleHornschuh, Melanie, Vivian Haas, Paul P. Winkel, Mira Y. Gökyildirim, Christina S. Mullins, Ida Maria Wrobel, Christian Manteuffel, and Elisa Wirthgen. 2022. "Negative Magnetic Sorting Preserves the Functionality of Ex Vivo Cultivated Non-Adherent Human Monocytes" Biology 11, no. 11: 1583. https://doi.org/10.3390/biology11111583
APA StyleHornschuh, M., Haas, V., Winkel, P. P., Gökyildirim, M. Y., Mullins, C. S., Wrobel, I. M., Manteuffel, C., & Wirthgen, E. (2022). Negative Magnetic Sorting Preserves the Functionality of Ex Vivo Cultivated Non-Adherent Human Monocytes. Biology, 11(11), 1583. https://doi.org/10.3390/biology11111583







