The Effect of Long-Term Moderate Static Magnetic Field Exposure on Adult Female Mice
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Static Magnetic Field
2.2. Complete Blood Count and Blood Biochemistry Analysis
2.3. H&E Staining
2.4. Behavioral Tests
2.4.1. Open Field Test
2.4.2. Elevated Plus Maze Test
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Total Antioxidant Capacity Assay (ABTS)
2.7. Lipid Peroxidation MDA Assay
2.8. Microbiota Analysis by 16S Sequencing
2.9. Statistical Analysis
3. Results
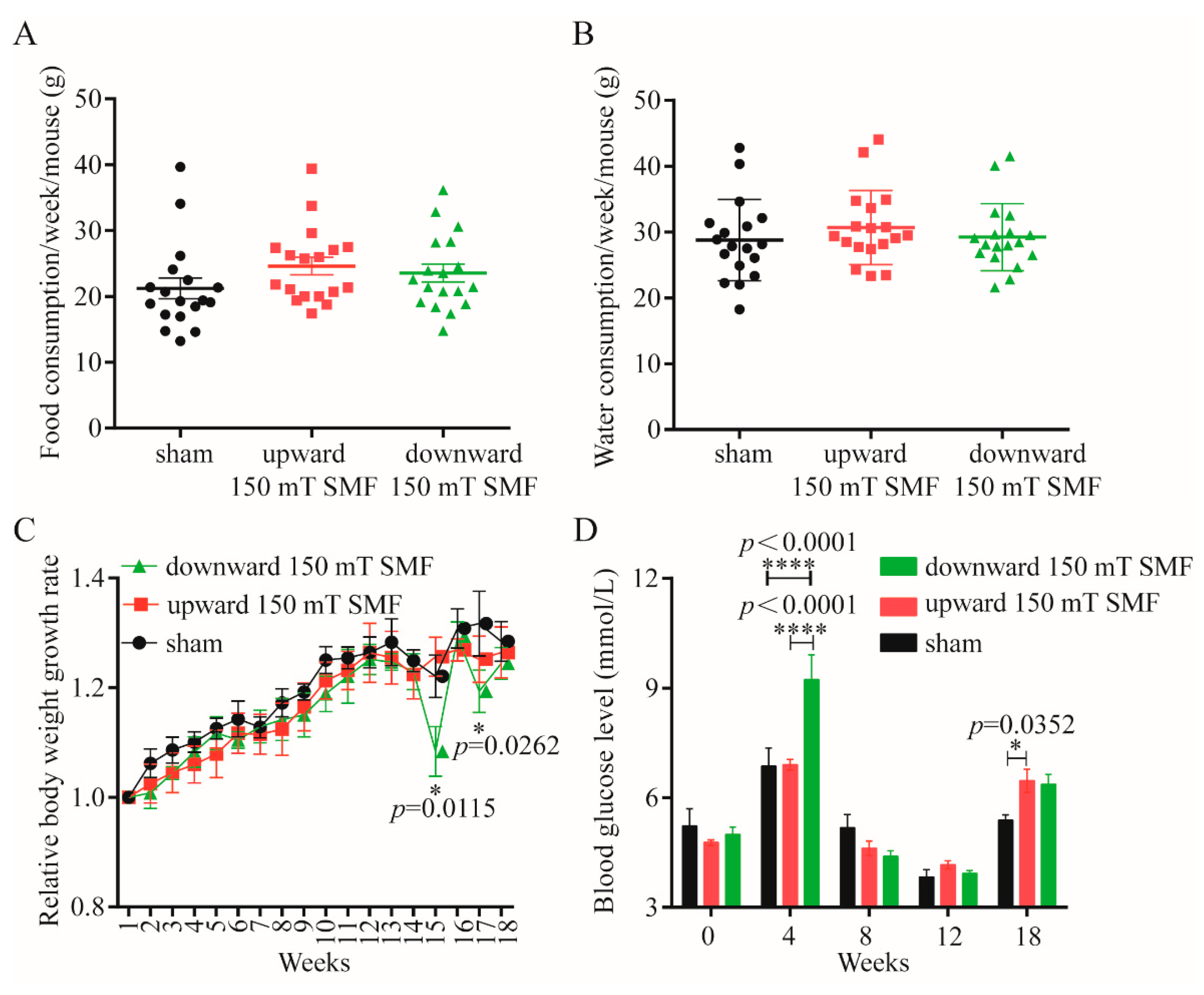
3.1. Long-Term 150 mT SMFs Exposure Did Not Affect Quality of Life of Female Mice
3.2. Long-Term 150 mT SMFs Exposure Enhanced Locomotive and Exploratory Activity in Adult Female Mice
3.3. Long-Term 150 mT SMFs Exposure Reducse Anxiety- and Depression-like Behavior in Adult Female Mice
3.4. Long-Term 150 mT SMF Exposure Improved the Function of the Ovaries and Uterus in Adult Female Mice
3.5. Long-Term 150 mT SMF Exposure Regulated Gut Microbiota and Metabolism in Adult Female Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SMF | Static magnetic field |
| SEM | standard error of mean |
| MRI | Magnetic resonance imaging |
| FDA | Food and drug administration |
| PBS | Phosphate-buffered saline |
| RBC | Red blood cell |
| HCT | Hematocrit |
| HGB | Hemoglobin |
| PLT | Platelet |
| WBC | White blood cell |
| MCV | Mean red blood cell volume |
| Gran | Granulocytes |
| MONO | Mononuclear cells |
| Lymph | Lymphocytes |
| ALT | Alanine transaminase |
| AST | Aspartate transaminase |
| UA | Uric acid |
| CREA | Creatinine |
| TBIL | Total bilirubin |
| TC | Total cholesterol |
| TG | Triglyceride |
| HDL-c | High-density lipoprotein cholesterol |
| LDL-c | Low-density lipoprotein cholesterol |
| OFT | Open field test |
References
- Sarracanie, M.; LaPierre, C.D.; Salameh, N.; Waddington, D.E.J.; Witzel, T.; Rosen, M.S. Low-Cost High-Performance MRI. Sci. Rep. 2015, 5, 15177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpowicz, J.; Gryz, K. The pattern of exposure to static magnetic field of nurses involved in activities related to contrast administration into patients diagnosed in 1.5 T MRI scanners. Electromagn. Biol. Med. 2013, 32, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi-Moghadam, A.; Mortazavi, S.M.J.; Hosseini-Moghadam, A.; Haghani, M.; Taeb, S.; Hosseini, M.A.; Rastegariyan, N.; Arian, F.; Sanipour, L.; Aghajari, S.; et al. Does Exposure to Static Magnetic Fields Generated by Magnetic Resonance Imaging Scanners Raise Safety Problems for Personnel? J. Biomed. Phys. Eng. 2018, 8, 333–336. [Google Scholar]
- Juhász, M.; Nagy, V.L.; Székely, H.; Kocsis, D.; Tulassay, Z.; László, J.F. Influence of inhomogeneous static magnetic field-exposure on patients with erosive gastritis: A randomized, self- and placebo-controlled, double-blind, single centre, pilot study. J. R. Soc. Interface 2014, 11, 20140601. [Google Scholar] [CrossRef]
- Colbert, A.P.; Markov, M.S.; Banerji, M.; Pilla, A.A. Magnetic mattress pad use in patients with fibromyalgia: A randomized double-blind pilot study. J. Back Musculoskelet. Rehabil. 1999, 13, 19–31. [Google Scholar] [CrossRef]
- Weintraub, M.I.; Wolfe, G.I.; Barohn, R.A.; Cole, S.P.; Parry, G.J.; Hayat, G.; Cohen, J.A.; Page, J.C.; Bromberg, M.B.; Schwartz, L.S.; et al. Static magnetic field therapy for symptomatic diabetic neuropathy: A randomized, double-blind, placebo-controlled trial. Arch. Phys. Med. Rehabil. 2003, 84, 736–746. [Google Scholar] [CrossRef]
- Hong, C.-Z.; Lin, J.C.; Bender, L.F.; Schaeffer, J.N.; Meltzer, R.J.; Causin, P. Magnetic necklace: Its therapeutic effectiveness on neck and shoulder pain. Arch. Phys. Med. Rehabil. 1982, 63, 462–466. [Google Scholar]
- Folkman, S.; Lazarus, R.S. Coping as a mediator of emotion. J. Pers. Soc. Psychol. 1988, 54, 466–475. [Google Scholar] [CrossRef]
- Toufexis, D.J.; Myers, K.M.; Davis, M. The effect of gonadal hormones and gender on anxiety and emotional learning. Horm. Behav. 2006, 50, 539–549. [Google Scholar] [CrossRef]
- Azevedo Da Silva, M.; Singh-Manoux, A.; Brunner, E.J.; Kaffashian, S.; Shipley, M.J.; Kivimäk, M.; Nabi, H. Bidirectional association between physical activity and symptoms of anxiety and depression: The Whitehall II study. Eur. J. Epidemiol. 2012, 27, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Kokoreva, L.V. [Physical endurance of the rat during multiple intensive exposures to a constant magnetic field]. Kosm. Biol. I Aviakosm. Med. 1986, 20, 61–63. [Google Scholar]
- Khan, M.H.; Huang, X.; Tian, X.; Ouyang, C.; Wang, D.; Feng, S.; Chen, J.; Xue, T.; Bao, J.; Zhang, X. Short- and long-term effects of 3.5-23.0 Tesla ultra-high magnetic fields on mice behaviour. Eur. Radiol. 2022, 32, 5596–5605. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Fan, Y.; Tian, X.; Yu, B.; Song, C.; Feng, C.; Zhang, L.; Ji, X.; Zablotskii, V.; Zhang, X. The Anti-Depressive Effects of Ultra-High Static Magnetic Field. J. Magn. Reason. Imaging 2022, 56, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Tasić, T.; Lozić, M.; Glumac, S.; Stanković, M.; Milovanovich, I.; Djordjevich, D.M.; Trbovich, A.M.; Japundžić-Žigon, N.; De Luka, S.R. Static magnetic field on behavior, hematological parameters and organ damage in spontaneously hypertensive rats. Ecotoxicol. Environ. Saf. 2021, 207, 111085. [Google Scholar] [CrossRef]
- Shuo, T.; Yumeng, Y.; Leilei, Y.; Yanhui, H.; Chao, Y.; Hua, Y.; Yuan, X.; Zhaoqian, J.; Cuicui, H.; Hongyan, Z.; et al. Static magnetic field induces abnormality of glucose metabolism in rats’ brain and results in anxiety-like behavior. J. Chem. Neuroanat. 2021, 113, 101923. [Google Scholar] [CrossRef] [PubMed]
- Houpt, T.A.; Pittman, D.W.; Barranco, J.M.; Brooks, E.H.; Smith, J.C. Behavioral effects of high-strength static magnetic fields on rats. J. Neurosci. 2003, 23, 1498–1505. [Google Scholar] [CrossRef] [Green Version]
- Cason, A.M.; DenBleyker, M.; Ferrence, K.; Smith, J.C.; Houpt, T.A. Sex and estrous cycle differences in the behavioral effects of high-strength static magnetic fields: Role of ovarian steroids. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R659–R667. [Google Scholar] [CrossRef]
- Kilkkinen, A.; Kao-Philpot, A.; O’Neil, A.; Philpot, B.; Reddy, P.; Bunker, S.; Dunbar, J. Prevalence of psychological distress, anxiety and depression in rural communities in Australia. Aust. J. Rural Health 2007, 15, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Tkáč, I.A.-O.; Benneyworth, M.A.-O.; Nichols-Meade, T.; Steuer, E.L.; Larson, S.N.; Metzger, G.A.-O.; Uğurbil, K.A.-O. Long-term behavioral effects observed in mice chronically exposed to static ultra-high magnetic fields. Magn. Reason. Med. 2021, 86, 1544–1559. [Google Scholar] [CrossRef]
- Taft, R.A.; Davisson, M.; Wiles, M.V. Know thy mouse. Trends Genet. 2006, 22, 649–653. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Houpt, T.A.; Houpt, C.E. Circular swimming in mice after exposure to a high magnetic field. Physiol. Behav. 2010, 100, 284–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoff, M.A.-O.; McKinney, A.t.A.-O.; Shellock, F.G.; Rassner, U.A.-O.; Gilk, T.; Watson, R.E.J.A.-O.; Greenberg, T.D.; Froelich, J.; Kanal, E.A.-O. Safety Considerations of 7-T MRI in Clinical Practice. Radiology 2019, 292, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Gorczynska, E.; Wegrzynowicz, R. Glucose homeostasis in rats exposed to magnetic fields. Invest. Radiol. 1991, 26, 1095–10100. [Google Scholar] [CrossRef]
- Chater, S.; Abdelmelek, H.; Pequignot, J.M.; Sakly, M.; Rhouma, K.B. Effects of sub-acute exposure to static magnetic field on hematologic and biochemical parameters in pregnant rats. Electromagn. Biol. Med. 2006, 25, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Sihem, C.; Hafedh, A.; Mohsen, S.; Marc, P.J.; Khmais, B.R. Effects of sub-acute exposure to magnetic field on blood hematological and biochemical parameters in female rats. Turk. J. Haematol. 2006, 23, 182–187. [Google Scholar]
- Elferchichi, M.; Mercier, J.; Coisy-Quivy, M.; Metz, L.; Lajoix, A.-D.; Gross, R.; Belguith, H.; Abdelmelek, H.; Sakly, M.; Lambert, K. Effects of exposure to a 128-mT static magnetic field on glucose and lipid metabolism in serum and skeletal muscle of rats. Arch. Med. Res. 2010, 41, 309–314. [Google Scholar] [CrossRef]
- Elferchichi, M.; Mercier, J.; Bourret, A.; Gross, R.; Lajoix, A.-D.; Belguith, H.; Sakly, M.; Lambert, K. Is static magnetic field exposure a new model of metabolic alteration? Comparison with Zucker rats. Int. J. Radiat. Biol. 2011, 87, 483–490. [Google Scholar] [CrossRef]
- Lahbib, A.; Elferchichi, M.; Ghodbane, S.; Belguith, H.; Chater, S.; Sakly, M.; Abdelmelek, H. Time-dependent effects of exposure to static magnetic field on glucose and lipid metabolism in rat. Gen. Physiol. Biophys. 2010, 29, 390–395. [Google Scholar] [CrossRef] [Green Version]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef] [Green Version]
- Zhong, F.; Wen, X.; Yang, M.; Lai, H.Y.; Momma, H.; Cheng, L.; Sun, X.; Nagatomi, R.; Huang, C. Effect of an 8-week Exercise Training on Gut Microbiota in Physically Inactive Older Women. Int. J. Sports Med. 2021, 42, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Queipo-Ortuño, M.I.; Seoane, L.M.; Murri, M.; Pardo, M.; Gomez-Zumaquero, J.M.; Cardona, F.; Casanueva, F.; Tinahones, F.J. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS ONE 2013, 8, e65465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erlandson, K.A.-O.; Liu, J.; Johnson, R.; Dillon, S.; Jankowski, C.M.; Kroehl, M.; Robertson, C.E.; Frank, D.N.; Tuncil, Y.; Higgins, J.; et al. An exercise intervention alters stool microbiota and metabolites among older, sedentary adults. Ther. Adv. Infect. Dis. 2021, 8, 20499361211027067. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wang, Y.; Liao, S.; Tang, W.; Peng, L.; Song, G. Bifidobacterium animalis subsp. lactis BB-12 Improves the State Anxiety and Sports Performance of Young Divers Under Stress Situations: A Single-Arm, Prospective Proof-of-Concept Study. Front. Psychol. 2021, 11, 570298. [Google Scholar] [CrossRef] [PubMed]
- Al-Akhras, M.A. Influence of 50 Hz magnetic field on sex hormones and body, uterine, and ovarian weights of adult female rats. Electromagn. Biol. Med. 2008, 27, 155–163. [Google Scholar] [CrossRef]
- Bakacak, M.; Bostancı, M.S.; Attar, R.; Yıldırım, Ö.K.; Yıldırım, G.; Bakacak, Z.; Sayar, H.; Han, A. The effects of electromagnetic fields on the number of ovarian primordial follicles: An experimental study. Kaohsiung J. Med. Sci. 2015, 31, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Türedi, S.; Hancı, H.; Çolakoğlu, S.; Kaya, H.; Odacı, E. Disruption of the ovarian follicle reservoir of prepubertal rats following prenatal exposure to a continuous 900-MHz electromagnetic field. Int. J. Radiat. Biol. 2016, 92, 329–337. [Google Scholar] [CrossRef]
- Jasmi, V.K.; Samadi, F.; Eimani, H.; Hasani, S.; Fathi, R.; Shahverdi, A. Follicle Development in Grafted Mouse Ovaries after Vitrification Processes Under Static Magnetic Field. Cryo Lett. 2017, 38, 166–177. [Google Scholar]
- Sirmatel, O.; Sert, C.; Sirmatel, F.; Selek, S.; Yokus, B. Total antioxidant capacity, total oxidant status and oxidative stress index in the men exposed to 1.5 T static magnetic field. Gen. Physiol. Biophys. 2007, 26, 86–90. [Google Scholar]
- Öztürk, B.; Durak, Z.E.; Büber, S.; Kocaoğlu, E.H. Effect of Static Magnetic Field on Oxidant/Antioxidant Parameters in Cancerous and Noncancerous Human Gastric Tissues. Scientifica 2016, 2016, 8608462. [Google Scholar] [CrossRef]






| Parameter | Sham (Mean ± SEM) | Upward 150 mT SMF (Mean ± SEM) | Downward 150 mT SMF (Mean ± SEM) |
|---|---|---|---|
| RBC (10−12/L) | 9.67 ± 2.0 | 8.79 ± 1.49 | 8.93 ± 2.61 |
| HCT (%) | 47.20 ± 10.15 | 44.47 ± 7.41 | 44.40 ± 13.43 |
| HGB (g/L) | 139.83 ± 29.56 | 129.00 ± 23.06 | 132.17 ± 39.35 |
| PLT (10−9/L) | 780.5 ± 148.45 | 986.83 ± 233.42 | 904.33 ± 284.16 |
| WBC (10−9/L) | 5.60 ± 1.31 | 6.05 ± 2.12 | 4.17 ± 1.58 |
| MCV (fL) | 48.73 ± 0.75 | 50.68 ± 0.95 | 49.55 ± 0.91 |
| Gran (%) | 15.90 ± 1.65 | 14.32 ± 1.38 | 18.32 ± 2.75 |
| MONO (%) | 3.10 ± 0.42 | 2.70 ± 0.23 | 3.25 ± 0.79 |
| Lymph (%) | 81.00 ± 1.96 | 82.98 ± 1.49 | 78.43 ± 3.47 |
| Blood Biochemical Analysis | Sham (Mean ± SEM) | Upward 150 mT SMF (Mean ± SEM) | Downward 150 mT SMF (Mean ± SEM) |
|---|---|---|---|
| ALT (IU/L) | 39.52 ± 10.38 | 35.08 ± 5.32 | 31.74 ± 4.32 |
| AST (IU/L) | 192.43 ± 41.45 | 166.15 ± 55.76 | 147.60 ± 44.31 |
| TBIL (μmol/L) | 19.05 ± 4.47 | 17.11 ± 2.94 | 21.61 ± 5.17 |
| UREA (mmol/L) | 21.31 ± 4.29 | 25.48 ± 3.45 | 34.63 ± 14.45 |
| CREA (mmol/L) | 30.81 ± 3.81 | 29.61 ± 2.28 | 26.41 ± 2.63 |
| UA (μmol/L) | 120.04 ± 8.02 | 117.69 ± 10.79 | 99.95 ± 14.21 * |
| TG (mmol/L) | 0.84 ± 0.26 | 1.13 ± 0.23 | 1.11 ± 0.54 |
| TC (mmol/L) | 2.92 ± 0.32 | 2.82 ± 0.24 | 2.93 ± 0.36 |
| HDL-c (mmol/L) | 1.36 ± 0.18 | 1.21 ± 0.08 | 1.20 ± 0.16 |
| LDL-c (mmol/L) | 0.42 ± 0.05 | 0.34 ± 0.08 | 0.37 ± 0.05 |
| Relative Organ Index | Sham (Mean ± SEM) | Upward 150 mT SMF (Mean ± SEM) | Downward 150 mT SMF (Mean ± SEM) |
|---|---|---|---|
| Heart | 0.0070 ± 0.0014 | 0.0071 ± 0.0009 | 0.0076 ± 0.0011 |
| Liver | 0.0444 ± 0.0050 | 0.0438 ± 0.0041 | 0.0450 ± 0.0051 |
| Spleen | 0.0046 ± 0.0006 | 0.0047 ± 0.0006 | 0.0049 ± 0.0006 |
| Lung | 0.0084 ± 0.0011 | 0.0082 ± 0.0005 | 0.0083 ± 0.0008 |
| Kidney | 0.0144 ± 0.0012 | 0.0137 ± 0.0006 | 0.0145 ± 0.0007 |
| Ovarian + uterine | 0.0110 ± 0.0026 | 0.0093 ± 0.0034 | 0.0092 ± 0.0024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Yu, B.; Song, C.; Feng, C.; Zhang, J.; Wang, X.; Cheng, G.; Yang, R.; Wang, W.; Zhu, Y. The Effect of Long-Term Moderate Static Magnetic Field Exposure on Adult Female Mice. Biology 2022, 11, 1585. https://doi.org/10.3390/biology11111585
Yang X, Yu B, Song C, Feng C, Zhang J, Wang X, Cheng G, Yang R, Wang W, Zhu Y. The Effect of Long-Term Moderate Static Magnetic Field Exposure on Adult Female Mice. Biology. 2022; 11(11):1585. https://doi.org/10.3390/biology11111585
Chicago/Turabian StyleYang, Xingxing, Biao Yu, Chao Song, Chuanlin Feng, Jing Zhang, Xinyu Wang, Guofeng Cheng, Rui Yang, Wei Wang, and Yong Zhu. 2022. "The Effect of Long-Term Moderate Static Magnetic Field Exposure on Adult Female Mice" Biology 11, no. 11: 1585. https://doi.org/10.3390/biology11111585
APA StyleYang, X., Yu, B., Song, C., Feng, C., Zhang, J., Wang, X., Cheng, G., Yang, R., Wang, W., & Zhu, Y. (2022). The Effect of Long-Term Moderate Static Magnetic Field Exposure on Adult Female Mice. Biology, 11(11), 1585. https://doi.org/10.3390/biology11111585






