The Blue Swimming Crab Portunus segnis in the Mediterranean Sea: Invasion Paths, Impacts and Management Measures
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
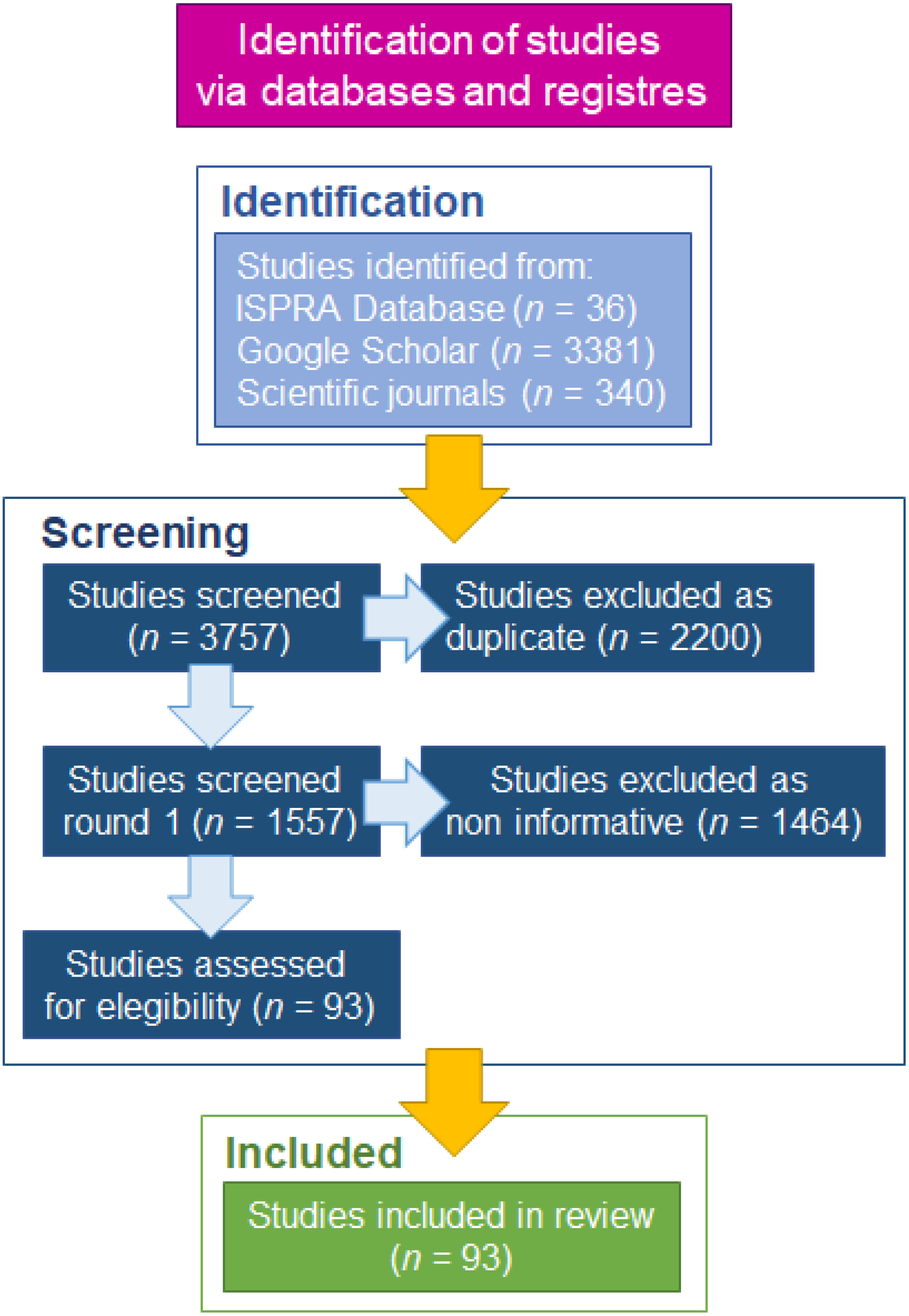
2.1. Systematic Review of P. segnis Invasion History in Mediterranean Sea
2.2. Distribution, Aggregation Patterns, and Spatial Structure Analyses
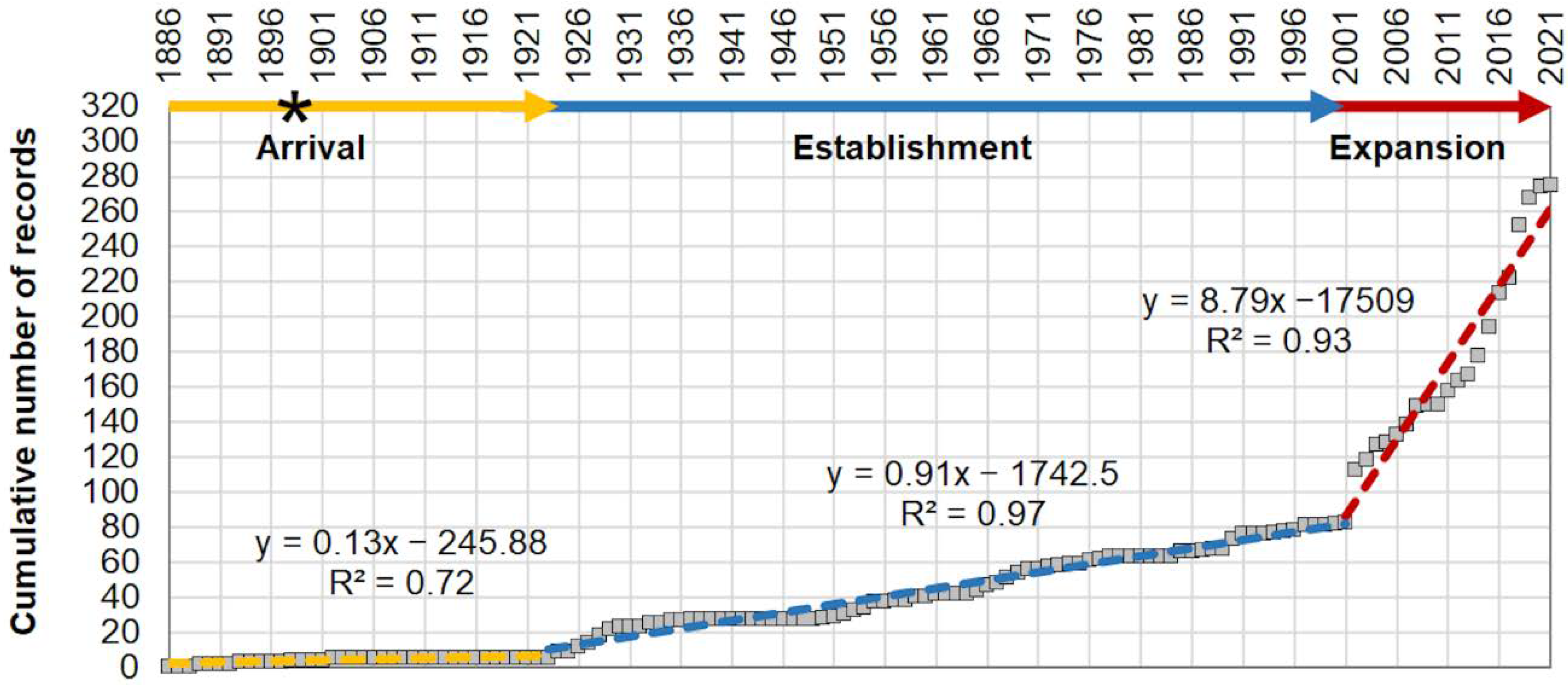
- the temporal pattern through the population’s increasing rate over time based on evident slope changes of the cumulative curve of all occurrences;
- the spatio–temporal pattern through cumulative kernel density of occurrences in order to investigate the population’s increasing rate over time and space, and the evolution of aggregation nuclei into persistent areas;
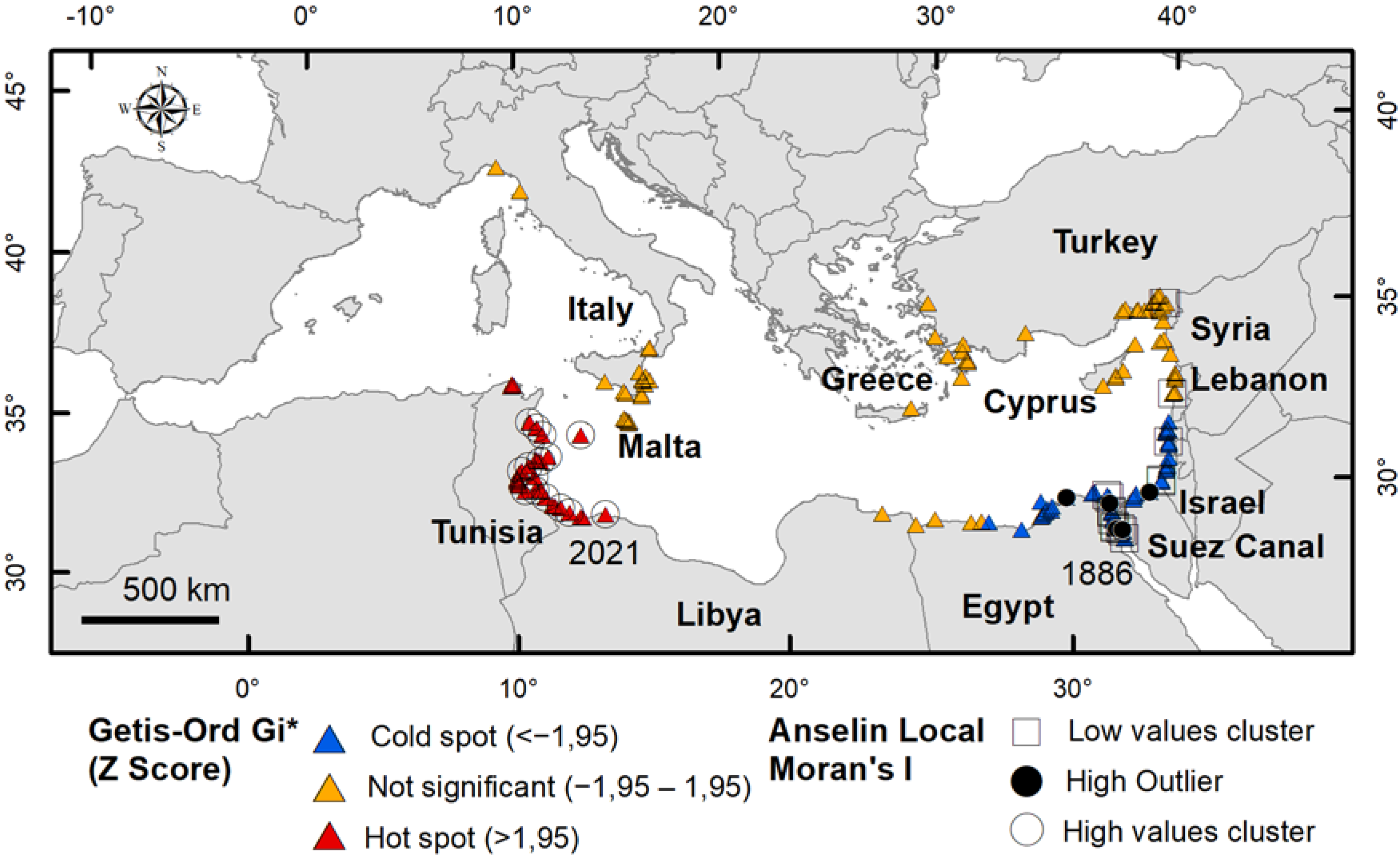
- the aggregation patterns and spatial structure via the spatial pattern at global scale (dispersion vs. random vs. cluster) and at local scale (hot spot, cold spot, clusters and outlier), in order to highlight the change over time, the direction of spread, the dispersion/settlement areas and outliers;
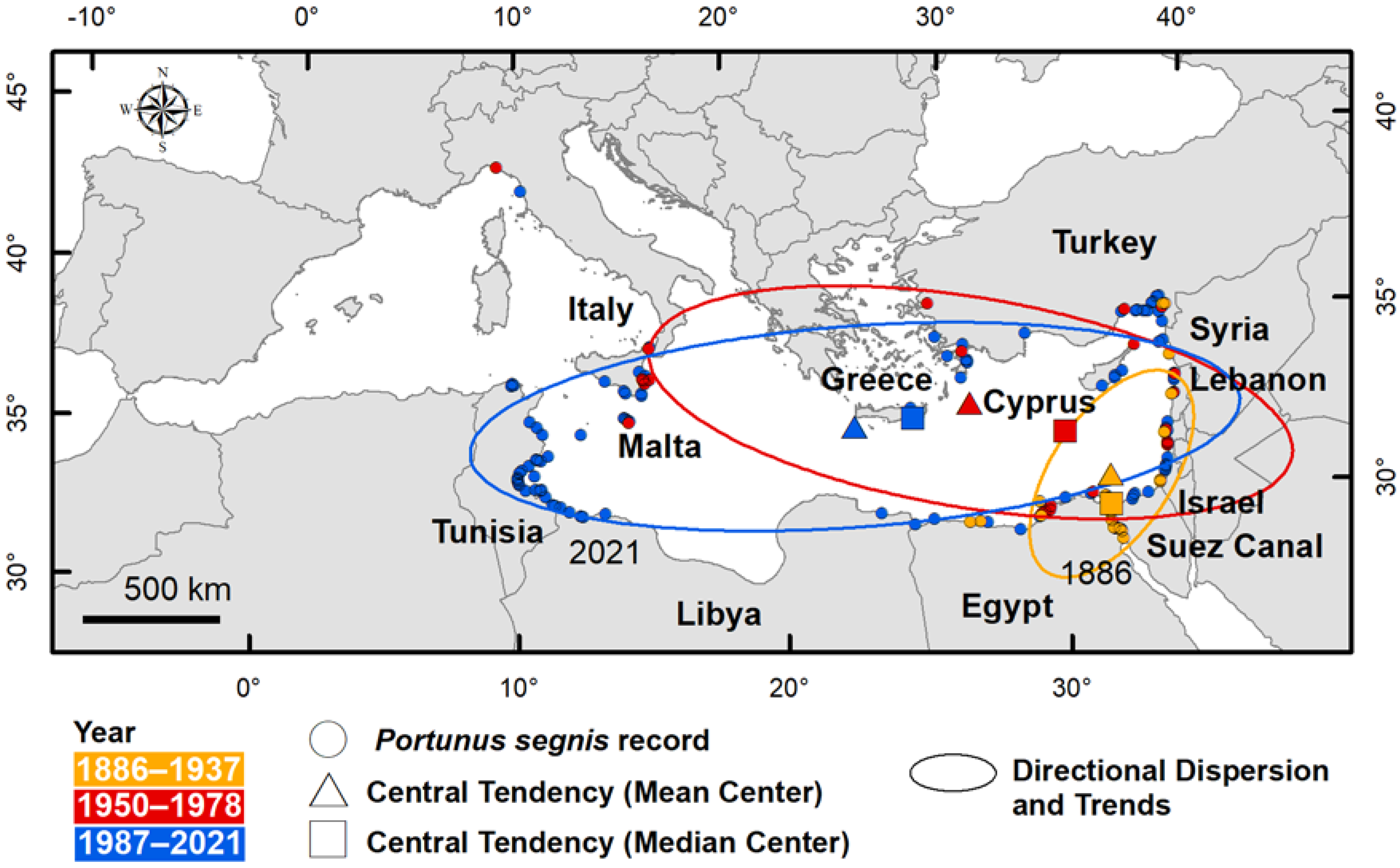
- the key characteristics of distribution (centre of gravity, directional dispersion and directional trends) by tracking changes in shape distribution (dispersed, compact, or elongated) over time and space and comparing the time group of occurrences with each other.
3. Results
3.1. Invasion History and Spatial–Temporal Patterns of P. segnis Distribution in the Mediterranean Sea
3.2. Aggregation Patterns and Spatial Structure
3.3. Key Characteristics of Distribution
4. Discussion
4.1. P. segnis Invasion Pathways
4.2. P. segnis Invasion: Impacts on Biodiversity and Ecosystem Services
4.3. P. segnis Invasion: Management Actions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef] [PubMed]
- Carlton, J.T. Biological invasions and cryptogenic species. Ecology 1996, 77, 1653–1655. [Google Scholar] [CrossRef]
- AquaNIS, Editorial Board. Information System on Aquatic Non–Indigenous and Cryptogenic Species. Available online: www.corpi.ku.lt/databases/aquanis (accessed on 27 May 2022).
- O’Donoghue, C.H.; White, K.M. A collection of marine molluscs, mainly opisthobranchs, from Palestina. Proc. Malac. Soc. Lond. 1940, 24, 92–96. [Google Scholar]
- Bazzicalupo, E.; Crocetta, F.; Estores–Pacheco, K.A.; Golestani, H.; Bazairi, H.; Giacobbe, S.; Jaklin, A.; Poursanidis, D.; Sneha Chandran, B.K.; Cervera, J.R.; et al. Population genetics of Bursatella leachii (De Blainville, 1817) and implications for the origin of the Mediterranean population. Helgol. Mar. Res. 2018, 72, 19. [Google Scholar] [CrossRef]
- Zenetos, A.; Albano, P.G.; Lopez Garcia, E.; Stern, N.; Tsiamis, K.; Galanidi, M. Established non–indigenous species increased by 40% in 11 years in the Mediterranean Sea. Mediterr. Mar. Sci. 2022, 23, 1. [Google Scholar] [CrossRef]
- Perzia, P.; Spinelli, A.; Interdonato, F.; Castriota, L. Ecological indicators from spatial statistics to describe the Atlantic fangtooth moray distribution in Mediterranean Sea. Trans. GIS 2022, 00, 1–16. [Google Scholar] [CrossRef]
- Maggio, T.; Perzia, P.; Falautano, M.; Visconti, G.; Castriota, L. From LEK to LAB: The case of the blue crab Portunus segnis in the Pelagie Islands Marine Protected Area, central Mediterranean Sea. Ocean. Coast. Manag. 2022, 219, 106043. [Google Scholar] [CrossRef]
- Falautano, M.; Perzia, P.; Castriota, L. First record of the Lessepsian fish Parexocoetus mento in Italian waters and GIS–based spatial and temporal distribution in Mediterranean Sea. J. Mar. Biol. Assoc. U.K. 2020, 100, 1163–1169. [Google Scholar] [CrossRef]
- Hosseini, M.; Pazooki, J.; Safaie, M.; Tadi–Beni, F. The biology of the blue swimming crab Portunus segnis (Forskal, 1775) along the Bushehr Coasts, Persian Gulf. Environ. Stud. Persian Gulf 2014, 1, 81–92. [Google Scholar]
- Giraldes, B.W.; Al–Maslamani, I.; Al–Ashwel, A.; Chatting, M.; Smyth, D. Basic assessment of Portunus segnis (Forskål, 1775)—A baseline for stock management in the Western Arabian Gulf. Egypt. J. Aquat. Res. 2016, 42, 111–119. [Google Scholar] [CrossRef][Green Version]
- Lai, J.C.Y.; Ng, P.K.L.; Davie, P.J.F. A revision of the Portunus pelagicus (Linnaeus, 1758) species complex (Crustacea: Brachyura: Portunidae), with the recognition of four species. Raffles Bull. Zool. 2010, 58, 199–237. [Google Scholar]
- Galil, B.; Froglia, C.; Noel, P.Y. Crustacean Decapods and Stomatopods. In CIESM Atlas of Exotic Species in the Mediterranean; Briand, F., Ed.; CIESM Publishers: Monaco, 2002; Volume 2, pp. 1–192. [Google Scholar]
- Holthuis, L.B.; Gottlieb, E. An annotated list of the Decapod Crustacea of the Mediterranean coast of Israel, with an appendix listing the Decapoda of the eastern Mediterranean. Bull. Res. Council Israel 1958, 7B, 1–126. [Google Scholar]
- Tureli, C.; Yesilyurt, I.N. Reproductive biology of blue swimming crab, Portunus segnis (Forskal, 1775) in Yumurtalık Cove, Northeastern Mediterranean, Turkey. Mediterr. Mar. Sci. 2017, 18, 424–432. [Google Scholar] [CrossRef]
- Fox, H.M. The migration of a Red Sea crab through the Suez Canal. Nature 1924, 113, 714–715. [Google Scholar] [CrossRef]
- AA.VV. Atlante Delle Specie Non Indigene Nei Mari Italiani e nel Mediterraneo. Progetto in Convenzione con il Ministero dell’Ambiente e Della Tutela del Territorio. 2011. Available online: www.medalien.isprambiente.it (accessed on 31 December 2021).
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, 160. [Google Scholar] [CrossRef] [PubMed]
- Olenin, S.; Gollasch, S.; Lehtiniemi, M.; Sapota, M.; Zaiko, A. Biological invasions. In Biological Oceanography of the Baltic Sea; Snoeijs-Leijonmalm, P., Schubert, H., Radziejewska, T., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 193–232. [Google Scholar]
- Lipej, L.; Mavric, B.; Paliska, D. New northernmost record of the blunthead pufferfish, Sphoeroides pachygaster (osteichthyes: Tetraodontidae) in the Mediterranean Sea/Nuova segnalazione a nord del pesce palla liscio, Sphoeroides pachygaster (Osteichthyes: Tetraodontidae), nel mare Mediterraneo. Ann. Ser. Hist. Nat. 2013, 23, 103–114. [Google Scholar]
- ESRI. ArcGIS Desktop Help: Release 10.3; Environmental Systems Research Institute: Redlands, CA, USA, 2011. [Google Scholar]
- Mitchell, A. The ESRI Guide to GIS Analysis; ESRI Press: Redlands, CA, USA, 2005; Volume 2, ISBN 1–58948–116–X. [Google Scholar]
- Scott, L.M.; Janikas, M.V. Spatial statistics in ArcGis. In Handbook of Applied Spatial Analysis: Software Tools, Methods and Applications; Fischer, M.M., Getis, A., Eds.; Springer–Verlag: Berlin/Heidelberg, Germany, 2010; pp. 27–41. [Google Scholar]
- Krukenberg, C.F.W. Die Durchflutung des Isthmus von Suez in Chorologischer, Hydrographischer und Historischer Bezeiehung; Carl Winter’s Univ. Buchhandlung: Heidelberg, Germany, 1888; pp. 1–156. [Google Scholar]
- Gruvel, A. Répartition géographique de quelques crustacés comestibles sur les côtes d’Egypte et de Syrie. C.R. Soc. Biogéographie 1928, 5, 45–46. [Google Scholar]
- Ekman, S. Zoogeography of the Sea; Sidgwick and Jackson: London, UK, 1953; pp. 1–417. [Google Scholar]
- Holthuis, L.B. Report on a collection of Crustacea Decapoda and Stomatopoda from Turkey and the Balkans. Zool. Verh. 1961, 47, 1–67. [Google Scholar]
- Ariani, A.P.; Serra, V. Sulla presenza del Portunus pelagicus (L.) in acque italiane, con osservazioni sulla morfologia della specie (Crustacea Decapoda). Arch. Bot. Biogeogr. Ital. 1969, 14, 187–206. [Google Scholar]
- Ghisotti, F. Il Callinectes sapidus Rathburn nel Mediterraneo (Crustacea, Decapoda). Nat. Milano 1966, 57, 177–180. [Google Scholar]
- Torchio, M. Il Callinectes sapidus Rathbun nelle acque siciliane. Natura 1967, 58, 81. [Google Scholar]
- Crocetta, F.; Agius, D.; Balistreri, P.; Bariche, M.; Bayhan, Y.K.; Çakir, S.; Ciriaco, S.; Corsini–Foka, M.; Deidun, A.; El Zrelli, R.; et al. New Mediterranean biodiversity records (October 2015). Mediterr. Mar. Sci. 2015, 16, 682–702. [Google Scholar] [CrossRef]
- Pessani, D.; Salton, L. Planktonic larval stages of Brachyura in the Gulf of Tigullio (Ligurian sea, Italy). Invertebr. Reprod. Dev. 1998, 33, 201–208. [Google Scholar] [CrossRef]
- Corsini–Foka, M.; Kondilatos, G.; Economidis, P.S. Occurrence of the lessepsian species Portunus pelagicus (Crustacea) and Apogon pharaonis (Pisces) in the marine area of Rhodes Island. Mediterr. Mar. Sci. 2004, 5, 83–90. [Google Scholar] [CrossRef][Green Version]
- Rifi, M.; Ounii–Ben Amor, K.; Ben Souissi, J.; Zaouali, J. Première mention du crabe lessepsien Portunus segnis (Forskål, 1775) (Décapode, Brachyoure, Portunidae) dans les eaux marines Tunisiennes. In Proceedings of the du 4ème congrès Franco–Maghrébin et 5èmes journées Franco–Tunisiennes de Zoologie, Korba, Tunisie, 13–17 November 2014; p. 9. [Google Scholar]
- Rabaoui, L.; Arculeo, M.; Mansour, L.; Tlig–Zouari, S. Occurrence of the lessepsian species Portunus segnis (Crustacea: Decapoda) in the Gulf of Gabès (Tunisia): First record and new information on its biology and ecology. Cah. Biol. Mar. 2015, 56, 169–175. [Google Scholar] [CrossRef]
- Por, F.D. Lessepsian Migration. The Influx of Red Sea Biota into the Mediterranean by Way of the Suez Canal; Springer: Berlin, Germany, 1978; pp. 1–228. [Google Scholar]
- Thorson, G. Animal migrations through the Suez Canal in the past, recent years and the future (a preliminary report). Vie Milieu suppl. 1971, 22, 841–846. [Google Scholar]
- Romano, N.; Zeng, C. The effects of salinity on the survival, growth and haemolymph osmolality of early juvenile blue swimmer crabs, Portunus pelagicus. Aquaculture 2006, 260, 151–162. [Google Scholar] [CrossRef]
- Osman, M.; Madkou, F.; Sallam, W.; Mohammed, S. Seasonal occurrence and distribution of brachyuran crabs caught along the Suez Canal, Egypt. Egypt. J. Aquat. Biol. Fish. 2015, 19, 21–28. [Google Scholar] [CrossRef]
- Kowarik, I. Time lags in biological invasions with regard to the success and failure of alien species. In Plant Invasions: General Aspects and Special Problems; Pysek, P., Prach, K., Rejmánek, M., Wade, M., Eds.; SPBAcademic Publishing: Amsterdam, The Netherlands, 1995; pp. 15–38. [Google Scholar]
- Hatira, S.; Fassatoui, C.; Romdhane, M.S. Fine–scale morphological and genetic variability of the invasive species of blue swimming crab Portunus segnis (Forskål, 1775) in the Gulf of Gabes (Southeastern Tunisia). Cah. Biol. Mar. 2020, 60, 207–218. [Google Scholar]
- Van Den Avyle, J.J.; Fowler, D.L. (1984) Species Profiles: Life Histories and Environmental Requirements of Coastal Fishes and Invertebrates (South Atlantic)—Blue Crab; U.S. Fish and Wildlife Service: Washington, DC, USA, 1984; pp. 1–16. [Google Scholar]
- Mili, S.; Ennouri, R.; Ghanem, R.; Rifi, M.; Jaziri, S.; Shaiek, M.; Ben Souissi, J. Additional and unusual records of bleu crabs Portunus segnis and Callinectes sapidus from the northeastern Tunisian waters (Central Mediterranean Sea). J. New Sci. 2020, 14, 303–311. [Google Scholar]
- Lejeusne, C.; Chevaldonné, P.; Pergent–Martini, C.; Boudouresque, C.F.; Pérez, T. Climate change effects on a miniature ocean: The highly diverse, highly impacted Mediterranean Sea. Trends Ecol. Evol. 2010, 25, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Corsini–Foka, M.; Abdulghani, A.; Al Mabruk, S.A.A.; Abdulrraziq, A.A.; Ibrahim, S.M.; Scannella, D.; Zava, B.; Deidun, A.; Gianguzza, P. Invasive portunid crabs in Libyan waters: First record of the Atlantic blue crab Callinectes sapidus Rathbun, 1896 and range expansion of the swimming blue crab Portunus segnis (Forskål, 1775). BioInvasions Rec. 2021, 10, 885–893. [Google Scholar] [CrossRef]
- Safaie, M.; Pazooki, J.; Kiabi, B.; Shokri, M.R. Reproductive biology of blue swimming crab, Portunus segnis (Forskal, 1775) in coastal waters of Persian Gulf and Oman Sea, Iran. Iran J. Fish. Sci. 2013, 12, 430–444. [Google Scholar]
- Safaie, M. Feeding habits of blue swimming crab Portunus segnis (Forskal, 1775) in the northern coastal waters of Iran. Mar. Biodivers. Rec. 2016, 9, 68. [Google Scholar] [CrossRef]
- Rabaoui, L.; Yacoubi, L.; Lin, Y.J.; Joydas, T.V.; Maneja, R.H.; Dagoy, J.; Qurban, M.A.; Roa–Ureta, R.H. Distribution, abundance, and life history traits of the blue swimming crab Portunus segnis (Forskål, 1775) in the Saudi waters of the Arabian Gulf. Reg. Stud. Mar. Sci. 2021, 46, 101895. [Google Scholar] [CrossRef]
- Ben Abdallah–Ben Hadj Hamida, O.; Ben Hadj Hamida, N.; Chaouch, H.; Nafkha, B.; Ben Ali, N.; Abidi, D.; Missaoui, H. Reproductive biology of the blue swimming crab Portunus segnis (Forskål, 1775) (Brachyura: Portunidae) in the Gulf of Gabes (southeastern Tunisia, central Mediterranean Sea). Afr. J. Mar. Sci. 2022, 44, 11–20. [Google Scholar] [CrossRef]
- Pancucci–Papadopoulou, M.A.; Raitsos, D.E.; Corsini–Foka, M. Biological invasions and climatic warming: Implications for south–eastern Aegean ecosystem functioning. J. Mar. Biol. Assoc. U.K. 2012, 92, 777–789. [Google Scholar] [CrossRef]
- Zainal, K.; Noorani, A. Temperature dependence of the heart rates in the blue swimming crab Portunus segnis (Forskal, 1775). Arab. J. Sci. Eng. 2019, 44, 6259–6265. [Google Scholar] [CrossRef]
- Ikhwanuddin, M.; Hayimad, T.; Ghazali, A.; Abdul Halim, S.; Abdullah, S. Resistance test at early larval stage of blue swimming crab, Portunus pelagicus. Songklanakarin J. Sci. Technol. 2016, 38, 83–90. [Google Scholar]
- Crocetta, F. First record of Portunus pelagicus (Linnaeus, 1758) (Decapoda, Brachyura, Portunidae) in the northern Tyrrhenian Sea. Crustaceana 2006, 79, 1145–1148. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Özcan, T. The swimming crab Portunus segnis (Forskål, 1775): Host for the barnacle Chelonibia platula (Ranzani, 1818) from the Turkish coast. J. Black Sea Mediterr. Environ. 2012, 18, 271–278. [Google Scholar]
- Ben Abdallah–Ben Hadj Hamida, O.; Ben Hadj Hamida, N.; Ammar, R.; Chaouch, H.; Missaoui, H. Feeding habits of the swimming blue crab Portunus segnis (Forskål, 1775) (Brachyura: Portunidae) in the Mediterranean. J. Mar. Biol. Assoc. U.K. 2019, 99, 1343–1351. [Google Scholar] [CrossRef]
- Liquete, C.; Piroddi, C.; Drakou, E.G.; Gurney, L.; Katsanevakis, S.; Charef, A.; Egoh, B. Current status and future prospects for the assessment of marine and coastal ecosystem services: A systematic review. PLoS ONE 2013, 8, e67737. [Google Scholar] [CrossRef] [PubMed]
- Ozgul, A.; Akyol, O. Occurrence of a Lessepsian Swimming Crab, Portunus segnis (Crustacea: Decapoda), in Southern Aegean Sea, Turkey. Ann.Ser. Hist. Nat. 2019, 29, 43–47. [Google Scholar] [CrossRef]
- Tsirintanis, K.; Azzurro, E.; Crocetta, F.; Dimiza, M.; Froglia, C.; Gerovasileiou, V.; Langeneck, J.; Mancinelli, G.; Rosso, A.; Stern, N.; et al. Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat. Invasions 2022, 17, 308–352. [Google Scholar] [CrossRef]
- Di Leonardo, R.; Mazzola, A.; Tramati, C.D.; Vaccaro, A.; Vizzini, S. Highly contaminated areas as sources of pollution for adjoining ecosystems: The case of Augusta Bay (Central Mediterranean). Mar. Poll. Bull. 2014, 89, 417–426. [Google Scholar] [CrossRef]
- Rotter, A.; Klun, K.; Francé, J.; Mozetič, P.; Orlando–Bonaca, M. Non–indigenous species in the Mediterranean Sea: Turning from pest to source by developing the 8Rs model, a new paradigm in pollution mitigation. Front. Mar. Sci. 2020, 7, 1–16. [Google Scholar] [CrossRef]
- Hoag, H. Bounty hunters. Nature 2014, 513, 294–295. [Google Scholar] [CrossRef]
- Kleitou, P.; Rees, S.; Cecconi, F.; Kletou, D.; Savva, I.; Cai, L.L.; Hall–Spencer, J.M. Regular monitoring and targeted removals can control lionfish in Mediterranean Marine Protected Areas. Aquat. Conserv. 2021, 31, 2870–2882. [Google Scholar] [CrossRef]
- Van Engel, W.A. The blue crab and its fishery in Chesapeake Bay Part 2—Types of gear for hard crab fishing. Commer. Fish. Rev. 1962, 24, 1–10. [Google Scholar]
- Hamdi, M.; Hajji, S.; Affes, S.; Taktak, W.; Maâlej, H.; Nasri, M.; Nasri, R. Development of a controlled bioconversion process for the recovery of chitosan from blue crab (Portunus segnis) exoskeleton. Food Hydrocoll. 2018, 77, 534–548. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and chitosans: Characteristics, eco–friendly processes, and applications in cosmetic science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, G.; Chainho, P.; Cilenti, L.; Falco, S.; Kapiris, K.; Katselis, G.; Ribeiro, F. The Atlantic blue crab Callinectes sapidus in southern European coastal waters: Distribution, impact and prospective invasion management strategies. Mar. Poll. Bull. 2017, 119, 5–11. [Google Scholar] [CrossRef] [PubMed]

| Analysis/Indicator Name | Tools | Spatial Scale | Time Scale | Ecological Meaning |
|---|---|---|---|---|
| Temporal and Spatial–Temporal Pattern | ||||
| Population increase | Cumulative curve of occurrence | Global | All years | Occurrences increasing over time |
| Population’s increasing rate | Evaluation of the slopes of the cumulative curve by Least Squares Method | Global | 1886–1923 1924–2001 2002–2021 | The rate of specimens’ increasing in time |
| Density hotspots | Kernel density | Global | 1886–1923 1886–1950 1886–1975 1886–2001 1886–2005 1886–2010 1886–2015 1886–2021 | Highest density areas; nuclei of record aggregation; occurrence persistent areas; space–time occurrences density increase. |
| Aggregation patterns and spatial structure | ||||
| Global Spatial Autocorrelation | Global Moran’s I (GMI) cutoff distance = 250 km | Global | All years | Distribution pattern: dispersion vs. random vs. clustering. Change in the spatial pattern over time |
| Statistically significant hot spots and cold spots | Getis—Ord Gi* (GOG*) Hot spot analysis cutoff distance = 250 km | Local | All years | Direction of spread and identification of dispersion/settle areas |
| Spatial outliers | Anselin local Moran’s I (AMI) Cluster and outlier analysis search threshold = 250 km | Local | All years | Direction of spread and identification of dispersion/settle areas and outliers |
| Key characteristics of distribution | ||||
| Centre of gravity | Central tendency (mean centre—median centre) | Global | 1886–1937 1950–1978 1987–2021 | Species concentration centre and its changing over time |
| Directional Dispersion | Standard deviational ellipse (1 standard deviation) | Global | 1886–1937 1950–1978 1987–2021 | Species distribution in X and Y directions |
| Directional Trends | Standard deviational ellipse (1 standard deviation) | Global | 1886–1937 1950–1978 1987–2021 | Directional trend of species dispersion |
| Indicator | Method | Unit | 1886–1937 | 1950–1978 | 1987–2021 |
|---|---|---|---|---|---|
| Central tendency | Mean centre | Longitude (DD) Latitude (DD) | 32.62 31.98 | 27.46 35.13 | 22.97 34.79 |
| Central tendency | Median centre | Longitude (DD) Latitude (DD) | 32.39 31.06 | 31.33 33.58 | 25.75 34.93 |
| Directional dispersion | Standard deviational ellipse | XStdDist (km) YStdDist (km) | 219 429 | 366 1405 | 1178 381 |
| Directional trends | Standard deviational ellipse | Rotation (°) | 33 | 99 | 86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castriota, L.; Falautano, M.; Maggio, T.; Perzia, P. The Blue Swimming Crab Portunus segnis in the Mediterranean Sea: Invasion Paths, Impacts and Management Measures. Biology 2022, 11, 1473. https://doi.org/10.3390/biology11101473
Castriota L, Falautano M, Maggio T, Perzia P. The Blue Swimming Crab Portunus segnis in the Mediterranean Sea: Invasion Paths, Impacts and Management Measures. Biology. 2022; 11(10):1473. https://doi.org/10.3390/biology11101473
Chicago/Turabian StyleCastriota, Luca, Manuela Falautano, Teresa Maggio, and Patrizia Perzia. 2022. "The Blue Swimming Crab Portunus segnis in the Mediterranean Sea: Invasion Paths, Impacts and Management Measures" Biology 11, no. 10: 1473. https://doi.org/10.3390/biology11101473
APA StyleCastriota, L., Falautano, M., Maggio, T., & Perzia, P. (2022). The Blue Swimming Crab Portunus segnis in the Mediterranean Sea: Invasion Paths, Impacts and Management Measures. Biology, 11(10), 1473. https://doi.org/10.3390/biology11101473






