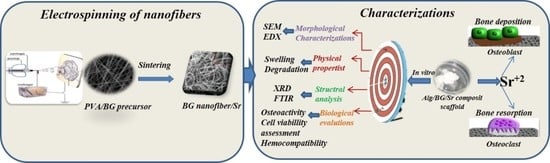
Nanofibrous Hydrogel Nanocomposite Based on Strontium-Doped Bioglass Nanofibers for Bone Tissue Engineering Applications
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bioglass Nanofibers and Sr-Doped Bioglass Nanofibers
2.2.1. Fabrication of Precursor Solutions of Electrospinning
2.2.2. Electrospinning Process
2.3. Fabrication of 3D Nanocomposite Scaffolds
2.4. Characterization Techniques
2.4.1. Morphological Observation
2.4.2. Crystallinity Assessment
2.4.3. Specific Functional Group Identification
2.4.4. Swelling Kinetics
2.4.5. Weight Loss Measurement
2.5. Biological Evaluations
2.5.1. Osteoactivity Investigation
2.5.2. Hemocompatibility Evaluation
2.5.3. Cell Viability Assessment
2.6. Statistical Analysis
3. Results
3.1. Characterizations
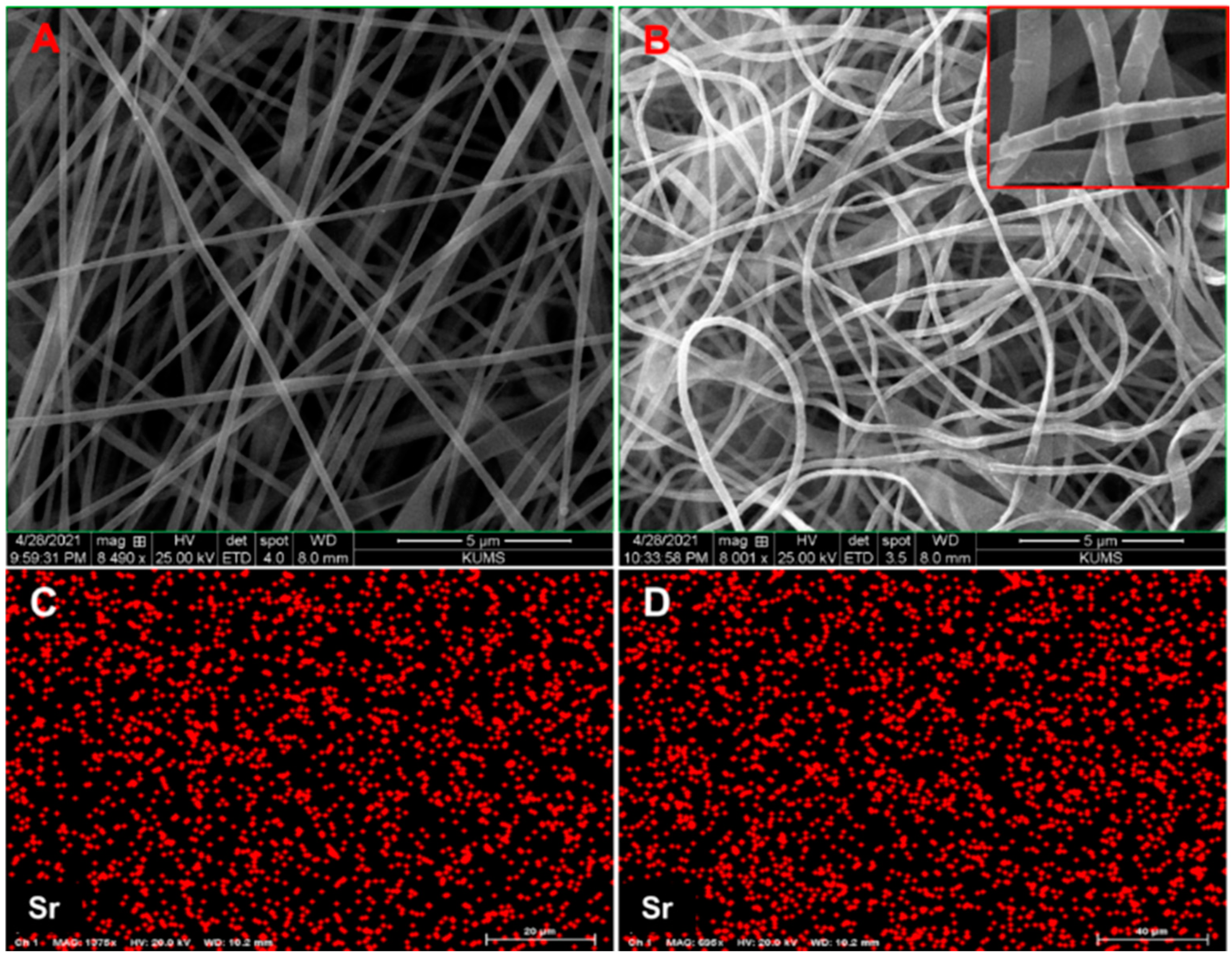
3.1.1. Morphology and Composition of Nanofibers and Nanocomposites
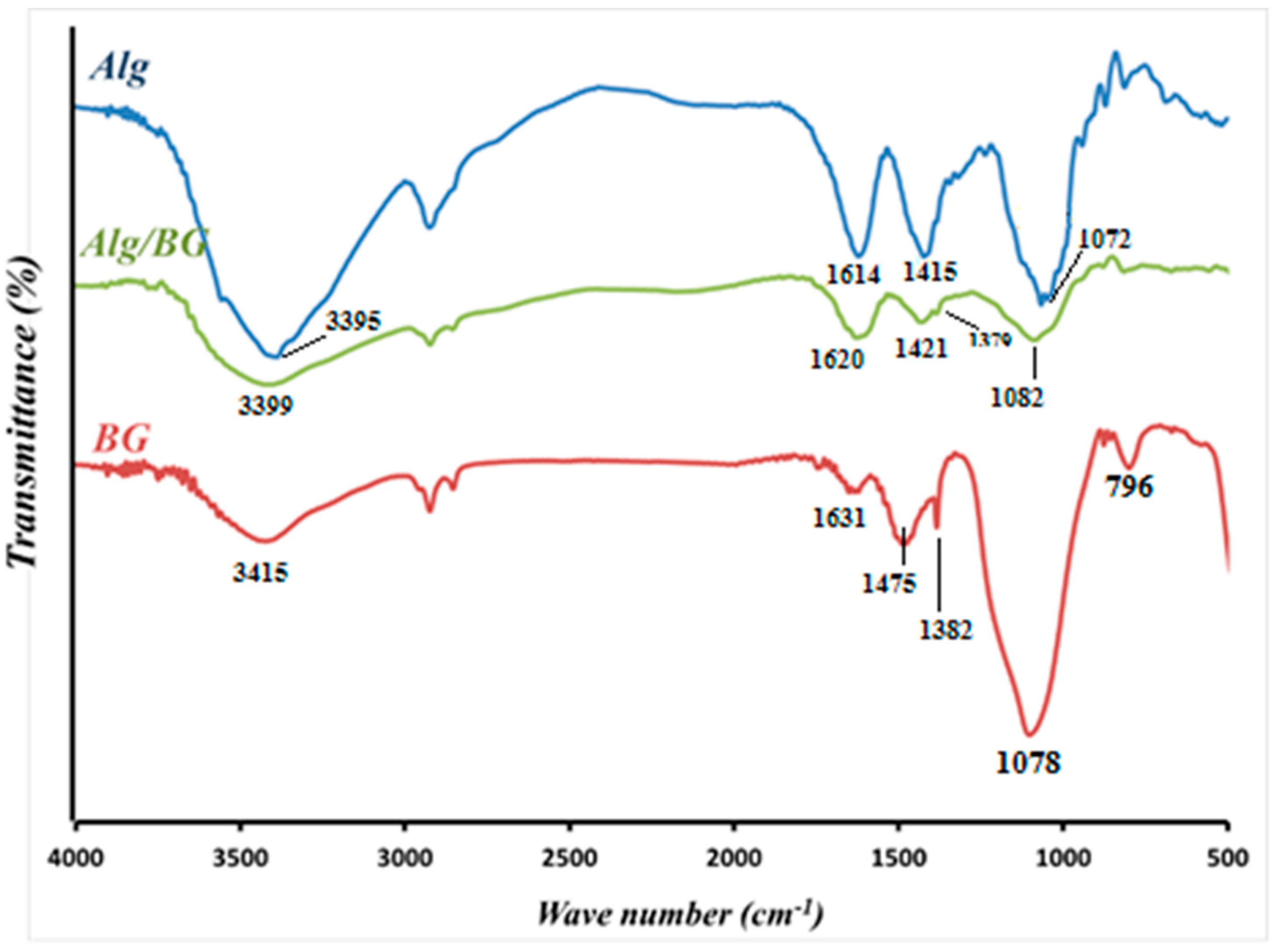
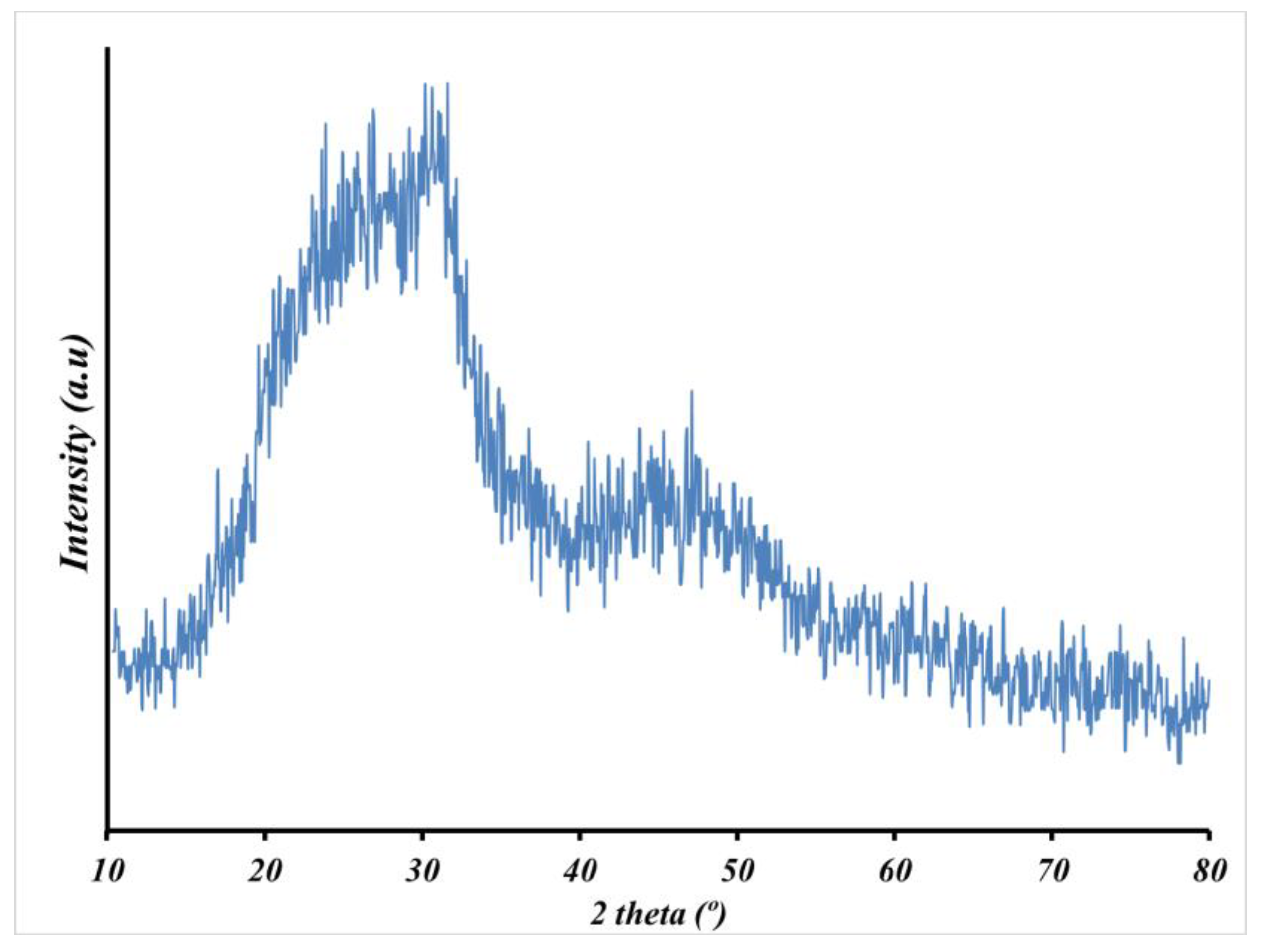
3.1.2. FTIR and XRD
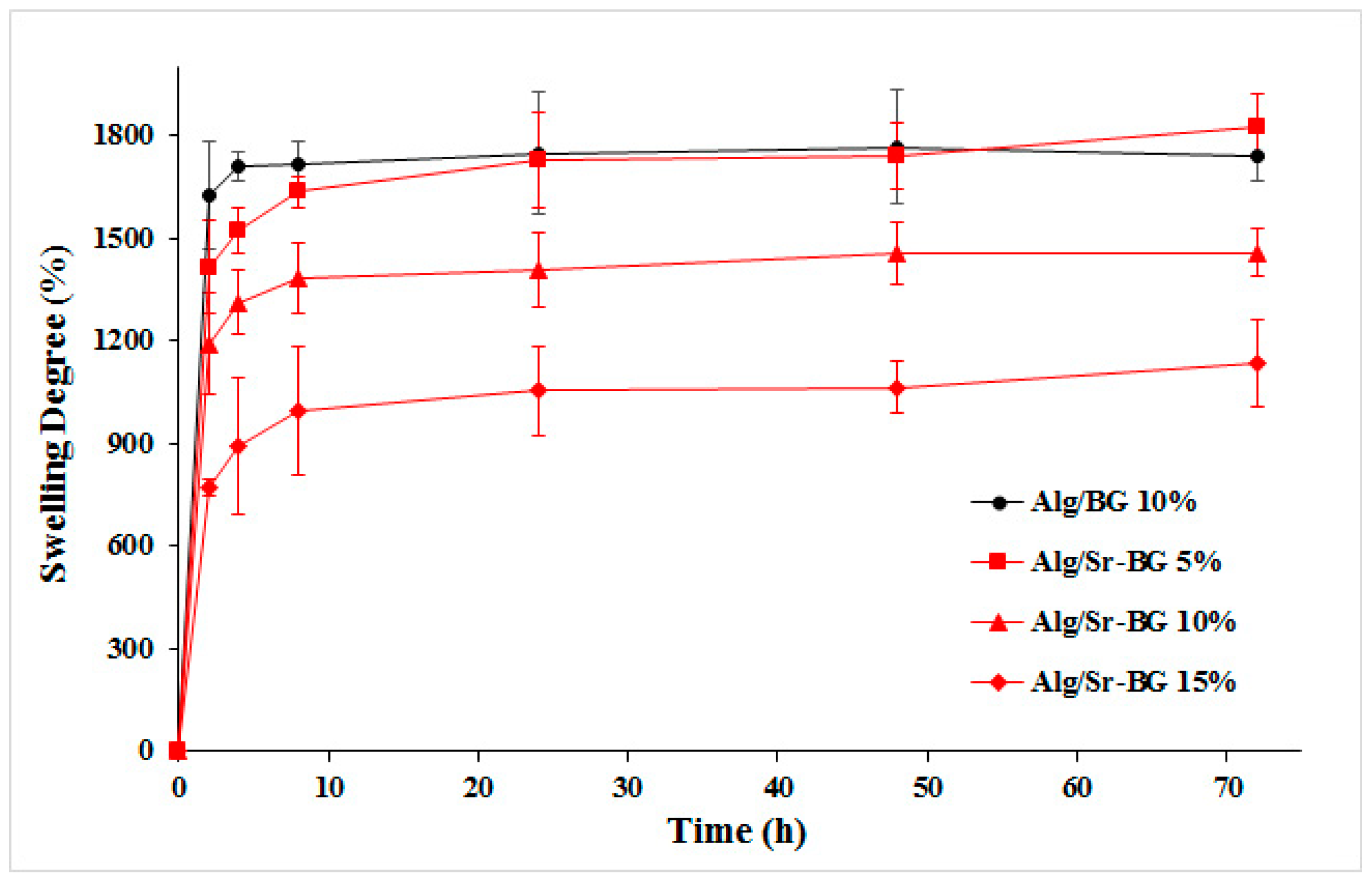
3.1.3. Swelling Kinetics
3.1.4. Biodegradation Profile
3.2. Biological Evaluation Findings
3.2.1. Osteoactivity Profile
3.2.2. Hemocompatibility Results
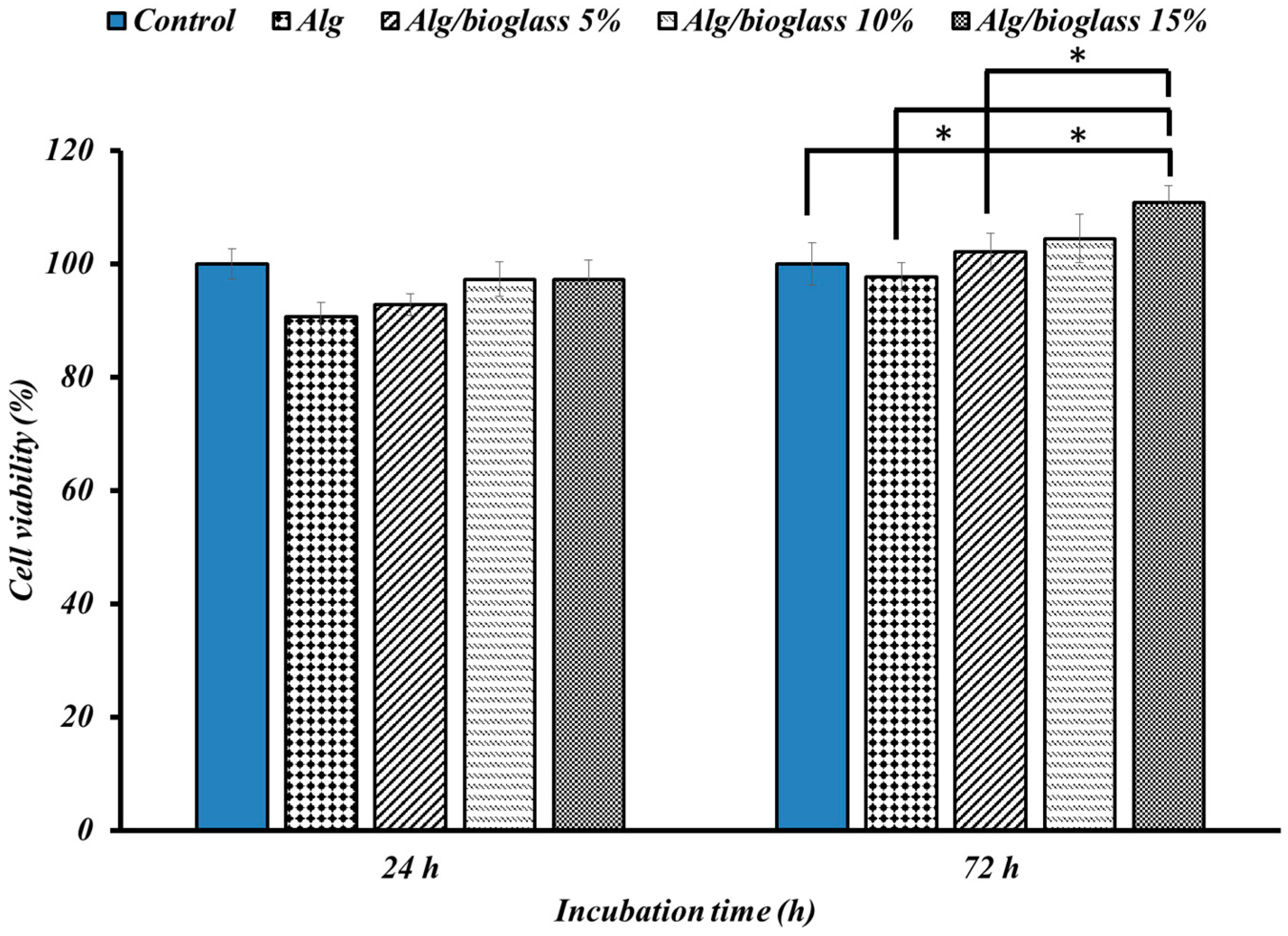
3.2.3. Cell Viability Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guerado, E.; Caso, E. Challenges of bone tissue engineering in orthopaedic patients. World J. Orthop. 2017, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Smrke, D.; Rožman, P.; Veselko, M.; Gubina, B. Treatment of bone defects—Allogenic platelet gel and autologous bone technique. In Regenerative Medicine and Tissue Engineering; IntechOpen: London, UK, 2013. [Google Scholar]
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-size bone defects: Is there a consensus for diagnosis and treatment? J. Orthop. Trauma 2018, 32, S7–S11. [Google Scholar] [CrossRef]
- Mohammadpour, M.; Samadian, H.; Moradi, N.; Izadi, Z.; Eftekhari, M.; Hamidi, M.; Shavandi, A.; Quéro, A.; Petit, E.; Delattre, C. Fabrication and Characterization of Nanocomposite Hydrogel Based on Alginate/Nano-Hydroxyapatite Loaded with Linum usitatissimum Extract as a Bone Tissue Engineering Scaffold. Mar. Drugs 2021, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Abbasian, M.; Massoumi, B.; Mohammad-Rezaei, R.; Samadian, H.; Jaymand, M. Scaffolding polymeric biomaterials: Are naturally occurring biological macromolecules more appropriate for tissue engineering? Int. J. Biol. Macromol. 2019, 134, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Mobasheri, H.; Azami, M.; Faridi-Majidi, R. Osteoconductive and electroactive carbon nanofibers/hydroxyapatite nanocomposite tailored for bone tissue engineering: In vitro and in vivo studies. Sci. Rep. 2020, 10, 14853. [Google Scholar] [CrossRef] [PubMed]
- Nekounam, H.; Kandi, M.R.; Shaterabadi, D.; Samadian, H.; Mahmoodi, N.; Hasanzadeh, E.; Faridi-Majidi, R. Silica nanoparticles-incorporated carbon nanofibers as bioactive biomaterial for bone tissue engineering. Diam. Relat. Mater. 2021, 115, 108320. [Google Scholar] [CrossRef]
- Gholivand, K.; Ardebili, S.A.A.; Mohammadpour, M.; Malekshah, R.E.; Hasannia, S.; Onagh, B. Preparation and examination of a scaffold based on hydroxylated polyphosphazene for tissue engineering: In vitro and in vivo studies. J. Appl. Polym. Sci. 2022, 139, 52179. [Google Scholar] [CrossRef]
- Ramdhan, T.; Ching, S.H.; Prakash, S.; Bhandari, B. Physical and mechanical properties of alginate based composite gels. Trends Food Sci. Technol. 2020, 106, 150–159. [Google Scholar] [CrossRef]
- Llorens-Gámez, M.; Salesa, B.; Serrano-Aroca, Á. Physical and biological properties of alginate/carbon nanofibers hydrogel films. Int. J. Biol. Macromol. 2020, 151, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Guastaferro, M.; Reverchon, E.; Baldino, L. Polysaccharide-based aerogel production for biomedical applications: A comparative review. Materials 2021, 14, 1631. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-H.; Li, B.; Guelcher, S.A. Gel microstructure regulates proliferation and differentiation of MC3T3-E1 cells encapsulated in alginate beads. Acta Biomater. 2012, 8, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wu, Z.; Zhao, H.; Cui, H.; Shen, J.; Chang, J.; Li, H.; He, Y. Bioactive injectable hydrogels containing desferrioxamine and bioglass for diabetic wound healing. ACS Appl. Mater. Interfaces 2018, 10, 30103–30114. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, K.M.; Mabrouk, M.; Soliman, I.E.; Beherei, H.H.; Aboelnasr, M.A. Novel alginate/hydroxyethyl cellulose/hydroxyapatite composite scaffold for bone regeneration: In vitro cell viability and proliferation of human mesenchymal stem cells. Int. J. Biol. Macromol. 2018, 112, 448–460. [Google Scholar] [CrossRef]
- Arepalli, S.K.; Tripathi, H.; Hira, S.K.; Manna, P.P.; Pyare, R.; Singh, S. Enhanced bioactivity, biocompatibility and mechanical behavior of strontium substituted bioactive glasses. Mater. Sci. Eng. C 2016, 69, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, E.; Fredholm, Y.C.; Jell, G.; Lotfibakhshaiesh, N.; O’Donnell, M.D.; Hill, R.G.; Stevens, M.M. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitro. Biomaterials 2010, 31, 3949–3956. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Närhi, T.O.; Hupa, L. Fiber glass–bioactive glass composite for bone replacing and bone anchoring implants. Dent. Mater. 2015, 31, 371–381. [Google Scholar] [CrossRef]
- Weng, L.; Boda, S.K.; Teusink, M.J.; Shuler, F.D.; Li, X.; Xie, J. Binary doping of strontium and copper enhancing osteogenesis and angiogenesis of bioactive glass nanofibers while suppressing osteoclast activity. ACS Appl. Mater. Interfaces 2017, 9, 24484–24496. [Google Scholar] [CrossRef]
- Fredholm, Y.C.; Karpukhina, N.; Brauer, D.S.; Jones, J.R.; Law, R.V.; Hill, R.G. Influence of strontium for calcium substitution in bioactive glasses on degradation, ion release and apatite formation. J. R. Soc. Interface 2012, 9, 880–889. [Google Scholar] [CrossRef]
- Gorustovich, A.A.; Steimetz, T.; Cabrini, R.L.; Porto López, J.M. Osteoconductivity of strontium-doped bioactive glass particles: A histomorphometric study in rats. J. Biomed. Mater. Res. Part A 2010, 92, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Durgalakshmi, D.; Balakumar, S. Phase separation induced shell thickness variations in electrospun hollow Bioglass 45S5 fiber mats for drug delivery applications. Phys. Chem. Chem. Phys. 2015, 17, 15316–15323. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kim, H.E.; Knowles, J.C. Production and potential of bioactive glass nanofibers as a next-generation biomaterial. Adv. Funct. Mater. 2006, 16, 1529–1535. [Google Scholar] [CrossRef]
- Nagrath, M.; Alhalawani, A.; Yazdi, A.R.; Towler, M.R. Bioactive glass fiber fabrication via a combination of sol-gel process with electro-spinning technique. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 521–538. [Google Scholar] [CrossRef]
- Samadian, H.; Mobasheri, H.; Hasanpour, S.; Faridi-Majid, R. Needleless electrospinning system, an efficient platform to fabricate carbon nanofibers. J. Nano Res. 2017, 50, 78–89. [Google Scholar] [CrossRef]
- Oyane, A.; Kim, H.M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. Part A 2003, 65, 188–195. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Shigematsu, M.; Nagashima, Y.; Tashiro, M.; Nakamura, T.; Yamamuro, T.; Higashi, S. Apatite-and wollastonite-containg glass-ceramics for prosthetic application. Bull. Inst. Chem. Res. Kyoto Univ. 1982, 60, 260–268. [Google Scholar]
- Samadian, H.; Khastar, H.; Ehterami, A.; Salehi, M. Bioengineered 3D nanocomposite based on gold nanoparticles and gelatin nanofibers for bone regeneration: In vitro and in vivo study. Sci. Rep. 2021, 11, 13877. [Google Scholar] [CrossRef] [PubMed]
- Tamburaci, S.; Tihminlioglu, F. Biosilica incorporated 3D porous scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C 2018, 91, 274–291. [Google Scholar] [CrossRef]
- Cheung, H.-Y.; Lau, K.-T.; Lu, T.-P.; Hui, D. A critical review on polymer-based bio-engineered materials for scaffold development. Compos. Part B Eng. 2007, 38, 291–300. [Google Scholar] [CrossRef]
- Baysal, K.; Aroguz, A.Z.; Adiguzel, Z.; Baysal, B.M. Chitosan/alginate crosslinked hydrogels: Preparation, characterization and application for cell growth purposes. Int. J. Biol. Macromol. 2013, 59, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Waseem, A.; Karpukhina, N.; Mohsin, S. Strontium-and zinc-containing bioactive glass and alginates scaffolds. Bioengineering 2020, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, M.; Safarian, S.; Khorasani, M.T.; Osman, N.A.A. Polyurethane/58S bioglass nanofibers: Synthesis, characterization, and in vitro evaluation. RSC Adv. 2016, 6, 35815–35824. [Google Scholar] [CrossRef]
- Zheng, K.; Taccardi, N.; Beltrán, A.M.; Sui, B.; Zhou, T.; Marthala, V.R.; Hartmann, M.; Boccaccini, A.R. Timing of calcium nitrate addition affects morphology, dispersity and composition of bioactive glass nanoparticles. RSC Adv. 2016, 6, 95101–95111. [Google Scholar] [CrossRef]
- Park, H.; Guo, X.; Temenoff, J.S.; Tabata, Y.; Caplan, A.I.; Kasper, F.K.; Mikos, A.G. Effect of swelling ratio of injectable hydrogel composites on chondrogenic differentiation of encapsulated rabbit marrow mesenchymal stem cells in vitro. Biomacromolecules 2009, 10, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Nazarnezhada, S.; Abbaszadeh-Goudarzi, G.; Samadian, H.; Khaksari, M.; Ghatar, J.M.; Khastar, H.; Rezaei, N.; Mousavi, S.R.; Shirian, S.; Salehi, M. Alginate hydrogel containing hydrogen sulfide as the functional wound dressing material: In vitro and in vivo study. Int. J. Biol. Macromol. 2020, 164, 3323–3331. [Google Scholar] [CrossRef] [PubMed]
- Serafin, A.; Murphy, C.; Rubio, M.C.; Collins, M.N. Printable alginate/gelatin hydrogel reinforced with carbon nanofibers as electrically conductive scaffolds for tissue engineering. Mater. Sci. Eng. C 2021, 122, 111927. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Han, Y.; Li, H.; Chang, J. Bioglass/alginate composite hydrogel beads as cell carriers for bone regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.R.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Tariq, M.; Hasan, A. Reduced Graphene Oxide Incorporated GelMA Hydrogel Promotes Angiogenesis for Wound Healing Applications [Corrigendum]. Int. J. Nanomed. 2022, 17, 2643–2645. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Jiang, G.; Hu, X.-L.; James, T.D.; He, X.-P.; Li, Y.; Tang, T. Biodegradable macroporous scaffold with nano-crystal surface microstructure for highly effective osteogenesis and vascularization. J. Mater. Chem. B 2018, 6, 1658–1667. [Google Scholar] [CrossRef]
- Lao, J.; Nedelec, J.-M.; Jallot, E. New strontium-based bioactive glasses: Physicochemical reactivity and delivering capability of biologically active dissolution products. J. Mater. Chem. 2009, 19, 2940–2949. [Google Scholar] [CrossRef]
- Amudha, S.; Ramya, J.R.; Arul, K.T.; Deepika, A.; Sathiamurthi, P.; Mohana, B.; Asokan, K.; Dong, C.-L.; Kalkura, S.N. Enhanced mechanical and biocompatible properties of strontium ions doped mesoporous bioactive glass. Compos. Part B Eng. 2020, 196, 108099. [Google Scholar] [CrossRef]
- Ramya, J.R.; Arul, K.T.; Elayaraja, K.; Kalkura, S.N. Physicochemical and biological properties of iron and zinc ions co-doped nanocrystalline hydroxyapatite, synthesized by ultrasonication. Ceram. Int. 2014, 40, 16707–16717. [Google Scholar] [CrossRef]
- Javanmard, S.H.; Anari, J.; Kharazi, A.Z.; Vatankhah, E. In vitro hemocompatibility and cytocompatibility of a three-layered vascular scaffold fabricated by sequential electrospinning of PCL, collagen, and PLLA nanofibers. J. Biomater. Appl. 2016, 31, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Salami, M.S.; Bahrami, G.; Arkan, E.; Izadi, Z.; Miraghaee, S.; Samadian, H. Co-electrospun nanofibrous mats loaded with bitter gourd (Momordica charantia) extract as the wound dressing materials: In vitro and in vivo study. BMC Complement. Med. Ther. 2021, 21, 111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gui, Q.; Yu, W.; Liao, S.; He, Y.; Tao, X.; Yu, Y.; Wang, Y. Interfacial diffusion printing: An efficient manufacturing technique for artificial tubular grafts. ACS Biomater. Sci. Eng. 2019, 5, 6311–6318. [Google Scholar] [CrossRef]
- Wu, X.; Li, H. Incorporation of bioglass improved the mechanical stability and bioactivity of alginate/carboxymethyl chitosan hydrogel wound dressing. ACS Appl. Bio Mater. 2021, 4, 1677–1692. [Google Scholar] [CrossRef]
- Govindan, R.; Karthi, S.; Kumar, G.S.; Girija, E.K. Development of Fe3O4 integrated polymer/phosphate glass composite scaffolds for bone tissue engineering. Mater. Adv. 2020, 1, 3466–3475. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Cui, X.; Sun, G.; Hu, J.; Xu, S.; Yang, F.; Zhang, L.; Wang, X.; Tang, P. Osteogenesis effects of magnetic nanoparticles modified-porous scaffolds for the reconstruction of bone defect after bone tumor resection. Regen. Biomater. 2019, 6, 373–381. [Google Scholar] [CrossRef]
- Srinivasan, S.; Jayasree, R.; Chennazhi, K.; Nair, S.; Jayakumar, R. Biocompatible alginate/nano bioactive glass ceramic composite scaffolds for periodontal tissue regeneration. Carbohydr. Polym. 2012, 87, 274–283. [Google Scholar] [CrossRef]
- Gönen, S.Ö.; Taygun, M.E.; Küçükbayrak, S. Fabrication of bioactive glass containing nanocomposite fiber mats for bone tissue engineering applications. Compos. Struct. 2016, 138, 96–106. [Google Scholar] [CrossRef]
- Rottensteiner, U.; Sarker, B.; Heusinger, D.; Dafinova, D.; Rath, S.N.; Beier, J.P.; Kneser, U.; Horch, R.E.; Detsch, R.; Boccaccini, A.R. In vitro and in vivo biocompatibility of alginate dialdehyde/gelatin hydrogels with and without nanoscaled bioactive glass for bone tissue engineering applications. Materials 2014, 7, 1957–1974. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ma, Z.; Kuang, X.; Zhang, Q.; Li, H.; Lai, D. Sodium alginate-bioglass-encapsulated hAECs restore ovarian function in premature ovarian failure by stimulating angiogenic factor secretion. Stem Cell Res. Ther. 2021, 12, 223. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zare, S.; Mohammadpour, M.; Izadi, Z.; Ghazanfari, S.; Nadri, S.; Samadian, H. Nanofibrous Hydrogel Nanocomposite Based on Strontium-Doped Bioglass Nanofibers for Bone Tissue Engineering Applications. Biology 2022, 11, 1472. https://doi.org/10.3390/biology11101472
Zare S, Mohammadpour M, Izadi Z, Ghazanfari S, Nadri S, Samadian H. Nanofibrous Hydrogel Nanocomposite Based on Strontium-Doped Bioglass Nanofibers for Bone Tissue Engineering Applications. Biology. 2022; 11(10):1472. https://doi.org/10.3390/biology11101472
Chicago/Turabian StyleZare, Soheila, Mahnaz Mohammadpour, Zhila Izadi, Samaneh Ghazanfari, Samad Nadri, and Hadi Samadian. 2022. "Nanofibrous Hydrogel Nanocomposite Based on Strontium-Doped Bioglass Nanofibers for Bone Tissue Engineering Applications" Biology 11, no. 10: 1472. https://doi.org/10.3390/biology11101472
APA StyleZare, S., Mohammadpour, M., Izadi, Z., Ghazanfari, S., Nadri, S., & Samadian, H. (2022). Nanofibrous Hydrogel Nanocomposite Based on Strontium-Doped Bioglass Nanofibers for Bone Tissue Engineering Applications. Biology, 11(10), 1472. https://doi.org/10.3390/biology11101472